Abstract
Stereochemical control is an important issue in carbohydrate synthesis. Glycosyl donors with participating acyl protective groups on 2-O have been shown to give 1,2-trans glycosides reliably under the pre-activation based reaction condition. In this work, the effects of additives and reaction solvents on stereoselectivity were examined using donors without participating protective groups on 2-O. While several triflate salt additives did not have major effects, the amount of AgOTf was found to significantly impact the reaction outcome. Excess AgOTf led to lower stereochemical control presumably due to its coordination with the glycosyl triflate intermediate and a more SN1 like reaction pathway. In contrast, the stereoselectivity could be directed by reaction solvents, with diethyl ether favoring the formation of α glycosides and dichloromethane leading to β isomers. The trend of stereochemical dependence on reaction solvent was applicable to a variety of building blocks including the selective formation of β-mannosides.
Keywords: stereoselectivity, pre-activation based glycosylation, additives, solvent effects
1 Introduction
During the past two decades, there has been tremendous growth in the development of novel glycosylation methodologies and reagents, resulting in our much improved abilities to form the glycosidic linkages [1, 2]. However, reliable stereochemical control remains as a major issue in carbohydrate synthesis. The glycosidic bond can be linked either in 1,2-cis or 1,2-trans manner. Synthesis of oligosaccharides containing 1,2-trans glycosidic linkages is typically accomplished by the installation of an acyl protective group on 2-O position of the glycosyl donor [1]. Upon donor activation, neighboring group participation of the 2-O acyl group will direct the formation of 1,2-trans linkages with a high degree of stereo-control. By contrast, efficient formation of 1,2-cis glycosidic linkages is a more difficult task [3]. Several innovative methods have been developed, which include benzylidene protected mannosyl donor [4, 5] and mannoside intramolecular aglycon delivery [6, 7] for β formation, thioether chiral auxiliary assisted formation of disarmed α-glucosides and galactosides [8]. Despite the successes in these special cases, in general, anomeric controls still mostly rely on the anomeric effect and/or steric hindrance, which can vary depending on structures of the coupling partners and often demand individual analysis [4, 9-14].
Recently, we have developed a pre-activation based one-pot glycosylation method using thioglycosides, where multiple sequential glycosylation reactions can be performed without intermediate purification [15]. This is a powerful strategy for glyco-assembly, which has been applied to the constructions of complex oligosaccharides containing both 1,2-cis and 1,2-trans linkages [16, 17]. Using donors bearing participating acyl groups on 2-O position, 1,2-trans glycoside products can be formed reliably [16, 17]. On the other hand, the formation of 1,2-cis linkages has not been systematically evaluated. Herein, we report our results on the exploration of various factors impacting stereo-selectivity using donors without participating neighboring groups on 2-O position.
2 Experimental
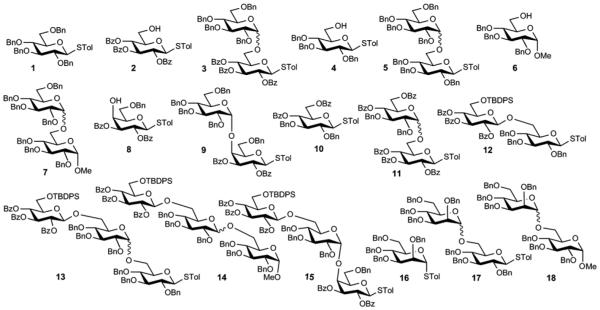
All reactions were carried out under nitrogen with anhydrous solvents in flame-dried glassware. All glycosylation reactions were performed in the presence of molecular sieves, which were flame dried right before the reaction under high vacuum. Glycosylation solvents were dried using a solvent purification system and used directly without further drying. Chemicals used were of reagent grade as supplied except where noted. Analytical thin-layer chromatography was performed using silica gel 60 F254 glass plate. Compound spots were visualized by UV light (254 nm) and by staining with a yellow solution containing Ce(NH4)2(NO3)6 (0.5 g) and (NH4)6Mo7O24·4H2O (24.0 g) in 6% H2SO4 (500 mL). Flash Column chromatography was performed on silica gel 60 (230–400 Mesh). NMR spectra were referenced using Me4Si (0 ppm), residual CHCl3 (δ1H NMR 7.24 ppm, 13C NMR 77.0 ppm). Peak and coupling constant assignments are based on 1H NMR, 1H-1H gCOSY and (or) 1H-13C gHMQC and 1H-13C gHMBC experiments. All optical rotations were measured at 25 °C using the sodium D line. ESI mass spectra were recorded in the positive ion mode. High-resolution mass spectra were recorded on a Micromass electrospray. Figure 1 shows the structures of compounds 1–18.
Figure 1.
Structures of 1-18.
2.1 Procedures for pre-activation based glycosylation
Method A
Donor (120 mg) was dissolved in Et2O (5 mL) and stirred at −78 °C with freshly activated molecular sieves MS 4 Å (100 mg) under nitrogen atmosphere for 30 min. AgOTf (1 equiv) dissolved in Et2O (1 mL) was added to the reaction mixture. After 5 min, p-TolSCl (1 equiv) was added via a microsyringe. As the reaction temperature was below the freezing point of p-TolSCl, p-TolSCl should be directly added to the reaction mixture without touching the flask wall. The characteristic yellow color of p-TolSCl dissipated within a few seconds. The donor was completely consumed after 1 min as confirmed by TLC analysis. Glycosyl acceptor (0.9 equiv) dissolved in Et2O (2 mL) was then added dropwise to the reaction mixture. The reaction was warmed up to −20 °C over 1 h under N2 at which point, the acceptor was completely consumed as confirmed by TLC. The reaction was quenched with Et3N then concentrated to dryness. The residue was diluted with CH2Cl2 (20 mL) and filtrated over Celite. The Celite was further washed with CH2Cl2 until no organic compound was present in the filtrate as determined by TLC. The fractions were combined, concentrated to dryness and the residue purified by silica gel flash chromatography.
Method B
Donor (120 mg) was dissolved in CH2Cl2 (5 mL) and stirred at −78 °C with freshly activated molecular sieves MS 4 Å (100 mg) under nitrogen atmosphere for 30 min. AgOTf (1 equiv) dissolved in acetonitrile/CH2Cl2 (v:v = 1:4, 0.5 mL) was added to the reaction mixture. After 5 min, p-TolSCl (1 equiv) was added via a microsyringe. As the reaction temperature was below the freezing point of p-TolSCl, p-TolSCl should be directed added to the reaction mixture without touching the flask wall. The characteristic yellow color of p-TolSCl dissipated within a few seconds. The donor was completely consumed after 5 min as confirmed by TLC analysis. Glycosyl acceptor (0.9 equiv) dissolved in CH2Cl2 (1 mL) was then added dropwise to the reaction mixture. The reaction was warmed up to −20 °C over 1 h under N2 at which point, the acceptor was completely consumed as confirmed by TLC. The reaction was quenched with Et3N then concentrated to dryness. This was followed by dilution with CH2Cl2 (20 mL) and filtration over Celite. The Celite was further washed with CH2Cl2 until no organic compounds was present in the filtrate. The fractions were combined then concentrated to dryness. The residue was purified by silica gel flash chromatography.
2.2 Characterization of anomeric stereochemistry
The stereochemistry of the newly formed glycosidic linkages are determined by 3JH1,H2 through 1H NMR and/or 1JC1,H1 through gHMQC 2-D NMR (without 1H decoupling). Smaller coupling constants of 3JH1,H2 (around 3 Hz) indicate α linkages and larger coupling constants 3JH1,H2 (7.2 Hz or larger) indicate β linkages for glucosides and galactosides. This can be further confirmed by 1JC1,H1 (~170 Hz) for α linkages and 1JC1,H1 (~160 Hz) for β linkages for all glycosidic linkages [32].
p-Tolyl 2,3,4-tri-O-benzyl-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-1-thio-β-d-glucopyranoside (3)
Using method A of general procedure for glycosylation, donor 1 reacted with acceptor 2 to give desired product 3 in 69% yield (α/β 6:1) after column purification (hexanes/ethyl acetate 3:1). 1H NMR (600 MHz, CDCl3) α anomer δ 2.15 (s, 3H), 3.48 (dd, 1H, J3 = 1.8, 10.8 Hz), 3.54 (dd, 1H, J3 = 3.0, 9.6 Hz), 3.62 (dd, 1H, J3 = 1.8, 10.2 Hz), 3.66 (t, 1H, J3 = 9.6 Hz), 3.71 (dd, 1H, J3 = 3.0, 10.8 Hz), 3.89 (dd, 1H, J3 = 7.8, 10.8 Hz), 3.93–3.98 (m, 2H), 4.05–4.12 (m, 1H), 4.43 (d, 1H, J3 = 12.0 Hz), 4.50 (d, 1H, J3 = 10.8 Hz), 4.58 (d, 1H, J3 = 12.0 Hz), 4.60 (d, 1H, J3 = 12.0 Hz), 4.65 (d, 1H, J3 = 3.6 Hz), 4.75 (d, 1H, J3 = 12.0 Hz), 4.79 (d, 1H, J3 = 10.8 Hz), 4.81 (d, 1H, J3 = 10.8 Hz), 4.92 (d, 1H, J3 = 10.8 Hz), 5.40 (t, 1H, J3 = 10.2 Hz), 5.42 (t, 1H, J3 = 10.2 Hz), 5.83 (t, 1H, J3 = 9.0 Hz), 6.98–7.02 (m, 2H), 7.10–7.98 (m, 37 H); 13C NMR (100.5 MHz, CDCl3) δ 21.31, 67.52, 68.63, 69.88, 70.42, 70.89, 73.62, 73.70, 74.64, 75.23, 75.98, 77.54, 77.96, 80.40, 82.23, 87.34, 97.54, 127.69, 127.78, 127.85, 128.02, 128.06, 128.12, 128.35, 128.44, 128.49, 128.62, 128.63, 128.67, 129.12, 129.58, 129.97, 130.13, 133.39, 133.49, 133.70, 133.93, 138.22, 138.48, 138.68, 138.74, 139.24, 165.32, 165.39, 166.00; HRMS C68H64NaO13S [M + Na+] calcd 1143.3965 found 1143.3999.
p-Tolyl 2,3,4-tri-O-benzyl-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-1-thio-β-d-glucopyranoside (5)
Using method A of general procedure for glycosylation, donor 1 reacted with acceptor 4 to give desired product 5 in 80% yield (α/β 5.7:1) after column purification (hexanes/ethyl acetate/CH2Cl2, 5.5:1:0.5). 1H NMR (400 MHz, CDCl3) δ α anomer 2.23 (s, 3H), 3.27 (t, 1H, J3 = 9.2 Hz), 3.42–3.92 (m, 10 H), 4.01 (t, 1H, J3 = 9.2 Hz), 4.47 (d, 1H, J3 = 12.0 Hz), 4.52 (d, 1H, J3 = 11.2 Hz), 4.58 (d, 1H, J3 = 9.6 Hz), 4.63 (d, 1H, J3 = 10.0 Hz), 4.64 (d, 1H, J3 = 12.0 Hz), 4.68 (d, 1H, J3 = 11.2 Hz), 4.76–4.95 (m, 8 H), 5.00 (d, 1H, J3 = 10.8 Hz), 5.04 (d, 1H, J3 = 3.2 Hz), 7.06–7.12 (m, 2H), 7.13–7.52 (m, 37H); β anomer 2.30 (s, 3H, SPhCH3), 3.46–3.56 (m, 4H), 3.63–3.70 (m,3H), 3.73–3.81 (m, 3H), 4.24 (dd, 1H, J3 = 1.8 Hz, 11.2 Hz,), 4.47 (d, 1H, J3 = 7.6 Hz), 4.57–4.67 (m, 4H, CH2Ph), 4.70 (d, 1H, J3 = 9.8 Hz), 4.75–5.02 (m, 10H, CH2Ph), 7.06–7.52 (m, 39H, aromatic); 13C NMR (100 MHz, CDCl3) δ α anomer 21.32, 68.79, 70.47, 72.70, 73.67, 75.18, 75.21, 75.71, 75.89, 75.92, 77.85, 77.93, 79.01, 80.42, 81.37, 82.03, 86.93, 88.73, 97.62, 127.78, 127.90, 127.93, 128.00, 128.08, 128.15, 128.23, 128.50, 128.59, 128.61, 128.65, 128.66, 128.71, 130.03, 133.15, 138.03, 138.26, 138.35, 128.45, 138.70, 138.75, 138.81, 139.14; β anomer 29.98, 60.65, 68.87, 69.18, 73.79, 75.08, 75.16, 75.63, 75.98, 76.00, 77.49, 78.13, 78.25, 79.12, 81.06, 82.48, 84.97, 86.99, 87.90, 100.05, 127.74, 127.84, 128.03, 128.20, 128.36, 128.44, 128.54, 129.94, 130.24, 130.27, 132.39, 137.66, 138.29, 138.60, 138.86. HRMS C68H70NaO10S [M + Na+] calcd. 1101.4587 found 1101.4531.
Methyl 2,3,4-tri-O-benzyl-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyranoside (7)
Using method B of general procedure for glycosylation, donor 1 reacted with acceptor 6 to give the desired product 7 in 79% yield (α/β 1:9) after column purification (hexanes/ethyl acetate 3:1). Comparison of 1H NMR spectra with literature values [33] confirmed the identity of compound 7. 1H NMR (500 MHz, CDCl3) δ 3.36 (s, 3H, OCH3), 3.44–3.47 (m, 2H), 3.51–3.71(m, 14H), 3.74 (d, 1H, J3 = 1.5 Hz), 3.76 (d, 1H, J3 = 2 Hz), 3.84–3.87 (m, 1H), 4.0–4.04 (m, 2H), 4.22 (dd, 1H, J3 = 2, 11 Hz), 4.39 (d, 1H, J3 = 8 Hz), 4.50–4.85 (m, 18H), 4.92–4.95 (m, 1H), 4.98–5.02 (m, 3H), 7.14–7.38 (m, 64H, aromatic), 7.81–7.86 (m, 6H, aromatic).
p-Tolyl 2,3,4-tri-O-benzyl-1-thio-β-d-glucopyranosyl-(1→4)-2,3-di-O-benzoyl-6-O-benzyl-1-thio-β-d-galatcopyranoside (9)
Using method A of general procedure for glycosylation, donor 1 reacted with acceptor 8 to give desired product (9) in 60 % yield as α isomer after column purification (hexanes/ethyl acetate 3:1). Comparison of 1H NMR with literature values [15] confirmed the identity of compound 9. 1H NMR (600 MHz, CDCl3) δ 2.14 (s, 3H SPhCH3), 2.88 (dd, 1H, J3 = 1.8, 11.4 Hz), 3.13 (dd, 1H, J3 = 1.8, 10.8 Hz), 3.49 (dd, 1H, J3 = 3.0, 9.6 Hz), 3.65(t, 1H, J3 = 9.6 Hz), 3.76 (m, 1H), 3.74–3.77. (m, 1H), 3.89–3.96 (m, 4H), 4.04 (d, 1H, J3 = 12 Hz, CH2Ph), 4.34–4.41 (m, 5H), 4.61 (d, 1H, J3 = 11.4 Hz, CH2Ph), 4.74–4.92 (m, 6H), 5.29 (dd, 1H, J3 = 3.0, 10.2 Hz), 5.65 (t, 1H, J3 = 10.2 Hz), 6.99–7.00 (m, 2H, aromatic), 7.10–7.14 (m, 4H, aromatic), 7.20–7.19 (m, 29H, aromatic), 7.89–7.94 (m, 4H, aromatic).
p-Tolyl 2,3-di-O-benzyl-4,6-di-O-benzoyl-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-1-thio-β-d-glucopyranoside (11)
Using method A of general procedure for glycosylation, donor 10 reacted with acceptor 2 to give the desired product 11 in 69% yield (α/β 6:1 as determined from SPhCH3 singlet and H-3 ratios) after column purification (hexanes/ethyl acetate 3:1). (c = 1.0, CH2Cl2); 1H NMR (600 MHz, CDCl3) δ 2.18 (s, 3H, SPhCH3, α), 2.27(s, SPhCH3 β), 3.56 (m, 1H), 3.72 (dd, 1H, J3 = 3.6, 9.6 Hz), 3.95–4.00 (m, 1H), 4.14–4.24 (m, 3H), 4.32–4.36 (m, 1H), 4.42 (dd, 1H, J3 = 2.4, 12 Hz), 4.64–4.68 (m, 2H), 4.74 (d, 1H, J3 = 3 Hz, H-1), 4.81 (d, 1H, J3 = 12 Hz, CH2Ph), 4.89 (d, 1H, J3 = 12 Hz, CH2Ph), 4.99 (d, 1H, J3 = 9.6 Hz, H-1), 5.38–5.45 (m, 3H), 5.84 (t, 1H, J3 = 12 Hz), 7.07–7.56 (m, 32H, aromatic), 7.77–8.04 (m, 11H, aromatic). 13C NMR (150 MHz, CDCl3), δ 21.07, 62.99,66.76, 68.01, 69.42, 70.54, 70.76, 73.62, 74.41, 75.69, 76.79, 77.0, 77.21, 79.42, 79.98, 87.07 (JC-1,H-1 = 159.07 Hz, C-1a), 97.01 (JC-1,H-1 = 169.76 Hz, C-1b) 114.77, 127.49, 127.79, 127.98, 128.13, 128.2, 128.26, 128.32, 128.37, 128.47, 128.52, 128.81, 128.89, 129.31, 129.54, 129.74, 129.78, 129.82, 129.83, 129.87, 132.44, 132.94,133.1,133.2, 133.3, 133.5, 138.06, 138.17, 138.2, 165.02, 165.08, 165.31, 165.77, 166.04. HRMS C68H60NaO15S [M + Na+] calcd 1171.3551 found 1171.3600.
p-Tolyl 2,3,4-tri-O-benzoyl-6-O-tert-butyldiphenylsilyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-1-thio-β-d-glucopyronoside (13)
Using method A of general procedure for glycosylation, donor 13 reacted with acceptor 4 to give the desired product 13 in 70 % yield exclusively as α-isomer after column purification (hexanes/ethyl acetate/CH2Cl2, 6:1:0.5). (c = 0.35, CH2Cl2); 1H NMR (600 MHz, CDCl3) δ 1.00 (s, 9H, (CH3)3CSi), 2.19 (s, 3H, SPhCH3), 3.18 (t, 1H, J3 = 9.6 Hz), 3.27–3.37 (m, 1H), 3.40–3.44 (m, 2H), 3.56–3.63 (m, 2H), 3.69–3.72 (m, 2H), 3.76–3.88 (m, 5H), 4.17 (d, 1H, J3 = 9 Hz), 4.26 (d, 1H, J3 = 11.4 Hz), 4.45 (d, 1H, J3 = 11.4 Hz), 4.50 (d, 1H, J3 = 9 Hz, H1c), 4.56 (d, 1H, J3 = 10.8 Hz), 4.59–4.64 (m, 4H), 4.70 (d, 1H, J = 8.4 Hz, H-1a), 4.71–4.90 (m, 5H), 5.01 (d, 1H, J3 = 3.6 Hz, H-1b), 5.54–5.58 (m, 2H), 5.81 (t, 1H, J3 = 10.2 Hz), 6.96–7.65 (m, 49H, aromatic), 7.67–7.71 (m, 2H, aromatic), 7.81–7.89 (m, 7H, aromatic); 13C NMR (125 MHz, CDCl3), δ 19.4, 21.3, 24.9, 26.86, 26.9, 29.9, 36.9, 63.1, 69.6, 69.9, 72.4, 72.6, 73.6, 74.8, 75.1, 75.6, 75.7, 75.8, 77.0, 77.3, 77.46, 77.5, 77.9, 78.0, 79.0, 80.3, 81.4, 81.7, 86.9, 89.0 (JC-1,H-1 = 170.04 Hz, C-1c), 97.5 (JC-1,H-1 = 170.72 Hz, C-1b), 101.5 (JC-1,H-1 = 161.68 Hz, C-1a), 127.5, 127.56, 127.6, 127.7, 127.8,127.83, 127.89, 127.92, 128.03, 128.06, 128.1, 128.43, 128.49, 128.53, 128.54, 128.58, 128.6, 128.65, 128.7, 129.2, 129.5, 129.58, 129.86, 129.89, 129.9,, 130.0,130.1, 130.5, 133.1, 133.2, 133.22, 133.28, 133.3, 133.4, 135.8, 135.9, 138.0, 138.4, 138.5, 138.7, 138.8, 138.9, 139.2, 165.16, 165.26, 166.19; HRMS C104-H108NO18SSi [M + NH4]+ calcd 1718.7135, found 1718.7137.
Methyl 2,3,4-tri-O-benzoyl-6-O-tert-butyldiphenylsilyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyronoside (14)
Compound 14 was synthesized from donor 12 and acceptor 6 in 76% yield as β isomer using method B of general procedure and purified by flash column chromatography (hexanes/ethyl acetate/CH2Cl2, 4:1:0.5). (c = 0.2, CH2Cl2); 1H NMR (500 MHz, CDCl3) δ 1.00 (s, 9H, (CH3)3CSi), 3.33 (d, 1H, J3 = 8.5 Hz), 3.35 (s, 3H, OCH3), 3.39–3.41 (m, 2H), 3.43–3.49 (m, 2H), 3.52–3.56 (m, 2H), 3.61–3.65 (m, 1H), 3.70–3.72 (m, 1H), 3.76–3.79 (m, 1H), 3.83–3.85 (m, 2H), 3.96 (t, 1H, J3 = 9.5 Hz), 4.00 (dd, 1H, J3 = 1.8, 10.8 Hz), 4.17 (d, 1H, J3 = b 7.5 Hz, H-1), 4.20 (dd, 1H J3 = 1.8, 11.4 Hz), 4.39 (d, 1H, J3 = 11 Hz, CH2Ph), 4.44 (d, 1H, J3 = 11 Hz, CH2Ph), 4 59 (d, 1H, J3 = 11 Hz, CH2Ph), 4.61 (d, 1H, J3 = 6 Hz, H-1a), 4.64 (d, 1H, J3 = 11 Hz, CH2Ph), 4.67 (d, 1H, J3 = 11 Hz, CH2Ph), 4.71 (d, 1H, J3 = 11 Hz, CH2Ph), 4.75–4.77 (m, 2H, CH2Ph), 4.82 (d, 1H, J3 = 11 Hz, CH2Ph), 4.85 (d, 1H, J3 = 8Hz, H-1c), 4.91 (d, 1H, J3 = 11 Hz, CH2Ph), 4.95 (d, 1H, J3 = 11 Hz, CH2Ph), 5.50–5.53 (m, 1H, H-2c), 5.61 (t, 1H, J3 = 9.5 Hz, H-4c), 5.78 (t, 1H, J3 = 9.5 Hz, H-3c), 7.08–7.40 (m, 49H aromatic), 7.49–7.52 (m, 1H, aromatic), 7.66–7.68 (m, 2H, aromatic), 7.79–7.81 (m, 2H, aromatic), 7.81–7.86 (m, 6H, aromatic); 13C NMR (125 MHz, CDCl3), δ 19.4, 26.9, 55.65, 55.7, 63.1, 68.1, 69.5, 69.9, 72.4, 73.5, 73.6, 74.9, 75.08, 75.1, 75.4, 75.77, 75.8, 77.0, 77.3, 77.46, 77.5, 77.9, 78.0, 79.95, 82.1, 82.2, 85.0, 98.4 (JC-1,H-1 = 168.69 Hz, C-1a), 101.7 (JC-1,H-1 = 159.14 Hz, C-1c), 103.7 (JC-1,H-1 = 159.32 Hz, C-1b), 127.68, 127.71, 127.74, 127.8, 127.9, 128.0, 128.13, 128.16, 128.38, 128.49, 128.53, 128.54, 128.56, 128.58, 128.61, 128.7, 129.2, 129.5, 129.7, 129.84, 129.88, 129.96, 130.0, 133.2, 133.31, 133.34, 133.4, 135.7, 135.9, 138.3, 138.4, 138.7, 138.8, 139.2, 165.20, 165.24, 166.1; HRMS C98H104NO19SSi [M + NH4]+ calcd 1626.6972, found 1626.6976.
p-Tolyl 2,3,4-tri-O-benzoyl-6-O-tert-butyldiphenylsilyl-β-d-glucopyranosyl-(1→6)-2,3,4-tri-O-benzyl-α-d-glucopyranosyl-(1→4)-2,3-di-O-benzoyl-6-O-benzyl-1-thio-β-d-galactopyranoside (15)
Using method A of general procedure for glycosylation, donor 12 reacted with acceptor 8 to give the desired product 15 in 74% yield exclusively as α isomer after column purification (hexanes/ethyl acetate/CH2Cl2, 6:1:0.5). (c = 0.55, CH2Cl2); 1H NMR (500 MHz, CDCl3) δ 0.94 (s, 9H, (CH3)3CSi), 2.10 (s, 3H, SPhCH3), 3.29 (dd, 1H, J3 = 3, 10.2 Hz), 3.35–3.42 (m, 2H), 3.46–3.50 (m, 1H), 3.66–3.70 (m, 1H), 3.73–3.74 (m, 2H), 3.82–3.91 (m, 4H), 4.17 (d, 1H, J3 = 11.5 Hz), 4.22 (d, 1H, J3 = 8 Hz, H-1c), 4.28–4.38 (m, 4H), 4.48 (d, 1H, J3 = 12 Hz, CH2Ph), 4.66 (d, 1H, J3 = 10.2 Hz), 4.67 (d, 1H, J3 = 9 Hz), 4.80 (d, 1H, J3 = 11 Hz), 4.82 (d, 1H, J3 = 10 Hz, H-1a), 4.90 (d, 1H, J3 = 3.5 Hz, H-1b), 5.34–5.43 (m, 2H), 5.50–5.69 (m, 3H), 6.95–7.03 (m, 4H, aromatic), 7.06–7.40 (m, 41H, aromatic), 7.44–7.51 (m, 4H aromatic), 7.64–7.65 (m, 2H, aromatic), 7.77–7.79 (m, 3H, aromatic), 7.82–7.85 (m, 4H, aromatic), 7.87–7.89 (m, 2H, aromatic); 13C NMR (125 MHz, CDCl3), δ 29.9, 26.9, 21.3 19.3, 21.3, 26.9, 29.9, 63.1, 67.8, 68.17, 68.2, 69.5, 70.5, 72.2, 73.5, 73.6, 74.1, 74.34, 74.39, 75.0, 75.34, 77.0, 77.3, 77.5, 78.5, 80.3, 81.6, 86.3 (JC-1,H-1 = 170.04 Hz, C-1b), 98.97 (JC-1,H-1 = 163.06 Hz, C-1c), 101.7 (JC-1,H-1 = 159.71Hz, C-1a), 127.55, 127.57, 127.64, 127.83, 127.84, 127.95, 127.97, 128.0, 128.02, 128.04, 128.40, 128.45, 128.48, 128.53, 128.57, 128.60, 128.64, 128.7, 129.24, 129.38, 129.6, 129.63, 129.8, 129.84, 129.88, 129.95, 129.99, 130.3, 133.0, 133.2, 133.24, 133.3, 133.34, 133.37, 133.4, 135.8,138.06, 138.1,138.7, 139.0, 139.2, 165.1, 165.2, 165.9, 166.2; HRMS C104H104NO20SSi [M + NH4]+ calcd 1746.6636 found 1746.6638.
p-Tolyl 2,3,4-tri-O-benzyl-α-d-mannopyranosyl-(1→6)-2,3,4-tri-O-benzoyl-1-thio-β-d-glucopyranoside (17)
Using method A of general procedure for glycosylation, donor 16 reacted with acceptor 4 to give the desired product 17 in 61% yield after column purification (hexanes/ethyl acetate/CH2Cl2, 5.5:1:0.5). (c = 0.15, CH2Cl2); 1H NMR (600 MHz, CDCl3) δ 2.20 (s, 3H, SPhCH3), 3.36–3.46 (m, 3H), 3.65–3.69 (m, 2H), 3.71–3.74 (m, 2H), 3.77–3.80 (m, 1H), 3.84–3.89 (m, 3H), 4.04 (t, 1H, J3 = 9.6 Hz), 4.47–4.53 (m, 3H), 4.58 (dd, 1H, J3 = 1.8, 10.2 Hz), 4.61–4.62 (m, 2H), 4.66 (d, 1H, J3 = 12 Hz), 4.73–4.84 (m, 5H), 4.89–4.95 (m, 3H), 5.05(s, 1H), 7.01–7.03 (m, 2H, aromatic), 7.14–7.20 (m, 2H, aromatic), 7.23–7.35 (m, 29H, aromatic), 7.41–7.43 (m, 6H, aromatic); 13C NMR (125 MHz, CDCl3), δ 21.3, 29.9, 66.6, 69.4, 72.2, 72.6, 73.5, 74.8, 75.1, 75.23, 75.3, 75.7, 76.1, 77.0, 77.3, 77.5, 77.8, 78.6, 79.9, 81.3, 86.9, 88.3 (JC-1,H-1 = 169.9 Hz, C-1b), 98.8 (JC-1,H-1 = 157.7 Hz, C-1a), 127.96, 127.63, 127.78, 127.81, 127.88, 127.9, 127.93, 127.95, 127.99, 128.01, 128.04, 128.05, 128.43, 128.45, 128.48, 128.49, 128.57, 128.60, 128.63, 128.64, 128.67, 128.69, 128.7, 129.89, 129.96, 130.1, 131.96, 133.1, 138.1, 138.27, 138.59, 138.62, 138.67, 138.95; HRMS C68H74NO10S [M + NH4]+ calcd 1096.5033 found 1096.5031.
Methyl 2,3,4-tri-O-benzyl-α-d-mannopyranosyl-(1→6)-2,3,4-tri-O-benzyl-1-methyl-α-d-glucopyranoside (18)
Using method A of general procedure for glycosylation, donor 16 reacted with acceptor 6 to give the desired product 18 in 79% yield after column purification (hexanes/ethyl acetate 3:1). α/β = 1:3 as determined by integration of OCH3 peaks and JC1-H1 coupling constants. Comparison with literature data confirmed its identity [34]. 1H NMR (600 MHz, CDCl3) δ 3.30 (s,OCH3-α), 3.31 (s, 3H, OCH3-β), 3.36–3.46 (m, 6H), 3.48 (dd, 1H, J3 = 3.6, 9.6 Hz), 3.59–3.87 (m, 10H), 3.96–4.02 (m, 2H), 4.11 (s, 1H), 4.13 (d, 1H, J3 = 10 Hz, H-1), 4.19–4.71 (m, 15H), 4.76–4.88 (m, 8H), 4.91(d, 1H, J3 = 12 Hz), 4.46 (d, 1H, J3 = 3 Hz, H-1), 4.51 (d, 1H, J3 = 12 Hz), 7.06–7.40 (m, 49H, aromatic).
3 Results
Our investigation started from the per-benzylated glucoside 1. As ethereal solvents can enhance α selectivity [19–23], we first performed the glycosylation in diethyl ether. Donor 1 dissolved in diethyl ether was pre-activated by 1 equiv of p-TolSOTf, formed in situ through the stoichiometric reaction of p-TolSCl [15] with AgOTf. 3 equiv of AgOTf was typically added as a standard protocol [16, 17, 24]. Upon the rapid complete activation of 1 within 1 min as judged by TLC, acceptor 2 was added, which led to the formation of disaccharide 3 with close to equal amounts of α and β anomers (α:β = 1.1:1) in 67% total yield (Table 1, entry 1). While 10 equiv of AgOTf produced more β anomer (α:β = 1:1.5) (Table 1, entry 2), interestingly, decreasing the amount of AgOTf to 1.1 equiv significantly improved α selectivity (α:β = 6:1) (Table 1, entry 3). The α selectivity could be further enhanced by performing the reaction under a more dilute condition. Increasing the volume of diethyl ether by 10 folds under otherwise identical conditions produced disaccharide 3 in a 10:1 α:β ratio (Table 1, entry 4).
Table 1.
Evaluation of stereoselectivity using donors with no 2-O acyl groups
| Entry | Donor | Acceptor | AgOTf | Reaction condition | Pdt | Yield % (α:β) |
|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 3 | a | 3 | 67 (1.1:1) |
| 2 | 1 | 2 | 10 | a | 3 | 66 (1:1.5) |
| 3 | 1 | 2 | 1.1 | a | 3 | 69 (6:1) |
| 4 | 1 | 2 | 1.1 | a* | 3 | 55 (10:1) |
| 5 | 1 | 2 | 3 | b | 3 | 92 (1:1.8) |
| 6 | 1 | 2 | 1.1 | b | 3 | 90 (1:8) |
| 7 | 1 | 4 | 1.1 | a | 5 | 80 (5.7:1) |
| 8 | 1 | 4 | 1.1 | b | 5 | 71 (1:1.7) |
| 9 | 1 | 6 | 1.1 | a | 7 | 86 (2:1) |
| 10 | 1 | 6 | 1.1 | b | 7 | 79 (1:9.4) |
| 11 | 1 | 8 | 1.1 | a | 9 | 69 (α only) |
| 12 | 10 | 2 | 1.1 | a | 11 | 69 (6:1) |
| 13 | 10 | 2 | 1.1 | b | 11 | 56 (1:1.2) |
| 14 | 12 | 4 | 1.1 | a | 13 | 70 (α only) |
| 15 | 12 | 4 | 1.1 | b | 13 | 90 (1:1.2) |
| 16 | 12 | 6 | 1.1 | a | 14 | 65 (2:1) |
| 17 | 12 | 6 | 1.1 | b | 14 | 92 (β only) |
| 18 | 12 | 6 | 1.1 | b** | 14 | 90 (β only) |
| 19 | 12 | 8 | 1.1 | a | 15 | 74 (α only |
| 20 | 16 | 4 | 1.1 | a | 17 | 61 (α only) |
| 21 | 16 | 6 | 1.1 | a | 18 | 58 (2:1) |
| 22 | 16 | 6 | 1.1 | b | 18 | 71 (1:3) |
a) Reaction was performed in diethyl ether (donor concentration was 50 mM). b) Reaction was performed in dichloromethane (donor concentration was 50 mM).
Donor concentration is 5 mM.
Toluene (5% of final volume) was added to the reaction to dissolve AgOTf.
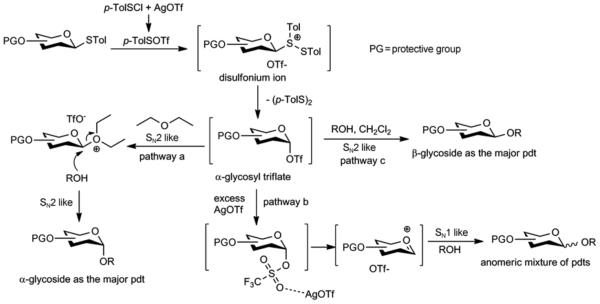
As α selectivity was obtained, it would be desirable that from the same glycosyl donor and acceptor, simple changes of the reaction condition can lead to a switch to β products. Following formation of p-TolSOTf, the thioglycosyl donor is first converted to a disulfonium ion (Figure 2), which can evolve into other intermediates such as glycosyl triflate. Crich and coworkers have reported that with benzylidene protected thio-glycosides including glucosides without 2-O acyl groups, the α glycosyl triflates were the predominant intermediates following pre-activation as observed by low temperature NMR studies [25, 26]. Similarly, in our low temperature NMR studies, we did not observe the presence of the glycosyl disulfonium ion [27], suggesting transient nature of this species. As the most likely intermediate is the glycosyl triflate and the stereochemical outcome in glycoside formation is dependent upon the balance between glycosyl triflates and the oxacarbenium ion [28], we envision that addition of exogenous triflate ion could potentially shift the equilibrium towards glycosyl triflate. This would favor the formation of the β glycoside product through a SN2 like reaction pathway, which could be supported by our observation that excess AgOTf led to more β products (Table 1, entry 2). To test this hypothesis, we explored the effects of triflate salts on stereoselectivity. The reaction between 1 and 2 was performed with 1.1 equiv of AgOTf in the presence of up to 10 equiv of triflate salts including NaOTf, Hf(OTf)4 and the more organic solvent soluble tetrabutylammonium triflate. However, none of these salts affected the stereoselectivity, which ruled out that the additional triflate anion could significantly influence the reaction pathway.
Figure 2.
Proposed mechanism of the effects of solvents and excess AgOTf on stereoselectivity.
Next, we tested the reactions in a variety of solvents including dichloromethane, cyclopentyl methyl ether, THF, toluene, toluene/1,4-dioxane [23] and acetonitrile. THF, toluene, toluene/1,4-dioxane and acetonitrile did not lead to productive coupling. Cyclopentyl methyl ether has been reported to improve cis selectivity [19]. However, in our reaction, it gave similar results as diethyl ether. Interestingly, a selectivity shift was observed when the reaction between 1 and 2 was performed in dichloromethane with 3 equiv of AgOTf, which gave disaccharide 3 with 92% total yield with the β anomer becoming the major product (α:β = 1:1.8) (Table 1, entry 5). Decreasing the amount of AgOTf to 1.1 eq greatly enhanced the β selectivity (α:β = 1:8) (Table 1, entry 6). Therefore, the stereochemical outcome of the reaction can be controlled by simply switching the reaction solvent, with diethyl ether favoring α glycoside and dichloromethane generating more β product.
With the stereoselective reaction conditions in hand, we examined their generality. Pre-activation of donor 1 ethyl ether by 1 equiv of p-TolSCl and 1.1 equiv of AgOTf followed by addition of the electron rich glucoside acceptor 4 gave disaccharide 5 in 90% yield with the α anomer as the major isomer (α:β = 5.7:1) (Table 1, entry 7). Exchanging the reaction solvent to dichloromethane produced the β isomer as the major product (α:β = 1:1.7) (Table 1, entry 8). The same trend held for a variety of building blocks, including glucoside acceptor 6 without the STol aglycon, galactoside acceptor 8 with a secondary hydroxyl group, electron poor glucosyl donor 10 and disaccharide donor 12 (Table 1, entries 7–19). β-Mannoside formation is a challenging problem for carbohydrate synthesis. The excellent methodology developed by Crich and coworkers for stereo-selective β-mannoside formation required the installation of a benzylidene moiety on the mannosyl donor [4]. It is noteworthy that under the β selective reaction condition, the per-benzylated electron rich mannosyl donor 16 without the benzylidene glycosylated glucoside 6 in dichloromethane forming β-mannoside 18 as the major product (Table 1, entry 22).
4 Discussion
As the glycosyl triflate is a likely intermediate formed after pre-activation, when the reaction is performed in diethyl ether, it is possible that it goes through a double inversion mechanism (Figure 2, pathway a). The diethyl ether can act as a nucleophile, displacing the triflate in an SN2 like fashion from the β-face. Subsequent SN2 like displacement of the ether molecule by the nucleophilic acceptor can lead to α glycoside as the major product. Under a dilute condition, the larger amount of diethyl ether can participate more effectively thus resulting in higher α selectivity. In the presence of excess AgOTf, it is most likely that AgOTf coordinates with the oxygen atom of the triflate, leading to its activation and glycosylation through a more SN1 like pathway (Figure 1, pathway b). This would result in the formation of anomeric mixtures.
When the glycosylation is performed in dichloromethane, due to the low solubility of AgOTf in dichloromethane, a solution of AgOTf in acetonitrile was added to the reaction. Although the amount of acetonitrile is small (2% of the final solvent volume for the reaction), it is possible that the β selectivity observed is a result of acetonitrile participating from the α face due to the known nitrile effect [29–31]. To test this possibility, we replaced acetonitrile with toluene in reaction of 13 with 6. The β linked disaccharide was the only product isolated (Table 1, entry 18), which suggests that acetonitrile does not play a significant role in determining the stereochemical outcome of the reaction. The β selectivity observed with dichloromethane as the reaction medium is thus likely due to the non-nucleophilic and non-polar nature of the solvent. The reaction goes through a more SN2 like pathway with the acceptor directly displacing the α-glycosyl triflate leading to β glycosides (Figure 1, pathway c).
In conclusion, glycosyl donors with participating acyl protective groups on 2-O have been shown previously to give 1,2-trans glycosides reliably under the pre-activation based reaction condition. In this work, we discovered that the stereoselectivity of the pre-activation based glycosylation using donors without participating protective group on 2-O can be controlled by the reaction solvent, with diethyl ether favoring the formation of α glycosides and dichloromethane leading to β isomers. Besides its role in generating the promoter p-TolSOTf, AgOTf can also exert significant impact on stereoselectivity presumably due to coordination with the glycosyl triflate intermediate. The stereochemical dependence can be applied to a variety of building blocks including the formation of β-mannosides. Other factors including donor and acceptor structures and protective groups also affect the outcome. A general strategy that can be applied to a wide range of building blocks for on demand stereospecific glycosylation requires further development and thorough understanding of the multiple factors guiding the stereoselectivity.
Supplementary Material
Acknowledgments
We are grateful for the National Institutes of Health (R01-GM-72667) for financial support of this work.
References
- 1.Demchenko AV, editor. Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance. Wiley-VCH; Weinheim: 2008. [Google Scholar]
- 2.Wang Z, Huang X. In: Comprehensive Glycoscience from Chemistry to Systems Biology. Kamerling JP, editor. Elsevier; 2007. pp. 379–413. [Google Scholar]
- 3.Demchenko A. Stereoselective chemical 1,2-cis O-glycosylation: from ‘sugar ray’ to modern techniques of the 21st century. Synlett. 2003:1225–1240. [Google Scholar]
- 4.Crich D, Li W, Li H. Direct chemical synthesis of the β-mannans: Linear and block syntheses of the alternating β-(1,3)-β-(1,4)-mannan common to Rhodotorula glutinis, Rhodotorula mucilaginosa, and Leptospira biflexa. J Am Chem Soc. 2004;126:15081–15086. doi: 10.1021/ja0471931. and references cited therein
- 5.Crich D, Sun S. Direct chemical synthesis of β-mannopyranosides and other glycosides via glycosyl triflates. Tetrahedron. 1998;54:8321–8348. [Google Scholar]
- 6.Fairbanks AJ. Intramolecular aglycon delivery (IAD): The solution to 1,2-cis stereocontrol for oligosaccharide synthesis? Synlett. 2003:1945–1958. [Google Scholar]
- 7.Jung K-H, Müller M, Schmidt RR. Intramolecular O-glycoside bond formation. Chem Rev. 2000;100:4423–4442. doi: 10.1021/cr990307k. [DOI] [PubMed] [Google Scholar]
- 8.Kim J-H, Yang H, Park J, Boons G-J. A general strategy for stereo-selective glycosylations. J Am Chem Soc. 2005;127:12090–12097. doi: 10.1021/ja052548h. and references cited therein
- 9.Chao C-S, Li C-W, Chen M-C, Chang S-S, Mong K-KT. Low-concentration 1,2-trans β-selective glycosylation strategy and its applications in oligosaccharide synthesis. Chem Eur J. 2009;15:10972–10982. doi: 10.1002/chem.200901119. [DOI] [PubMed] [Google Scholar]
- 10.Tanifum CT, Chang C-WT. Sonication-assisted oligomannoside synthesis. J Org Chem. 2009;74:634–644. doi: 10.1021/jo8019835. [DOI] [PubMed] [Google Scholar]
- 11.Teumelsan N, Huang X. Synthesis of branched Man5 oligosaccharides and an unusual stereochemical observation. J Org Chem. 2007;72:8976–8979. doi: 10.1021/jo7013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Zhou L, El-boubbou K, Ye X-S, Huang X. Multi-component one-pot synthesis of the tumor-associated carbohydrate antigen globo-H based on preactivation of thioglycosyl donors. J Org Chem. 2007;72:6409–6420. doi: 10.1021/jo070585g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohman GJS, Seeberger PH. A Stereochemical surprise at the late stage of the synthesis of fully N-differentiated heparin oligosaccharides containing amino, acetamido, and N-sulfonate groups. J Org Chem. 2004;69:4081–4093. doi: 10.1021/jo035732z. [DOI] [PubMed] [Google Scholar]
- 14.Orgueira HA, Bartolozzi A, Schell P, Litjens REJN, Palmacci ER, Seeberger PH. Modular synthesis of heparin oligosaccharides. Chem Eur J. 2003;9:140–169. doi: 10.1002/chem.200390009. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Huang L, Wang H, Ye X-S. Iterative one-pot oligosaccharide synthesis. Angew Chem Int Ed. 2004;43:5221–5224. doi: 10.1002/anie.200460176. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Kamat M, Huang L, Huang X. Chemical synthesis of a hyaluronic acid decasaccharide. J Org Chem. 2009;74:7608–7617. doi: 10.1021/jo9016925. and references cited therein
- 17.Sun B, Srinivasan B, Huang X. Pre-activation based one-pot synthesis of an α-(2,3)-sialylated core-fucosylated complex type Bi-antennary N-glycan dodecasaccharide. Chem Eur J. 2008;14:7072–7081. doi: 10.1002/chem.200800757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Huang X. Highly efficient syntheses of hyaluronic acid oligosaccharides. Chem Eur J. 2007;13:529–540. doi: 10.1002/chem.200601090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokimoto H, Fujimoto Y, Fukase K, Kusumoto S. Stereoselective glycosylation using the long-range effect of a [2-(4-phenylbenzyl) oxycarbonyl] benzoyl group. Tetrahedron Assym. 2005;16:441–447. [Google Scholar]
- 20.Chiba H, Funasaka S, Mukaiyama T. Catalytic and stereoselective glycosylation with glucosyl thioformimidates. Bull Chem Soc Jpn. 2003;76:1629–1644. [Google Scholar]
- 21.Adinolfi M, Barone G, Iadonisi A, Schiattarella M. Efficient activation of glycosyl N-(phenyl)trifluoroacetimidate donors with ytterbium(iii) triflate in the glycosylation reaction. Tetrahedron Lett. 2002;43:5573–5577. [Google Scholar]
- 22.Jona H, Mandai H, Chavasiri W, Takeuchi K, Mukaiyama T. Protic acid catalyzed stereoselective glycosylation using glycosyl fluorides. Bull Chem Soc Jpn. 2002;75:291–309. [Google Scholar]
- 23.Demchenko A, Stauch T, Boons G-J. Solvent and other effects on the stereoselectivity of thioglycoside glycosidations. Synlett. 1997:818–819. [Google Scholar]
- 24.Huang L, Wang Z, Li X, Ye X-S, Huang X. Iterative one-pot syntheses of chitotetraoses. Carbohydr Res. 2006;341:1669–1679. doi: 10.1016/j.carres.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crich D, Cai W. Chemistry of 4,6-O-benzylidene-d-glycopyranosyl triflates: contrasting behavior between the gluco and manno series. J Org Chem. 1999;64:4926–4930. doi: 10.1021/jo990243d. [DOI] [PubMed] [Google Scholar]
- 26.Crich D, Sun S. Are glycosyl triflates intermediates in the sulfoxide glycosylation method? A chemical and 1H, 13C, and 19F NMR spectroscopic investigation. J Am Chem Soc. 1997;119:11217–11223. [Google Scholar]
- 27.Zeng Y, Wang Z, Whitfield D, Huang X. Installation of electron donating protective groups, a strategy for glycosylating unreactive thioglycosyl acceptors using the pre-activation based glycosylation method. J Org Chem. 2008;73:7952–7962. doi: 10.1021/jo801462r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crich D, Chandrasekera NS. Mechanism of 4,6-O-benzylidene-directed β-mannosylation as determined by α-deuterium kinetic isotope effects. Angew Chem Int Ed. 2004;43:5386–5389. doi: 10.1002/anie.200453688. [DOI] [PubMed] [Google Scholar]
- 29.Crich D, Patel M. On the nitrile effect in l-rhamnopyranosylation. Carbohydr Res. 2006;341:1467–1475. doi: 10.1016/j.carres.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braccini I, Derouet C, Esnault J, du Penhoat CH, Mallet J-M, Michon V, Sinaÿ P. Conformational analysis of nitrilium intermediates in glycosylation reactions. Carbohydr Res. 1993;246:23–41. [Google Scholar]
- 31.Schmidt RR, Behrendt M, Toepfer A. Nitriles as solvents in glycosylation reactions: highly selective β-glycoside synthesis. Synlett. 1990:694–696. [Google Scholar]
- 32.Bock K, Pedersen C. A study of 13CH coupling constants in hexopyranoses. J Chem Soc, Perkin Trans 2. 1974:293–297. [Google Scholar]
- 33.Sasai H, Hosono S, Kim W-S, Shibasaki M. Rare earth salts promoted glycosidation of glycosyl fluorides. Heterocycles. 1996;42:795–809. [Google Scholar]
- 34.Chang XG, Lowary LT. A glycosylation protocol based on activation of glycosyl 2-pyridyl sulfones with samarium triflate. Org Lett. 2000;2:1505–1508. doi: 10.1021/ol005579k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.