Abstract
Protein-polymer conjugates exhibit superior properties to unmodified proteins, generating a high demand for these materials in the fields of medicine, biotechnology, and nanotechnology. Multimeric conjugates are predicted to surpass the activity of monomeric conjugates. Herein, we report a straightforward method to synthesize multimeric polymer-conjugates. Four armed poly(N-isopropylacrylamide) (pNIPAAm) was synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization in the presence of a tetra-functionalized trithiocarbonate chain transfer agent (CTA). The polymer molecular weight, architecture and polydispersity index (PDI) were verified by gel permeation chromatography (GPC), dynamic light scattering gel permeation chromatography (DLS-GPC), and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. This approach afforded well-defined polymers (PDI's < 1.06) and the ability to target various molecular weights. Maleimide functional groups were introduced at the chain ends by heating the polymers in the presence of a furan-protected azo-initiator. This allowed for site-specific conjugation of V131C T4 lysozyme to the polymers to generate multimeric protein-polymer conjugates. MALDI-TOF mass spectrometry, electrospray ionization gas-phase electrophoretic-mobility macromolecule analysis (ESI-GEMMA), gel electrophoresis, and liquid chromatography tandem mass spectrometry (LC-MS/MS) of the trypsin digests demonstrated that multimeric protein-polymer conjugates had formed. This simple strategy provides ready access to star protein-polymer conjugates for application in the fields of drug discovery, drug delivery, and nanotechnology.
Keywords: RAFT, N-isopropylacrylamide, star polymer, protein-polymer conjugate, multimeric
INTRODUCTION
Recent advances in controlled radical polymerization (CRP) techniques have provided access to polymeric architectures containing a single branch point and three or more chains, called star polymers. Traditionally, these were synthesized by living anionic polymerization. CRPs are tolerant to a larger variety of monomers than anionic polymerization, thus allowing for a broader range of functional materials to be synthesized.1–6 The most widely used techniques include nitroxide-mediated polymerization (NMP),3, 7 atom transfer radical polymerization (ATRP),8–10 and reversible addition-fragmentation chain transfer (RAFT) polymerization.5, 11–21 Two distinct approaches for the synthesis of star polymers are (1) grafting of linear polymers to a core and (2) growth of the polymers from a core. The grafting approach can suffer from variable conjugation efficiencies of the arms to the core due to steric crowding.5 Growth of the arms from a functionalized core is advantageous because it allows for precise control over the chain length and number of arms.2–4, 12
Star polymers made by CRP techniques contain end groups that allow for additional modification.2–4, 12 High retention of the end group is essential for generating a variety of well-defined polymeric architectures. For example, Gao et al. demonstrated polymerization of styrene from a trifunctional ATRP initiator to generate well-defined, three-armed polystyrene (PS) containing a high retention of bromine on the arm termini post-polymerization. The bromine was displaced with an azido-group and efficiently clicked to alkyne functionalized polyethylene oxide (PEG) to generate well-defined, PEG-b-PS three-armed stars.22 Hao et al. utilized a triol core, first generation dendrimer, and second generation dendrimer to generate three, six, and twelve armed PS and polybutylacrylate (pBA) by RAFT polymerization. The resulting polymers exhibited high retention of trithiocarbonates at the chain ends, thus allowing for multi-armed block-copolymer stars of PS-b-pBA and pBA-b-PS to be synthesized from the macroinitiators.23 In another example, Chen et al. synthesized a tetrafunctional chain transfer agent (CTA) to mediate the polymerization of styrene by RAFT, followed by polymerization of 2-(dimethylamino) ethyl methacrylate (DMAEMA) resulting in well-defined PS-b-PDMAEMA four-armed star polymers. These block copolymers self-assembled into spherical micelles and were utilized for the controlled release of chlorambucil, a chemotherapeutic.24
As demonstrated in the above examples, star polymers can provide access to a variety of architectures with interesting properties. Herein, we sought to utilize star polymers to generate scaffolds to produce multimeric protein conjugates. Protein-polymer conjugates are fundamentally interesting materials with far reaching applications as therapeutics,25 nanoassemblies,26 and smart materials.27 After linking the polymer to the protein, the properties of the biomolecule are altered advantageously. Because polymers with narrow molecular weight distributions are superior for many applications, CRPs3, 9, 28–31 have emerged as excellent methods to produce biomolecule conjugates.32, 33 Polymers with protein-reactive end groups have been synthesized by both ATRP and RAFT polymerization.34–42 Conjugates have also been prepared by polymerizing directly from the proteins.43–49 All of these examples consist of one protein attached to one or more polymer chains. Multimeric protein conjugates, where more than one protein is attached to a single polymer chain, can have superior properties for many applications. Enzymes often oligomerize in the active state in which homotetramers are highly prevalent.50 Ion channels often consist of multiple proteins acting in concert. Antibodies are oligomers consisting of either two, four, six, or ten epitopes.51 Thus, fixation of multiple proteins to a polymer scaffold can result in a conjugate that exhibits higher activities then the monomeric conjugate.52 We disclose in this report for the first time a straightforward CRP strategy to access star protein-polymer conjugates.
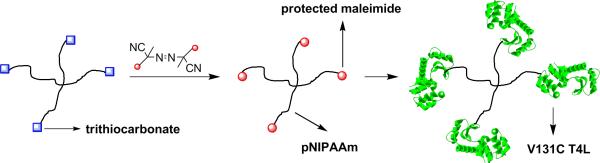
We recently reported a strategy to access homodimeric conjugates.53 We have also disclosed heterodimeric conjugates by CRP.54 The key step involves exchange of the macroCTA chain ends with a protein-reactive azoinitiator. This radical coupling method was first disclosed to remove thiocarbonylthio moieties by Perrier.55 Specifically in this report, we describe the synthesis of a star polymer by RAFT polymerization and the installment of maleimides at the chain ends (Scheme 1). Maleimides react with free cysteines providing a means for site-specific functionalization of proteins.56 Indeed, successful conjugation to a model protein containing a free cysteine, V131C T4 lysozyme (T4L), was performed resulting in star protein-polymer conjugates (Scheme 1). Details of the synthesis, characterization of the polymers and mass spectrometry analysis of the conjugates are discussed below.
Scheme 1.
Synthesis of protein star polymer-conjugates.
EXPERIMENTAL
Materials
2,2′-Azobisisobutyronitrile (AIBN) (Aldrich, 98%) was recrystallized twice from acetone. N-Isopropylacrylamide (NIPAAm, Acros, 99%) was recrystallized twice from hexane. The V131C T4-lysozyme (T4L) mutant expression vector was kindly provided by Professor Wayne Hubbell (UCLA), and the protein was expressed from E. coli and purified as previously described.46, 57, 58 Pentaerythritol tetrakis(2-bromopropionate)59 and the furan-protected maleimide azo-initiator 454 were synthesized as previously described. All other solvents were obtained at the highest purity available from Aldrich Chemical Company and used without further purification.
Methods
Polymerization and radical coupling reactions were carried out using standard Schlenk techniques under an inert atmosphere of argon. TLC was performed using pre-coated silica gel 60 F254 and developed in the solvent system indicated. Compounds were visualized by use of UV light (254 nm) or a basic solution (10% K2CO3 aqueous solution) of KMnO4. Merck 60 (230–400 Mesh) silica gel was used for column chromatography. Gel permeation chromatography (GPC) analysis of the polymers was conducted on a Shimadzu HPLC system equipped with a refractive index detector RID-10A, a UV-Vis detector SPD-10A VP and two Polymer Laboratories PLgel 5 μm mixed D columns (with guard column). LiBr (0.1 M) in DMF at 40 °C was used as the eluent (flow rate: 0.80 mL/min). Near-monodisperse poly(methyl methacrylate) (PMMA) standards (Polymer Laboratories) were employed for calibration. Chromatograms were processed with the EZStart 7.2 chromatography software. NMR spectra were obtained on Bruker ARX500 and Bruker DRX400 spectrometers. Monomer conversions were calculated by 1H NMR by monitoring the disappearance of the peaks corresponding to the vinylic protons with the CH of the poly(NIPAAm) as internal standard. The number-average molecular weight (Mn) by dynamic light scattering (DLS) was collected from a Viscotek GPCmax VE2001 GPC solvent/sample module with 270 Dual detector and VE 3580 RI detector. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with 4–20% Tris-Glycine gels (Invitrogen, 1.0 mm × 12 well). Matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry was performed on an Applied Biosystems Voyager-DE STR and operated in linear mode with an external calibration. Polymer samples were prepared by mixing 5:1:5 v/v/v ratios of trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene]malononitrile (DTCB) matrix (40 mg/mL in THF) to potassium trifluoroacetate (5 mg/mL in THF) to polymer (1 mg/mL in THF). Infrared absorption spectra were recorded on a PerkinElmer FTIR instrument equipped with an ATR accessory.
Synthesis of tetrameric CTA (1)
A solution of ethanethiol (4.0 mL, 54.0 mol) in 8.0 mL of water was kept at 0 °C for 10 min. A 50% NaOH aqueous solution (4.32 g, containing 2.16 g, 54.0 mol of NaOH) was then added followed by 3.0 mL of acetone. The clear colorless solution was stirred for 30 min and treated with carbon disulfide (4.45 g, 59.0 mol) to give a bright orange solution. The system was stirred at 23 °C for 30 min and cooled down to 0 °C. Then 20 mL dichloromethane containing pentaerythritol tetrakis(2-bromopropionate) (6.0 g, 8.9 mmol) was added slowly and the mixture was stirred at 23 °C for 48 h. After filtration to remove the suspended solid, the solvent was distilled under vacuum. The remaining crude was purified by column chromatography on silica gel (diethyl ether: hexane = 1: 4) to give product as yellow oil (2.7 g, yield: 33.6%). 1H NMR (400.13 MHZ, CDCl3)/ppm: 4.82 (q, J = 7.5 Hz, 4H, CHS), 4.11–4.00 (m, 8H, CCH2), 3.41–3.31 (m, 8H, SCH2), 1.58 (d, J = 7.5 Hz, 12H, CHCH3), 1.35 (t, J = 7.6 Hz, 12H, CH2CH3); 13C NMR (100.61 MHZ, CDCl3)/ppm: 221.76 (C=S), 170.75 (C=O), 62.92 (CCH2), 47.40 (CHCH3), 42.41 (CCH2), 31.88 (SCH2), 16.63 (CHCH3), 12.75 (CH2CH3). IR (cm−1): 2929, 1736, 1448, 1374, 1147, 1028, 807. MALDI-TOF MS: M+Na+ expected (observed): 926.96 (926.98).
Preparation of four-armed poly(NIPAAm) (3)
NIPAAm (2.22 g, 20.0 mmol), 1 (22.6 mg, 2.5 × 10−2 mmol) and AIBN (0.82 mg, 5.0 × 10−3 mmol) were dissolved in 5 mL of DMF, the Schlenk tube was subjected to three freeze-pump-thaw cycles, and placed into a 70 °C oil bath. Samples were removed periodically using a degassed syringe for conversion analysis by 1H NMR. After the conversion reached 52%, the polymerization was halted by cooling the reaction mixture in an ice-water bath. Most of the DMF was removed under high vacuum at 40 °C, and the polymer was purified by dialysis against methanol (MWCO: 6–8,000). 1H NMR (400.13 MHZ, CDCl3)/ppm: 7.10–5.62 (main chain, NH), 3.99 (main chain, CH), 3.34 (chain end, SCH2), 2.07 (main chain, CH), 1.83–1.60 (main chain, CH2), 1.12 (main chain, CH(CH3)2). Using the Mn obtained by DLS and the calculated molar extinction coefficient of 54804 M−1cm−1 the % of chain-end trithiocarbonate was calculated as 95 % by UV-Vis analysis in 1,2-dichloroethane (see Electronic Supporting Information, ESI for details).
Aminolysis of core
Four-armed poly(NIPAAm) (2) (15 mg, 3.00 × 10−4 mmol) was dissolved in 5 mL of methanol, 0.10 mL of butylamine was added and the solution was refluxed for 20 h. The volatile organics were removed by vacuum distillation. The residue was redissolved in DMF and employed for GPC analysis directly.
Radical coupling of polymer with functionalized azo-initiator (4)
Four armed poly(NIPAAm) (3) (50 mg, Mn~50,000, 10−3 mmol), and 4 (69 mg, 0.10 mmol) were dissolved in 2 mL of dioxane/DMF (1:1). The Schlenk tube was subjected to three freeze-pump-thaw cycles, and placed into a 70 °C bath for 4 h. The dioxane and DMF were removed under vacuum at 40 °C, and the crude was dissolved in 10 mL of methanol. The insoluble portion was removed by centrifugation. The methanol solution was then dialyzed against ethyl acetate/methanol (1:1, MWCO: 6–8,000) for 72 h to give the clean furan-protected maleimide functionalized four-arm polyNIPAAm (5). 1H NMR (400.13 MHZ, CDCl3)/ppm: 7.10–5.66 (main chain, NH), 6.48 (chain end, CH=CH), 5.23 (chain end, CHOCH), 3.97 (main chain, CH), 3.54 (chain end, NCH2), 2.82 (chain end, CHOCH) CHC=O), 2.07 (main chain, CH), 1.79–1.59 (main chain, CH2), 1.10 (main chain, CH(CH3)2.
Retro Diels-Alder Reaction (6)
The four armed poly(NIPAAm) with furan-protected maleimide end group (5) was heated at 105 °C under vacuum for 2 h to give four armed poly(NIPAAm) terminated with maleimide (6). 1H NMR (400.13 MHZ, CDCl3)/ppm: 6.96–5.66 (main chain, NH), 6.72 (chain end, CH=CH), 4.00 (main chain, CH), 3.63–3.49 (chain end, NCH2), 2.07 (main chain, CH), 1.81–1.60 (main chain, CH2), 1.13 (main chain, CH(CH3)2.
Conjugation of T4L with 6
A typical conjugation was carried out as follows. Star polymer 6 (0.33 mg, 4.3 mg/mL) in PBS, pH 7.5, with 10 mM of EDTA and 10 mM of TCEP was added to a T4L solution (1.69 mg/mL) in Tris-buffer at pH 7.4. The mixture was maintained at 4 °C with gentle stirring for 16 h. After concentration by centrifugal filtration (Amicon Ultra, MWCO 10,000) the solution was used directly for SDS PAGE analysis.
Electrospray Ionization – Gas-Phase Electrophoretic-Mobility Macromolecule Analysis (ESI-GEMMA)
Samples were prepared in 20 mM ammonium acetate, pH 8 or 20 mM acetic acid. Amicon Ultra-4 centrifugal filter devices (Millipore) or Micro Bio-Spin 6 chromatography columns (Bio-Rad) were used for sample buffer exchange. The ESI-GEMMA device60 (TSI Inc.) consists of an Electrospray Aerosol Generator 3480, Electrostatic Classifier 3080 with Nano-DMA (Differential Mobility Analyzer) 3085, and Ultrafine Condensation Particle Counter 3025A. The DMA separates the particles based on their electrophoretic mobility in air. Sample solutions were introduced in the ESI source through a fused silica capillary tube at a flow rate of 70 nL/min and voltage of 2 to 3 kV. To promote particle charge reduction, the gases present inside the ESI neutralizing chamber were ionized by a 210Po α-radiation source. The DMA sheath flow was set to 20 L/min and the voltage was scanned −10 to −10,000 volts for 120 sec. to sample the electrophoretic mobility diameter (EMD) range of 2–56 nm. The DMA voltage was scanned and data recorded by Aerosol Instrument Manager Software (TSI Inc). PeakFit Version 4.12 (SeaSolve Software Inc.) was used to centroid EMD peaks of interest. Data points were smoothed using Igor Pro 4.0.8.0 (Wavemetrics, Inc.). Molecular weights were calculated using the formula for density and the volume of a sphere: MW=V*d*Na where d is the effective density, Na is Avogadro's number and the volume, V = (EMD)3π/6.
RESULTS AND DISCUSSION
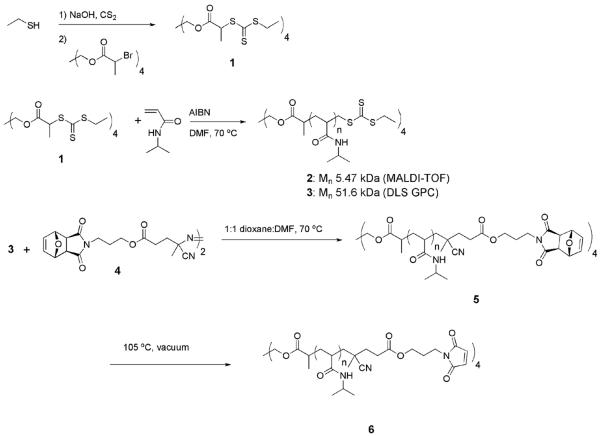
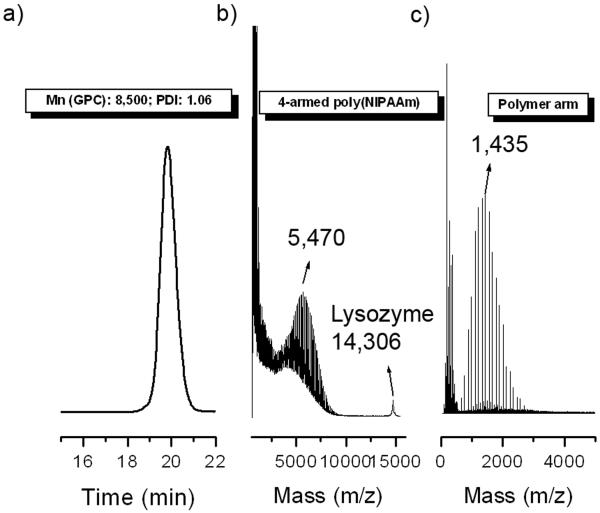
The four armed CTA (1) was synthesized by displacement of the bromine on pentaerythritol tetrakis(2-bromopropionate) with the trithiocarbonate anion (Scheme 2) in 34% yield. A polyNIPAAm star polymer was then prepared by RAFT polymerization using 1. Initially, a low molecular weight polymer (2) was targeted in order to be able to analyze the efficiency of the reaction by mass spectrometry. Ratios of [NIPAAm]:[1]:[AIBN] of 100:1:0.2 were used. To avoid unwanted cross-linking reactions the polymerization was stopped at 45%. The number-average molecular weight (Mn) by GPC (trace shown as Figure 1a) was 8,500 Da, and the polydispersity index (PDI) was 1.06, demonstrating that a well-defined star had been synthesized. To further characterize the star, polymer 2 was analyzed by MALDI-TOF mass spectrometry. This gave a MW of 5,470 Da (Figure 1b), which was close to the theoretical target (6,000 Da). Aminolysis with butyl amine was utilized to cleave the arms from the core to confirm that the polymer contained four arms of similar molecular weights. The mass of the cleaved arms was 1,435 Da (Figure 1c). Due to the heterogeneity intrinsic to a polymer sample, this result was considered within the uncertainty of the expected value (1,351 Da) and suggested that the desired four-arm star had been synthesized.
Scheme 2.
Synthesis of 4-armed maleimide poly(NIPAAm).
Figure 1.
GPC and mass spectra of four-arm poly(NIPAAm) (2). GPC trace of the 4-armed poly(NIPAAm) (left); MALDI-TOF spectra before (middle) and after (right) aminolysis.
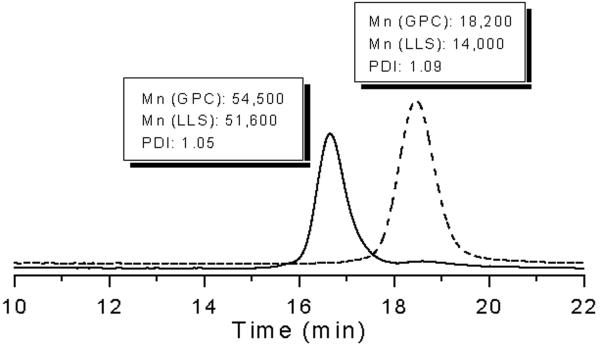
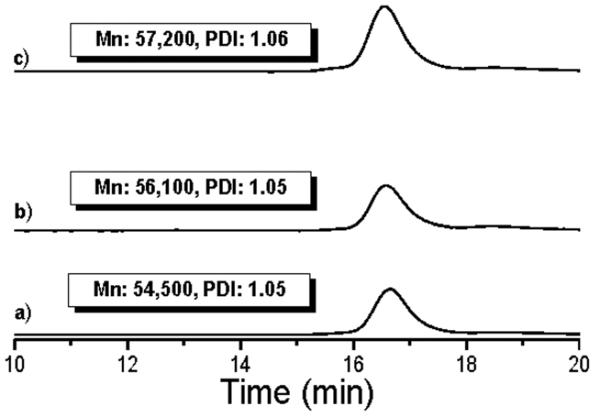
These data indicated that the designed tetrafunctional CTA was facilitating homogenous growth of the arms; thus a larger molecular weight polymer was next pursued. This polymer was synthesized with [NIPAAm]:[1]:[AIBN] ratios of 800:1:0.2 to 52% conversion, and purified by dialysis to give 3. DLS GPC indicated a Mn of 51,600 Da and a PDI of 1.05. 1H NMR indicated a Mn of 50,000 Da. Both were close to the targeted molecular weight of 48,900 Da. Hydrolysis of 3 produced linear polymers with a Mn of 14,000 Da by DLS GPC. This was slightly larger than the expected mass (12,900 Da) and the PDI broadened to some extent to 1.09 (Figure 2). This result suggested that the star contained 3.7 arms, indicating that the larger polymer was a mixture of three and four arm stars had been obtained.
Figure 2.
GPC traces of 4-armed poly(NIPAAm) and polymer arm after aminolysis.
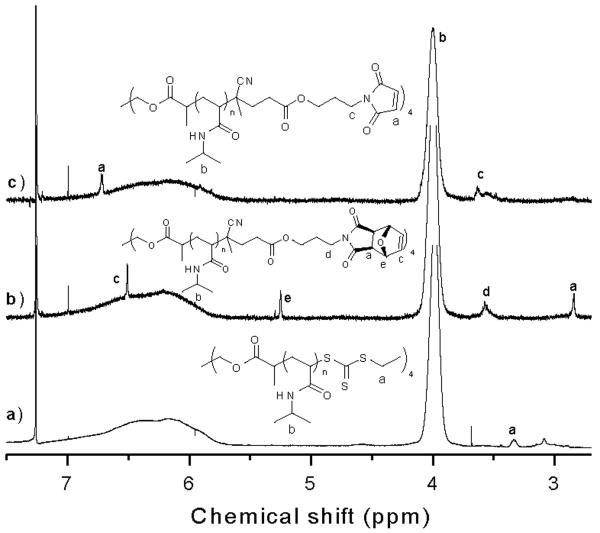
Fortunately, the end groups of the star polymer were available for further elaboration; UV-Vis analysis of the star polymer (see SI) demonstrated retention of the trithiocarbonate CTA end group ~95%. After heating in the presence of functionalized azoinitiator 4 and performing the retro Diels-Alder reaction, the desired four-arm polymer with a maleimide chain end was obtained as observed by the maleimide peak at 6.8 ppm (a, Figure 3c) and the disappearance of the protected maleimide peaks (a, c, and e, Figure 3b). Analysis by GPC indicated that post polymerization (Figure 4a), radical coupling (Figure 4b), and maleimide deprotection (Figure 4c) the polymer remained well-defined (PDI = 1.05–1.06).
Figure 3.
1H NMR (CDCl3) spectra of a) four-armed polyNIPAAm 3, b) four-armed furan-protected polyNIPAAm 5, and c) four-armed maleimide polyNIPAAm 6.
Figure 4.
GPC traces of a) four-armed polyNIPAAm 3, b) four-armed furan-protected polyNIPAAm 5, and c) four-armed maleimide polyNIPAAm 6.
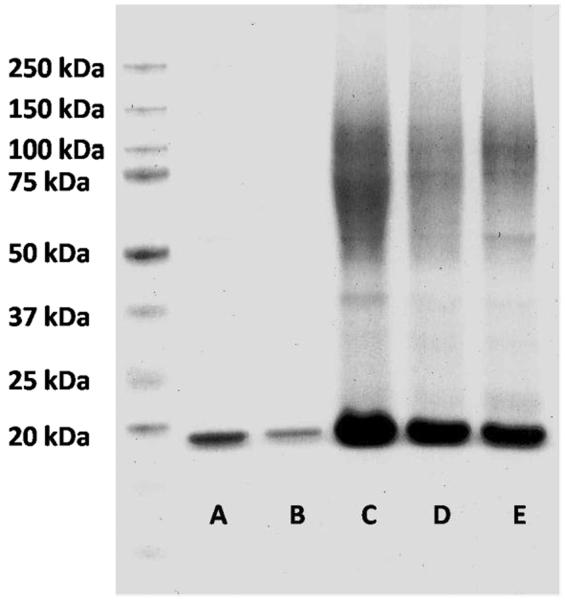
Immobilization of V131C T4L on 6 was pursued at 4°C. The conjugation was investigated using different ratios of protein to polymer (1:2, 2:1, and 8:1). In all three systems a shift to higher molecular weight was observed by SDS-PAGE (Figure 5, lanes C, D, and E respectively). Importantly, when the maleimides were protected and not available for conjugation, the observed band was exactly the same as for unmodified T4L (Figure 5, lanes B and A, respectively). LC-MS/MS of the trypsin digests of some of the fractions verified that these bands contained T4L and were likely the polymer conjugate (see SI for details, Table S1). A ratio of T4L to polymer of 8 to 1 gave the highest molecular weight band in the gel. Although gels of polymer conjugates cannot be expected to provide accurate molecular weight values when compared to the protein standards, the mass determined by comparison to protein markers of known molecular weight was reasonably close to the expected molecular weight of a tetramer at ~124 kDa. In addition, the band was broad, suggesting that a mixture of conjugates was obtained.
Figure 5.
SDS-page analysis. A) native T4L; B) T4L + furan protected polymer 5; C) T4L + maleimide poymer 6 1:2; D) T4L/6 2:1; E) T4L/6 8:1.
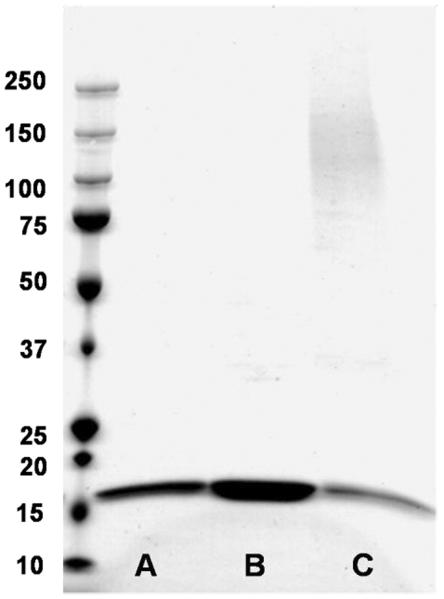
MALDI-TOF mass spectrometry failed to reveal the molecular mass of the conjugates, possibly because of the large size of the expected macromolecule. Thus, to determine the actual number of proteins conjugated to the polymer, characterization by ESI-GEMMA was pursued. First, the conjugate was prepared using the optimized conjugation conditions and purified by Sephadex column (G-100). The SDS PAGE of the product (Figure 6) displayed a higher molecular weight band shown in lane C; once again the furan protected polymer did not react and only T4L was observed as shown in lane B. This conjugate (spectrum provided as Figure S3), the star polymer, and the star polymer post-hydrolysis (arms) were analyzed by ESI-GEMMA. Table 1 lists the EMD of the peaks identified in the ESI-GEMMA spectra of the protein-polymer conjugate sample.
Figure 6.
SDS-PAGE analysis. A) native T4L; B) T4L + 5; C) T4L + 6 (ratio of T4L/6 = 1:8).
Table 1.
Calculated molecular weights of protein-polymer conjugate determined from ESI-GEMMA data.
| Protein-Polymer Conjugate (four armed polymer) | ||
|---|---|---|
| EMD (nm) | Apparent MW (Da, d=0.67 g/cc)1–3 | Assignment4 |
| 3.9 | P (hydrolyzed) | |
| 6.1 | P4 | |
| 8.0 | 11 × 104 | P4 + 3L |
| 10.1 | 21 × 104 | 2 (P4 + 3L) |
Based on the standard deviation of the effective density, a mass accuracy of ±10% is estimated.
Molecular weight was calculated using the formula: MW=V*d*Na where d is the effective density, Na is Avogadro's number and the volume, V = (EMD)3π/6.
Density of the polymer-protein conjugate is based on the predicted estimate that a polymer with 3 lysozymes would be 53% lysozyme and 47% polymer. Density of protein and protein complexes is from [61]. Density of the polymer is based on mass estimated from NMR of 50000 and the apparent EMD using the formula d=MW/V.
Abbreviations are defined as follows: L is T4 lysozyme; P (hydrolyzed) is the four armed polymer after hydrolysis; P4 is the four armed polymer; P4 + 3L is the trimer linked four armed polymer; 2 (P4 + 3L) is the dimer of the trimer linked four armed polymer.
The EMD of the star polymer and star polymer post-hydrolysis were 6.0 nm and 3.8 nm, respectively (data not shown). Given the structure of the four armed polymer, the density of the star polymer was expected to exhibit a different density than that of a linear polymer. Based on the EMD and the mass estimated by NMR (~50,000 Da), the density (d=MW/V) of the star polymer was calculated to be 0.73 g/cc.60, 61 Peaks consistent with the size of the star polymer (6.1 nm) and star polymer post-hydrolysis (3.9 nm) were identified (Figure S3). The 8.0 nm and 10 nm peaks were assigned to the protein-polymer conjugate. Based on ESI-GEMMA measurements, the average effective density of proteins and protein complexes is 0.58 g/cc.61 The density of the polymer-protein conjugate was calculated to be 0.67 g/cc based on the prediction that a polymer with three lysozymes would be 53% lysozyme and 47% polymer by weight. The EMD and calculated molecular weights for the protein-polymer conjugate of three lysozymes (8.0 nm, 11 × 104 Da) was consistent with what was observed by SDS-PAGE. A dimer of this protein-polymer conjugate was also observed (10 nm, 21 × 104 Da).
Using ESI-GEMMA it was determined that an average of three proteins was attached to each star polymer. From the GPC data of the cleaved arms, the star was likely a mixture of three and four arms. Hydrolysis of the maleimide over the course of the conjugation reaction or steric crowding around the star polymer core could be additional factors as to why tetrameric conjugates were not observed. For the former, use of a more stable Michael acceptor, such as a vinyl sulfone, may prove to be advantageous. For the latter, stars with longer polymer arms could be explored.
Yet, these data do demonstrate that multimeric protein-polymer conjugates are readily accessible by this technique. Site-selective binding of proteins to polymers is important for bioactivity, and maleimides provide this. In addition, a wide variety of end groups can be envisioned as a number of azo initiators could be synthesized. Moreover, this approach should be amenable to the range of architectures and polymer identities accessible by RAFT polymerization. For many biological applications, tethering multiple proteins together onto a single polymer chain is expected to provide constructs with superior activities than simple protein-polymer conjugates. This approach immediately provides access to trimer conjugates. It also offers a synthetic strategy to target different valency constructs, depending on the desired application.
CONCLUSIONS
A multimeric star poly(NIPAAm)-lysozyme conjugate was successfully synthesized utilizing a combination of RAFT, radical coupling with a designed initiator, and site specific bioconjugation. Different molecular weights were readily accessed. Mass spectrometry analysis of the star and cleaved arms indicated that the CTA was efficient, and that the desired 4 armed star polymers had been prepared when the molecular weight was low. With a larger molecular weight star, an average of 3.7 armed stars was prepared. 1H NMR analysis and GPC indicated that the maleimide was readily incorporated utilizing a radical reaction with an azo-initiator, followed by heating. Using SDS PAGE, the reaction conditions, specifically the optimal ratios for conjugating protein to polymer, were explored. A conjugate prepared using an 8 to 1 ratio of polymer to T4L was further analyzed by ESI-GEMMA. This measurement revealed that three proteins were attached to each conjugate. We anticipate that this method to produce site-selective multimeric conjugates will be generally useful for preparing materials for applications in nanotechnology and medicine.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NSF (CHE-0809832). HDM thanks Amgen (New Faculty Award) and the Sloan Foundation for further support. The UCLA Functional Proteomics Center was established and equipped by a grant from the W. M. Keck Foundation. JAL acknowledges support from the National Institutes of Health (RR20004). CSK acknowledges support from the Chancellor's Dissertation Year Fellowship. GNG thanks the Christopher S. Foote Graduate Fellowship in Organic Chemistry and the NIH-sponsored Biotechnology Training in Biomedical Sciences and Engineering Program for funding. The authors appreciate Dr. Chien-Wen Chang and Dr. Karina L. Heredia for preparing some mutant T4L and for helpful conversations. Dr. Pinmanee Boontheung is thanked for LC-MS/MS analysis of the tryptic digests of the SDS-PAGE gel bands.
Footnotes
SUPPORTING INFORMATION Supporting Information Available: UV-vis analysis, LC-MS/MS data, and ESI-GEMMA spectrum are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Gao HF, Matyjaszewski K. Prog. Polym. Sci. 2009;34:317–350. [Google Scholar]
- 2.Hadjichristidis N, Iatrou H, Pitsikalis M, Mays J. Prog. Polym. Sci. 2006;31:1068–1132. [Google Scholar]
- 3.Hawker CJ, Bosman AW, Harth E. Chem Rev. 2001;101:3661–3688. doi: 10.1021/cr990119u. [DOI] [PubMed] [Google Scholar]
- 4.Moad G, Rizzardo E, Thang SH. Polymer. 2008;49:1079–1131. [Google Scholar]
- 5.Moad G, Rizzardo E, Thang SH. Acc. Chem. Res. 2008;41:1133–1142. doi: 10.1021/ar800075n. [DOI] [PubMed] [Google Scholar]
- 6.Zhou YF, Yan DY. Chem. Commun. 2009:1172–1188. doi: 10.1039/b814560c. [DOI] [PubMed] [Google Scholar]
- 7.Miura Y, Dairoku M. J. Polym. Sci., Part A: Polym. Chem. 2007;45:4364–4376. [Google Scholar]
- 8.Gao HF, Matyjaszewski K. Macromolecules. 2006;39:7216–7223. [Google Scholar]
- 9.Matyjaszewski K, Xia JH. Chem. Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 10.Zhao YL, Chen YM, Chen CF, Xi F. Polymer. 2005;46:5808–5819. [Google Scholar]
- 11.Barner L, Davis TP, Stenzel MH, Barner-Kowollik C. Macromol. Rapid Commun. 2007;28:539–559. [Google Scholar]
- 12.Barner-Kowollik C, Davis TP, Stenzel MH. Aust. J. Chem. 2006;59:719–727. [Google Scholar]
- 13.Boschmann D, Edam R, Schoenmakers PJ, Vana P. Polymer. 2008;49:5199–5208. [Google Scholar]
- 14.Boschmann D, Manz M, Poppler AC, Sorensen N, Vana P. J. Polym. Sci., Part A: Polym. Chem. 2008;46:7280–+. [Google Scholar]
- 15.Boschmann D, Vana P. Macromolecules. 2007;40:2683–2693. [Google Scholar]
- 16.Chaffey-Millar H, Hart-Smith G, Barner-Kowollik C. J. Polym. Sci., Part A: Polym. Chem. 2008;46:1873–1892. [Google Scholar]
- 17.Frohlich MG, Vana P, Zifferer G. Macromol. Theory Simul. 2007;16:610–618. [Google Scholar]
- 18.Lu DR, Wang Y, Wu TY, Tao K, An LJ, Bai RK. J. Polym. Sci., Part A: Polym. Chem. 2008;46:5805–5815. [Google Scholar]
- 19.McDowall L, Chen GJ, Stenzel MH. Macromol. Rapid Commun. 2008;29:1666–1671. [Google Scholar]
- 20.Perrier S, Takolpuckdee P. J. Polym. Sci., Part A: Polym. Chem. 2005;43:5347–5393. [Google Scholar]
- 21.Yang LP, Zhou HX, Shi GY, Wang Y, Pan CY. J. Polym. Sci., Part A: Polym. Chem. 2008;46:6641–6653. [Google Scholar]
- 22.Gao HF, Min K, Matyjaszewski K. Macromol. Chem. Phys. 2007;208:1370–1378. [Google Scholar]
- 23.Hao XJ, Malmstrom E, Davis TP, Stenzel MH, Barner-Kowollik C. Aust. J. Chem. 2005;58:483–491. [Google Scholar]
- 24.Chen WX, Fan XD, Huang Y, Liu YY, Sun L. React. Funct. Polym. 2009;69:97–104. [Google Scholar]
- 25.Duncan R. Nat. Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 26.Vandermeulen GWM, Klok HA. Macromol. Biosci. 2004;4:383–398. doi: 10.1002/mabi.200300079. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman AS, Stayton PS. Macromol. Symp. 2004;207:139–151. [Google Scholar]
- 28.Kamigaito M, Ando T, Sawamoto M. Chemical Reviews. 2001;101:3689–3745. doi: 10.1021/cr9901182. [DOI] [PubMed] [Google Scholar]
- 29.Moad G, Rizzardo E, Thang SH. Australian Journal of Chemistry. 2005;58:379–410. [Google Scholar]
- 30.Perrier S, Takolpuckdee P. Journal Of Polymer Science Part A-Polymer Chemistry. 2005;43:5347–5393. [Google Scholar]
- 31.Barner L, Davis TP, Stenzel MH, Barner-Kowollik C. Macromol. Rapid Commun. 2007;28:539–559. [Google Scholar]
- 32.Heredia KL, Maynard HD. Organic & Biomolecular Chemistry. 2007;5:45–53. doi: 10.1039/b612355d. [DOI] [PubMed] [Google Scholar]
- 33.Nicolas JM,G, Haddleton DM. Macromol. Rapid Commun. 2007;28:1038–1111. [Google Scholar]
- 34.Bontempo D, Heredia KL, Fish BA, Maynard HD. J. Am. Chem. Soc. 2004;126:15372–15373. doi: 10.1021/ja045063m. [DOI] [PubMed] [Google Scholar]
- 35.Bontempo D, Li RC, Ly T, Brubaker CE, Maynard HD. Chem. Commun. 2005:4702–4704. doi: 10.1039/b507912h. [DOI] [PubMed] [Google Scholar]
- 36.Hong CY, Pan CY. Macromolecules. 2006;39:3517–3524. [Google Scholar]
- 37.Liu J, Bulmus V, Barner-Kowollik C, Stenzel MH, Davis TP. Macromol. Rapid Commun. 2007;28:305–314. [Google Scholar]
- 38.Mantovani G, Lecolley F, Tao L, Haddleton DM, Clerx J, Cornelissen J, Velonia K. J. Am. Chem. Soc. 2005;127:2966–2973. doi: 10.1021/ja0430999. [DOI] [PubMed] [Google Scholar]
- 39.Pound G, McKenzie JM, Lange RFM, Klumperman B. Chem. Commun. 2008:3193–3195. doi: 10.1039/b803952f. [DOI] [PubMed] [Google Scholar]
- 40.Qi K, Ma QG, Remsen EE, Clark CG, Wooley KL. J. Am. Chem. Soc. 2004;126:6599–6607. doi: 10.1021/ja039647k. [DOI] [PubMed] [Google Scholar]
- 41.Sen Gupta S, Raja KS, Kaltgrad E, Strable E, Finn MG. Chem. Commun. 2005:4315–4317. doi: 10.1039/b502444g. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Dorbatt V, Maynard HD. Biomacromolecules. 2006;7:2297–2302. doi: 10.1021/bm060105f. [DOI] [PubMed] [Google Scholar]
- 43.Bontempo D, Maynard HD. J. Am. Chem. Soc. 2005;127:6508–6509. doi: 10.1021/ja042230+. [DOI] [PubMed] [Google Scholar]
- 44.Boyer C, Bulmus V, Liu JQ, Davis TP, Stenzel MH, Barner-Kowollik C. J. Am. Chem. Soc. 2007;129:7145–7154. doi: 10.1021/ja070956a. [DOI] [PubMed] [Google Scholar]
- 45.De P, Li M, Gondi SR, Sumerlin BS. J. Am. Chem. Soc. 2008;130:11288–11289. doi: 10.1021/ja804495v. [DOI] [PubMed] [Google Scholar]
- 46.Heredia KL, Bontempo D, Ly T, Byers JT, Halstenberg S, Maynard HD. J. Am. Chem. Soc. 2005;127:16955–16960. doi: 10.1021/ja054482w. [DOI] [PubMed] [Google Scholar]
- 47.Le Droumaguet B, Velonia K. Angew. Chem. Int. Ed. 2008;47:6263–6266. doi: 10.1002/anie.200801007. [DOI] [PubMed] [Google Scholar]
- 48.Lele BS, Murata H, Matyjaszewski K, Russell AJ. Biomacromolecules. 2005;6:3380–3387. doi: 10.1021/bm050428w. [DOI] [PubMed] [Google Scholar]
- 49.Zeng QB, Li T, Cash B, Li SQ, Xie F, Wang Q. Chem. Commun. 2007:1453–1455. doi: 10.1039/b617534a. [DOI] [PubMed] [Google Scholar]
- 50.Marianayagam NJ, Sunde M, Matthews JM. Trends Biochem. Sci. 2004;29:618–625. doi: 10.1016/j.tibs.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Mammen M, Choi SK, Whitesides GM. Angew. Chem., Int. Ed. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Griffith BR, Allen BL, Rapraeger AC, Kiessling LL. J. Am. Chem. Soc. 2004;126:1608–1609. doi: 10.1021/ja037646m. [DOI] [PubMed] [Google Scholar]
- 53.Tao L, Kaddis CS, Loo RRO, Grover GN, Loo JA, Maynard HD. Chem. Commun. 2009:2148–2150. doi: 10.1039/b822799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heredia KL, Grover GN, Tao L, Maynard HD. Macromolecules. 2009;42:2360–2367. doi: 10.1021/ma8022712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrier S, Takolpuckdee P, Mars CA. Macromolecules. 2005;38:2033–2036. [Google Scholar]
- 56.Hermanson GT. Bioconjugate Techniques. Academic Press; New York: 1996. [Google Scholar]
- 57.Columbus L, Kalai T, Jeko J, Hideg K, Hubbell WL. Biochemistry (Mosc) 2001;40:3828–3846. doi: 10.1021/bi002645h. [DOI] [PubMed] [Google Scholar]
- 58.Mchaourab HS, Lietzow MA, Hideg K, Hubbell WL. Biochemistry (Mosc) 1996;35:7692–7704. doi: 10.1021/bi960482k. [DOI] [PubMed] [Google Scholar]
- 59.Matyjaszewski K, Miller PJ, Pyun J, Kickelbick G, Diamanti S. Macromolecules. 1999;32:6526–6535. [Google Scholar]
- 60.Kaddis CS, Lomeli SH, Yin S, Berhane B, Apostol MI, Kickhoefer VA, Rome LH, Loo JA. J. Am. Soc. Mass Spec. 2007;18:1206–1216. doi: 10.1016/j.jasms.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ude S, Fernandez de la Mora J, Thomson BA. J. Am. Chem. Soc. 2004;126:12184–12190. doi: 10.1021/ja0381306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.