Abstract
We previously showed that medullary thyrotropin-releasing hormone (TRH) or the stable TRH agonist, RX-77368 administered intracisternally induces vagal-dependent activation of gastric myenteric neurons and prevents post surgery-induced delayed gastric emptying in rats. We investigated whether abdominal surgery alters intracisternal (ic) RX-77368 (50 ng)-induced gastric myenteric neuron activation. Under 10 min enflurane anesthesia, rats underwent an ic injection of saline or RX-77368 followed by a laparotomy and a 1-min cecal palpation, or no surgery and were euthanized 90 min later. Longitudinal muscle/myenteric plexus whole-mount preparations of gastric corpus and antrum were processed for immunohistochemical detection of Fos alone or double labeled with protein gene-product 9.5 (PGP 9.5) and vesicular acetylcholine transporter (VAChT). In the non surgery groups, ic RX-77368 induced a 17 fold increase in Fos-expression in both gastric antrum and corpus myenteric neurons compared to saline injected rats. PGP 9.5 ascertained the neuronal identity of myenteric cells expressing Fos. In the abdominal surgery groups, ic RX-77368 induced a significant increase in Fos-expression in both the corpus and antrum myenteric ganglia compared with ic saline injected rats which has no Fos in the gastric myenteric ganglia. However, the response was reduced by 73–78% compared with that induced by ic RX 77368 without surgery. Abundant VAChT positive nerve fibers were present around Fos positive neurons. These results indicate a bidirectional interaction between central vagal stimulation of gastric myenteric neurons and abdominal surgery. The modulation of gastric vagus-myenteric neuron activity could play an important role in the recovery phase of postoperative gastric ileus.
Keywords: abdominal surgery, gastric myenteric neurons, Fos, TRH agonist, PGP 9.5, vesicular acetylcholine transporter
1. Introduction
It is well established, clinically and experimentally, that abdominal surgery suppresses effective transit of gastrointestinal content including gastric emptying [10,19,24]. The etiology of abdominal surgery-induced gastric ileus is complex. It involves initially the activation of neural reflex driven by capsaicin-sensitive afferent neurons, and the recruitment of brain stress circuitries including corticotropin releasing factor (CRF) signaling pathways that alters the autonomic nervous system regulating the gastrointestinal motor function [2,3,5,6,15]. Convergent clinical studies indicate that gum chewing improves postsurgical ileus, likely through pathways recruited by sham feeding [7,8]. Experimental studies established that brain medullary thyrotropin-releasing hormone (TRH) plays a physiological role in the central vagal regulation of gastric motor function including the vagally mediated gastric response to sham feeding [25]. Our previous studies also established that intracisternal (ic) injection of TRH- or the stable TRH agonist RX-77368-induced stimulation of gastric vagal efferent discharges, is associated with vagal-cholinergic dependent activation of gastric myenteric neurons in antrum and corpus in rats [18,20,25,27]. In addition we recently reported that ic injection of RX-77368 or activation of brain TRH medullary pathway during acute cold exposure [17,25] prevents postoperative gastric ileus in rats as monitored 90 min post surgery [24]. These data suggest that ic injection of RX-77368 may influence the activity of gastric myenteric neurons in rats undergoing abdominal surgery.
Therefore the present study aimed to do investigate the occurrence of the nuclear phospho-protein Fos in gastric corpus and antrum myenteric neurons as a marker of neuronal activity [18,27] in response to ic RX-77368 in rats which underwent abdominal surgery compared to those not subjected to abdominal surgery. In addition, dual immunohistochemical labeling of Fos with the neuronal marker, protein-gene product 9.5 (PGP 9.5) [14] and vesicular acetylcholine transporter (VAChT), an established marker for the cholinergic nervous system innervation, [22] was examined to determine whether (i) gastric ganglionic myenteric cells expressing Fos as the result of ic RX-77368 alone or combined with abdominal surgery are neurons, and (ii) receive cholinergic innervation.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats weighing 250–300 g (Harlan Sprague-Dawley, Indianapolis, IN) were maintained under standard environmental conditions for at least 1 week before being used. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committee at Veterans Affairs Greater Los Angeles Healthcare System (Animal protocol # 05058-02).
2.2. Surgery and treatment
2.2.1. Abdominal surgery
Abdominal surgery was performed in rats anesthetized with enflurane (5% vapor concentration in oxygen; Éthrane, Ohmeda Pharmaceutical, Liberty Corner, NJ, USA) as in our previous studies [24]. Briefly after a median laparotomy (2–3 cm), the cecum was exteriorized, placed in saline-soaked gauze and gently manipulated between two fingers for 1 min. Thereafter, the cecum was replaced into the abdominal cavity and the midline incision closed in two layers. Anesthesia and surgery lasted for approximately 10 min and animals regained consciousness within 2–3 min after the removal of enflurane. The control groups consisted of rats anesthetized as above for 10 min but without surgery. As the study aim is to assess the impact of abdominal surgery per se on the central initiated mechanisms that modulate gastric myenteric neuronal activity, to avoid any possible confounding influence of anesthesia, rats in the control groups were exposed to same duration of anesthesia as in the abdominal surgery groups but without surgery.
2.2.2. Intracisternal injection
The ic injection of RX-77368 solution or saline was delivered in 10 µl and performed in enflurane anesthetized rats mounted on ear bars of a Kopf stereotaxic frame (model 900) as previously described [18]. The atlanto-occipital membrane was punctured using a Hamilton microliter syringe, and confirmation of needle placement in the cisterna magna was assessed by aspirating cerebrospinal fluid into the syringe. The procedure is performed within 2–3 min.
2.3. Experimental protocols
Four groups of rats were singly housed and fasted for 17 h with free access to drinking water prior to the experiment carried out between 9.00 h and 11.00 h. Two groups of rats were anesthetized with enflurane for 10 min and underwent ic injection of saline or RX-77368 (50 ng in sterile saline; Ferring Pharmaceuticals, Feltham, Middlesex, UK) followed immediately by abdominal surgery. The two other groups were also anesthetized with enflurane for 10 min and injected intracisternally with saline or RX-77368 (50 ng) only. After recovering from anesthesia (2–3 min) all rats were housed singly without access to food or water and euthanized by decapitation 90 min after the end ic injection. The stomach was immediately harvested and processed for Fos, Fos/PGP 9.5 and Fos/VAChT immunohistochemistry. The dose of RX-77368 (50 ng) was based on previous studies showing maximal Fox expression in gastric myenteric neurons [18,27].
2.4. Immunohistochemistry
2.4.1. Gastric tissue preparation
The stomach was placed in phosphate-buffered saline (PBS; pH 7.4) containing nifedipine (10−6 M) to avoid muscle contraction, opened along the small curvature, pinned flat in a Sylgard-coated Petri dish and fixed overnight in buffered 4% paraformaldehyde (pH 7.4) at 4°C. The longitudinal muscle/myenteric plexus (LMMP) whole mount preparations of ~ 0.25 cm2 each were dissected from the gastric antrum and corpus and collected separately in PBS supplemented with 0.1% sodium azide.
2.4.2. Fos immunohistochemistry
Briefly, gastric LMMP whole mount preparations of gastric corpus and antrum were processed for immunohistochemical labeling using an indirect immunofluorescence technique [18]. LMMP whole mount preparations from the corpus or antrum were rinsed (3 × 10 min) in PBS and incubated for 1 h at room temperature with normal goat serum (3%) in PBS containing 0.3% Triton X-100 (PBS-T) before being placed in primary antibody. Tissues were then incubated for 48 h at 4°C with polyclonal rabbit anti-Fos Ab-5 (1:10,000; Oncogene Research Products, MA) diluted in PBS-T. Thereafter, tissues were rinsed (3 × 10 min) in PBS and incubated for 2 h at room temperature with goat anti-rabbit IgG conjugated to TRITC (1:100; Jackson ImmunoResearch, PA) diluted in PBS. Finally tissues underwent a last rinse (3 × 10 min) with PBS, were mounted in bicarbonate-buffered glycerol and examined using the Zeiss LSM 510 laser scanning microscope as detailed elsewhere [18]. Immunohistochemical controls routinely performed involved incubation of tissues in blocking solution followed by antibody diluent in place of primary antibody and processed as described above. No positive staining was observed in controls.
2.4.3. Double immunolabeling Fos/PGP 9.5 and Fos/VAChT
Briefly gastric tissues were rinsed in PBS (3 × 10 min) and incubated for 1 h at room temperature with PBS-T before being placed separately for 48h at 4°C in primary antibody constituted of (i) polyclonal rabbit anti-Fos Ab-5 (1:10,000) combined with monoclonal mouse anti-human PGP 9.5 (1:500; Ultraclone, UK) and (ii) monoclonal mouse anti-Fos (TF161, 1:500, Dr. K. Riabowol, University of Calgary, Canada) combined with rabbit anti-VAChT (1:500, Phoenix Pharmaceuticals, CA). Tissues from both immunohistochemical experimental groups were rinsed separately in PBS (3 × 10 min) and incubated for 2h at room temperature with (i) goat anti-rabbit IgG conjugated to TRITC (1:100) combined with donkey anti-mouse IgG conjugated to FITC (1:100; Jackson ImmunoResearch, PA) for Fos/PGP 9.5 double labeling and (ii) donkey anti-mouse IgG conjugated to FITC (1:100) combined with goat anti-rabbit IgG conjugated to TRITC (1:100) for Fos/VAChT double labeling. Finally tissues underwent a last rinse (3 × 10 min) with PBS, were mounted in bicarbonate-buffered glycerol and examined using the Zeiss LSM 510 laser scanning microscope [18].
2.5. Quantitative analysis and statistics
The number of Fos-immunoreactive (IR) nuclei was blindly counted in 25 myenteric ganglia randomly selected in each examined LMMP whole mount preparation from the gastric antrum or corpus and expressed as a mean number per myenteric ganglion. The mean number of Fos-IR nuclei per myenteric ganglion from each rat was used to generate a mean number for each experimental group. Data are expressed as mean ± SEM and analyzed by One Way ANOVA followed by Newman-Keuls test. The power to detect at least a 15% difference in mean Fos positive number with n=3–6 per group and alpha of 0.05 (one tail) is 90%. Differences between groups were considered significant when p <0.05.
3. Results
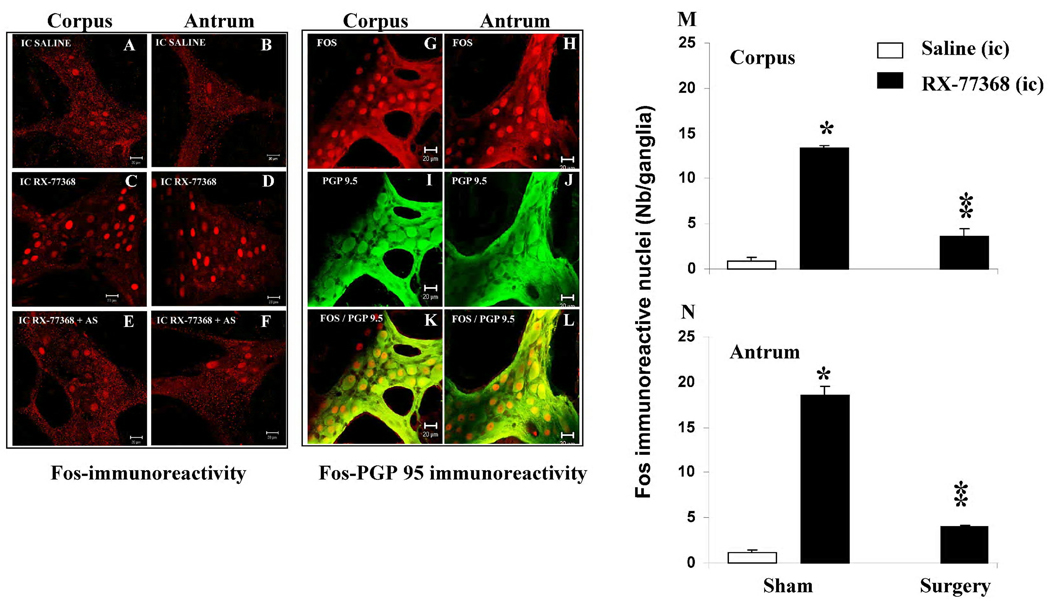
Rats injected intracisternally with saline exhibited a very small proportion of gastric ganglionic myenteric cells expressing Fos in the corpus (Fig. 1A &M) and antrum (Fig. 1B & N) while ic injection of RX-77368 resulted in a widespread Fos-IR nuclei in the corpus (Fig. 1C & M) and antrum (Fig. 1D & N) myenteric ganglia as monitored 90 min post injection. Quantitative analysis of Fos-IR nuclei in these no surgery groups (10 min anesthesia alone) showed that ic injection of RX-77368 resulted in 16.8 fold increased in Fos-IR nuclei in the corpus (13.4 ± 0.3 vs 0.8 ± 0.5 nuclei/ganglion, p<0.05 Fig. 1M) and 16.5 fold increase in Fos-IR nuclei in the antrum (18.5 ± 1.0 vs 1.1 ± 0.4 nuclei/ganglion, p<0.05, Fig. 1N) compared to ic saline injection. Double labeling of Fos with the neuron-specific protein PGP 9.5 ascertained the neuronal identity of gastric ganglionic myenteric cells expressing Fos (Fig. 1G–L).
Fig. 1.
A–F. Representative confocal microscope images showing Fos-IR nuclei in the gastric myenteric ganglia in rats. A–B: ic saline and under 10 min anesthesia; C–D: ic RX-77368 (50 ng) and under 10 min anesthesia. E–F ic RX-77368 (50 ng) + abdominal surgery (AS) under 10 min anesthesia. Fos immunoreactive nuclei are rare in gastric corpus (A) and antrum (B) myenteric ganglia from control rats (ic saline under anesthesia no surgery). Intracisternal injection of RX-77368 induces widespread Fos expression in myenteric ganglia of corpus (C) and antrum (D) in rats from control group (no abdominal surgery) and a modest neuronal activation in corpus (E) and antrum (F) from rats that underwent abdominal surgery. Bars: 20 µm. Fig.1G–L. Double labeling of Fos with PGP 9.5 shows that Fos positive cells in the corpus (G,I & K) and antrum (H,J & L) are mainly neurons. Fig. 1M–N. Quantitative data showing the number of Fos positive nuclei in the corpus (M) and antrum (N). Each bar represents the mean ± SEM Fos-IR nuclei counted in 25 myenteric ganglia/animal (n= 3–6 rats/group) ; * p <0.05; ** p< 0.01
In rats injected intracisternally with saline followed by abdominal surgery, there was no Fos-IR nuclei in both the corpus (Fig. 1M) and antrum (Fig. 1N) myenteric ganglia. The ic injection of RX-77368 (50 ng) before surgery resulted in Fos expression in the corpus (3.6 ± 0.9 vs 0.0 ± 0.0 nuclei/ganglion, Fig. 1E & M) and antrum (4.0 ± 0.1 vs 0.0 ± 0.0 nuclei/ganglion, P <0.05 Fig. 1F & N) myenteric ganglia. However, the response was reduced by 73% and 78% in the corpus and antrum respectively, compared to rats injected with RX-77368 and not subjected to surgery (Fig. 1M & N, p<0.05).
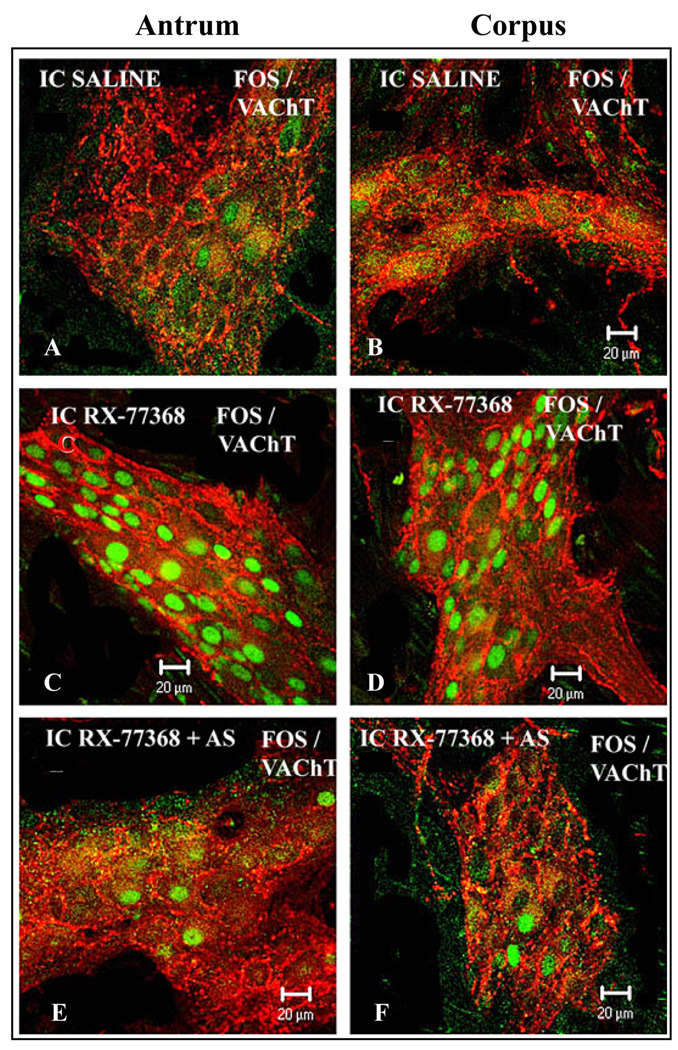
Double labeling of Fos/VAChT showed VAChT-IR varicosities and nerve cells bodies in the gastric corpus and antrum myenteric ganglia from ic RX-77368-injected rats without (Fig. 2C–D) or with abdominal surgery (Fig. 2E–F). VAChT-IR varicosities surrounded all cells bodies including those displaying Fos positive nuclei (Fig. 2C–D and Fig 2E–F). VAChT-IR varicosities and nerve cells bodies were also found in the gastric myenteric ganglia from rats injected intracisternally with saline followed or not by abdominal surgery (Fig. 2A,B) emphasizing the abundance and importance of cholinergic innervation in the gastric myenteric plexus.
Fig. 2.
Representative confocal microscope images showing the double labeling of Fos (green) with VAChT (red) in the myenteric ganglia of gastric corpus (A,C & E) and antrum (B,D & F). A few positive nuclei are seen in the myenteric ganglia from rats injected intracisternally with saline (no surgery) which exhibit abundant VAChT-IR varicosities and nerve cells bodies (A–B). Rats injected intracisternally with RX-77368 show VAChT as well as robust Fos immunoreactivity in the myenteric ganglia (C–D). Rats that received RX-77368 (ic) followed by abdominal surgery (E–F) show abundant VAChT and a few Fos immunoreactivity in the myenteric ganlia. Note that Fos-IR nuclei in the myenteric ganglia after ic RX-77368 (C–D or E–F) were present in VAChT positive nerve cell bodies encircled by abundant varicose fibers-IR to VAChT. Scale bar: 20 µm.
4. Discussion
The present study showed that central vagal activation by ic RX-77368 induces Fos expression in the gastric myenteric neurons in rats, and that abdominal surgery inhibits basal and significantly reduces gastric myenteric neuronal activation induced by RX-77368. These findings provide the first evidence of a bidirectional interaction between central vagal activation of gastric myenteric neurons and abdominal surgery.
In rats under 10 min enflurane anesthesia, RX-77368 injected intracisternally at a low dose (50 ng) induces a widespread Fos expression in the gastric corpus and antrum myenteric ganglia compared to ic injection of saline which shows only minor Fos expression in gastric myenteric neurons monitored 90 min post injection. The neuronal identity of gastric myenteric cells expressing Fos was ascertained by the double labeling of Fos with PGP 9.5, an established neuronal marker [14]. Consistent with the present findings, we previously reported that ic injection of RX-77368 (50 ng) performed under 2–3 min of enflurane anesthesia induces Fos expression in the vast majority (>90%) of neurons in the gastric (corpus and antrum) myenteric ganglia as shown by the double labeling of Fos with PGP 9.5 or cuprolinic blue [18,27]. We also established that such neuronal activation encompasses the entire population of ganglionic cholinergic neurons identified using the antibody selective for peripheral choline acetyltransferase (pChAT) [27]. Moreover, previous anatomical, electrophysiological and pharmacological studies have delineated the involvement of central vagal activation and peripheral nicotinic receptors as the pathways that mediate Fos expression in gastric myenteric neurons in response to ic RX-77368 [18,20]. In addition, previous [18] and present data showed that all Fos positive neurons in gastric myenteric neurons are encircled or make putative contacts with a dense network of VAChT-positive nerve fibers as revealed with anti-VAChT antibody. The observed VAChT innervation is consistent with tracing studies establishing that gastric vagal efferent fibers target all gastric myenteric neurons [12]. Under these conditions, we demonstrate that abdominal surgery consisting of laparotomy followed by a 1-min cecal palpation inhibits Fos expression in gastric corpus-antrum myenteric ganglia by 73–78% as assessed at 90 min post RX-77368 injection. This represents a specific effect of abdominal surgery since gastric myenteric ganglia from rats exposed to the same duration of anesthesia (10 min) without surgery exhibited a widespread Fos expression in the corpus and antrum myenteric ganglia in response to ic RX-77368.
The sites of action and underlying mechanisms through which abdominal surgery dampens ic RX-77368-induced activation of gastric myenteric neurons is yet to be established. However it can be speculated that abdominal surgery may exert this inhibitory action by recruiting both central and peripheral inhibitory pathways. There is evidence that abdominal surgery performed under similar conditions activates brain stress-related circuitries, including CRF signaling pathway [1,4,5,9,28]. Activation of brain CRF circuitry is known to inhibit RX-77368 excitatory input to the prevagal motor neurons [11,21,26] leading to decreased vagal excitatory discharges [13] directed to gastric myenteric neurons [12]. On the other hand, abdominal surgery may also recruit peripheral inhibitory mechanisms at presynaptic levels to modulate the cholinergic vagal efferent output conveyed to gastric myenteric neurons or directly on myenteric neurons. In the present study the distribution of VAChT immunoreactivity in nerves surrounding the gastric myenteric neurons remained unchanged irrespectively of treatments (saline vs. RX-77368 with or without surgery). Although VAChT immunoreactivity delineates the cholinergic innervation of gastric myenteric neurons, this may not be a sensitive marker to detect changes in the activity of cholinergic system due to abundance of VAChT positive nerve fibers and cell bodies in the gastric myenteric plexus in a quiescent state. However, irrespectively of the mechanisms or sites of action, these observations provide functional evidence at the cellular level that abdominal surgery is able to interfere with central vagally mediated stimulation of gastric myenteric activity.
Importantly, the present study demonstrates that in rats undergoing abdominal surgery, ic RX-77368 is still able to activate a non-neglectable proportion of ganglionic myenteric neurons in the gastric corpus and antrum as revealed with Fos immunohistochemistry while ic injection of saline fails to do so. Such changes in neuronal myenteric activity may have functional implications. Silencing the activity of gastric myenteric neurons is known to inhibit gastric emptying [23] and we previously showed that under similar conditions of surgery, gastric emptying is delayed [1,2,24]. Moreover, our previous study showed that ic injection of RX-77368 under similar conditions prevented abdominal surgery-induced inhibition of gastric emptying of a viscous non-nutrient solution monitored during the first post operative hour [24]. Taken together the present and previous functional study [24] suggest that restoring some level of gastric myenteric activity may be sufficient to normalize postoperative gastric ileus.
In summary, we showed using an immunohistochemical approach that the activity of gastric ganglionic myenteric neurons induced by central vagal cholinergic stimulation via the ic injection of stable TRH agonist RX-77368 in rats is markedly reduced by abdominal surgery. However the level of activation of gastric myenteric neurons in rats undergoing abdominal surgery after ic injection of RX-77368 is significantly above that observed after ic injection of saline. This demonstrates for the first time that there is a bidirectional interaction between central vagal stimulation and abdominal surgery in the regulation of gastric myenteric neuron activity. The present findings may also have relevance towards mechanisms through which gum chewing (used as a kind of sham feeding) enhanced the recovery from postoperative ileus in a clinical setting [7,8], as brain medullary TRH that plays a role in the gastric response to sham feeding [16].
Acknowledgments
This work was supported by the Veterans Administration Research Career Scientist Award, VA Merit Award, R01 NIH DK 33061 (YT) and Center Grant NIH DK-41301 (Animal Core, YT, MM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No conflict of interest exists.
References
- 1.Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am J Physiol. 1996;270:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- 2.Barquist E, Zinner M, Rivier J, Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992;262:G616–G620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- 3.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol. Motil. 2004;16 Suppl 2:54–60. doi: 10.1111/j.1743-3150.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonaz B, Plourde V, Taché Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J. Comp. Neurol. 1994;349:212–222. doi: 10.1002/cne.903490205. [DOI] [PubMed] [Google Scholar]
- 5.Bonaz B, Taché Y. Corticotropin-releasing factor and systemic capsaicin-sensitive afferents are involved in abdominal surgery-induced Fos expression in the paraventricular nucleus of the hypothalamus. Brain Res. 1997;748:12–20. doi: 10.1016/s0006-8993(96)01281-4. [DOI] [PubMed] [Google Scholar]
- 6.Calogero AE, Norton JA, Sheppard BC, Listwak SJ, Cromack DT, Wall R, Jensen RT, Chrousos GP. Pulsatile activation of the hypothalamic-pituitary-adrenal axis during major surgery. Metabolism. 1992;41:839–845. doi: 10.1016/0026-0495(92)90164-6. [DOI] [PubMed] [Google Scholar]
- 7.Choi H, Kang SH, Yoon DK, Kang SG, Ko HY, Moon DG, Park JY, Joo KJ, Cheon J. Chewing gum has a stimulatory effect on bowel motility in patients after open or robotic radical cystectomy for bladder cancer: A Prospective Randomized Comparative Study, Urology. 2010 doi: 10.1016/j.urology.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald JE, Ahmed I. Systematic review and meta-analysis of chewing-gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World J. Surg. 2009;33:2557–2566. doi: 10.1007/s00268-009-0104-5. [DOI] [PubMed] [Google Scholar]
- 9.Gourcerol G, Gallas S, Mounien L, Leblanc I, Bizet P, Boutelet I, Leroi AM, Ducrotte P, Vaudry H, Jegou S. Gastric electrical stimulation modulates hypothalamic corticotropin-releasing factor-producing neurons during post-operative ileus in rat. Neuroscience. 2007;148:775–781. doi: 10.1016/j.neuroscience.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood-Van Meerveld B. Emerging drugs for postoperative ileus. Expert. Opin. Emerg. Drugs. 2007;12:619–626. doi: 10.1517/14728214.12.4.619. [DOI] [PubMed] [Google Scholar]
- 11.Herman MA, Cruz MT, Sahibzada N, Verbalis J, Gillis RA. GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am. J. Physiol Gastrointest. Liver Physiol. 2009;296:G101–G111. doi: 10.1152/ajpgi.90504.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J. Comp Neurol. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Kosoyan HP, Wei JY, Taché Y. Intracisternal sauvagine is more potent than corticotropin-releasing factor to decrease gastric vagal efferent activity in rats. Peptides. 1999;20:851–858. doi: 10.1016/s0196-9781(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 14.Krammer HJ, Karahan ST, Rumpel E, Klinger M, Kuhnel W. Immunohistochemical visualization of the enteric nervous system using antibodies against protein gene product (PGP) 9.5. Anat. Anz. 1993;175:321–325. doi: 10.1016/s0940-9602(11)80029-4. [DOI] [PubMed] [Google Scholar]
- 15.Luckey A, Wang L, Jamieson PM, Basa NR, Million M, Czimmer J, Vale W, Taché Y. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125:654–659. doi: 10.1016/s0016-5085(03)01069-2. [DOI] [PubMed] [Google Scholar]
- 16.Martinez V, Barrachina MD, Ohning G, Taché Y. Cephalic phase of acid secretion involves activation of medullary TRH receptor subtype 1 in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1310–G1319. doi: 10.1152/ajpgi.00222.2002. [DOI] [PubMed] [Google Scholar]
- 17.Martinez V, Wu SV, Taché Y. Intracisternal antisense oligodeoxynucleotides to the thyrotropin-releasing hormone receptor blocked vagal-dependent stimulation of gastric emptying induced by acute cold in rats. Endocrinology. 1998;139:3730–3735. doi: 10.1210/endo.139.9.6195. [DOI] [PubMed] [Google Scholar]
- 18.Miampamba M, Yang H, Sharkey KA, Taché Y. Intracisternal TRH analog induces Fos expression in gastric myenteric neurons and glia in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G979–G991. doi: 10.1152/ajpgi.2001.280.5.G979. [DOI] [PubMed] [Google Scholar]
- 19.Miedema BW, Johnson JO. Methods for decreasing postoperative gut dysmotility. Lancet Oncol. 2003;4:365–372. doi: 10.1016/s1470-2045(03)01118-5. [DOI] [PubMed] [Google Scholar]
- 20.O-Lee TJ, Wei JY, Taché Y. Intracisternal TRH and RX 77368 potently activate vagal efferent discharge in rats. Peptides. 1997;18:213–219. doi: 10.1016/s0196-9781(96)00281-1. [DOI] [PubMed] [Google Scholar]
- 21.Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 22.Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84:361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- 23.Sobreira LF, Zucoloto S, Garcia SB, Troncon LE. Effects of myenteric denervation on gastric epithelial cells and gastric emptying. Dig. Dis. Sci. 2002;47:2493–2499. doi: 10.1023/a:1020508009213. [DOI] [PubMed] [Google Scholar]
- 24.Stengel A, Goebel M, Luckey A, Yuan PQ, Wang L, Taché Y. Cold ambient temperature reverses abdominal surgery-induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides. 2010;31:2229–2235. doi: 10.1016/j.peptides.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taché Y, Yang H, Miampamba M, Martinez V, Yuan PQ. Role of brainstem TRH/TRH-R1 receptors in the vagal gastric cholinergic response to various stimuli including sham-feeding. Auton. Neurosci. 2006;125:42–52. doi: 10.1016/j.autneu.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Cardin S, Martinez V, Taché Y. Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 1996;736:44–53. doi: 10.1016/0006-8993(96)00726-3. [DOI] [PubMed] [Google Scholar]
- 27.Yuan PQ, Kimura H, Million M, Bellier JP, Wang L, Ohning GV, Taché Y. Central vagal stimulation activates enteric cholinergic neurons in the stomach and VIP neurons in the duodenum in conscious rats. Peptides. 2005;26:653–664. doi: 10.1016/j.peptides.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zittel TT, De Giorgio R, Brecha NC, Sternini C, Raybould HE. Abdominal surgery induces c-fos expression in the nucleus of the solitary tract in the rat. Neurosci Lett. 1993;159:79–82. doi: 10.1016/0304-3940(93)90803-s. [DOI] [PubMed] [Google Scholar]