Abstract
Insulin-like growth factor-I (IGF-I), a functionally important neurotrophic factor, impacts tissues throughout the body including the central nervous system. In addition to the significant proportion of IGF-I that is synthesized in the liver and released into the plasma, IGF-I is expressed locally in tissues. The present study investigated the relationship between plasma and local brain levels of IGF-I in two well-characterized models of decreased IGF-I. The first is an adult-onset growth hormone deficiency (AOGHD) model, and the second is a caloric restriction (CR) model. In the first cohort of animals from both models, the hippocampus was removed from the brain immediately following decapitation, and in the second cohort, the animals were perfused transcardially with phosphate buffered saline to remove cerebral blood prior to harvesting the hippocampus. Our results demonstrated that although the plasma IGF-I levels were decreased in the CR and AOGHD rats compared to controls, the hippocampal IGF-I levels did not differ among the groups. These data suggest that local brain IGF-I levels are regulated in a different manner than plasma IGF-I levels.
Keywords: Caloric restriction, dwarf rat, hippocampus, Fischer 344 × Brown Norway rat, growth hormone
Introduction
Insulin-like growth factor-I (IGF-I) is a polypeptide hormone that plays a major role in the regulation of cellular processes in tissues throughout the body including, but not limited to, protein synthesis, maintenance of bone mass, and both immune and cardiovascular function (Xu and Sonntag 1996; Sonntag et al. 2005a). Particularly important for the central nervous system, IGF-I is associated with neurotrophic effects and the amelioration of aging-related cognitive impairment (Sonntag et al. 2005b).
Circulating IGF-I is produced primarily in the liver and mediates the endocrine effects of growth hormone (GH). GH produced in the anterior pituitary, is secreted in a pulsatile manner and then binds to its receptor in the liver, stimulating the synthesis and secretion of IGF-I (Le Roith et al. 2001). Although approximately 95% of the IGF-I that acts on the brain is derived from the liver (Yamamoto and Murphy 1995), there also is evidence for local synthesis of IGF-I. Specifically, IGF-I synthesis in the brain has been documented in glia (Breese et al. 1996; Walter et al. 1997), neurons (Bondy et al. 1990; Sonntag et al. 1999) and cerebral vasculature (Delafontaine et al. 1991; Sonntag et al. 1997). Thus, in addition to endocrine effects mediated by circulating IGF-I that crosses the blood–brain barrier (Reinhardt and Bondy 1994; Pan and Kastin 2000), local production provides an avenue for autocrine and paracrine effects of IGF-I in the brain.
In light of the neurobiological importance of IGF-I, the question arises of what relationship, if any, exists between the plasma levels of IGF-I and the levels produced in the brain. Therefore, the present study was designed to determine whether the hippocampal levels of IGF-I reflect the plasma levels of IGF-I in two well-characterized models of IGF-I manipulation. The first is a model of adult-onset growth hormone deficiency (AOGHD) in which IGF-I levels are reduced significantly due to a spontaneous mutation that causes a decrease in GH secretion from the anterior pituitary with a subsequent decrease in plasma IGF-I levels (Carter et al. 2002a,b). The second is a caloric restriction (CR) model, a dietary manipulation that has been well-documented to result in decreased plasma levels of IGF-I compared to ad libitum (AL) fed controls (Masoro 2005). In both models, we used an ELISA to detect IGF-I in the plasma and in the brains of decapitated rats and in rats perfused with dextrose saline to clear blood from the cerebral vasculature. Our results indicate that we can reliably detect IGF-I levels in the brains of perfused rats, and further suggest that in both models there is a remarkable stability of local brain IGF-I levels despite significantly lowered plasma IGF-I levels.
Materials and methods
Experimental subjects
A total of 47 male Lewis and 26 F1 male Fischer 344 × Brown Norway (F344 × BN) hybrid rats were used in the present study. Homozygous AOGHD rats originally derived from the Lewis strain were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN, USA) and F344 × BN rats were obtained from the NIA Caloric Restriction Colony (Harlan Sprague Dawley, Inc.). All animals were maintained in our facility in a climate-controlled room on a 12-h light/dark cycle (lights on 6 am and off 6 pm). The animal facility at Wake Forest University Health Sciences is fully accredited by the American Association for Accreditation of Laboratory Animal Care and complies with all Public Health Service, National Institutes of Health and institutional policies and standards for laboratory animal care. All experiments were conducted in accordance with Guidelines for the Care and Use of Experimental Animals and approved by the Institutional Animal Care and Use Committee.
Adult-onset GH deficiency model
The AOGHD model used in this study is a Lewis strain with a recessive mutation in the transcription factor necessary for the development of the somatotroph, resulting in reduced pituitary GH and plasma IGF-I levels without changes in other anterior pituitary hormones (Carter et al. 2002a,b). Homozygous dwarf male rats were bred with wild type females of the Lewis strain to produce heterozygous (HZ) offspring of normal size. Thereafter, HZ females were mated with homozygous males to produce HZ and homozygous littermates. At 4 weeks of age, homozygous males were identified by reduced body weight compared with HZ males, and were divided into treatment groups. Homozygous rats were administered subcutaneous injections of GH (200 mg of recombinant porcine GH; Alpharma, Victoria, Australia) twice daily from 4 to 14 weeks of age to provide GH during normal development (see Sonntag et al. 2005a for GH in overall development; see Nyberg 2000 for GH in brain development). At 14 weeks of age, the young adult homozygous rats were assigned randomly to either the GH deficient (GH−) or the GH replete (GH+) group. GH− animals then were administered vehicle (saline) from 14 weeks until 8 months of age during which time GH+ animals continued to receive GH twice daily. An age-matched group of HZ animals received twice daily saline injections from 4 weeks until 8 months of age and served as controls. Animals in all 3 groups were killed at 8 months of age, and pituitaries were collected for measurement of GH levels to validate group classifications. Animals were pair-housed starting at 4 weeks of age with food and water AL and weighed every other week to monitor health.
Caloric restriction model
In the NIA CR Colony, F344 × BN rats were fed NIH-31 diet AL until 14 weeks of age. The CR regimen then was initiated by incremental calorie reduction of 10% per week over four weeks, reaching full 60% calorie reduction by week 17. The vitamin-fortified NIH-31 diet fed to CR rats provided 60% of the calories and the 100% of the vitamins consumed by AL rats. After arriving at our facility, age-matched groups of AL and CR rats were fed the appropriate NIH-31 diet daily at 4 pm, had free access to water, were weighed once each week, and were killed at 26 months of age. In order to facilitate monitoring of daily food intake, animals were housed individually.
Plasma and hippocampal tissue collection
The three groups of AOGHD rats (GH+, GH−, and HZ) and the two groups of CR rats (AL and CR) were divided into two cohorts, “non-perfused” and “perfused”, in order to evaluate the effect of removing blood from the cerebral vasculature on the brain levels of IGF-I. All animals were anesthetized with sodium pentobarbital (150 mg/kg, i.p.) prior to euthanasia. The non-perfused cohort of each group was decapitated and the trunk blood collected for analysis of plasma levels of IGF-I. Trunk blood was collected into tubes with 38 units porcine heparin added per milliliter, incubated on ice 1–3 h, centrifuged at 300g for 15 min and plasma collected and stored in aliquots at −80°C. The brain was removed rapidly and chilled for 3 minutes in cold artificial cerebrospinal fluid (140mM NaCl, 5mM KCl, 1mM MgCl2, 1mM CaCl2, 24mM dextrose, 10mM HEPES, pH 7.4). The left hippocampus was dissected from the brain, carefully avoiding contamination with surface blood, weighed, and immediately frozen on dry ice. The perfused cohort of each group was perfused transcardially with phosphate buffered saline containing 5mM dextrose. Prior to perfusion, 1ml of cardiac blood was removed from the left ventricle of the heart using a 1 cm3 tuberculin syringe containing 38 units of porcine heparin for determination of the plasma levels of IGF-I. Samples were centrifuged at 300g for 15 min and plasma stored as described above. The brain and the left hippocampus then were removed, processed and stored as described above.
Extraction of IGF-I from hippocampal tissue
Several protocols were tested to maximize recovery of IGF-I from hippocampal tissue samples. The optimized protocol used here was derived mainly from the method used by D'Ercole (1984) with modifications to improve the consistency of extraction among samples. One molar sodium acetate homogenization buffer (NaA-HB), pH 3.6, was prepared by adding 7.5 ml of 1M sodium acetate to 92.5 ml 1M acetic acid in water and stored at 4°C. Hippocampal tissue from AOGHD or CR rats was homogenized in small sets (n = 6–9) representing all groups and containing both non-perfused and perfused cohorts. Frozen hippocampi were added to 1ml (AOGHD rats; mean Hp 58.3 + 4 mg) or 5ml (CR rats, mean Hp 69.2 + 5.2mg) ground glass dounces on ice containing 15μl NaA-HB/mg tissue and homogenized to uniform appearance. Homogenates were transferred to 1.5 ml microtubes on ice. Each sample was vortexed and two 250 μl aliquots of homogenate were removed and placed into new 1.5 ml microtubes for IGF-I extraction. To reveal potential loss of IGF-I immunoreactivity from the extraction procedure, the second 250 μl aliquot for each homogenate was spiked with 5 μl of 25 ng/ml recombinant rat IGF-I (rrIGF-I; Novozymes Gropep, Adelaide, Australia; receptor grade in 10mM HCl plus 1 mg/ml Sigma-Aldrich (Poole, UK) A7030 BSA carrier protein) for a final dilution of 500 pg/ml in the homogenate. Blank controls, composed of NaA-HB with the addition of 1 mg/ml BSA (A7030) carrier protein, and without or with rrIGF-I spike, were included with each tissue homogenate set. Samples were incubated 2 h at 4°C shaking at 1400 rpm on an Eppendorf Mixmate™, centrifuged for 10 min at 3000g and supernatants were collected and stored 1–2 days at −20°C. For a subset of samples, the pellets were placed on ice and a second extraction was performed by adding 250 μl NaA-HB, re-homogenizing the pellet using microtube pellet pestles, and continuing the 2 h shaking incubation. Frozen supernatants in batches of 32–40 were dried with heat for 2.5 h using a speed vacuum (Savant GMI Inc., Ramsey, MN, USA), capped and stored at −20°C until assayed for IGF-I (within 1 week).
IGF-I measurements in plasma and tissue homogenates
Total IGF-I levels in plasma and hippocampal tissue homogenates were determined using R&D Systems Quantikine® mouse IGF-I Immunoassay (MG100; Minneapolis, MN, USA). Cross-species reactivity to rat IGF-I was confirmed by running a dilution series of rrIGF-I in kit calibrator diluent buffer at equivalent concentrations to the recombinant mouse IGF-I standard series (31–2000 pg/ml). Multiple linear regression confirmed essentially 96% cross-species reactivity in this assay [Rat Abs = −0.0185 + (0.966 × Ms Abs); p < 0.001]. The recombinant mouse IGF-I standard linear regression trend line was y = 0.00041x + 0.0094 (R2 = 1) compared to y = 0.00043x − 0.0095 (R2 = 1) observed for the rrIGF-I test material.
Plasma samples were thawed to room temperature, gently vortexed, diluted serially in kit calibrator diluent at 1:2091 final and assayed according to the manufacturer's instructions. Results are reported as ng IGF-I per milliliter plasma.
Dried supernatants from hippocampal homogenate acid extracts were brought to room temperature, reconstituted in 250 μl of 0.1M HEPES buffer, pH 7.8, gently vortexed and homogenized using microtube pellet pestles. Insoluble material was removed by centrifugation for 10 min at 3000g and supernatants transferred to new tubes. Neutral pH was confirmed in representative samples using pH strips. Supernatants were sampled undiluted according to the manufacturer's instructions. Results are reported as pg IGF-I per milligram hippocampus. Recovery of the 500pg/ml rrIGF-I added in spike homogenate samples was calculated as a percentage of the level measured for spike blank controls after subtraction of the endogenous tissue level from each unspiked sample homogenate pair.
Pituitary GH levels
Measurements of pituitary GH levels were performed to confirm the homozygous or HZ status of the AOGHD rats. Pituitaries were collected from all GH+, GH−, HZ rats immediately following brain removal. Samples were weighed in 0.6 ml microtubes, frozen on dry ice and stored at −80°C. For GH measurement, pituitaries were homogenized at a ratio of 50μl buffer per mg tissue in 20mMHEPES buffer, pH 7.5, containing 300mMNaCl, 2mM EDTA and 1:250 fresh protease inhibitor cocktail (P8340; Sigma-Aldrich) using ground glass dounces on ice. Homogenates were centrifuged at 16,000g for 15 min at 4°C. Supernatants were aliquoted, frozen on dry ice, and stored at −80°C. Measurements of GH levels were made using the Linco rat/mouse GH ELISA (Millipore, Billerica, MA, USA). The Linco rat/mouse GH ELISA was developed to measure rat GH with high specificity and uses purified rat GH as the standard. Supernatants required dilution in kit assay buffer prior to sampling; HZ rat samples were diluted 1:60,000 and dwarf rat samples were diluted 1:4500. The assay was performed according to the manufacturer's instructions with the modification that the GH standard curve was represented by seven levels from 2 to 50 ng/ml (kit 0.07–50 ng/ml) and concentrations for sample unknowns were calculated from a second-order polynomial curve fit of rat GH standards using absorbance measurements made at 630 nm. Results are presented as μg GH per rat pituitary.
Results
The purpose of the current study was 2-fold. The first goal was to establish that local brain IGF-I levels could be measured precisely and independently of plasma IGF-I levels, and the second was to determine whether the plasma and brain levels of IGF-I are regulated in the same manner in two well-characterized models of decreased IGF-I.
Plasma and hippocampal IGF-I immunoassay
Plasma
Preliminary tests confirmed the performance of the R&D Systems Quantikine® mouse IGF-I Immunoassay for direct measurement of total IGF-I in heparinized rat plasma. For these tests, representative heart (perfused group) and trunk plasma samples (non-perfused group) fromF344 × BN AL and CR rats were serially diluted in kit calibrator diluent and tested for IGF-I detection. Absorbance values showed a linear reduction of signal with increasing dilution in parallel to the mouse IGF-I standard provided with the assay. This indicates sufficient inhibition of potential quenching agents within the rat plasma samples (i.e., IGF binding proteins or receptors) by the kit calibrator diluent, yielding a constant prediction for IGF-I levels over a broad range of dilutions. The exceptional sensitivity of this colorimetric ELISA allows very high dilution of samples, further enabling reduction of binding protein interference and supporting reliable measurements of total plasma IGF-I. A final assay dilution of 1:2091 for all experimental plasma samples placed absorbance values within the central region of the mouse IGF-I standard curve. In order to test for the possibility that genetic variance in the AOGHD model might alter the composition or levels of circulating quenching agents, a small test group of plasma samples representing each group (GH+, GH−, and HZ) and including both heart and trunk plasma samples, were diluted 1:2091 in kit calibrator diluent and an aliquot was spiked with 500 pg/ml rrIGF-I. One hundred percent of the rrIGF-I spike signal was recovered for each experimental status (data not shown), indicating reliable relative measures of total plasma IGF-I in potentially diverse states of endogenous quenching.
Hippocampus
It is well-established that immunodetection of IGF-I protein in plasma is hindered by the presence of soluble IGF-I binding proteins, and the presence ofIGF-I binding proteins and receptors in brain tissue would be expected to pose similar problems for IGF-I measurements in homogenates of hippocampal tissue. As the R&D ELISA was developed for direct measurement of total IGF-I in plasma, it was necessary also to evaluate the efficacy of that ELISA for direct measurement of total IGF-I in brain tissue homogenized in a neutral buffered solution. To evaluate the efficacy of measurements, we also followed the method of D'Ercole et al. (1984) and extracted IGF-I away from binding components in hippocampal tissue samples by homogenizing in 1M acetic acid (1M AA), but our results indicated poor IGF-I recovery. As D'Ercole et al. (1984) reported that pH levels below 3.6 resulted in reduced IGF-I immunoreactivity, we modified the protocol to include an extraction buffer, specifically 1M NaA-HB to maintain pH at 3.6.
Initial trials of IGF-I extraction from hippocampal tissue (1:10) revealed approximately 3-fold greater levels of immunodetectable IGF-I measured in tissue homogenized using NaA-HB compared to either 1M AA or 0.1M Tris–HCl (pH 7.8). A 5-fold dilution of these extracts in the ELISA kit calibrator diluent (to evaluate quenching) increased detection of IGF-I by only 1.09-fold in the 1M NaA-HB-extracted samples compared to 1.72- and 2.02-folds for 1M AA and 0.1M Tris (pH 7.8) homogenates, respectively. The reduced levels and poor recovery with dilution observed for the 1M AA extraction probably reflects loss of immunoreactive IGF-I at low pH. It is possible that increasing dilution of the 0.1M Tris-HCl (pH 7.8) homogenate supernatants in the kit calibrator diluent would result in immunodetectable IGF-I levels comparable to the 1M NaA-HB extraction, however, the low levels present in the hippocampus did not allow sufficient dilution before exceeding the sensitivity of the ELISA. Subsequent assay development trials using only 1M NaA-HB (pH 3.6) at 15 μl per mg hippocampus for extraction led to the final protocol described in Materials and methods. Importantly, serial dilution of NaA-HB hippocampal homogenate test samples predicted relatively constant immunodetectable levels of IGF-I (96% when diluted by 1/2 and 94% when diluted by 1/4 in kit calibrator diluent), indicating a lack of interference by quenching agents. All experimental samples were assayed undiluted to place measured absorbance values within the central region of the recombinant mouse IGF-I standard curve.
Potential biochemical and procedural loss of IGF-I during extraction was examined using rrIGF-I. Hippocampal tissue was homogenized and immediately divided into two equal-volume aliquots. One aliquot for each sample was spiked with 500 pg/ml rrIGF-I and the pair of samples then was treated identically through the remainder of extraction procedures. A reference rrIGF-I spike blank sample (BSA carrier protein, no tissue) was included with each homogenization group. Potential interference in rrIGF-I signal by the presence of tissue (i.e., binding proteins, receptor, and enzymes) was indicated by calculating the recovery of the rrIGF-I spike in tissue homogenates (spiked minus endogenous level for each pair) relative to the spike blank control. In the AOGHD model, two-way ANOVA showed no significant main effect of group (perfusion, perfused or non-perfused; GH status, GH+, GH−, or HZ) and no significant group interactions. The AOGHD overall mean tissue rrIGF-I spike recovery was 84.4 + 2.1% (SEM, n = 56). For the CR model, two-way ANOVA also showed no significant main effect of group (perfusion, perfused or non-perfused; diet, AL, or CR) and no significant group interactions. The CR overall mean tissue rrIGF-I spike recovery was 100 + 2.4% (SEM, n = 24). The reason for the lower rrIGF-I spike recovery in the AOGHD model is unclear, but may reflect a difference between the Lewis and F344 × BN strains. In order to estimate the loss of IGF-I immunoreactivity due to the extraction procedure, rrIGF-I levels in the experimental spike blank samples were compared to those in a freshly diluted 500 pg/ml rrIGF-I sample run in each ELISA assay. This comparison revealed no significant difference in procedural loss of rrIGF-I measured from spike blank reference samples for either AOGHD or CR groups, with an overall recovery equal to 75 + 3.6% (SEM) of the freshly diluted rrIGF-I. Thus, values reported here for hippocampal samples underestimate actual IGF-I levels minimally by 25%.
IGF-I levels in adult-onset GH deficiency
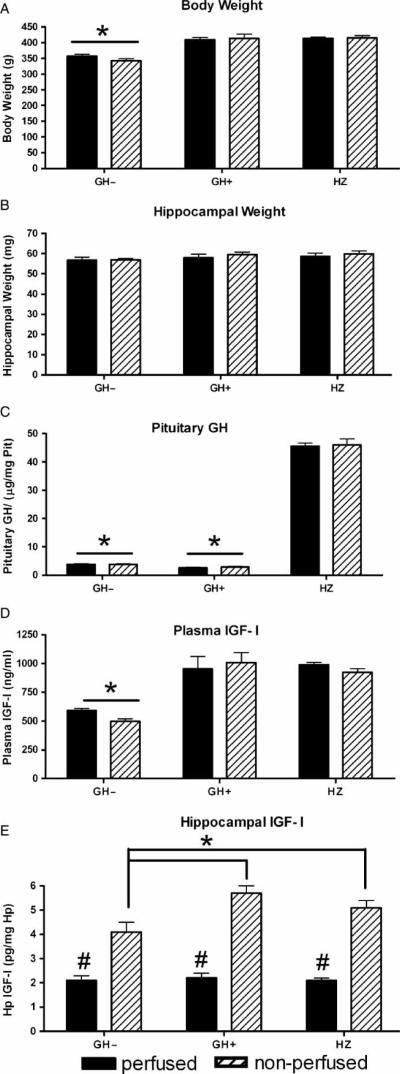
Weekly monitoring of animal weight indicated similar body weights in the GH+ and HZ rats (Figure 1(A)). In contrast, the mean body weight of GH− rats was significantly less than that of either the GH+ or HZ groups (Figure 1(A); p < 0.001). We also examined hippocampal weight and found it to be similar across groups and cohorts (perfused and non-perfused, Figure 1(B)). Finally, we confirmed the dwarf status of all animals using an ELISA assay to determine GH levels in the pituitary. Perfusion with phosphate buffered saline had no significant impact on pituitary GH measurements, and the small amount of blood in the pituitary homogenates in non-perfused rats did not contribute to the GH signal at the dilutions tested in this assay (Figure 1(C)). Both GH− and GH+ groups had minimal levels of pituitary GH compared to the HZ rats (Figure 1(C), p < 0.001).
Figure 1.
Adult-onset GH deficiency model. (A) GH deficient (GH2−) groups have significantly lower mean body weights than heterzygous (HZ) and GH replete (GH+) groups. Perfused and non-perfused cohorts do not differ in any group. *p < 0.001 GH− compared to GH+ and HZ. (B) Hippocampal weight does not differ among HZ, GH+ and GH− groups. (C) Pituitary levels of GH in GH+ and GH− groups are significantly lower compared to the HZ group. Perfused and non-perfused cohorts do not differ in any group. *p < 0.001 GH− and GH+ compared to HZ. (D) Plasma IGF-I levels are significantly lower in GH− than in GH+ and HZ rats, but do not differ between perfused (cardiac blood) and non-perfused (trunk blood) in any group. *p < 0.001 GH− compared to GH+ and HZ. (E) In non-perfused hippocampus, the IGF-I level in GH− rats is significantly lower than in GH+ and HZ rats. In contrast, in perfused hippocampus, IGF-I levels do not differ among groups, but in each group are significantly lower than in non-perfused hippocampus. *p < 0.001 non-perfused hippocampus GH− compared to GH+ and HZ; #perfused GH−, GH+, and HZ groups compared to non-perfused GH−, GH+, and HZ groups.
GH regulates the liver production of IGF-I, and GH deficiency lowers the levels of circulating IGF-I (Carter et al. 2002a,b). Consistent with this relationship, our data revealed that both the cardiac (perfused cohort) and trunk (nonperfused cohort) plasma samples had significantly lower IGF-I levels in GH− animals compared to GH+ and HZ rats (Figure 1(D); p < 0.001), and that those samples did not differ from each other in any of the three groups. In order to determine whether brain levels of IGF-I are similarly regulated by GH, we measured hippocampal IGF-I levels in the GH−, GH+, and HZ groups in two cohorts. The first cohort of animals in each group was decapitated, and the second cohort was perfused transcardially with phosphate buffered saline. We observed that in the decapitated, nonperfused cohort, the hippocampal levels of IGF-I were significantly lower in the GH− than in either the GH+ or HZ rats (Figure 1(E); p < 0.001). In perfused animals, however, the hippocampal levels of IGF-I did not differ among groups (Figure 1(E)), and were significantly lower than in non-perfused tissue in all groups. These results suggested that: (i) the observed difference in hippocampal IGF-I levels in the non-perfused GH− compared to GH+ and HZ groups is a reflection of plasma, rather than actual brain levels of IGF-I and (ii) local brain levels of IGF-I are not regulated by systemic levels of GH.
IGF-I levels in CR
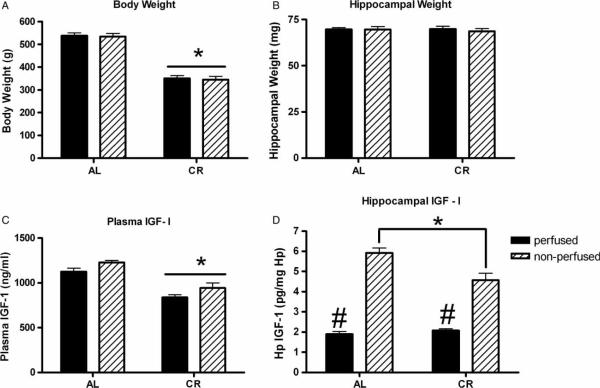
Daily monitoring of food consumption ensured that the required calories were consumed, and body weight was checked weekly to monitor health. The differences in mean weight varied significantly between groups and the weight of CR rats was 40% lower than that of AL rats (Figure 2(A); p < 0.001). No differences were observed in the hippocampal weight between AL and CR rats (Figure 2(B)). Neither body weight nor hippocampal weight differed between perfused and non-perfused cohorts.
Figure 2.
Caloric restriction model. (A) CR groups have significantly lower mean body weights than AL fed groups. Perfused and non-perfused cohorts do not differ in any group. *p < 0.001 CR compared to AL. (B) Hippocampal weight does not differ between the AL and CR groups. (C) Plasma IGF-I levels are significantly lower in CR than in AL rats, but do not differ between perfused (cardiac blood) and non-perfused (trunk blood) in any group. *p < 0.001 CR compared to AL. (D) In non-perfused hippocampus, the IGF-I level in CR rats is significantly lower than AL rats. In contrast, in perfused hippocampus, IGF-I levels do not differ between CR and AL rats, but in both groups are significantly lower than in non-perfused hippocampus. *p < 0.001 non-perfused hippocampus CR compared AL; #p < 0.001 perfused AL and CR groups compared to non-perfused AL and CR groups.
CR has been shown to reduce circulating levels of IGF-I (Masoro 2005). Corroborating those findings, results in the present study indicated that the plasma levels of IGF-I in both perfused and non-perfused rats were significantly lower in the CR compared to the AL group (Figure 2(C); p < 0.001). In addition, plasma IGF-I levels in perfused and non-perfused cohorts were similar to each other in both groups (Figure 2(C)). Analysis of hippocampal levels of IGF-I revealed significantly lower levels in the non-perfused hippocampus of CR compared to AL rats (Figure 2(D), p < 0.001). IGF-I levels in the perfused hippocampus were significantly lower than in the non-perfused for both groups and IGF-I levels in the perfused hippocampus did not differ between AL and CR groups (Figure 2(D)). These data are consistent with those from the AOGHD model and support the notion that the higher IGF-I levels in the non-perfused hippocampus of AL rats are due to the contribution of the plasma and may not reflect actual brain levels of IGF-I.
Discussion
IGF-I has functionally significant effects on the adult brain. For example, intracerebroventricular (ICV) infusion of IGF-I improved performance on the Morris water maze test of spatial learning and memory, a task that is dependent on the hippocampus (Markowska et al. 1998). Moreover, ICV infusion of IGF-I increased neurogenesis in the dentate gyrus of the hippocampus in aged rats (Lichtenwalner et al. 2001), as well as facilitated the maintenance and/or formation of synapses (Nieto-Bona et al. 1997; Shi et al. 2005). IGF-I also affects synaptic proteins, synaptic strength, and membrane excitability (Le Grevès et al. 2005; Ramsey et al. 2005). Levels of the N-methyl-D-aspartate (NMDA) type of glutamate receptor decrease in the aging brain and short-term ICV infusion of IGF-I reverses this decrease (Sonntag et al. 2000). Moreover, it has been demonstrated recently that acute application of IGF-I to hippocampal slices from young and old rats increases the peak amplitude response of the NMDA currents in the CA1 region (Molina et al. 2008).
IGF-I has been shown to cross the blood–brain barrier (Reinhardt and Bondy 1994; Pan and Kastin 2000), providing an endocrine source for the neurotrophic effects of IGF-I. In addition, IGF-I expression has been documented in the brain (Jafferali et al. 2000; Sonntag et al. 2000; Le Grevès et al. 2005) suggesting the potential for autocrine and/or paracrine control of brain levels of IGF-I. Moreover, the expression of IGF-I in the brain raises the issue of whether local levels of IGF-I in the brain are controlled in a similar fashion to the circulating IGF-I levels. In the present study, we asked whether hippocampal levels of IGF-I vary in the same direction and to the same extent as plasma levels in two animal models with low circulating IGF-I, a genetic model of AOGHD and a model of lifelong CR. In AOGHD rats plasma IGF-I levels are altered by an endogenous, genetic change producing a GH deficiency (Carter et al. 2002a,b). In CR plasma IGF-I levels are lowered by an environmental factor, a diet consisting of 60% AL calories beginning at 4 months of age (Masoro 2005).
The first step in the present study was to establish that IGF-I within the brain could be measured accurately, and independently of the IGF-I in the plasma. To this end, we critically evaluated the efficacy of an ELISA designed for direct measurement of plasma IGF-I, for use in the measurement of IGF-I in brain tissue. Our findings revealed consistent direct measurement of IGF-I in the plasma using this assay. In addition, we were able to consistently measure IGF-I levels in hippocampus using a pH-controlled acid extraction to separate IGF-I from quenching agents. Importantly, pH is an essential variable in the extraction procedure, with diminishing levels of immunodetectable IGF-I present at lower pH (D'Ercole et al. 1984). Accordingly, we modified the protocol to include an acid extraction buffer, specifically NaA-HB at pH 3.6, in order to reduce the potential loss of IGF-I immunoreactivity due to low pH.
The second focus of the present study was to evaluate whether the biological conditions that regulate plasma IGF-I result in parallel changes in local brain levels of IGF-I. In both the AOGHD and CR models, plasma levels of IGF-I followed expectations. In the AOGHD model, GH− rats had significantly lower plasma levels than both GH+ and HZ rats. In the CR model, CR rats had significantly lower plasma levels than AL rats. Because IGF-I levels are so much higher in plasma (500–1000 ng/ml) than in hippocampus (2 pg/mg), it is not surprising that hippocampal IGF-I levels in non-perfused cohorts demonstrate the same relationship across groups in both models as do plasma IGF-I levels. Strikingly, however, in both models if the cerebral blood is washed out, the hippocampal levels of IGF-I are stable across groups and independent of either GH status (AOGHD model) or diet (CR model). Taken together these results suggest that the autocrine and/or paracrine mechanisms that determine local levels of IGF-I in brain are regulated in a different manner than the endocrine control of plasma IGF-I.
Conclusion
Our data suggest that local IGF-I levels in brain are regulated in a different manner than plasma IGF-I levels. Furthermore, hippocampal levels of IGF-I appear to be stable across conditions regulating plasma IGF-I as demonstrated in two different models of plasma IGF-I reduction. Notably, our results indicate that plasma IGF-I must be cleared from the tissue in order to accurately determine local brain levels of IGF-I. This stability and tight regulation of brain IGF-I levels may serve to protect the brain and also to provide a means for separate homeostatic regulation of brain function via autocrine and/or paracrine mechanisms.
Acknowledgements
This work was supported by NIA P01AG11370, R01AG019886, and K01AG027828.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Bondy CA, Werner H, Roberts CT, Jr, LeRoith D. Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: Comparison with IGF-II gene expression. Mol Endocrinol. 1990;4:1386–1398. doi: 10.1210/mend-4-9-1386. [DOI] [PubMed] [Google Scholar]
- Breese CR, D'Costa A, Rollins YD, Adams C, Booze RM, Sonntag WE, Leonard S. Expression of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 2 (IGF-BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J Comp Neurol. 1996;369:388–404. doi: 10.1002/(SICI)1096-9861(19960603)369:3<388::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Ingram RL, Cashion AB, Cefalu WT, Wang ZQ, Sonntag WE. Models of growth hormone and IGF-1 deficiency: Applications to studies of aging processes and life-span determination. J Gerontol A Biol Sci Med Sci. 2002a;57:B177–B188. doi: 10.1093/gerona/57.5.b177. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002b;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- D'Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: Further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA. 1984;81:935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafontaine P, Lou H, Alexander RW. Regulation of insulin-like growth factor I messenger RNA levels in vascular smooth muscle cells. Hypertension. 1991;18:742–747. doi: 10.1161/01.hyp.18.6.742. [DOI] [PubMed] [Google Scholar]
- Jafferali S, Dumont Y, Sotty F, Robitaille Y, Quirion R, Kar S. Insulin-like growth factor-I and its receptor in the frontal cortex, hippocampus, and cerebellum of normal human and alzheimer disease brains. Synapse. 2000;38:450–459. doi: 10.1002/1098-2396(20001215)38:4<450::AID-SYN10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Le Grevès M, Le Grevès P, Nyberg F. Age-related effects of IGF-I on the NMDA-, GH- and IGF-I-receptor mRNA transcripts in the rat hippocampus. Brain Res Bull. 2005;65:369–374. doi: 10.1016/j.brainresbull.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Molina DP, Weiner JL, Ariwodola SJ, Sonntag WE, Adams MM, Brunso-Bechtold JK. Effect of growth hormone (GH) supplementation on age-related changes in excitatory synaptic transmission in CA1. Soc Neurosci Abstr. 2008;537:18. [Google Scholar]
- Nieto-Bona MP, Garcia-Segura LM, Torres-Alemán I. Transynaptic modulation by insulin-like growth factor I of dendritic spines in Purkinje cells. Int J Dev Neurosci. 1997;15:749–754. doi: 10.1016/s0736-5748(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Nyberg F. Growth hormone in the brain: Characteristics of specific brain targets for the hormone and their functional significance. Front Neuroendocrinol. 2000;21:330–348. doi: 10.1006/frne.2000.0200. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Interactions of IGF-I with the blood–brain barrier in vivo and in situ. Neuroendocrinology. 2000;72:171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J Neurophysiol. 2005;94:247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood–brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb Cortex. 2004;15:571–577. doi: 10.1093/cercor/bhh158. Epub 2004 Aug 18. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, McShane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269–279. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor sub-type expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor 1 deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005a;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005b;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Walter HJ, Berry M, Hill DJ, Logan A. Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology. 1997;138:3024–3034. doi: 10.1210/endo.138.7.5284. [DOI] [PubMed] [Google Scholar]
- Xu X, Sonntag WE. Growth hormone and aging: Regulation, signal transduction and replacement therapy. Trends Endocrinol Metab. 1996;7:145–150. doi: 10.1016/1043-2760(96)00043-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Murphy LJ. Enzymatic conversion of IGF-I to des(1–3)IGF-I in rat serum and tissues: A further potential site of growth hormone regulation of IGF-I action. J Endocrinol. 1995;146:141–148. doi: 10.1677/joe.0.1460141. [DOI] [PubMed] [Google Scholar]