Abstract
Exposure to a spatial location leads to habituation of exploration such that, in a novelty preference test, rodents subsequently prefer exploring a novel location to the familiar location. According to Wagner's (1981) theory of memory, short-term and long-term habituation are caused by separate and sometimes opponent processes. In the present study, this dual-process account of memory was tested. Mice received a series of exposure training trials to a location before receiving a novelty preference test. The novelty preference was greater when tested after a short, rather than a long, interval. In contrast, the novelty preference was weaker when exposure training trials were separated by a short, rather than a long interval. Furthermore, it was found that long-term habituation was determined by the independent effects of the amount of exposure training and the number of exposure training trials when factors such as the intertrial interval and the cumulative intertrial interval were controlled. A final experiment demonstrated that a long-term reduction of exploration could be caused by a negative priming effect due to associations formed during exploration. These results provide evidence against a single-process account of habituation and suggest that spatial habituation is determined by both short-term, recency-based memory and long-term, incrementally strengthened memory.
Keywords: mice, recognition memory, spatial learning, novelty, familiarity
Habituation can reflect both short-term and long-term reductions in unconditioned responding, but often, both forms are claimed to reflect the same qualitative, nonassociative process in memory (e.g., Kandel & Schwartz, 1985). In contrast to this view, Wagner (1981) proposed that habituation is caused by both nonassociative and associative processes. Specifically, short-term habituation is caused by a nonassociative process and long-term habituation is caused by an associative process. Wagner (1981) also predicts that, under certain conditions, there is competition between these separate processes.
Wagner (1976, 1981) proposed that habituation is caused by a representation of a stimulus being active in a refractory memory state at the time when a stimulus is presented. A representation can become active by either a recent presentation of a stimulus or by associative retrieval of the representation. More formally, Wagner (1981) proposed that a stimulus is represented by a set of elements. When a stimulus is presented it is able activate a proportion of its elements into a primary activity state (A1). Elements rapidly decay from the A1 state into a secondary activity state (A2) where they remain before gradually decaying back to an inactive state. Whereas elements in the A1 state receive processing and can generate strong levels of responding, elements in the A2 state cannot be processed and are less able to generate responding. If elements are in the A2 state when a stimulus is presented they are not able to return to the A1 state. Consequently, there is a reduction in the number of elements that can be activated into the A1 state, which results in a reduction in responding. Thus, habituation occurs to the degree to which a stimulus' elements are in the A2 state. Habituation can occur simply because a recent presentation of a stimulus results in its elements being active in the A2 state (self-generated priming). However, if enough time has passed after a stimulus presentation, such that a stimulus' elements have returned to the inactive state, then habituation will not occur, because the stimulus will be able to fully activate its elements into the A1 state. Therefore, a short interval between stimulus exposures can result in a short-term form of habituation.
Whereas short-term habituation reflects a time-dependent decay process, long-term habituation reflects an associative retrieval process. Wagner (1981) suggested that long-term habituation occurs because associations formed between stimuli can result in elements being directly activated into the A2 state (retrieval-generated priming). Thus, if a stimulus (e.g., X) has formed an association with the context in which it is presented, then presentation of the context can associatively activate X's elements into the A2 state. Associative activation of elements into the A2 state results in a long-term form of habituation, because rather than being dependent on how recently a stimulus was experienced (as for short-term habituation), the level of A2 activation is dependent on the strength of the association between stimuli.
Associative learning occurs to the extent that elements of different stimuli are concurrently active in the A1 state. If there is a short interval between stimulus exposures, then A2 activation caused by self-generated priming will reduce the number of elements that are active in the A1 state. This will reduce the amount of associative learning that can take place on a particular trial. Therefore, the associative process that underlies long-term habituation occurs more readily when stimulus exposures are separated by a long interval. Thus, while self-generated and retrieval-generated priming independently cause habituation they can compete with one another such that self-generated priming can reduce long-term habituation (Wagner, 1981).
Evidence for competition between short-term and long-term habituation has been provided by experiments examining the effect of the interval between stimulus exposures. It has typically been observed that short intervals between exposures to a stimulus result in greater habituation of unconditioned responding than long intervals (Thompson & Spencer, 1966; Groves & Thompson, 1970). However, Davis (1970) demonstrated that short and long intervals between stimulus exposures can in fact have opposite effects on short-term and long-term habituation of the startle response in rats. In this experiment one group of rats received habituation training with a short interval (2 s) between stimulus presentations (massed training) and another group received similar training, but with a long interval (16 s) between stimulus presentations (spaced training). Both groups then received a test session after a 1-min interval. During training the massed training group showed greater habituation than the spaced training group. However, in the test session the pattern of performance of the two groups had reversed, and now the spaced training group showed greater habituation than the massed training group. Therefore, short intervals between training trials resulted in a stronger, short-term form of habituation than long intervals, but long intervals between training trials produced a more durable, long-term form of habituation (Davis, 1970). Similar results demonstrating that temporally spaced stimulus presentations can cause greater long-term habituation than massed presentations have been found in a range of species. These include the orienting response in humans (Gatchel, 1975), reverse swimming response in worms (Beck & Rankin, 1997), gill withdrawal response in aplysia (Carew, Pinsker, & Kandel, 1972) and exploratory response in rats (Anderson, Jablonski, & Klimas, 2008).
Wagner's theory (1981) can explain Davis' (1970) results by suggesting that a short interval between stimulus exposures during training increased self-generated priming. This would result in strong short-term habituation, but would also reduce the associative process underlying long-term habituation. In contrast to Wagner's (1981) account, Mackintosh (1987) has suggested that there are other explanations of Davis' (1970) results that do not need to appeal to a dual-process model. First, it is possible that short intervals between stimulus exposures may increase the likelihood that nonmnemonic factors such fatigue may reduce responding. This in turn may reduce the amount of processing that the stimulus receives during training. Thus, when tested after an interval that is long enough to allow recovery from fatigue, responding will return (i.e., dishabituation). Therefore, short-term and long-term habituation need not both reflect stimulus-specific memory processes. Second, the overall length of exposure to the experimental context in which the habituated stimulus was presented was different between the groups that received massed and spaced stimulus presentations. A less familiar context may cause greater arousal than a more familiar context. This may cause dishabituation to the target stimulus during the final test. Last, performance during the test session may be affected by a generalization decrement between differences in the training and test session in terms of the perception of the stimulus or the state of the animal. Therefore, it is possible that animals that received spaced training showed greater habituation during the test session conducted after a relatively long interval, because the conditions at the test more closely matched the conditions during training compared to the group that received massed training. Given that these factors may affect performance, Mackintosh (1987) suggested that Davis' (1970) results may reflect a single, short-term memory process that shows incomplete transfer over time.
The purpose of the following set of experiments was to test the prediction that short-term and long-term habituation reflect separate processes that can compete with one another. Experiments 1 and 2 examined habituation in mice using a spatial exploration task. In Experiment 1 mice were tested using a design similar to that used by Davis (1970), but that adopted procedures to rule out potential confounds as suggested by Mackintosh (1987). Experiments 2a and 2b sought to provide further evidence for long-term habituation that is consistent with a dual-process account of habituation. Finally, Experiment 3 endeavored to find evidence that an associative process results in long-term spatial habituation.
Experiment 1
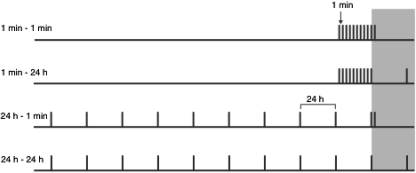
The aim of Experiment 1 was to test the effect of the interval between stimulus exposures on preference for a novel spatial location using a design that is similar to Davis' (1970) experiments (Figure 1). Mice were tested using a spatial novelty preference task that has been demonstrated to rely on the use of extramaze, allocentric cues (Sanderson et al., 2007). Mice were given ten 2-min exposure trials to two arms of Y-shaped maze and then received a novelty preference test in which they were now allowed to explore the two previously visited arms and the third, novel arm. In different conditions the exposure training trials were either separated by a short, 1-min interval (massed training) or by a long, 24-h interval (spaced training). Mice from each training inter-trial interval (ITI) condition then received a novelty preference test after either 1 min (Group 1 min–1 min, Group 24 h–1 min) or 24 h (Group 1 min–24 h, Group 24 h–24 h; see Figure 1). Thus, mice were tested in one of four conditions in which the effects of the training ITI and test interval were assessed in a factorial design.
Figure 1. Design of Experiment 1. The black bars on the white background represent exposure training trials and black bars on the gray background represent the novelty preference test. A short gap between the bars represents a 1-min interval, and a long gap represents a 24-h interval (not to scale). Two groups received exposure training trials separated by a 1-min inter-trial interval (ITI) (Groups 1 min–1 min, and 1 min–24 h) and another two groups received training with a 24-h ITI (Groups 24 h–1 min, and 24 h–24 h). A group from each training condition was then tested after either a 1-min interval (Groups 1 min–1min, 24 h–1 min) or after a 24-h interval (Groups 1 min–24 h, and 24 h–24 h).
The design of the task differed from that of Davis' (1970) in two important ways. First, in the present experiment habituation was assessed by a stimulus-specific novelty preference test. Thus, if habituation has occurred to the familiar arm, then mice should show greater exploration of the novel arm than the familiar arm. This stimulus-specific test rules out an account of habituation in terms of a global decline in responding to all stimuli of a particular modality. Second, the present experiment provides a means of assessing whether differences in the long-term consequences of massed and spaced habituation simply reflect dishabituation caused by differences in the training and test conditions. This would be indicated by mice, tested after an interval that is different from the interval between training trials (i.e., Group 1 min–24 h, and Group 24 h–1 min), showing a smaller novelty preference than mice trained with an interval that is common throughout training and testing (Group 1 min–1 min, and Group 24 h–24 h). This pattern of effects would result in a Training ITI × Test interval interaction. However, main effects of test interval and training ITI would indicate independent effects of the interval on short-term and long-term habituation, respectively.
Method
Subjects
Thirty-six female C57BL/6J/Ola mice Mus musculus obtained from Harlan OLAC Ltd (Oxon, United Kingdom) were used. The mice were approximately 10 weeks old and a mean weight of 17.6 g (range = 16.5–19.5 g) at the start of testing. Mice were caged in groups of six, in a temperature-controlled housing room (light–dark cycle; 0700–1900). Mice were tested during the light period between 10 a.m. and 2 p.m. Throughout testing, mice had ad libitum access to food and water.
Apparatus
A Y-maze constructed from transparent Perspex was mounted on an opaque square Perspex board (64.5 cm × 56.5 cm). The walls of the Y-maze were 20 cm high and 0.5 cm thick. Each arm was 30 cm long and 8 cm wide. The maze was placed in a room containing a variety of extramaze cues. During the exposure trials, the entrance to a given arm could be blocked using a sheet of opaque Perspex. The floor of the maze was covered by a thin layer (~1 mm) of sawdust bedding that was replaced daily.
Procedure
Mice received repeated exposures to two arms of the Y-maze (defined as the Start and Familiar arms). At the end of exposure training, mice received a novelty preference test in which they were now allowed to explore all three arms (i.e., the previously exposed, Start and Familiar arms and the previously unexplored, Novel arm). The experimenter sat facing the end of an arm of the maze, so that the two remaining arms were at an equal distance from the experimenter. The arm of the Y-maze located closest to the experimenter (arm 1) was always used as the Start arm. From the remaining arms (arms 2 and 3), mice were allocated one arm as the pre-exposed Familiar arm and one arm as the Novel arm.
Mice received ten 2-min exposure training trials. At the start of a trial the mouse was placed at the end of the Start arm, facing the end wall, and was allowed to explore the Start arm and Familiar arm. The 2-min period started as soon as the mouse was placed in the maze. Access to the Novel arm was blocked during the exposure trials. At the end of a trial the mouse was returned to its homecage. For short intertrial intervals, the homecage remained in the testing room, but for long intervals the homecage was returned to the holding room. At the end of every trial the sawdust bedding was mixed and randomly redistributed throughout the maze so as to minimize the effects of intramaze odor cues.
After the completion of exposure training mice received a novelty preference test. At the start of the novelty preference test the mouse was placed at the end of the Start arm, facing the end wall, and now allowed to explore the Start, Familiar, and Novel arms. The time spent in each arm was recorded for 2 min once the mouse had left the Start arm (by placing all four paws outside of the arm). Mice were considered to have entered an arm when all four paws were placed inside an arm. Similarly, mice were considered to have left an arm once all four paws were placed outside of the arm. At the end of the 2-min test period, the mouse was removed from the maze and returned to its homecage.
The time spent in the arms and the number of arm entries throughout training and testing were scored manually by the experimenter. Previous work (Sanderson and Bannerman, unpublished data) has demonstrated that there was a significant correlation between the scores generated by the experimenter and an observer who was blind to the group allocation of the mice and the arm contingencies (r = .96, p < .0005), thus indicating robust interrater reliability.
Mice were trained and tested in one of the four groups (i.e., 1 min–1 min, 1 min–24 h, 24 h–1 min, 24 h–24 h; N = 9 per group; see Figure 1 for depiction of the experimental design). Within each group approximately half of the mice were allocated arm 2 as the Familiar arm and arm 3 as the Novel arm. The opposite was true for the remaining mice. This was done in such a manner so that across the groups trained with the same ITI and the groups tested after the same interval there was an equal number of mice allocated arms 2 and 3 as the Novel arm.
Statistical analyses
For all reported experiments data that met the assumptions of parametric analyses were analyzed using analysis of variance (ANOVA). Where appropriate both between subjects and within subjects factors were included in multifactorial analyses. Where relevant, additional factors were included as covariates. Significant interactions were further analyzed using simple main effects analysis that used the pooled error term from the original ANOVA. Data that did not meet the assumptions of parametric analyses were analyzed using Mann–Whitney U tests for comparisons of two independent groups and the Kruskal-Wallis test for comparisons between three independent groups. Group differences indicated by a significant Kruskal-Wallis test were analyzed using a post hoc test that corrected for multiple comparisons (Langley, 1979). Within-subject comparisons were made using the Wilcoxon's rank sum test and categorical data were analyzed using the χ2 test.
Results and Discussion
Exposure training
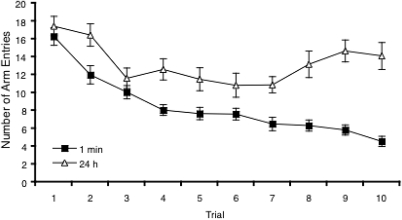
Across exposure training the number of arm entries declined in both groups trained with a 1-min and a 24-h ITI. However, consistent with the idea that short intervals between trials leads to a greater response decrement, the habituation of activity was greater in mice trained with a short, 1-min ITI than mice trained with a long, 24-h ITI (Figure 2). In the following analyses of the exposure training data, to determine whether there were any random differences between the groups allocated to the two test intervals (i.e., 1 min or 24 h), test interval was included as a factor. Thus, activity was analyzed using a 2 (training ITI = 1 min, 24 h) by 2 (test interval = 1 min, 24 h) by 10 (trial = 1–10) ANOVA. Although within the factors of training ITI and test interval there were an equal number of mice allocated either arm 2 or arm 3 as the Novel arm, within the groups that formed each cell of the factorial design, there was unequal Novel arm allocation. To control for any variance caused by this factor, Novel arm allocation was included as a covariate in all the following ANOVAs. There was a significant main effect of trial, F(9, 279) = 2.48, p < .02, and training ITI, F(1, 31) = 24.96, p < .0005. The test interval factor was not significant (F < 1). There was also a significant Trial × Training ITI interaction, F(9, 279) = 6.11, p < .0005. Simple main effects analysis revealed that this was because of a significant effect of training ITI that emerged over trials (Figure 2). Whereas there was no effect of training ITI on Trial 1 (F < 1) and Trial 3, F(1, 31) = 1.46, p > .2 there was for the rest of the trials (all p values <0.05). There was also a significant Trial × Test interval interaction, F(9, 279) = 2.19, p < .05. This indicated a near significant difference on Trial 3, F(1, 31) = 3.93, p = .06, in which mice in the 24-h test interval condition showed a greater number of arm entries than mice in the 1-min test interval condition. However, there were no other significant effects of test interval on other trials (all p values >0.09). The effect of Novel arm allocation was not significant (F < 1). The training ITI by test interval was not significant (F < 1), nor was the three-way interaction, F(1, 31) = 1.75, p > .07.
Figure 2. The number of arm entries across exposure training trials in Experiment 1. The results are shown collapsed across Test interval conditions to show the effect of Training inter-trial interval (ITI) on activity. Error bars indicate ± SEM.
Of importance, the four groups spent a similar total length of time in the Familiar arm during the exposure training trials (1 min–1 min, 495 s ± 32 SEM; 1 min–24 h, 410 s ± 41 SEM; 24 h–1 min, 456 s ± 17 SEM; and 24 h–24 h, 435 s ± 33 SEM). There was no significant effect of training ITI (F < 1), nor test interval, F(1, 31) = 3.09, p > .08, and no significant interaction of these factors, F(1, 31) = 1.19, p > .2. Novel arm allocation was not significant, F(1, 31) = 1.94, p > .1.
Test trial
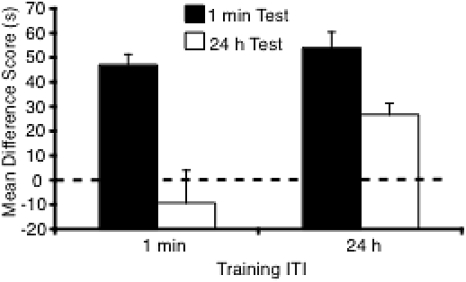
To simplify the results of the test trial, preference for the Novel arm was calculated as a difference score (time in Novel arm minus time in Familiar arm). Therefore, scores greater than zero indicate a novelty preference. Mice tested after a 1-min interval showed a greater preference for the Novel arm than mice tested after 24 h. In contrast to the effect of test interval, mice trained with 24-h ITI showed a greater preference for the Novel arm than mice trained with a 1-min ITI (Figure 3). This pattern of results was confirmed by a 2 (training ITI = 1 min, 24h) × 2 (test interval = 1 min, 24 h) ANOVA. There was a significant effect of test interval, F(1, 31) = 29.08, p < .0005, and training ITI, F(1, 31) = 7.6, p < .02, but no significant interaction between the factors, F(1, 31) = 2.78, p > .1. Novel arm allocation failed to reach significance, F(1, 31) = 3.4, p > .07. Analyses of the novelty preference calculated as a ratio (time in Novel/total time in Novel and Familiar) yielded an identical pattern of results (data not shown).
Figure 3. Novelty preference results shown as a difference score (time in Novel arm minus time in Familiar arm) for groups in Experiment 1. The dashed line indicates chance performance. Error bars indicate SEM. ITI = inter-trial interval.
Analyses of the combined time spent exploring the Novel and Familiar arms during the test showed that while there was no significant effect of training ITI (F < 1), there was a significant effect of test interval, F(1, 31) = 9.13, p < .01, that significantly interacted with training interval, F(1, 31) = 4.74, p < .05 (Table 1). Although mice tested after a 1-min interval showed greater combined levels of time in the Novel and Familiar arms during the novelty preference test, simple main effects analysis of the interaction showed that this effect was significant only for mice trained with a 1-min ITI, F(1, 31) = 13.5, p < .005, but not for mice trained with a 24-h ITI (F < 1). The effect of Novel arm allocation failed to reach significance, F(1, 31) = 3.97, p > .05.
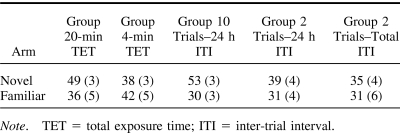
Table 1. The Mean (SEM) Exploration Time (s) in the Novel and Familiar Arms During the Novelty Preference Test for Each Condition in Experiment 1.
The results of this novelty preference test are consistent with the hypothesis that a short interval between training and test leads to greater short-term habituation. In addition, it was found that a short interval between training trials actually led to weaker habituation than when training trials were separated by a long interval. This pattern of results replicates the findings of Davis (1970) using a stimulus-specific test of habituation and demonstrates that short and long intervals between stimulus exposures have opposite effects on short-term and long-term habituation. The results fail to provide support for the idea that differences in the long-term effects of massed and spaced habituation training reflect dishabituation caused by a mismatch between the conditions during training and testing. Instead they provide further support for the idea that habituation is determined by separate short-term and long-term processes in memory. Moreover, the results demonstrate that short-term memory can reduce long-term memory, thus, implying that there is competition between these short-term and long-term processes.
Experiments 2a and 2b
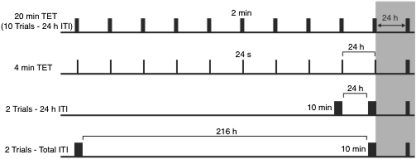
The purpose of Experiments 2a and 2b was to further examine the conditions that cause long-term habituation and to rule out two potential confounds that may have led to the detrimental effect of massed exposure training in Experiment 1. Long-term habituation was assessed by testing the spatial novelty preference of mice after a long, 24-h interval. Groups of mice were tested in conditions that differed in either the amount of stimulus exposure (Experiment 2a) or the rate of stimulus exposure (Experiment 2b). In both experiments performance of the experimental groups was compared with a control group that received ten 2-min exposure training trials, each separated by a 24-h interval (Figure 4). Thus, the training and test conditions of the control group were identical to Group 24 h–24 h in Experiment 1.
Figure 4. Design of Experiments 2a and 2b. Black bars on the white background represent exposure training trials and black bars on the gray background represent the novelty preference test. The top panel illustrates the time line (not to scale) of trials for the control group used in both Experiments 2a and 2b. In Experiment 2a, performance of the control group (20-min total exposure time [TET]) was compared with a group that received similar training and testing conditions, except that the training trials were a duration of only 24 s (4-min TET, shown in the panel second from the top). In Experiment 2b, performance of the control group (10 Trials–24 h inter-trial interval [ITI]) was compared with two groups that received two 10-min trials separated by either a 24-h interval (2 trials–24 h ITI, shown in second panel from the bottom), or by an interval of 216 h (i.e., 9 days; 2 trials–total ITI, shown in the bottom panel).
In Experiment 2a, the experimental group received identical training to the control group, except that the duration of the exposure training trials was only 24 s. Thus, whereas the control group received a total exposure time (TET) of 20 min (Group 20-min TET), the experimental group received only 4 min (Group 4-min TET; Figure 4).
In Experiment 2b, two experimental groups received the same amount of exposure training as the control group, but these groups differed in the number of exposure training trials. Whereas the control group received ten 2-min trials (Group 10 trials–24 h ITI), the experimental groups received two 10-min trials. For one group the trials were separated by a 24-h interval (Group 2 trials–24 h ITI), thus matching the ITI used for the control group. The other group received the two trials separated by 9 days (Group 2 trials—total ITI; Figure 4), thus matching the total ITI used for the control group.
While both a single, short-term process account and a dual-process account of habituation would predict that the duration of exposure (Experiment 2a) would affect the strength of habituation, the two accounts differ in their predictions for the effect of the number of exposure trials (Experiment 2b). A single, short-term process account would predict that habituation caused by the effect of a recent stimulus exposure would show some, but incomplete, transfer over trials, depending on the duration of the intertrial interval. So, although both accounts predict that the distribution of exposure over time will affect habituation, in contrast to the predictions of Wagner (1981), a single, short-term process account would assume that massed exposure training rather than spaced training would lead to greater habituation. If massed exposure increases habituation then Group 2 trials–24 h ITI would show greater habituation than Group 10 trials–24 h ITI and Group 2 trials—total ITI in Experiment 2b. However, if massed training reduces habituation then Group 2 trials–24 h ITI should show weaker habituation than Group 10 trials–24 h ITI that receives training that is more spaced. Although Group 10 trials–24 h ITI and Group 2 trials—total ITI receive the same total amount of exposure distributed over the same length of time, Group 2 trials—total ITI receives fewer trials with a greater duration than Group 10 trials–24 h ITI. Thus, exposure is more relatively massed in the Group 2 trials—total ITI. If a decrease in the number of trials results in exposure being relatively more massed then 10 training trials should result in greater habituation than two trials regardless of whether the ITI or the total ITI is controlled.
Experiments 2a and 2b provide a means of ruling out two confounds that may have contributed to the detrimental effect of trial massing in Experiment 1. First, in Experiment 1 the interval between exposure training trials was confounded with the rate in which mice were handled across training. It is possible that the more frequent rate of handling in the massed, 1 min Training ITI condition caused a level of stress that reduced the amount of learning that resulted in long-term habituation. Second, mice in the massed and spaced training conditions were exposed to different stimuli during the intertrial interval. While mice from both groups were returned to their homecage at the end of the trial, mice in the spaced condition spent the majority of the intertrial interval in the holding room, whereas mice in the massed condition remained in the testing room during the intertrial interval. It is possible that this qualitative rather quantitative difference in the massed and spaced training conditions could have led to the pattern of results in Experiment 1. These confounds are ruled out in Experiments 2a and 2b by either matching the rate of handling (Experiment 2a) or by confounding spaced training with a greater rate of handling (Experiment 2b, i.e., Group 10 trials–24 h ITI was handled more often than Group 2 trials–24 h ITI and Group 2 trials—total ITI, and at a faster rate than Group 2 trials—total ITI). Also, in Experiments 2a and 2b mice were returned to the holding room after each exposure trial, thus equating the stimuli that mice were exposed to in the inter-trial interval.
Method
Subjects and apparatus
In Experiment 2a, 32 female C57BL/6J/Ola mice were used. Mice were approximately 10 weeks old and a mean weight of 17.2 g (range = 15.6–19.1 g) at the start of testing. The same mice that were used in Experiment 1 were used in Experiment 2b. In Experiment 2a training was carried out in three rooms (A, B, and C), with distinct spatial cues making them different to one another. In Experiment 2b, training was carried out in a room with distinct spatial cues making it different to the room used for Experiment 1. Experiment 2b commenced four days after the completion of Experiment 1. All other details for Experiments 2a and 2b were the same as for Experiment 1.
Procedure
For Experiment 2a, mice were divided into two groups (N = 16 per group) and were trained in one of two conditions: 20-min TET and 4-min TET (Figure 4). In the 20-min TET condition mice received ten 2-min exposure trials. In the 4-min TET condition, mice received ten 24-s exposure trials. In both conditions, trials were spaced with a 24-h ITI, and mice were tested 24 h after the last exposure trial. All other procedural details were the same as for Experiment 1.
Initial testing revealed that there was an apparent difference between the groups that failed to reach significance. To examine whether the difference between groups was reliable, the training and testing procedures were repeated twice more. Therefore, mice were tested three times: first in room A, then room B, and finally in room C. The interval between each repetition was approximately one week. The allocations of the Novel arm and the Familiar arms to arms 2 and 3 were counterbalanced with respect to condition (20-min TET; 4-min TET) and room (A, B, and C). Also, the order of Novel arm allocation across the first, second, and third tests was counterbalanced across the three tests. Thus, within each condition (20-min TET; 4-min TET) there were an equal number of mice allocated to each possible combination of Novel arm allocations.
For Experiment 2b, mice were assigned to one of three groups: 10 trials–24 h ITI; 2 trials 24 h ITI; 2 trials–total ITI (N = 12 per group). The allocation of mice to the three groups was counterbalanced so that an equal number of mice from each of the previous training regimes used in Experiment 1 were in each group. The allocations of Novel arm and Familiar arms (to arms 2 and 3) for individual mice were the same as for Experiment 1. This resulted in the allocation of arms being fully counterbalanced within each group.
Group 10 trials–24 h ITI received ten 2-min exposure trials separated by a 24-h ITI. Group 2 trials–24 h ITI received two 10-min trials separated by a 24 h ITI. Group 2 trials–total ITI received two 10-min trials separated by 216 h (9 days; Figure 4). All groups were tested 24 h after their last training trial. All other procedural details were the same as Experiment 1.
Results and Discussion
Experiment 2a
In all three tests, Group 20-min TET showed a greater novelty preference than Group 4-min TET (median difference score: Test 1: Group 20-min TET = 38 s, Group 4-min TET = 11 s; Test 2: Group 20-min TET = 20 s, Group 4-min TET = 2 s; Test 3: Group 20-min TET = 11 s, Group 4-min TET = −1 s). To simplify the analysis the difference scores were calculated from the mean time spent in the Novel and Familiar arms across the three tests. Group 20-min TET displayed a significantly greater preference for the Novel arm than Group 4-min TET (Group 20-min TET: median = 21 s, interquartile range = 8–30 s; Group 4-min TET: median = 5 s, interquartile range = −14–15 s; Mann–Whitney U (16, 16) = 72.5, p < .05). Calculation of the novelty preference as a ratio (time in Novel/total time in Novel and Familiar) similarly demonstrated that Group 20-min TET showed a numerically greater preference than Group 4-min TET (data not shown). There was no significant difference in the combined time spent exploring the Novel and Familiar arms during the test (F < 1; Table 2).
Table 2. The Mean (SEM) Exploration Time (s) in the Novel and Familiar Arms During the Novelty Preference Test for Each Condition in Experiments 2a and 2b.
All the mice in Group 4-min TET spent time in the Familiar arm across training trials, N = 16, χ2(1) = 16, p < .0001. This suggests that it is unlikely that Group 4-min TET showed a smaller novelty preference than Group 20-min TET simply because they failed to enter the Familiar arm during the exposure trials.
For the 4-min TET group the mean length of time exploring the Familiar arm per trial in the first five trials (10 s ± 1 SEM) and the last five training trials (11 s ± 1 SEM) did not significantly differ, F(1, 15) = 2.00, p > .1, thus suggesting that exploration was stable across exposure training. It is, therefore, unlikely that across training trials mice became slower to leave the Start arm such that the 4-min TET group may have failed to enter the Familiar arm as training progressed. If this happened it would have meant that the actual test interval would be greater than 24 h for the 4-min TET group. Also, calculation of the total time that each mouse spent exploring in the familiar arm, during training, as a proportion of total available exploration time (i.e., 20-min TET = 1,200 s; 4-min TET = 240 s) showed that the two groups showed similar relative durations of exploration (20-min TET = 0.43 ± 0.03 SEM; 4-min TET = 0.43 ± 0.05 SEM, F < 1). Thus, the manipulation of the training trial duration was successful in resulting in a fivefold difference in the total exposure to the familiar arm between the two groups.
Experiment 2b
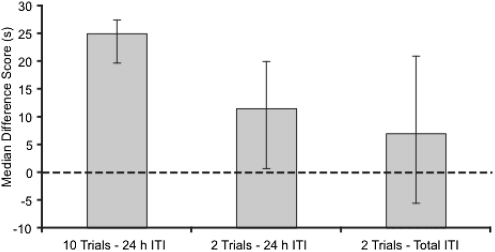
Preference for the Novel arm was calculated as a difference score. The results are shown in Figure 5. Group 10 trials–24 h ITI spent more time exploring the Novel arm than the Familiar arm. However, the two groups that received just two trials showed little preference for exploring the Novel arm over the Familiar arm. The data were analyzed using a Kruskal-Wallis nonparametric, one-way analysis of variance. There was a significant effect of group, H(2) = 7.88, p < .02. Post hoc comparisons for a significant Kruskal-Wallis test (Langley, 1979) revealed that Group 10 trials–24 h ITI showed a significantly greater novelty preference than Group 2 trials–24 h ITI (p < .05) and Group 2 trials—total ITI (p < .05). There was no significant difference between the two 2 trials conditions. Calculation of the novelty preference as a ratio (time in Novel/total time in Novel and Familiar) demonstrated a similar ordinal relationship between the groups (data not shown). There was no significant difference between the groups in the combined time spent exploring the Novel and Familiar arms during the test, F(2, 33) = 2.8, p > .07 (Table 2).
Figure 5. Novelty preference results shown as difference score (time in Novel arm minus time in Familiar arm) for groups in Experiment 2b. The dashed line indicates chance performance. Error bars indicate the interquartile range. ITI = inter-trial interval.
It is possible that groups differed during the test phase because the different training conditions affected the amount of exposure to the cues associated with the Familiar arm. However, analysis of the time spent exploring the Familiar arm across exposure training trials failed to reveal a significant difference between the groups (median time: Group 10 trials–24 h ITI, 523 s, interquartile range = 501–540; Group 2 trials–24 h ITI, 470 s, interquartile range = 407–539; Group 2 trials—total ITI, 449 s, interquartile range = 390–573) H(2) = 2.38, p > .3. Similarly, there was no difference in the total number of arm entries across training (median arm entries: Group 10 trials–24 h ITI, 125, interquartile range = 98–150; Group 2 trials–24 h ITI, 109, interquartile range = 92–119; Group 2 trials—total ITI, 95, interquartile range = 85–118) H(2) = 3.05, p > .2, suggesting that the levels of activity during exposure training were similar between the groups.
The results of Experiments 2a and 2b demonstrate that the amount of exposure and the number of exposures independently determine the strength of habituation. Thus, there are both within-trial and between-trial increments in the strength of long-term habituation. Along with the results of Experiment 1, the results of Experiments 2a and 2b provide evidence against a single, short-term process account of habituation and alternatively, suggest that there are separate processes that contribute to short-term and long-term habituation. Collectively, the results of Experiments 1 and 2 demonstrate that the detrimental effect of massed exposure training is not due to confounds in the rate of handling or because of differences in the cues that mice are exposed to in the intertrial interval.
While the finding that habituation is affected by the amount of exposure training (Experiment 2a) is not that surprising, the finding that habituation is affected by the number of trials (Experiment 2b) is more informative. Similar to Experiment 1, Experiment 2b tested the effect of massed versus spaced exposure training on habituation, but whereas Experiment 1 tested this by manipulating the ITI, Experiment 2b tested this by manipulating the number of trials. Thus, even though the groups that received two exposure trials received these trials separated by intervals that either matched the ITI or the total ITI of the control group in Experiment 2b, the reduction in the number of trials compared with the control group resulted in exposure being relatively more massed. Thus, massing exposure by either reducing the ITI or the number of trials has a detrimental effect on habituation. This demonstrates that the number of trials determines the strength of long-term habituation even when factors such as the amount of exposure, ITI and total ITI are controlled. These findings parallel those examining the effect of the number of trials on conditioning (Gottlieb & Rescorla, 2010), and provide support for trial-based models of learning (e.g., Rescorla & Wagner, 1972) rather than time-accumulation based models (e.g., Gallistel & Gibbon, 2000).
Experiment 3
Experiments 1 and 2 collectively demonstrate that there are both short-term and long-term processes that contribute to habituation. However, while short-term habituation of spatial exploration can be explained by assuming that a memory of a stimulus decays over time, the mechanism underlying long-term spatial habituation is less clear. Wagner (1976, 1981) suggested that long-term habituation of unconditioned responding occurs because of the formation of long-term associative memory. If two stimuli (e.g., A and B) have formed an association such that presentation of A leads to the retrieval of the memory of B, then this retrieved memory will result in habituation of unconditioned responding to B in a similar manner to that of the memory of a recently experienced stimulus. Support for this claim has come from experiments examining conditioned diminution of the unconditioned response. For example, pairings of a conditioned stimulus (CS) with a shock can lead to a reduction in the unconditioned response (UR) to the shock if immediately preceded by the CS, compared with presentation of the shock alone, or to the shock preceded by a CS that is not associated with shock (Donegan, 1981). Similarly, pairings of an auditory stimulus with a visual stimulus (e.g., A1 and V1; A2 and V2) subsequently led to a reduction of the orienting response to the visual stimulus if preceded by the same auditory cue as used in training (i.e., A1 − V1; A2 − V2), compared with when the visual stimulus is preceded by an auditory cue with which it has not been paired (i.e., A1–V2; A2–V1) (Honey, 2000; Honey & Good, 2000a; Honey & Good, 2000b; Honey, Good, & Manser, 1998; Honey, Watt, & Good, 1998).
It is possible that long-term spatial novelty preference is also caused by an associative retrieval mechanism. In Experiments 1 and 2, associations may have formed between spatial locations that are experienced in close temporal proximity such that exposure to a spatial location leads to retrieval of a representation of another spatial location. Another possibility is that during exposure training an association formed between a spatial location and the response that was made when leaving the spatial location. For example, if a mouse turned left when leaving the Start arm to enter the Familiar arm, then the Start arm may have become associated with the turn left response.
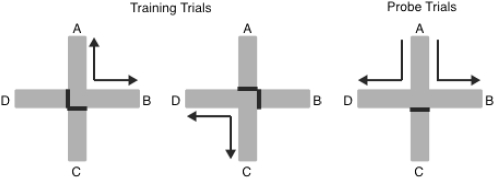
To test the prediction that an associative process contributes to long-term spatial habituation mice were tested in the following manner. Mice were allowed to repeatedly explore two pairs of arms (e.g., A and B; C and D; Figure 6) of a cross-shaped maze. Mice received a food reward at the end of each of the arms, so as to motivate and maintain exploration between the pairs of arms throughout training. The maze was rotated regularly so that only extramaze cues were relevant for discriminating between arms of the maze. After a period of training, mice received probe trials in which they were placed in an arm, for example A, and then allowed to enter either arm B or D (Figure 6). If an association was made between a given spatial location and either another location, or the response made when leaving the location, then it would be predicted that exposure to A would lead to less exploration of the primed arm, B than the unprimed arm, D.
Figure 6. Design of Experiment 3. Mice were trained to collect food reward from two pairs of arms of a plus-maze on separate trials. The pairs were formed from arms that formed a right-angle (e.g., A and B, left panel; C and D, middle panel). While exploring one pair of arms, access was blocked to the other pair. Mice received probe trials (right panel) in which they were placed in an arm (e.g., arm A) and were allowed to explore the previously paired arm (e.g., B, primed arm) or the previously unpaired arm (e.g., D, unprimed arm). Mice received an equal number of probe trials starting from each arm of the maze.
Method
Subjects
Twelve mice that were previously used in Experiment 2a were used in the current experiment. Six mice from each of the two conditions in Experiment 2a (i.e., 20-min TET and 4-min TET) were chosen at random. Testing was carried out in a room with distinct spatial cues that made it different from the testing rooms used in Experiment 2a. Throughout testing mice were maintained at 90% of their free-feeding weight by receiving a restricted diet.
Apparatus
A cross-shaped maze made from wood that was painted gray was used. All four arms were 10 cm wide. The north- (arm A) and south-facing (arm C) arms were 47 cm long, and the east- (arm B) and west-facing (arm D) arms were 35 cm long. Each arm was surrounded by a 10-cm high wall. A food well was situated at the end of each arm, which contained 0.1 ml sweetened, condensed milk (Nestle, York, United Kingdom), diluted 1:1 with water. During training and testing, the entrance to particular arms could be blocked with wooden partitions that were painted the same shade of gray as the maze. The maze was situated in a room that contained extramaze cues that were visible above the height of the wall of the maze.
Procedure
Mice were trained to traverse pairs of arms to collect food rewards. Each pair of arms consisted of a north- or south-facing arm (i.e., arms A or C) and an east- or west-facing arm (i.e., arms B or D) such that the two allocated arms formed a right angle (Figure 6). When exploring a pair of arms, access to the other two arms was blocked. Each mouse was allocated two pairs of arms. Half of the mice received training with pairs formed from arms A and B (A-B), and arms C and D (C-D). The remaining mice received training with pairs formed from arms A and D (A-D) and arms C and B (C-B). An equal number of mice from each condition in Experiment 2a were allocated to the two groups of arm pairings.
At the start of a trial, the mouse was placed facing the end of an arm and was allowed to eat from the food well. The mouse was then allowed to traverse the arm and enter the other arm in the pair and eat from the food well at the end of that arm. Once the food in the second arm had been eaten, the mouse was removed from the maze. Mice received two trials a day (one with each pair of arms) separated by approximately 30 min. The order of arm pairs within each day of testing was reversed on each consecutive day (e.g., in an XY-YX order). Within pairs of arms, trials were started equally often from each arm. For example, on half of A-B trials mice were started from arm A, with the remaining trials started from arm B. Within a pair of arms, the order of trials starting from different arms was pseudorandom with the constraint that there was an equal number of each trial type across 2-day blocks.
Mice initially received eight days of training. They then received probe trials that were intermixed with additional days of training trials. On a probe trial a mouse was placed in an arm of the maze (start arm) and allowed to consume the food reward (e.g., arm A; see Figure 6). The mouse was then allowed to choose between the two arms that were either to the left or to the right of the start arm (e.g., arms B and D; see Figure 6). Therefore, the mouse was required to choose between the arm that had been paired with the start arm (primed arm) and the arm that had not been paired with the start arm (unprimed arm; Figure 6). Food reward was present in both the primed and unprimed arms during probe trials. The remaining arm (the arm opposite to the start arm, e.g., arm C; see Figure 6) was blocked during the probe trial.
Mice received two probe trials starting from each of the four arms, thus totaling eight probe trials. Probe trials were carried out one per day. Between each day with a probe trial, mice received two further days of normal training trials. Therefore, prior to the last probe trial mice had received a total of 22 days of training (i.e., 44 trials). On the first probe trial mice were randomly allocated one of the four arms as the start arm. On subsequent probe trials the start arm was the arm that was 90° clockwise from the arm that had been used as the start arm on the previous probe trial. Throughout training, and prior to each probe test, the maze was regularly rotated 180° so as to make the intramaze cues irrelevant for performance in the probe tests.
Results and Discussion
Across the eight probe trials mice showed a strong preference to choose the unprimed arm that was not previously paired with the start arm. All 12 mice showed a preference for the unprimed arm (i.e., proportion of unprimed arm choices >50%) χ2(1) = 12.00, p < .0005. The median percentage of unprimed arm choices in the first four probe tests (75%, interquartile range = 50–75) and the last four probe trials (75%, interquartile range = 75–75) did not significantly differ (Wilcoxon's rank sum test: z = −0.97, p > .3), thus suggesting that performance was stable across testing.
While the results of Experiment 3 provide support for the theory that associative learning underlies long-term habituation, alternative accounts of the results should first be considered. For example, it is possible that during a probe trial, reward may have been predicted to occur in the primed arm, but not necessarily in the unprimed arm. Thus, the experience of reward in an unprimed arm may have led to a subsequent increase in the tendency to choose this arm, because an unexpected or surprising US is able to support a greater increment in associative strength (e.g., Rescorla & Wagner, 1972). This would lead to a greater tendency to choose this arm on subsequent trials. The fact that performance was stable across testing suggests that this was not the case. The tendency to choose the unprimed arm did not change across the first and second block of trials. Also, each arm was equally assigned as the unprimed and primed arm across trials. Therefore, if experience of reward in an unprimed arm led to that specific arm having greater associative strength then it would be predicted that mice would fail to show a preference for a different unprimed arm when the previously unprimed arm was assigned as the primed arm. The fact that the mice showed a significant preference for the unprimed arm suggests that this was not the case.
The present experiment, therefore, demonstrates that an associative process can result in habituation of exploration. However, one shortcoming of the experiment is that it is not clear what association has been formed during the training trials. First, it is possible that the spatial locations that form the pairs of arms during training have become associated such that experience of one location leads to the retrieval of a memory of the other location. Second it is possible that spatial locations become associated with the response that is made when leaving the location throughout training. Thus, if the south and the west arm have been paired during training, then the south arm may have become associated with a left body turn response that would have resulted in the mouse entering the west arm. It is important to note that these two accounts both assume that associative retrieval of a representation will lead to a reduction in the unconditioned response to the relevant stimuli. For example, retrieval of a spatial location will lead to a subsequent reduction of exploration of that location so that mice are more likely to explore a different location. Or alternatively, retrieval of an egocentric body turn will lead to a reduction in the tendency to make that response such that the mice are more likely to make the opposite response.
While both accounts are possible, there are reasons to suggest that the place priming account is more likely than the response priming account. If associative activation of a representation has the same effect as direct activation of a representation by experience of the stimulus, then it would be predicted that direct activation of either allocentric or egocentric information would result in habituation. This has been tested by examining whether animals demonstrate alternation behavior due to place or to response information. For example, Montgomery (1952) placed rats in the south arm of a plus maze and then forced the rats to turn left to the west arm. Rats were then subsequently placed in the north arm and allowed to choose between either the west and east arm. If during the forced trial, entering the west arm activated a representation of the west arm then it would be predicted that on the choice trial rats should be more likely to enter the east arm. In contrast, if entering the west arm activated a representation of the turn left response then it would be predicted that in the choice trial rats should be more likely to turn right and enter the west arm. It was found that rats alternated according to the place information rather than response information regardless of whether they are rewarded in the forced trial (Montgomery, 1952) or not (Glanzer, 1953). Furthermore, attempts to maximize the use of egocentric cues in alternation have shown that the use of place information still dominates performance during initial training, and after extensive training there is only a very small effect of egocentric information (Futter & Aggleton, 2006; see also Baird, Futter, Muir, & Aggleton, 2004). These results suggest that it is more likely that associative activation of a place caused the priming effect seen in Experiment 3.
General Discussion
The results of the present set of experiments demonstrate that habituation of spatial exploratory behavior is determined by separate short-term and long-term processes that can compete with one another. In Experiment 1, whereas a short interval between training and test resulted in greater habituation, a short interval between exposure training trials actually reduced habituation. This massed exposure effect also occurred when the number of trials was manipulated and the intertrial interval and the cumulative intertrial interval were controlled (Experiment 2b). Experiment 3 provided evidence that long-term spatial habituation can be caused by an associative process. In accordance with evidence that short-term memory can interfere with conditioning (Sunsay, Stetson, & Bouton, 2004), it is possible that short-term memory caused by massing exposure training reduced an associative process underlying long-term habituation in Experiments 1 and 2b.
Together with the results of the current experiments, there is converging evidence that there are competitive short-term and long-term processes underlying spatial habituation that comes from studies examining the role of synaptic plasticity in learning. Sanderson et al., (2009) tested genetically modified mice lacking the GluA1 subunit of the AMPA receptor on short-term and long-term spatial habituation. Similar to the present experiments, mice were repeatedly exposed to a spatial location before receiving a novelty preference test in which mice were allowed to explore the previously exposed, familiar location and a novel location. In one condition the interval between each exposure trial and prior to the test was short, whereas in another condition this interval was long. It was found that in the short interval Condition GluA1 knockout mice were impaired in contrast to controls and failed to show a novelty preference. However, in the long interval Condition GluA1 knockout mice did show a novelty preference, which was significantly greater than that of controls. Therefore, depending on the interval between stimulus exposures GluA1 deletion both impaired and enhanced habituation (Sanderson et al., 2009).
The present results help to provide an explanation of the pattern of results with GluA1 knockout mice (Sanderson et al., 2009). If there are separate, competitive processes that contribute to short-term and long-term habituation, then it is possible that under certain conditions a reduction in the process underlying short-term habituation can lead to an increase in the process underlying long-term habituation. If it is assumed that GluA1 contributes to memory for recently experienced stimuli, then it is possible that GluA1 deletion will have the consequences of both reducing short-term habituation and also, under certain conditions, increasing long-term habituation. These seemingly paradoxical results are uniquely accounted for by Wagner's (1981) model which assumes that there is competition between the processes underlying habituation.
The results of the present set of experiments may have implications for theories of spontaneous recognition memory in rodents. Experiments 1 and 2 used a spatial novelty preference task that is analogous in design to the spontaneous object recognition task used in rodents (Ennaceur & Delacour, 1988). In the spontaneous object recognition task animals are exposed to an object before receiving a test in which they are allowed explore the previously exposed, familiar object and a novel object. Typically rodents show a preference for exploring the novel object over the familiar object. Dissociations between the contributions of different brain regions to the spontaneous object recognition task have been used to provide evidence for a dual-process account of recognition memory (e.g., Aggleton & Brown, 2005, 2006). However, rather than reflecting qualitatively different forms of memory, these neuroanatomical dissociations have been argued to reflect quantitative differences in memory strength, which can be explained by a single-process account (Squire, Wixted, & Clark, 2007). If Experiments 1 and 2 reflect a form of spontaneous spatial recognition memory, then the data may provide evidence against the single-process, memory strength argument. For example differences in memory strength could not explain the opposite effects of short and long intervals between exposure training trials and prior to testing on spatial novelty preference in Experiment 1. Thus, the competitive short-term and long-term processes that result in spatial novelty preference require a dual-process account.
To conclude, these results provide evidence against a single-process account of habituation. Instead, the results suggest that there are separate processes underlying short-term and long-term habituation. Whereas short-term habituation is caused by a recent stimulus exposure, long-term habituation is caused by incrementally strengthened memory that likely reflects an associative process.
Acknowledgments
The work was funded by the Wellcome Trust (grant no. 074385).
References
- Aggleton J. P., & Brown M. W. (2005). Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Quarterly Journal of Experimental Psychology B, Comparative and Physiological Psychology, 58, 218–233. [DOI] [PubMed] [Google Scholar]
- Aggleton J. P., & Brown M. W. (2006). Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Science, 10, 455–463. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Jablonski S. A., & Klimas D. B. (2008). Spaced initial stimulus familiarization enhances novelty preference in Long-Evans rats. Behavioural Processes, 78, 481–486. [DOI] [PubMed] [Google Scholar]
- Baird A. L., Futter J. E., Muir J. L., & Aggleton J. P. (2004). On the transience of egocentric working memory: Evidence from testing the contribution of limbic brain regions. Behavioral Neuroscience, 118, 785–797. [DOI] [PubMed] [Google Scholar]
- Beck C. D. O., & Rankin C. H. (1997). Long-term habituation is produced by distributed training at long ISIs and not by massed training or short ISIs in Caenorhabditis elegans. Animal Learning & Behavior, 25, 446–457. [Google Scholar]
- Carew T. J., Pinsker H. M., & Kandel E. R. (1972). Long-term habituation of a defensive withdrawal reflex in aplysia. Science, 175, 451–454. [DOI] [PubMed] [Google Scholar]
- Davis M. (1970). Effects of interstimulus interval length and variability on startle-response habituation in the rat. Journal of Comparative Physiology and Psychology, 72, 177–192. [DOI] [PubMed] [Google Scholar]
- Donegan N. H. (1981). Priming-produced facilitation or diminution of responding to a Pavlovian unconditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes, 7, 295–312. [PubMed] [Google Scholar]
- Ennaceur A., & Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research, 31, 47–59. [DOI] [PubMed] [Google Scholar]
- Futter J. E., & Aggleton J. P. (2006). How rats perform spatial working memory tasks: Limitations in the use of egocentric and idiothetic working memory. Quarterly Journal of Experimental Psychology, 59, 77–99. [DOI] [PubMed] [Google Scholar]
- Gallistel C. R., & Gibbon J. (2000). Time, rate, and conditioning. Psychological Review, 107, 289–344. [DOI] [PubMed] [Google Scholar]
- Gatchel R. J. (1975). Effects of interstimulus interval length on short-term and long-term habituation of autonomic components of orienting response. Physiological Psychology, 3, 133–136. [DOI] [PubMed] [Google Scholar]
- Glanzer M. (1953). The role of stimulus satiation in spontaneous alternation. Journal of Experimental Psychology, 45, 387–393. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. A., & Rescorla R. A. (2010). Within-subject effects of number of trials in rat conditioning procedures. Journal of Experimental Psychology: Animal Behavior Processes, 36, 217–231. [DOI] [PubMed] [Google Scholar]
- Groves P. M., & Thompson R. F. (1970). Habituation: A dual-process theory. Psychological Review, 77, 419–450. [DOI] [PubMed] [Google Scholar]
- Honey R. C. (2000). Associative priming in Pavlovian conditioning. Quarterly Journal of Experimental Psychology, 53B, 1–23. [DOI] [PubMed] [Google Scholar]
- Honey R. C., & Good M. (2000a). Associative components of recognition memory. Current Opinion in Neurobiology, 10, 200–204. [DOI] [PubMed] [Google Scholar]
- Honey R. C., & Good M. (2000b). Associative modulation of the orienting response: Distinct effects revealed by hippocampal lesions. Journal of Experimental Psychology: Animal Behavior Processes, 26, 3–14. [DOI] [PubMed] [Google Scholar]
- Honey R. C., Good M., & Manser K. L. (1998). Negative priming in associative learning: Evidence from a serial-habituation procedure. Journal of Experimental Psychology: Animal Behavior Processes, 24, 229–237. [DOI] [PubMed] [Google Scholar]
- Honey R. C., Watt A., & Good M. (1998). Hippocampal lesions disrupt an associative mismatch process. Journal of Neuroscience, 18, 2226–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., & Schwartz J. H. (1985). Principles of neural science (2nd ed.). New York: Elsevier. [Google Scholar]
- Langley R. (1979). Practical statistics simply explained (2nd ed.). London: Pan Books. [Google Scholar]
- Mackintosh N. J. (1987). Neurobiology, psychology and habituation. Behaviour Research and Therapy, 25, 81–97. [DOI] [PubMed] [Google Scholar]
- Montgomery K. C. (1952). A test of two explanations of spontaneous alternation. Journal of Comparative Physiology and Psychology, 45, 287–293. [DOI] [PubMed] [Google Scholar]
- Rescorla R. A., & Wagner A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Black A. H. & Prokasy W. F. (Eds.), Classical conditioning, II: Current research and theory (pp. 64–99). New York: Appleton-Century-Crofts. [Google Scholar]
- Sanderson D. J., Good M. A., Skelton K., Sprengel R., Seeburg P. H., Rawlins J. N., & Bannerman D. M. (2009). Enhanced long-term and impaired short-term spatial memory in GluA1 AMPA receptor subunit knockout mice: Evidence for a dual-process memory model. Learning and Memory, 16, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson D. J., Gray A., Simon A., Taylor A. M., Deacon R. M., Seeburg P. H., Sprengel R., et al. (2007). Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behavioral Neuroscience, 121, 559–569. [DOI] [PubMed] [Google Scholar]
- Squire L. R., Wixted J. T., & Clark R. E. (2007). Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience, 8, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunsay C., Stetson L., & Bouton M. E. (2004). Memory priming and trial spacing effects in Pavlovian learning. Learning and Behavior, 32, 220–229. [DOI] [PubMed] [Google Scholar]
- Thompson R. F., & Spencer W. A. (1966). Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review, 73, 16–43. [DOI] [PubMed] [Google Scholar]
- Wagner A. R. (1976). Priming in STM: An information processing mechanism for self-generated or retrieval-generated depression in performance. In Tighe T. J. & Leaton R. N. (Eds.), Habituation: Perspectives from child development, animal behavior, and neurophysiology (pp. 95–128). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Wagner A. R. (1981). SOP: A model of automatice memory processing in animal behavior. In Spear N. E. & Miller R. R. (Eds.), Information processing in animals: Memory mechanisms (pp. 5–47). Hillsdale, NJ: Erlbaum Inc. [Google Scholar]