Introduction
For presurgical evaluation of resection of a mass or other lesion, noninvasive neuroimaging aids in the surgical planning and in counseling patients about possible risks of the surgery. MEG performs the most common types of surgical planning that the neurosurgeon faces, including localization of epileptic discharges, determination of the hemispheric dominance of verbal processing, and the ability to locate eloquent cortex. MEG is most useful when it is combined with structural imaging, most commonly combined with structural MRI and MR diffusion imaging. We review the history of clinical MEG, introduce the basic concepts about biophysics of MEG, and outline the basic neurosurgical applications of MEG.
Brief History of MEG
In 1968, David Cohen (see Figure 1) used a room-temperature copper coil as a detector to recorded the first magnetoencephalogram at the university of Illinois [1]. Later, at the Massachusetts Institute of Technology, he built a more elaborate shielded room. At about the same time, James Zimmerman and colleagues developed the Superconducting Quantum Interference Device (SQUID), which uses the Josephson junction to measure tiny magnetic fields. It requires cooling to liquid helium temperatures, and has a sensitivity of several hundreds of times that of a copper coil. Zimmerman brought this detector to Cohen's room, and this combination of shielding and detector allowed the first clear measurements of the body's magnetic fields. After they measured the heart, Cohen next recorded the first MEG measured with a SQUID [2].
Figure 1.
Dr. David Cohen, Ph.D performed the first magnetic fields from the brain. His laboratory at Massachusetts Institute of Technology in Boston, Massachusetts USA pioneered MEG experiments and applications.
For the initial measurements of magnetic brain activity, physicists and neuroscientists used a single or a just few magnetometers. Localizing activity with such a low number of sensors required moving them in order to sampling from various locations over the head. This was time consuming and not practical for routine clinical use. Clinical MEG became possible when MEG systems were developed that provided coverage of approximately 7–12 cm, enough to produce a field map large enough to visualize the magnetic field lines from a single dipole source. This made it possible to localize the activity in a registered brain MRI.
Biophysical Principles of Magnetoencephalography
Modern MEG systems now provide hundreds of channels that can provide whole-head coverage (See Figure 2). This makes it possible to map activity throughout the cerebral cortex and is critical for presurgical mapping of language areas, and for detecting propagating or widespread epileptic activity. Due to interference from extraneous magnetic fields, all MEG measurements must be performed in a magnetically shielded room, which typically consists of two to four layers of aluminum and multiple layers of ferromagnetic shielding.
Figure 2.
Typical modern MEG device positioned in the upright position facing a back projection screen.
Unlike other hemodynamic techniques (fMRI and PET), both MEG and electroencephalography (EEG) directly measures electric brain activity. The neural generators of the MEG and EEG are identical. There are critical differences that make them both useful and complementary. MEG preferentially detects activity in superficial, non-radial areas of cortex, such as the fissural cortex of the cerebral hemispheres. This is particularly advantageous if the area of the area of interest is also radial, such as the primary somatosensory cortex, which lies in the walls of the sulci.
Much of the neural activity measured by MEG originates as the post-synaptic activity in the pyramidal cells of the cerebral cortex. MEG measures the vector sum of post-synaptic potentials, as contrasted with BOLD fMRI and some form of PET imaging that reflects neural activity indirectly through changes in blood flow. MEG localizes neural activity more accurately than EEG because magnetic fields are less perturbed than electrical potentials by overlying brain structures: scalp, skull, cerebrospinal fluid, meninges, and vascular structures. Recently, statistical combination of structural MRI, functional MRI, with MEG have taken a great stride forward by yielding the maximum benefit from each technique into a single image [3, 4].
The calculation of the magnetic field is more straightforward than that of the electric field because of the symmetries and conductivity distribution of the human head. Since the EEG also is influenced by the extracellular volume current, which are difficult to model accurately. All currents, both intracellular and extracellular, generate magnetic fields, but, due to the near spherical shape of the head, one can calculate the resultant magnetic fields due to primary currents without taking into account the conductivity layers of the head.
Interpretation of MEG
Intelligent interpretation of MEG requires the examination of the waveforms recorded by the SQUIDS sensors, followed by a source estimate that reflects the site of activity in the brain. The raw measurement of MEG is a time varying magnetic field. For epilepsy discharge mapping, segments of the activity is used for source analysis, often without any signal averaging. In order to improve the signal-to-noise ratio of measured signals for evoked activity, such as language laterality, however, it is often necessary to average several (typically around 100 trials) responses from identical or similar stimuli. The signal is typically averaged based on the timing of the presented stimulus, the subject's response, or the peak of activity, such as the peak of an epileptic spike. Averaging lowers the effects of extraneous activity, effectively improving the overall signal-to-noise ratio of the desired activity.
Source Modeling
Source modeling determines the origin of the measured neuromagnetic fields, which is the goal of most MEG measurements. The mathematics behind this analysis is known as the inverse problem. Generally, the inverse solution of electromagnetic measurements is non-unique and ill-posed. If proper assumptions are made, however, the solution becomes solvable. We will discuss some of most commonly used source analysis methods used clinically.
Equivalent current dipole
In order to make the inverse solution tractable, one can approximate that the activity from primary sensory or motor cortex originates as a single equivalent current dipole (ECD). This is physiologically plausible, given that a limited patch of cortex is synchronously activated and that the sensors are at least a few centimeters from the source. The ECD provides spatial information, magnitude (current dipole moment), and direction. It is typically computed using a standard iterative least-square algorithm [5], which can also provide a measure of dipole parameter confidence, as well as the best-fitting parameters such as goodness of fit measure (GOF) [6].
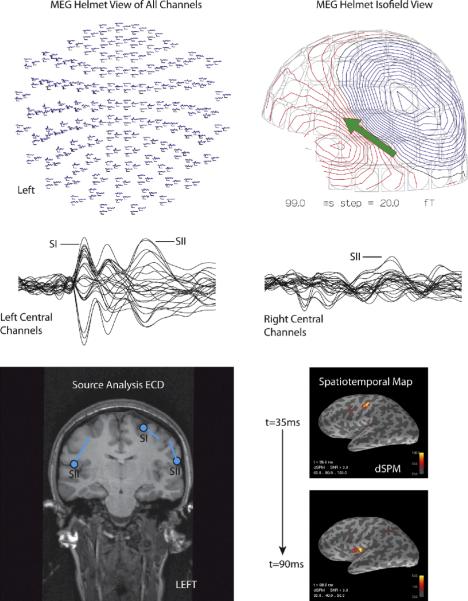
Thus, by assuming a single source, the inverse problem has a unique solution. This works particularly well for primary sensory areas, focal epilepsies, and for higher cognitive areas that have a focal source. Further advances in the ECD approach, for both EEG and MEG, has made it possible to find multiple ECDs with a multidipole approach, such as that developed by Hari and colleges for somatosensory activation [7]. One approach to investigate temporal changes in different areas of the brain is known as the time-varying dipole model. In this model, a series of dipoles are modeled such that the locations are fixed, but allowing the amplitudes to vary over time (See Figure 3). This ECD approach works quite well for sequentially or simultaneously activated cortical sources, although the fine spatial details are lost due to the fact that the measurements are obtained at least 3 cm from the brain sources [6].
Figure 3.
MEG somatosensory response from median nerve electrical stimulation. (A) equivalent current dipole fittings of the primary somatosensory SI (axial T1-wieghted MRI) and secondary SII areas (left lower). The 2 locations for SI are for ipsilateral (20ms latency) and contralateral (35ms) stimulation. (B) The distributed source solution using a noise-normalized minimum norm estimate (MNE) shown at 35 ms and 90 ms after stimulation (lower right).
Distributed solutions
Minimum Norm Estimate (MNE)
If a large patch of cortex is activated, or if there are several areas of cortex activity simultaneously, the single ECD solution may be misleading. In practice this may be suspected when a dipole localizes too deep to be physiologically plausible, i.e., in the deep white matter. In such cases, distributed solutions such as the minimum norm estimate (MNE), the minimum current solution (MCE), or a beamformer solution maybe more accurate. Although numerous other inverse solutions exist [8, 9], this review concentrates on the MNE and beamformer solutions.
Originally pioneered by Hämäläinen [10], and improved upon by Dale and colleges [3, 4], the minimum norm estimate (MNE) is now available in various software packages, from freeware to commercially available software. The MNE does have some important limitations that must be kept in mind especially when used clinically: a depth bias, and a difficulty in determining the extent of activation. In its most elementary expression, the source variance is assumed to be equal throughout the volume, and the MNE solution is biased toward the most superficial currents. One approach to lessen this effect is to use a cortical constraint obtained from the anatomic MRIs [11]. In addition to a depth bias, determining the extent of the sources is also problematic. Simulations [11] show that the point-spread function of estimates is a function of location. Further, the spread depends on the assumed source variance.
In order to compensate for the superficial bias of MNE current sources, Dale has suggested producing a dynamic statistical parametric map (dSPM) by normalizing the estimate MNE by the signal noise [4]. Using the dSPM, the point-spread function is more uniform across the brain [12] and removes the superficial bias (See Figure 3). Additionally, the dSPM is F-distributed, allowing it to be used for hypothesis testing [4].
Beamformer
Another distributed source solution is commonly termed the beamform, which is based on the principal of spatial filters. Beamformers are typically are applied without a cortical constraint, although they can be modified to do so. The synthetic-aperture magnetometry (SAM) [13] is a beamformer approach that can be applied to both raw data and averaged (evoked) MEG or EEG. More sophisticated methods, such as Adaptive spatial filtering [14, 15] and Dynamic Imaging of Coherent Sources (DICS) [16], use a spectral analysis with coherence or other correlations which make it possible to measure functional connectivity in the brain within a particular spectral band. This makes it possible to localize changes in spectral power or functional connectivity that can be used to make evoked, such as language and motor mapping, or resting state functional connectivity.
Eloquent Cortex Evaluation
Current presurgical methods to evaluate eloquent cortex
In patients with epilepsy or brain masses, lateralization and localization of language functioning may be critical for preserving the quality of life. Currently, there are several methods to evaluate eloquent cortex, including neuropsychological testing, fMRI, MEG, the Intracarotid Amobarbital Procedure (IAP), and invasive electrode mapping [17–20]. Precise localization of the motor cortex and somatosensory cortex (central sulcus) is commonly required for frontal masses or for frontal lobe epilepsy. Verbal memory and language are the most common functions to be affected by a L-ATL, a common surgery for medial temporal lobe epilepsy [21–24]. Testing whether a temporal lobe is dominant for verbal function is often referred to as simply determining language lateralization (or verbal memory lateralization). Language cortex, typically the posterior language cortex, also needs to be precisely localized when a lesion is located in the posterior temporal lobe. We will briefly discuss the evaluation of language function in order to understand how MEG current evaluation of the surgical patient.
Neuropsychological testing
Neuropsychological testing assesses the functional status of the hippocampus and predicts the neurocognitive outcome of after resection of the hippocampus, but its use as a sole predictor of outcomes is limited. Neuropsychological test performance correlates with MTLE status and neuropsychological test performance before and after surgery, although the overall specificity is moderate [23, 24]. Material-specific memory loss as measured by neuropsychological test performance is observed in 20–30% of patients following unilateral ATL [24–26]. Material-specific memory loss is far more common following left- than right-sided ATL, with 25–50% of left anterior temporal lobectomy patients demonstrating verbal memory decline This is particularly true for patients without significant sclerosis on the surgical side. Declines in visual memory following right-sided ATL are less consistently observed but have been reported on tests that are highly spatial in nature. Accordingly, preoperative scores on material specific memory tests have demonstrated some utility in the prediction of cognitive morbidity following anterior temporal lobectomy; however, the sensitivity of prediction is modest (56%).
Intracarotid Amobarbital Procedure (IAP)
The Intracarotid Amobarbital Procedure (IAP) is used to predict language and memory outcomes following an ATL or for resections near cortical language area. For an IAP, amobarbital is injected into the internal carotid artery (ICA) through an arterial catheter and brief neuropsychological tests are performed. The IAP generally assesses verbal memory and, to a lesser extent, memory for visual items that are not easily encoded verbally such as scenes or faces [27, 28].
Limitations of the IAP
Despite its widespread use, some shortcomings to the IAP are widely known and limit its use [29–31]. It is invasive with a small but measurable risk of stroke, vascular damage, and infection [29, 31, 32]. The results of the IAP have been shown to be unreliable in test-retest studies, which may be due to flow of anesthetic to the opposite hemisphere (trans-hemispheric cross-flow) and variable penetration of the vasculature to the mesial temporal lobe from the internal carotid artery. Recently, there has been a shortage of amobarbital that has also reduced the use of the procedure, at least temporally [33]. Pelletier, et al. [34] suggests four requirements needed before a test could replace the IAP: (1) A high predictive power for the presence or absence of critical functions in specific brain regions. (2) A user-independent statistical methodology. (3) High-spatial resolution (4) Production of reliable activation maps at the individual level. MEG meets these criteria and, in our experience, its routine use has lead to a reduction in the number of patients who undergo the IAP.
Language Evaluation
In part due to advancements in functional neuroimaging, the IAP is being used less and less frequently [29]. Despite the IAP being the consensus gold standard for verbal functional, it has been criticized because of potential cross-flow to the contra-lateral hemisphere, and due to typical lack of testing of territory supplied by the posterior circulation. Since the mid 1990s, MEG is being used to determine both hemispheric dominance for language, as well as regional language mapping of individual language areas [35–43]. MEG of language areas provides an accurate non-invasive method of mapping language areas. In practice, MEG can be used to plan for an IAP and stimulation-based intraoperative mapping techniques.
Determining the location of language processing in a subject requires applying the task that best activates the desired stream of language processing. Specific language processes are complex and often include phonological, lexical, and also semantic processes. [44]. Verbal memory encoding and retrieval occurs concurrently in virtually any task, making it difficult to separate `language processing' from `memory'. Further supporting language processes are attention, motor planning (speech), and visual or auditory functions. In fMRI, semantic decision and stem completion tasks are popular as they require a response from the patient, which ensures the quality of the patient's responses. For MEG, covert responses are more desirable, as overt (spoken) responses may lead to unacceptable motion artifacts. Still, some passive sensory paradigms requiring no patient response have been reported in the literature to be successful (see below).
Hemispheric Dominance for Language
Determination of the language dominant hemisphere is critical in the presurgical evaluation of patients who will have temporal or frontal lobe resections. For left anterior temporal lobectomies (L-ATL), the determination of language laterality is done routinely with MEG and fMRI. Wada and Rasmussen determined that over 93% of patients are left hemisphere dominant for language, as are over 96% of right-handed patients, although more recent studies indicate that many patients have more bilateral representation of language than the original studies [45]. In left-handed epilepsy patients, only about 70% of patients demonstrate left hemispheric dominance for language, with about 15% of patients demonstrating bilateral language lateralization.
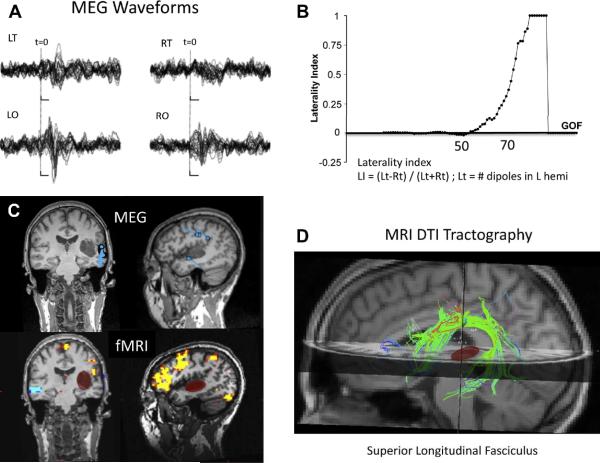
First proposed by Papanicolaou [46], sequential dipole fitting is a robust methods to determine language laterality (See Figure 5). The stimuli used by Papanicolaou were both auditory and visual words. The results concur with Wada test results [35] and electrical stimulation mapping [47]. Szymanski et al [42] reported using simple phonetic stimuli, such as the vowel sounds /a/ and /u/, for determining the language hemispheric dominance by summing the number of selective dipoles in the late auditory magnetic field on each hemisphere, and calculating a lateralization index. Multiple groups report a strong correlation with both intraoperative mapping techniques and the results of the Wada test [39, 42].
Figure 5.
Multiple imaging modalities for language mapping (MEG, fMRI, and DTI). (A) MEG waveforms for left and right temporal (LT and RT, respectively) and occipital (LO, RO) sensors, showing several peaks related to sensory and language activity. (B) Laterality index as a function of the goodness of fit (GOF )of a sequential equivalent current dipole (ECD) fit to the MEG data, suggesting left-hemisphere dominance. (C) Locations of the ECDs for the MEG data (top) and functional MRI (bottom) for a visual reading task, displayed in coronal and sagittal slices of of anatomical MRI. Note the cluster of MEG dipoles in the posterior superior temporal gyrus, presumably including Wernicke's area. Functional MRI shows largest activation in the inferior lateral left frontal cortex. The location of a tumor in the left temporal lobe is highlighted with the red oval. (D) MRI tractography showing the superior longitudinal fasciculus (SLF) that connects temporal and frontal language areas. Note that the tumor (red) does not interrupt or displace the SLF white matter fiber bundle.
Regional Language Mapping
In addition to lateralizing language, MEG also can accurately spatially locate language cortex. MEG localizes, with a high temporal resolution, both receptive and productive brain areas. The spatial resolution can be further increased by combining with a fMRI data [4]. For localizing language specific areas in the cortex, there are several criteria that are important for patient studies compared with the more frequent use for cognitive neuroscience. First, the neurosurgical application requires precise localization in individual subjects, although for group studies the neuroscientist can average responses over several subjects. Second, presurgical evaluation usually requires mapping the essential language areas, not just participating areas. Removal of essential language areas may result in a language deficit. Participating areas are activated during language paradigms, but do not result in a post-operative language deficit after resection, either because there are areas of redundant processing or because other areas learn to take over the same functions. Currently, there is no reliable neuroimaging method to distinguish essential from participating areas with non-invasive imaging, and remains a major goal of clinical functional imaging. Combining MEG with other technologies such as transcranial magnetic stimulation (TMS) may make this possible in the near future.
By simply applying a source localization procedure, the same techniques described in determining hemispheric dominance can be used for regional language mapping. The mapping of equivalent current dipoles of the late auditory evoked fields can be used for both posterior temporal and frontal operculum mapping [46, 48] (Figure 5). Temporal maps of activation have similar profiles as determined by invasive electro-corticography (ECoG). On MEG tracing, the latency of Wernicke's area is typically between 210–420 msec, and Broca's area 400 msec – 700 msec, depending on the particular language task and differences in individual subjects. Generally, the peak activation of Wernicke's area precedes Broca's area, although occasionally other temporal profiles have been reported [49]. The beamformer technique, such as SAM (Figure 6), can map decreases specific spontaneous activity power bands, such as beta band decreases (~20Hz), known as event related desynchronization [50].
Figure 6.
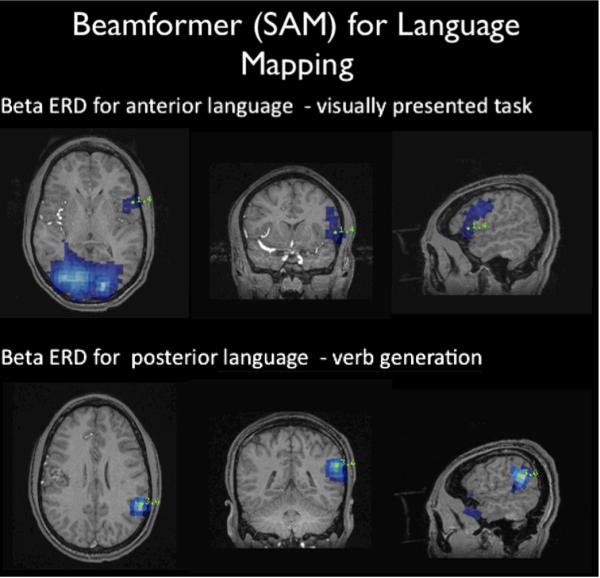
Beamformer for presurgical mapping. The upper panel shows the map for language using a visually presented word with robust activation of anterior language cortex, comprising Broca's area. A verb generation paradigm was used to map the posterior language areas (Wernicke's area). [Figure courtesy of Dr. Erin Simon Schwartz and Dr. Timothy Roberts, Children's Hospital Philadelphia].
A common clinical use of MEG is in the presurgical imaging of epilepsy or brain tumor patients is to aid in the localization of the central sulcus. Even with histologically low-grade tumors, the central sulcus can be distorted by tumor infiltration and/or mass effect, obscuring it on an anatomic MRI. Using a tactile stimulator, the somatosensory cortex homunculus can be easily mapped by successively stimulating finger digits, foot digits, and lip using MEG. Alternatively, an electrical nerve stimulator can be used to map the median, tibial nerve, and lip representative areas. If an electrical nerve stimulator is used, the electrodes are placed, and the intensity set, such that thumb twitching or toe twitching is elicited. After signal averaging, both the primary (SI) and secondary (SII) somatosensory cortices are detectable with MEG (Figure 3).
After recording the evoked magnetic fields, the primary somatosensory cortex is localized typically, such as with an ECD method [51–53] or distributed source [50, 54]. If electrical median nerve stimulation technique is used, the N20m—the first identifiable component of the evoked magnetic field—is easily evoked in most patients, including ones under deep anesthesia or in a coma [55, 56]. The N20m generator is located in the anterior wall of the somatosensory gyrus (Brodmann area 3b), with a non-radial orientation which is ideal for detection with MEG [7, 51, 53, 55, 57]. It is a pre-conscious field that does not synapse in the thalamus and, due to a high signal-to-noise ratio, has a very repeatable localization, with localization accuracy on the order of millimeters.
MEG identification of the central sulcus has been validated by several groups using intraoperative measurements using a variety of methods including equivalent current dipoles [45, 58–60] and the beamformer approach [54]. Schiffbauer et al [60], for example, found that regardless of tumor grade, intra-axial brain tumors may border on or invade the somatosensory and auditory cortex. Importantly, low-grade tumors were more likely than high-grade tumors to invade and involve the functionally viable cortex. The authors concluded that low-grade tumors, due to slow growth, more often demonstrate functional activity within the radiologically abnormal areas, than high-grade tumors. High-grade tumors, on the other hand, often show functional activity at the margin of the contrast-enhancing area. They suggested these findings were caused by physical displacement of the functional tissue due to mass effect of the high-grade tumor. Firsching et al [61] reported that in 30 patients, ECD localization of the tactile neuromagnetic response localized in the somatosensory cortex, and was in agreement with phase reversal measurements at the time of surgery without exception. Further, they concluded that MEG-based functional neuronavigation was practical and reliable. Recent reports of non-invasive multimodal technologies—MEG, fMRI, and others—have noted that when combined enhance the reliability of identification of the central sulcus. Some have suggested using a functional risk profile (FRP), based on MEG findings to improve surgical decision-making [62]. It should be kept in mind that brain tumor patients with known sensory or motor deficits may have diminished evoked fields [62]. Nagaragan et al [54] demonstrated that an adaptive spatial filter, a type of beamformer, can accurately identify the motor cortex and the somatosensory cortex. They further demonstrated that it is be more accurate in some circumstances than the equivalent current dipole approach.
Motor
As noted above, many functional imaging techniques, such as fMRI and PET, can accurately identify the central sulcus [63]. However, isolating pure motor activity for example, of the precentral gyrus with fMRI is difficult due to inevitable activation of the adjacent somatosensory cortex. Unlike fMRI and PET, MEG can be used to isolate motor activity due to high temporal resolution. In practice, it requires precise timing of the onset of motor movement in order to produce an averaged evoked field. This can be achieved by a self-paced button press, or by use of a trigger a photo-optic switch. Activity that peaks between 20–50ms before the onset of movement reflects activity in the primary motor cortex [64].
Alternatively, the motor cortex may be mapped by quantifying the functional connectivity between an electromyogram (EMG) and the MEG sensors, as suggested by Makela et al. [65]. Placing bipolar electrodes over the first interosseous muscle and instructing the patient to slowly adduct generates large signal in both the EMG and MEG. Calculation of the coherence of the MEG-EMG yields a spectrum of a spike centered on or near 20Hz, strongest over the central sensors. This represents synchronization of the muscle twitches with the 20Hz `mu' rhythm, which is known to originate in the motor cortex [65]. By localizing the functional connectivity, either by single ECD or distributed source method [xxxGross], identifies the primary motor cortex. This isolates the primary motor cortex from the somatomotor network, something that can be difficult with fMRI. Further, MEG identifies the entire neural network activated during the planning and the act of motor movement, including supplementary cortex [66, 67] and premotor cortex [16, 68].
Visual Cortex
In patients with lesions lying near visual areas, MEG can evaluate the function of visual cortex. Mapping of the visual areas is theoretically difficult withm MEG due to synchronously active sources that may result in magnetic field cancellation at the scalp. The primary visual cortex can be mapped by a simple ECD of the first visual evoked field from nearly any strong visual stimulus, typically a portion of a visual quadrant is stimulated with a checkerboard. In practice, however, it does yield valid results [69–71]. Mapping of the magnetic equivalent of visual evoked potentials N75, P100, and N145 components is robust with large visual stimuli with phase reversal techniques, and can be performed to detect visual field deficits [69–71].
Spontaneous Activity: Epileptic Spike Localization
Localization of epileptic discharges may be performed in the context of a low-grade brain tumor or as part of the workup of surgical epilepsy. Interictal activity is easily captured with MEG. Ictal activity, although more difficult, can also be detected and localized with whole head MEG systems—particularly if the instrument is located in a hospital where antiepileptic medicines can be tapered. The most effective MEG measurement of epilepsy is with a whole-head system with simultaneously recorded EEG. A standard EEG electrode array, manufactured with no magnetic material, provides a standard EEG classification of ictal or interictal activity. High-density electrode caps are also available if EEG source localization is needed.
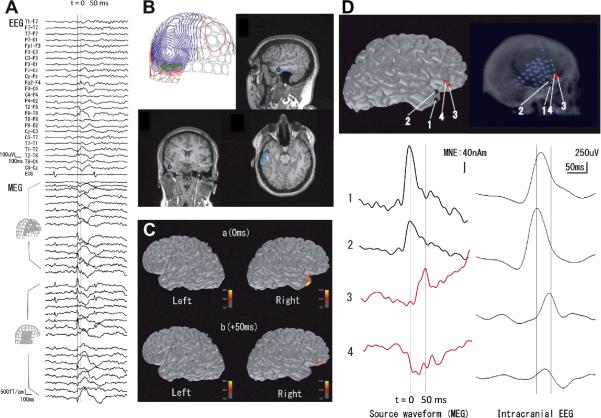
Presurgical evaluation of patients with epilepsy typically involves simultaneous whole-head MEG and EEG recording during rest, which records basic rhythmic activity, sleep-related activity, and other spontaneous brain activity, in addition to the epileptic discharges. Localizing the neural source of interictal spikes present in the MEG data (see Figure 7) localizes the epileptogenic zone even in the event of propagation [72–75] The equivalent current dipole (ECD) is an important source model for epileptic discharges, typically for localizing the peak of an ictal or inter-ictal epileptic discharge. However, the ECD at times may not match the location of the seizure onset. In particular, source analysis using a distributed source model, such as minimum norm estimate (MNE) [76], and beamformer techniques [77] may be helpful in demonstrating the time-course of cortical activation and thereby reveal the generation and propagation of epileptic activity [78].
Figure 7.
Localization of epileptic spikes. (A) Simultaneously acquired EEG (top) and MEG (bottom) signals from a patient with epilepsy. An epileptic spike is seen in MEG sensors over the right temporal and frontal regions. (B) An equivalent current dipole (ECD) computed at the peak of the spike (“0 ms”; the corresponding isocontour map of the MEG data, with the ECD as a green arrow, is shown at top left) is localized in the temporal lobe (blue dots superimposed on the anatomical MRI). (C) Distributed source estimates, the noise-normalized minimum-norm estimate (MNE), also known as dynamic statistical parametric map (dSPM), for the MEG data are displayed on the cortical surface representation reconstructed from anatomical MRI. The source estimates suggest that the activity propagates from a right temporal region (“0 ms”) to the right frontal region (“50 ms”). (D) Comparison of MEG data with intracranial EEG (iEEG). The left panel shows the estimated MEG source waveforms (MNE) at four locations (“1” and “2” temporal, “3” and “4” frontal). The right panel shows the iEEG of an epileptic spike at corresponding locations. The MEG and iEEG are consistent in suggesting temporal activity propagating to the frontal lobe over a 50 ms time period. [Figures created by Dr. Naoro Tanaka, M.D., Ph.D.]
MEG Improves Clinical Treatment of Surgical Epilepsy Patients
A number of recent studies demonstrate that MEG improves the quality clinical care in refractory epilepsy patients. Including the MEG in the presurgical evaluation of epilepsy influences clinical decisions and increases the likelihood of surgical success (making a person seizure free) [79–81]. MEG also provides non-redundant clinical information in about one third of the epilepsy cases that were performed during the presurgical evaluation [82].
Functional Connectivity
Functional connectivity defines correlations of activity in regions across brain regions, whereas effective connectivity measures the influence of brain regions on each other. Corticomuscular coherence is a type of functional connectivity between the motor cortex and the muscle subunits that is used clinically to identify primary motor cortex. Functional connectivity using temporal correlations in fMRI has made it possible to map several neural networks, such as the motor system [83]. The high temporal resolution of MEG allows connectivity analysis to be performed at a high temporal resolution. Other abnormalities in functional connectivity include epilepsy. For example, abnormally high functional or effective connectivity across small distances could influence epileptic spike generation. Increased connectivity across brain areas has been hypothesized to be responsible for the propagation and generalization of epilepsy. On the other hand, a failure of integration of perceptions might suggest a failure in “binding” object features within and across brain regions, may lead to psychiatric diseases such as schizophrenia. Functional connectivity, using MEG may help define epileptogenic cortex, especially in multifocal epilepsy [84].
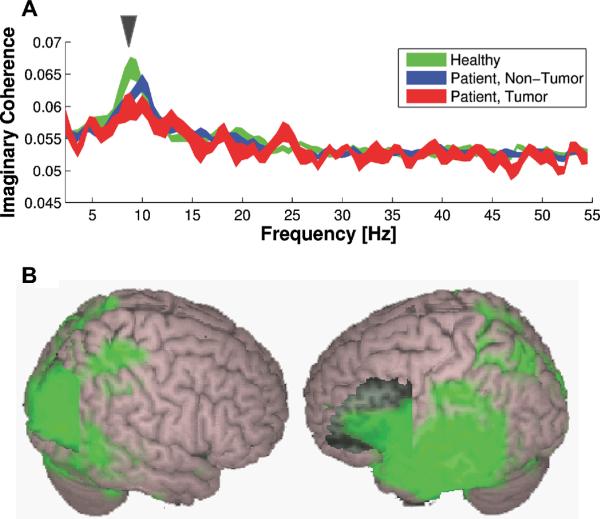
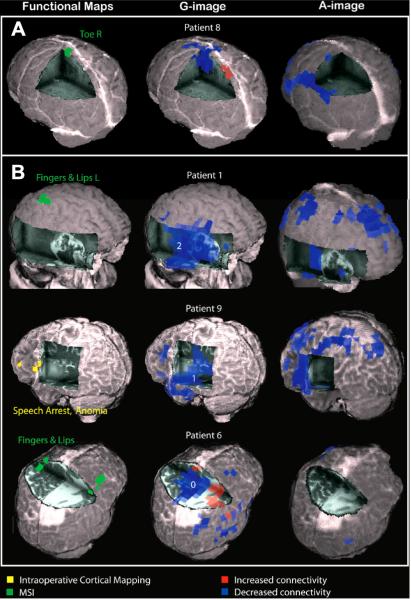
Recent work has shown that abnormalities in brain connectivity as measured by MEG have clinical value, such as in patients with brain tumors [54]. Patients with brain tumors have abnormal connectivity compared to healthy controls (Figure 8). In patients with brain tumors, eloquent cortex found within abnormal resting-state functional connectivity have an increased risk of post-operative deficits (Figure 9). These are some potentially new useful clinical applications of MEG in neurosurgical patients.
Figure 8.
Frequency spectrum and spatial distribution of imaginary coherence (IC). (A) Tumor tissue has lower functional connectivity in the alpha frequency range than nontumor tissue of lesion patients and healthy control subjects. (B) The functional connectivity from healthy subjects superimposed on 3D brain atlas. High functional connectivity is found in Broca's area and right visual cortex. [From {Guggisberg, 2008 #595}. Used with permission]
Figure 9.
Functional maps obtained with MEG connectivity and ESM (yellow) and funcitonal connectivity images of four patients with brain tumors are superimposed over their three-dimensional-rendered individual brain. (A) Twenty-five-year-old woman with a right foot with grade III astrocytoma grade infiltrating the left medial sensorimotor cortex. Functional connectivity in the right foot area of the sensorimotor cortex was decreased. (B) MEG functional connectivity in three tumor patients without presurgical functional deficits, sugesting functional disconnection (blue) of the corresponding tumor tissue (graded 0–2, with 0 indicating smallest proportion of decreased functional connectivity). In agreement with the MEG functional connectivity images and the clinical status, eloquent cortex was mapped outside of decreased connectivity (blue) areas by MEG and cortical mapping in all patients. MEG functional connectivity predicts post-surgery function: whereas Patient 6 suffered from postsurgical sensible deficits in the left arm and leg, Patients 1 and 9 had no post-operative deficits. Red areas indicate increased connectivity. [From {Guggisberg, 2008 #595}. Used with permission]
Neuronavigation and MEG
Co-registration of MEG and structural images with skin fiducial markers makes it makes it possible to delimit the volume containing the lesion and the surgical field [85–87]. Frameless stereotactic systems assist with resection of small, deep-seated tumors during neurosurgery, with a precision approaching 2mm. Ganslandt et al [88] combined MEG somatosensory mapping with a free-hand stereotactic pointing device in 25 cases of peri-rolandic tumors and masses. They found agreement with intraoperative somatosensory mapping in all cases. Further, they eliminated 11 patients who were not available for open surgery because of tumor infiltration of the motor cortex [88]. Rezai et al [89, 90] report the use of a combination of MEG-derived functional mapping, and CT scans, MRI, and digital angiography reduces surgical risk. In order to overcome the errors associated with shift of brain contents during an operation requires sulcal landmarks on 3-D reconstructions with fMRI, MEG, iEEG and tractography aid in surgical planning.
Conclusions & Future Directions
MEG is now an accepted method of presurgical evaluation in patients with brain tumors and epilepsy. Beyond epileptic discharge mapping and non-invasive mapping of eloquent cortex, clinical research studies suggest that MEG is ready for other neurosurgical applications, including head trauma, Parkinson's disease, and other disorders. Combining MEG, EEG, fMRI and new methods of connectivity are also being evaluated for clinical applications. These studies will continue to change how MEG is applied in neurosurgical evaluations.
Figure 4.
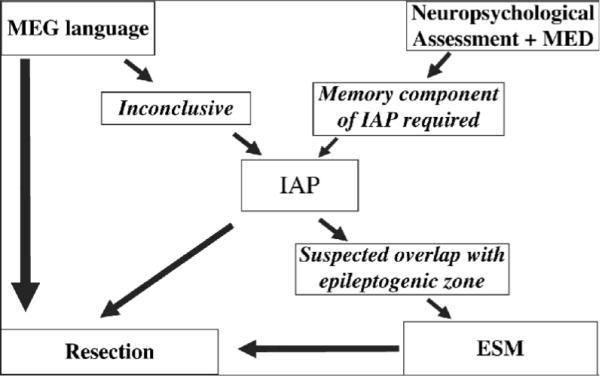
Current algorithm for presurgical lateralization of language and memory. ESM is electrical stimulation map with the grid This diagram depicts language and memory assessment used at many centers, including ours. In this algorithm, noninvasive language and memory tests are sometimes followed by IAP and, if needed, subdural grid evaluation prior to resection. Modified after {Loddenkemper, 2008 #2470}.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Cohen D. Magnetoencephalography: evidence of magnetic fields produced by alpha-rhythm currents. Science. 1968;161(843):784–6. doi: 10.1126/science.161.3843.784. [DOI] [PubMed] [Google Scholar]
- 2.Cohen D. Magnetoencephalography: detection of the brain's electrical activity with a superconducting magnetometer. Science. 1972;175(22):664–6. doi: 10.1126/science.175.4022.664. [DOI] [PubMed] [Google Scholar]
- 3.Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Curr Opin Neurobiol. 2001;11(2):202–8. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 4.Dale AM, Liu AK, Fischl BR, et al. Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- 5.Marquardt DW. An Algorithm for Least-Squares Estimation of Nonlinear Parameters SIAM. J. Appl. Math. 1963;11(2):431–441. [Google Scholar]
- 6.Hamalainen M, Hari R, Ilmoniemi RJ, et al. Magnetoencephalography -theory, instrumentation, and application to noninvasive studies of the working human brain. Review of Modern Physics. 1993;65:413–497. [Google Scholar]
- 7.Hari R, Karhu J, Hamalainen M, et al. Functional organization of the human first and second somatosensory cortices: a neuromagnetic study. Eur J Neurosci. 1993;5(6):724–34. doi: 10.1111/j.1460-9568.1993.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 8.Mosher JC, Leahy RM, Lewis PS. EEG and MEG: forward solutions for inverse methods. IEEE Trans Biomed Eng. 1999;46(3):245–59. doi: 10.1109/10.748978. [DOI] [PubMed] [Google Scholar]
- 9.Mosher JC, Leahy RM. Recursive MUSIC: a framework for EEG and MEG source localization. IEEE Trans Biomed Eng. 1998;45(11):1342–54. doi: 10.1109/10.725331. [DOI] [PubMed] [Google Scholar]
- 10.Hamalainen MS, Ilmoniemi RJ. Vol. Report TKK-F-A559. Helsinki Univ. of Technology; Helsinki, Finland: 1984. Interpreting measured magnetic fields of the brain: estimates of current distributions. [Google Scholar]
- 11.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J. Cog. Neurosci. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 12.Liu AK, Dale AM, Belliveau JW. Monte Carlo simulation studies of EEG and MEG localization accuracy. Hum Brain Mapp. 2002;16(1):47–62. doi: 10.1002/hbm.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25(2):249–71. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- 14.Sekihara K, Sahani M, Nagarajan SS. A simple nonparametric statistical thresholding for MEG spatial-filter source reconstruction images. Neuroimage. 2005;27(2):368–76. doi: 10.1016/j.neuroimage.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekihara K, Nagarajan SS, Poeppel D, et al. Performance of an MEG adaptive-beamformer technique in the presence of correlated neural activities: effects on signal intensity and time-course estimates. IEEE Trans Biomed Eng. 2002;49(12 Pt 2):1534–46. doi: 10.1109/tbme.2002.805485. [DOI] [PubMed] [Google Scholar]
- 16.Gross J, Kujala J, Hamalainen M, et al. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 2001;98(2):694–9. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojemann GA. Individual variability in cortical localization of language. J Neurosurg. 1979;50(2):164–9. doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- 18.Bell BD, Davies KG, Haltiner AM, et al. Intracarotid amobarbital procedure and prediction of postoperative memory in patients with left temporal lobe epilepsy and hippocampal sclerosis. Epilepsia. 2000;41(8):992–7. doi: 10.1111/j.1528-1157.2000.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 19.Perrine K, Westerveld M, Sass KJ, et al. Wada memory disparities predict seizure laterality and postoperative seizure control. Epilepsia. 1995;36(9):851–6. doi: 10.1111/j.1528-1157.1995.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 20.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. 1960. J Neurosurg. 2007;106(6):1117–33. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- 21.Vaz SA. Nonverbal memory functioning following right anterior temporal lobectomy: a meta-analytic review. Seizure. 2004;13(7):446–52. doi: 10.1016/j.seizure.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Loring DW. Amobarbital effects and lateralized brain function : the Wada test. Springer-Verlag; New York: 1992. p. xiii.p. 138. [Google Scholar]
- 23.Loring DW, Hermann BP, Meador KJ, et al. Amnesia after unilateral temporal lobectomy: a case report. Epilepsia. 1994;35(4):757–63. doi: 10.1111/j.1528-1157.1994.tb02507.x. [DOI] [PubMed] [Google Scholar]
- 24.Loring DW, Meador KJ, Lee GP. Determinants of quality of life in epilepsy. Epilepsy Behav. 2004;5(6):976–80. doi: 10.1016/j.yebeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Loring DW, Meador KJ, Lee GP, et al. Structural versus functional prediction of memory change following anterior temporal lobectomy. Epilepsy Behav. 2004;5(2):264–8. doi: 10.1016/j.yebeh.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Loring DW, Murro AM, Meador KJ, et al. Wada memory testing and hippocampal volume measurements in the evaluation for temporal lobectomy. Neurology. 1993;43(9):1789–93. doi: 10.1212/wnl.43.9.1789. [DOI] [PubMed] [Google Scholar]
- 27.Griffith HR, Richardson E, Pyzalski RW, et al. Memory for famous faces and the temporal pole: functional imaging findings in temporal lobe epilepsy. Epilepsy Behav. 2006;9(1):173–80. doi: 10.1016/j.yebeh.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Powell HW, Richardson MP, Symms MR, et al. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. J Neurol Neurosurg Psychiatry. 2008;79(6):686–93. doi: 10.1136/jnnp.2007.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loddenkemper T. Quo vadis Wada? Epilepsy Behav. 2008;13(1):1–2. doi: 10.1016/j.yebeh.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Loddenkemper T, Moddel G, Dinner DS, et al. Language assessment in Wada test: comparison of methohexital and amobarbital. Seizure. 2009;18(9):656–9. doi: 10.1016/j.seizure.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Loddenkemper T, Morris HH, Moddel G. Complications during the Wada test. Epilepsy Behav. 2008;13(3):551–3. doi: 10.1016/j.yebeh.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Baxendale SA, Thompson PJ, Duncan JS. Evidence-based practice: a reevaluation of the intracarotid amobarbital procedure (Wada test) Arch Neurol. 2008;65(6):841–5. doi: 10.1001/archneur.65.6.841. [DOI] [PubMed] [Google Scholar]
- 33.Jones-Gotman M, Sziklas V, Djordjevic J, et al. Etomidate speech and memory test (eSAM): a new drug and improved intracarotid procedure. Neurology. 2005;65(11):1723–9. doi: 10.1212/01.wnl.0000187975.78433.cb. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier I, Sauerwein HC, Lepore F, et al. Non-invasive alternatives to the Wada test in the presurgical evaluation of language and memory functions in epilepsy patients. Epileptic Disord. 2007;9(2):111–26. doi: 10.1684/epd.2007.0109. [DOI] [PubMed] [Google Scholar]
- 35.Breier JI, Simos PG, Zouridakis G, et al. Lateralization of cerebral activation in auditory verbal and non-verbal memory tasks using magnetoencephalography. Brain Topogr. 1999;12(2):89–97. doi: 10.1023/a:1023458110869. [DOI] [PubMed] [Google Scholar]
- 36.Breier JI, Simos PG, Zouridakis G, et al. Language dominance determined by magnetic source imaging: a comparison with the Wada procedure. Neurology. 1999;53(5):938–45. doi: 10.1212/wnl.53.5.938. [DOI] [PubMed] [Google Scholar]
- 37.Castillo EM, Simos PG, Venkataraman V, et al. Mapping of expressive language cortex using magnetic source imaging. Neurocase. 2001;7(5):419–22. doi: 10.1076/neur.7.5.419.16249. [DOI] [PubMed] [Google Scholar]
- 38.Floel A, Knecht S, Lohmann H, et al. Language and spatial attention can lateralize to the same hemisphere in healthy humans. Neurology. 2001;57(6):1018–24. doi: 10.1212/wnl.57.6.1018. [DOI] [PubMed] [Google Scholar]
- 39.Simos PG, Breier JI, Maggio WW, et al. Atypical temporal lobe language representation: MEG and intraoperative stimulation mapping correlation. Neuroreport. 1999;10(1):139–42. doi: 10.1097/00001756-199901180-00026. [DOI] [PubMed] [Google Scholar]
- 40.Simos PG, Castillo EM, Fletcher JM, et al. Mapping of receptive language cortex in bilingual volunteers by using magnetic source imaging. J Neurosurg. 2001;95(1):76–81. doi: 10.3171/jns.2001.95.1.0076. [DOI] [PubMed] [Google Scholar]
- 41.Simos PG, Papanicolaou AC, Breier JI, et al. Localization of language specific cortex by using magnetic source imaging and electrical stimulation mapping. J Neurosurg. 1999;91(5):787–96. doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- 42.Szymanski MD, Perry DW, Gage NM, et al. Magnetic source imaging of late evoked field responses to vowels: toward an assessment of hemispheric dominance for language. J Neurosurg. 2001;94(3):445–53. doi: 10.3171/jns.2001.94.3.0445. [DOI] [PubMed] [Google Scholar]
- 43.Szymanski MD, Rowley HA, Roberts TP. A hemispherically asymmetrical MEG response to vowels. Neuroreport. 1999;10(12):2481–6. doi: 10.1097/00001756-199908200-00009. [DOI] [PubMed] [Google Scholar]
- 44.Poeppel D, Idsardi WJ, van Wassenhove V. Speech perception at the interface of neurobiology and linguistics. Philos Trans R Soc Lond B Biol Sci. 2008;363(1493):1071–86. doi: 10.1098/rstb.2007.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beisteiner R, Gomiscek G, Erdler M, et al. Comparing localization of conventional functional magnetic resonance imaging and magnetoencephalography. Eur J Neurosci. 1995;7(5):1121–4. doi: 10.1111/j.1460-9568.1995.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 46.Papanicolaou AC, Simos PG, Breier JI, et al. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90(1):85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- 47.Panagiotis B. Grand mal seizures with liver toxicity in a case of clozapine treatment. J Neuropsychiatry Clin Neurosci. 1999;11(1):117–8. doi: 10.1176/jnp.11.1.117a. [DOI] [PubMed] [Google Scholar]
- 48.Breier JI, Simos PG, Wheless JW, et al. Language dominance in children as determined by magnetic source imaging and the intracarotid amobarbital procedure: a comparison. J Child Neurol. 2001;16(2):124–30. doi: 10.1177/088307380101600211. [DOI] [PubMed] [Google Scholar]
- 49.Kober H, Moller M, Nimsky C, et al. New approach to localize speech relevant brain areas and hemispheric dominance using spatially filtered magnetoencephalography. Hum Brain Mapp. 2001;14(4):236–50. doi: 10.1002/hbm.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holodny A. Functional Neuroimaging: A Clinical Approach. Informa Healthcare: 2008. [Google Scholar]
- 51.Hari R. On brain's magnetic responses to sensory stimuli. J Clin Neurophysiol. 1991;8(2):157–69. doi: 10.1097/00004691-199104000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Hari R. Magnetic evoked fields of the human brain: basic principles and applications. Electroencephalogr Clin Neurophysiol Suppl. 1990;41:3–12. doi: 10.1016/b978-0-444-81352-7.50005-4. [DOI] [PubMed] [Google Scholar]
- 53.Hari R, Forss N. Magnetoencephalography in the study of human somatosensory cortical processing. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1145–54. doi: 10.1098/rstb.1999.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagarajan S, Kirsch H, Lin P, et al. Preoperative localization of hand motor cortex by adaptive spatial filtering of magnetoencephalography data. J Neurosurg. 2008;109(2):228–37. doi: 10.3171/JNS/2008/109/8/0228. [DOI] [PubMed] [Google Scholar]
- 55.Hoshiyama M, Kakigi R, Koyama S, et al. Somatosensory evoked magnetic fields following stimulation of the lip in humans. Electroencephalogr Clin Neurophysiol. 1996;100(2):96–104. doi: 10.1016/0013-4694(95)00241-3. [DOI] [PubMed] [Google Scholar]
- 56.Kakigi R. Somatosensory evoked magnetic fields following median nerve stimulation. Neurosci Res. 1994;20(2):165–74. doi: 10.1016/0168-0102(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 57.Hari R, Hamalainen H, Hamalainen M, et al. Separate finger representations at the human second somatosensory cortex. Neuroscience. 1990;37(1):245–9. doi: 10.1016/0306-4522(90)90210-u. [DOI] [PubMed] [Google Scholar]
- 58.Inoue T, Shimizu H, Nakasato N, et al. Accuracy and limitation of functional magnetic resonance imaging for identification of the central sulcus: comparison with magnetoencephalography in patients with brain tumors. Neuroimage. 1999;10(6):738–48. doi: 10.1006/nimg.1999.0501. [DOI] [PubMed] [Google Scholar]
- 59.Kober H, Nimsky C, Moller M, et al. Correlation of sensorimotor activation with functional magnetic resonance imaging and magnetoencephalography in presurgical functional imaging: a spatial analysis. Neuroimage. 2001;14(5):1214–28. doi: 10.1006/nimg.2001.0909. [DOI] [PubMed] [Google Scholar]
- 60.Schiffbauer H, Ferrari P, Rowley HA, et al. Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery. 2001;49(6):1313–20. doi: 10.1097/00006123-200112000-00005. discussion 1320–1. [DOI] [PubMed] [Google Scholar]
- 61.Firsching R, Bondar I, Heinze HJ, et al. Practicability of magnetoencephalography-guided neuronavigation. Neurosurg Rev. 2002;25(1–2):73–8. doi: 10.1007/s101430100161. [DOI] [PubMed] [Google Scholar]
- 62.Hund M, Rezai AR, Kronberg E, et al. Magnetoencephalographic mapping: basic of a new functional risk profile in the selection of patients with cortical brain lesions. Neurosurgery. 1997;40(5):936–42. doi: 10.1097/00006123-199705000-00011. discussion 942–3. [DOI] [PubMed] [Google Scholar]
- 63.Bittar RG, Olivier A, Sadikot AF, et al. Presurgical motor and somatosensory cortex mapping with functional magnetic resonance imaging and positron emission tomography. J Neurosurg. 1999;91(6):915–21. doi: 10.3171/jns.1999.91.6.0915. [DOI] [PubMed] [Google Scholar]
- 64.Lewine JD, Orrison WW., Jr. Magnetic source imaging: basic principles and applications in neuroradiology. Acad Radiol. 1995;2(5):436–40. doi: 10.1016/s1076-6332(05)80351-4. [DOI] [PubMed] [Google Scholar]
- 65.Makela JP, Kirveskari E, Seppa M, et al. Three-dimensional integration of brain anatomy and function to facilitate intraoperative navigation around the sensorimotor strip. Hum Brain Mapp. 2001;12(3):180–92. doi: 10.1002/1097-0193(200103)12:3<180::AID-HBM1014>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erdler M, Beisteiner R, Mayer D, et al. Supplementary motor area activation preceding voluntary movement is detectable with a whole-scalp magnetoencephalography system. Neuroimage. 2000;11(6 Pt 1):697–707. doi: 10.1006/nimg.2000.0579. [DOI] [PubMed] [Google Scholar]
- 67.Erdler M, Windischberger C, Lanzenberger R, et al. Dissociation of supplementary motor area and primary motor cortex in human subjects when comparing index and little finger movements with functional magnetic resonance imaging. Neurosci Lett. 2001;313(1–2):5–8. doi: 10.1016/s0304-3940(01)02167-x. [DOI] [PubMed] [Google Scholar]
- 68.Gross J, Tass PA, Salenius S, et al. Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol. 2000;527(Pt 3):623–31. doi: 10.1111/j.1469-7793.2000.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatanaka K, Nakasato N, Seki K, et al. Striate cortical generators of the N75, P100 and N145 components localized by pattern reversal visual evoked magnetic fields. Tohoku J Exp Med. 1997;182(1):9–14. doi: 10.1620/tjem.182.9. [DOI] [PubMed] [Google Scholar]
- 70.Nakasato N, Seki K, Fujita S, et al. Clinical application of visual evoked fields using an MRI-linked whole head MEG system. Front Med Biol Eng. 1996;7(4):275–83. [PubMed] [Google Scholar]
- 71.Nakasato N, Yoshimoto T. Somatosensory, auditory, and visual evoked magnetic fields in patients with brain diseases. J Clin Neurophysiol. 2000;17(2):201–11. doi: 10.1097/00004691-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Tang L, Mantle M, Ferrari P, et al. Consistency of interictal and ictal onset localization using magnetoencephalography in patients with partial epilepsy. J Neurosurg. 2003;98(4):837–45. doi: 10.3171/jns.2003.98.4.0837. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka N, Hamalainen MS, Ahlfors SP, et al. Propagation of epileptic spikes reconstructed from spatiotemporal magnetoencephalographic and electroencephalographic source analysis. Neuroimage. 50(1):217–22. doi: 10.1016/j.neuroimage.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shiraishi H, Ahlfors SP, Stufflebeam SM, et al. Application of magnetoencephalography in epilepsy patients with widespread spike or slow-wave activity. Epilepsia. 2005;46(8):1264–72. doi: 10.1111/j.1528-1167.2005.65504.x. [DOI] [PubMed] [Google Scholar]
- 75.Shiraishi H, Stufflebeam SM, Knake S, et al. Dynamic statistical parametric mapping for analyzing the magnetoencephalographic epileptiform activity in patients with epilepsy. J Child Neurol. 2005;20(4):363–9. doi: 10.1177/08830738050200041601. [DOI] [PubMed] [Google Scholar]
- 76.Shiraishi H, Watanabe Y, Watanabe M, et al. Interictal and ictal magnetoencephalographic study in patients with medial frontal lobe epilepsy. Epilepsia. 2001;42(7):875–82. doi: 10.1046/j.1528-1157.2001.042007875.x. [DOI] [PubMed] [Google Scholar]
- 77.Robinson SE, Nagarajan SS, Mantle M, et al. Localization of interictal spikes using SAM(g2) and dipole fit. Neurol Clin Neurophysiol. 2004;2004:74. [PMC free article] [PubMed] [Google Scholar]
- 78.Shiraishi H, Takano K, Shiga T, et al. Possible involvement of the tip of temporal lobe in Landau-Kleffner syndrome. Brain Dev. 2007;29(8):529–33. doi: 10.1016/j.braindev.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 79.Knowlton RC. Can magnetoencephalography aid epilepsy surgery? Epilepsy Curr. 2008;8(1):1–5. doi: 10.1111/j.1535-7511.2007.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knowlton RC, Elgavish RA, Bartolucci A, et al. Functional imaging: II. Prediction of epilepsy surgery outcome. Ann Neurol. 2008;64(1):35–41. doi: 10.1002/ana.21419. [DOI] [PubMed] [Google Scholar]
- 81.Knowlton RC, Elgavish RA, Limdi N, et al. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol. 2008;64(1):25–34. doi: 10.1002/ana.21389. [DOI] [PubMed] [Google Scholar]
- 82.Sutherling WW, Mamelak AN, Thyerlei D, et al. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology. 2008;71(13):990–6. doi: 10.1212/01.wnl.0000326591.29858.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 84.Lin FH, Hara K, Solo V, et al. Dynamic Granger-Geweke causality modeling with application to interictal spike propagation. Hum Brain Mapp. 2009;30(6):1877–86. doi: 10.1002/hbm.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grunert P, Muller-Forell W, Darabi K, et al. Basic principles and clinical applications of neuronavigation and intraoperative computed tomography. Comput Aided Surg. 1998;3(4):166–73. doi: 10.1002/(SICI)1097-0150(1998)3:4<166::AID-IGS6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 86.Jannin P, Fleig OJ, Seigneuret E, et al. A data fusion environment for multimodal and multi-informational neuronavigation. Comput Aided Surg. 2000;5(1):1–10. doi: 10.1002/(SICI)1097-0150(2000)5:1<1::AID-IGS1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 87.Jannin P, Morandi X, Fleig OJ, et al. Integration of sulcal and functional information for multimodal neuronavigation. J Neurosurg. 2002;96(4):713–23. doi: 10.3171/jns.2002.96.4.0713. [DOI] [PubMed] [Google Scholar]
- 88.Ganslandt O, Fahlbusch R, Nimsky C, et al. Functional neuronavigation with magnetoencephalography: outcome in 50 patients with lesions around the motor cortex. J Neurosurg. 1999;91(1):73–9. doi: 10.3171/jns.1999.91.1.0073. [DOI] [PubMed] [Google Scholar]
- 89.Rezai AR, Hund M, Kronberg E, et al. The interactive use of magnetoencephalography in stereotactic image-guided neurosurgery. Neurosurgery. 1996;39(1):92–102. doi: 10.1097/00006123-199607000-00018. [DOI] [PubMed] [Google Scholar]
- 90.Rezai AR, Mogilner AY, Cappell J, et al. Integration of functional brain mapping in image-guided neurosurgery. Acta Neurochir Suppl. 1997;68:85–9. doi: 10.1007/978-3-7091-6513-3_16. [DOI] [PubMed] [Google Scholar]