Abstract
Purpose
17-Allylamino-17-Demethoxygeldanamycin (17-AAG) is a benzoquinone ansamycin antibiotic with anti-proliferative activity in several mouse xenograft models including prostate cancer models. A two-stage phase II study was conducted to assess the activity and toxicity profile of 17-AAG administered to patients with metastatic, hormone-refractory prostate cancer.
Experimental Design
Patients with at least one prior systemic therapy and a rising PSA were eligible. Patients received 17-AAG at a dose of 300 mg/m2 IV weekly for three out of four weeks. The primary objective was to assess the PSA response. Secondary objectives were to determine overall survival, to assess toxicity, to measure IL-6, IL-8 and maspin levels and quality of life.
Results
Fifteen eligible patients were enrolled. The median age was 68 years and the median PSA was 261 ng/mL. Patients received 17-AAG for a median number of 2 cycles. Severe adverse events included: grade 3 fatigue (4 pts), grade 3 lymphopenia (2 pts) and grade 3 back pain (2 pts). The median PSA progression free survival was 1.8 months (95% CI: 1.3–3.4 months). The six-month overall survival was 71% (95% CI: 52%–100%).
Conclusion
17-AAG did not show any activity with regards to PSA response. Due to insufficient PSA response, enrollment was stopped at end of first stage per study design. The most significant severe toxicity was grade 3 fatigue. Further evaluation of 17-AAG at a dose of 300 mg/m2 IV weekly as a single agent in patients with metastatic, hormone-refractory prostate cancer who received at least one prior systemic therapy is not warranted.
Keywords: prostate cancer, hormone-refractory, 17-AAG
Introduction
The Androgen Receptor (AR) is a member of the steroid receptor family that binds to testosterone and dihydrotestosterone upon cellular entry [1]. AR is also important for the growth of male urogenital structures and for spermatogenesis. In hormone-refractory prostate cancer, increased AR activity may result from mutations, increased AR phosphorylation by upstream signaling pathways, or by increased transcription of AR. The AR function may be further regulated through conformational changes due to its dynamic partnership with heat shock proteins. In its inactive state, AR is bound to at least three heat shock proteins (Hsp90, Hsp70 and Hsp56) [2]. Upon activation, AR is released from heat shock proteins, interacts with other cellular proteins and ultimately, activates target genes.
Docetaxel-based chemotherapy regimens are now considered the standard of care for the treatment of men with metastatic, hormone-refractory prostate cancer [3, 4]. Treatment options for those patients who fail docetaxel-based chemotherapy are limited. We postulate that targeting multiple mitogenic signaling pathways may delay or block the progression of hormone-refractory metastatic prostate cancer. To this end, multiple mitogenic signaling pathways (including the AR pathway) depend on the chaperoning activity of heat shock protein, especially Hsp90. Predominantly a cytoplasmic protein during normal conditions, Hsp90 may be accumulated and continue to act as a chaperon in the nuclei in response to stressful cellular environment [5, 6]. In addition to AR, Hsp90 client proteins include Akt kinase, Raf-1 kinase, Bcr-Abl kinase, HER2, and HIF-1alpha. The activity of Hsp90 can be regulated through its association with different sets of interacting molecules. Interestingly, tumor suppressive protein maspin is recently shown to interact with Hsp90 [7]. Furthermore, maspin expression in prostate cancer is inversely correlated with tumor grade and AR, but positively correlated with disease free survival of patients who received hormonal ablation therapies [8, 9].
The ability of Hsp90 to chaperone protein kinases or transcription factors depends on the binding and hydrolysis of ATP at its binding domain [10]. Accordingly, multiple mitogenic pathways may be blocked simultaneously by synthetic inhibitors of the Hsp90 ATPase activity, such as 17-allylamino-17-demthoxygeldanamycin (17-AAG) [11–13]. 17-AAG is a benzoquinone ansamycin antibiotic with antiproliferative activity. Its parent compound, geldanamycin showed promising antitumor properties in preclinical studies. 17-AAG proved to be less hepatotoxic than its parent compound. Both compounds are believed to act biologically similar by binding to the hydrophobic ATP/ADP-binding site on Hsp90.
In preclinical studies, 17-AAG was found to be active in several mouse xenograft models including breast cancer, melanoma, ovarian cancer and prostate cancer. Solit et al. reported growth inhibition of both androgen-sensitive and androgen-insensitive tumors in prostate cancer xenografts treated with 17-AAG [14]. In addition, 17-AAG caused the down-regulation and reduction in HER2, HER3, wild-type and mutant AR expression.
Phase 1 clinical trials of 17-AAG were conducted in patients with advanced solid tumors [15–21]. In a Phase I trial of 17-AAG involving patients with advanced prostate cancer, one patient treated with twice weekly 17-AAG treatment achieved a PSA response (25% decline)[21]. Based on promising pre-clinical and clinical data and its unique mechanism of action, 17-AAG was evaluated in a multi-center, phase II trial in poor prognosis, metastatic, hormone-refractory prostate cancer patients.
Methods
Eligibility Criteria
Men with histologically confirmed prostate adenocarcinoma with metastasis were eligible if they met the following criteria: Objective disease progression or rising PSA despite androgen deprivation therapy and antiandrogen withdrawal; Patients with rising PSA must demonstrate a rising trend with 2 successive elevations at a minimum interval of 1 week; A minimum PSA of 5 ng/ml or new areas of bony metastases on bone scan are required for patients with no measurable disease; Baseline imaging for disease assessment must be done ≤ 28 days prior to registration; All patients must have had at least 1 prior regimen of chemotherapy for metastatic disease; All patients must be documented to be castrate with a testosterone level of < 50 ng/ml. LHRH agonist therapy must be continued, if required to maintain castrate levels of testosterone. Patients must be off antiandrogens for ≥ 4 weeks for flutamide and 6 weeks for bicalutamide or nilutamide; Prior radiation therapy completed ≥ 28 days prior to registration; Life expectancy of ≥ 12 weeks; ≥18 years of age; ANC ≥ 1,500/mm3; PLT ≥ 100,000/ mm3; Hgb ≥8.0 g/dL; Total bilirubin ≤1.5 × UNL; SGOT and/or SGPT ≤2.5 × UNL if alk phos ≤UNL OR alk phos ≤4 x UNL if SGOT and/or SGPT are ≤UNL; Calculated creatinine clearance of ≥60 ml/min or serum creatinine ≤ULN; ECOG Performance Status of 0, 1, or 2.
Contraindications to enrollment into the study included the following: Men of childbearing potential or their sexual partners who are unwilling to employ adequate contraception (condoms, diaphragm, birth control pills, injections, intrauterine device [IUD], surgical sterilization, subcutaneous implants, or abstinence, etc.); Use of investigational agents for treatment of prostate cancer ≤ 28 days prior to registration; Current treatment with any other anti-neoplastic agent. Patients may continue to receive zoledronic acid for bone metastases or hypercalcemia; Known brain metastatic disease requiring active therapy; Any of the following conditions ≤ 6 months prior to registration: myocardial infarction, severe/unstable angina, symptomatic congestive heart failure, cerebrovascular accident or transient ischemic attack, coronary/peripheral artery bypass grafting or Patients who have experienced a pulmonary embolus, deep venous thrombosis or other clinically significant thromboembolic event ≤6 months prior to registration are eligible if they are clinically stable on anticoagulation therapy; Significant cardiac disease including heart failure that meets New York Heart Association classification III or IV, history of myocardial infarction ≤one year of study entry, uncontrolled dysrhythmias, or poorly controlled angina.; Current active infection, including known human immunodeficiency virus (HIV) positivity. For HIV patients on highly active antiretroviral therapy (HAART), the pharmacokinetics of 17-AAG may be seriously affected. When appropriate, 17-AAG will be studied in patients with HIV on HAART; Serious allergy to eggs (i.e. hypotension, dyspnea, anaphylaxis, edema).
This study was approved by the local institutional review board at all clinical centers and written informed consents were obtained from all patients prior to registration.
Patients underwent a complete medical examination prior to registration and every other cycle. The medical examination included measurement of PSA, indicator lesion(s) (every other cycle if done by radiologic method), hematologic and chemistry laboratory tests (Hgb, ANC, PLT, WBC, sodium, potassium, calcium, BUN, creatinine, alkaline phosphatase, SGOT, and SGPT), exam, history, weight, performance status assessment and digital rectal examination.
Treatment Plan
Patients were treated with 17-AAG at 300 mg/m2 IV on days 1, 8, and 15 every 28 days. Every 28 days was considered one cycle. Patients underwent laboratory testing (including PSA and testosterone) and imaging studies (CT scan of abdomen/pelvis, bone scan, MRI of brain, etc.) at baseline and after every 2 cycles.
Dose Reductions
No dose modifications were made for ≤ grade 1 toxicity. For grade 3 neutropenia, the dose of 17-AAG was reduced to 240 mg/m2 IV (dose level -1). For grade 4 neutropenia, the regular dose was held and re-administered at dose level -1 when toxicity has resolved to ≤ grade 1. For grade 2 thrombocytopenia, the dose was reduced to dose level -1, but for grades 3 and 4 thrombocytopenia, the regular dose was held and readministered at dose level-1 when toxicity has resolved to ≤ grade 1. For grade 2 diarrhea and/or transaminitis, the dose was reduced to dose level -1. For grade 3 diarrhea and/or transaminitis, the dose was held and readministered at dose level -1 when toxicity has resolved to ≤ grade 1. For grade 4 diarrhea and/or transaminitis, 17-AAG therapy was discontinued. For other grade 3 toxicities, the regular dose was held and readministered at dose level -1 upon resolution of toxicity. For other grade 4 toxicities, treatment with 17-AAG was discontinued. For any cardiac toxicities, specific guidelines regarding QTc prolongation, atrial/ventricular dysrhythmia, and left ventricular ejection fraction were followed.
Statistical Methods
The primary objective of this study was to assess the PSA response as defined by the Prostate-Specific Antigen Working Group (19) to 17-AAG in patients with metastatic, hormone-refractory prostate cancer, who have failed front-line chemotherapy [22]. The secondary objectives were to determine overall survival, disease free survival, response of 17-AAG on measurable disease and evaluate correlative serum markers. QOL assessments were obtained only in patients treated at the Karmanos Cancer Institute (KCI), utilizing the QLQ-30 EORTC questionnaire. The emotional functioning and physical functioning scales are scored with a valid score between 0 and 100 points.
The study utilized a Simon minmax two-stage design where a maximum of 25 evaluable patients would be accrued unless undue toxicity was encountered [23]. An interim analysis was to be conducted after 16 patients were accrued and observed for 6 months. If at most 1 success was observed in the first 16 patients (stage 1), this was considered early evidence of an ineffective treatment regimen in this patient population, leading to termination of accrual. If at least two successes were observed, the study was able to proceed to the next stage (stage 2) to accrue 9 additional evaluable patients. Success was defined as a PSA response, which was confirmed by a second value at least 4 weeks later.
The distribution of overall survival and disease-free survival are estimated using the method of Kaplan-Meier. Overall survival time is defined as the time from registration to death due to any cause. Disease-free survival is defined as the time from registration to documentation of disease progression. Disease progression was defined as a 25% or greater PSA increase over the nadir (or baseline if PSA never decreased) and an increase in PSA at least 5 ng/ml, which was confirmed by a 2nd value obtained approximately 1 week later. If the PSA did not show progression, but a bone scan did show progressive disease (defined as development of at least 2 new lesions), the patient was also considered to have progression of disease.
IL-6, IL-8 and maspin were obtained at baseline, on day 15, and at the end of treatment. Changes from pre-treatment levels were assessed using the Wilcoxon signed rank test. The relationship of each marker with PSA progression was also evaluated. The percent change in each marker at treatment failure was compared to the % change in PSA at treatment failure using the Spearman rank correlation coefficient.
Correlative Markers
The correlative markers evaluated in this study included serum levels of IL-6, IL-8 in peripheral blood lymphocytes, and serum levels of maspin. The correlative markers were selected to help determine potential effects of 17-AAG on the mechanism of hormone-refractory prostate cancer.
Immunoassay for quantitation of IL-6/IL-8
For the determination of IL-6/IL-8 in human serum samples, the blood samples were centrifuged for 15 minutes at 1000 × g. The serum was collected, and analyzed for IL-6 or IL-8 using ELISA kit (R&D Systems, Minneapolis, MN) following standard procedures supplied by the manufacture. Briefly, for ELISA, 100 μl of assay diluent’s RD1W or RD1-85 was added to 96 well plate coated with mouse monoclonal antibodies against IL-6 or IL-8. About 50–100 μl of either standard or sample was added to each well and incubated for 2 h at room temperature. After washing four times with washing buffer, conjugate solution was added to each well, and incubated for 2 h at room temperature. Wells were washed four times with washing buffer, and 200 μl of substrate solution containing a mixture of color reagent A and B was added and plate was incubated at room temperature for 20 minutes. About 50 μl of stop solution was added to each well and optical density was measured at 450nm within 30 minutes. Standard curve was generated and concentration of IL-6 or IL-8 was calculated from the standard curve. Appropriate assay blanks, and control well were also included during the assay.
Quantitative real-time PCR Detection of Maspin
Total RNA was extracted from the patient blood samples as described [24]. The quality of the RNA was verified by agarose gel electrophoresis showing intact 18S and 28S rRNA, and by UV spectrophotometry showing an A260nm/A280nm ratio between 1.8 and 2. One microgram of each RNA sample was reverse-transcribed in a 20-μL reaction as described [24]. For real-time PCR, 1 μL of the resulting cDNA was mixed with SYBR Green PCR Mastermix (Stratagene) (LaJolla, CA) and 300 nM of the PCR primers. The primers for maspin coding sequence were: 5′-CTACTTTGTTGGCAAGTGGATGAA-3′ and 5′-ACTGGTTTGGTGTCTGTCTTGTTG-3′. The primers for GAPDH were as previously described [25]. The real-time PCR thermal profile was: 1 cycle of 95 °C/10 min, 40 cycles of 95 °C/30 sec →55 °C/1 min →72 °C /30 sec, 1 cycle of 95 °C/1 min, and finally 41 cycles of 95 °C→ (55 °C + 1°C /cycle)/30 sec. Critical threshold cycle numbers (Ct) were obtained using the built-in software of the Stratagene Mx4000™ Multiplex Quantitative PCR System. The measurement of maspin-specific cDNA species were normalized by the measurement of internal control GAPDH. Data represent the average of three repeats.
Quality of Life (QOL)
Participants at KCI were recruited independently from the larger study to provide QOL assessments in conjunction with their treatment. Patients were asked by physicians about their interest in participating in a companion quality of life study. A member of the research team explained study procedures to any interested patients. Patients who agreed to participate provided consent in the form of a signature on a consent form, which described the nature of the study and explicitly stated that participants may discontinue their involvement with the study at any time. Refusal to participate in the quality of life study did not impact their eligibility or participation in the larger clinical trial.
Participants in the QOL arm of the study completed the EORTC Quality of Life questionnaire (QLQ-30—C30, version 3). For this study, the global Physical Functioning (PF) and Emotional Functioning (EF) subscales were used in analyses. A baseline QOL assessment was conducted at the same time as the pre-study evaluation for the larger clinical trial. Subsequent assessments were conducted every 2 weeks (corresponding to Day 1 and Day 15 of each treatment cycle) for the first 2 cycles of the trial. QOL assessments were completed using a computer-administered questionnaire via a laptop computer.
Results
Seventeen patients were enrolled between January 11, 2005 and April 10, 2006. One patient never started treatment due to high potassium levels. Another patient was deemed ineligible since the patient was concurrently taking contraindicated medications. Six of the 15 evaluable patients completed QOL assessments. This study was permanently closed to patient enrollment per protocol design at the time of interim analysis due to insufficient PSA response.
The characteristics of the 15 evaluable patients are presented in Table 1. The median age of the cohort was 68 (range: 52–78). The median number of days from the last chemotherapy treatment was 91 (range: 28–925). Thirteen (87%) patients received prior taxane-based chemotherapy. The median PSA was 261 (range: 46–1705). A median of 2 cycles (range 1–5) of treatment was given. Only 1 patient received more than 2 cycles of treatment. All 15 eligible patients have discontinued study treatment due to disease progression (13 pts, 87%), patient refusal (1 pt, 7%), and other medical problems (1 pt, 7%). See Table 2 for more details on 17-AAG dosage.
Table 1.
Patient Characteristics at Baseline
| Characteristic | N=15 | % |
|---|---|---|
| Median age (range) | 68 (52–78) | |
|
| ||
| Median PSA (range) | 261 (46–1705) | |
|
| ||
| Median Gleason Score (range) | 8 (5–10) | |
|
| ||
| Median Hemoglobin (range) | 11.6 (9.3–14.3) | |
|
| ||
| Median Alkaline Phosphate (range) | 154 (52–323) | |
|
| ||
| Median Days Since Last Chemotherapy (range) | 91 (28–925) | |
|
| ||
| Performance Status | ||
| 0 | 4 | 27 |
| 1 | 10 | 67 |
| 2 | 1 | 6 |
|
| ||
| Receiving concurrent zoledronic acid | ||
| yes | 9 | 60 |
| no | 6 | 40 |
|
| ||
| Race | ||
| white | 12 | 80 |
| black | 3 | 20 |
|
| ||
| Hypercalcemia | ||
| no | 15 | 100 |
|
| ||
| Prostatectomy | ||
| yes | 5 | 33 |
| no | 10 | 67 |
|
| ||
| Chemotherapy | ||
| Taxane based | 13 | 87 |
| Other | 2 | 13 |
|
| ||
| Radiation Therapy | ||
| yes | 12 | 80 |
| no | 3 | 20 |
|
| ||
| Androgen ablation | ||
| yes | 14 | 93 |
| no | 1 | 7 |
|
| ||
| Other Treatment | ||
| yes | 9 | 60 |
| no | 6 | 40 |
|
| ||
| Site of Disease - Visceral | ||
| yes | 6 | 40 |
| no | 9 | 60 |
|
| ||
| Site of Disease - Bone | ||
| yes | 11 | 73 |
| no | 4 | 27 |
|
| ||
| Site of Disease – Soft Tissue | ||
| yes | 4 | 27 |
| no | 11 | 73 |
|
| ||
| Other Site | ||
| yes | 9 | 60 |
| no | 6 | 40 |
|
| ||
| Disease Status | ||
| measurable | 2 | 13 |
| measurable & evaluable | 9 | 60 |
| evaluable, not measurable | 4 | 27 |
Table 2.
17-AAG Dose Administered
| 17-AAG | ||||
|---|---|---|---|---|
| Cycle | N | Median Total Dose (mg/m2) Administered | Median Dose Level (mg/m2) Administered | % Receiving Full Dose |
| 1 | 15 | 1710 | 300 | 93.3* |
| 2 | 10 | 1795 | 300 | 100 |
| 3 | 1 | 1872 | 300 | 100 |
| 4 | 1 | 1881 | 300 | 100 |
| 5 | 1 | 1254 | 300 | 100 |
One patient reduced for AST/ALT
Response
No patient achieved a confirmed PSA response or an objective disease response as evaluated by radiologic imaging studies. One patient was able to maintain a stable PSA response for 5 cycles of treatment. This patient had a baseline PSA of 99 and maintained their PSA from 74 to 112 for the 1st four cycles before progressing after the 5th cycle of treatment.
Survival and Disease Progression
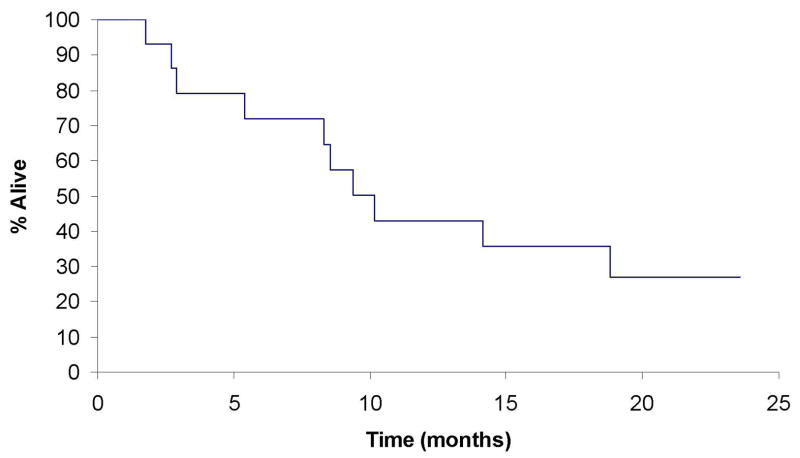
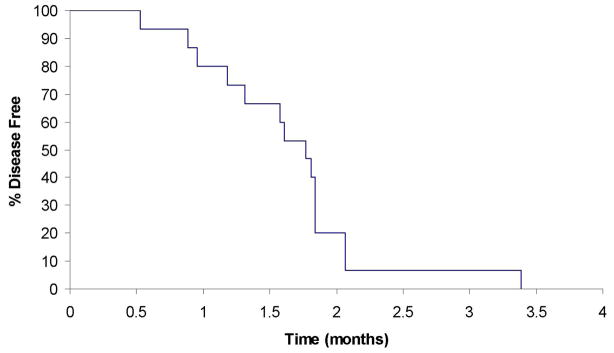
Patients were followed until death or a median of 10.1 months among living patients. At last contact, 5 (33%) of the 15 eligible patients were still alive with survival follow-up ranging from 1.7 to 23.5 months. Median time to disease progression was 1.8 months (95% CI: 1.3–3.4 months). The 6-month overall survival was 71% (95% CI: 52–100). (See Figures 1 and 2)
Fig 1.
Overall survival in patients with hormone refractory metastatic prostate cancer.
Fig 2.
Disease-free survival in patients with hormone refractory metastatic prostate cancer.
Adverse Events
Adverse event data was available on 15 patients. Overall, 60% (9/15) of patients experienced a grade 3 adverse event. There have been no grade 4 or 5 adverse events reported. The most common severe (grade 3) adverse event reported in the study was fatigue with 4 (27%) events reported. (See Table 3)
Table 3.
Severe Hematologic/Non-hematologic Adverse Event (regardless of attribution)
| Toxicity | Grade 3
|
|
|---|---|---|
| N | % | |
| PAIN-ABDOMINAL | 1 | 7 |
| ANOREXIA | 1 | 7 |
| SGOT (AST) | 1 | 7 |
| PAIN-BACK | 2 | 13 |
| QTC INTERNAL PROLONG | 1 | 7 |
| FATIGUE | 4 | 27 |
| ANEMIA | 1 | 7 |
| HYPOPHOSPHATEMIA | 1 | 7 |
| LEUKOPENIA | 1 | 7 |
| LYMPHOPENIA | 2 | 13 |
| NEURO-MOTOR | 1 | 7 |
| THROMBOSIS | 1 | 7 |
IL-6 and IL-8 and Maspin
No significant changes in IL-6, IL-8 and Maspin were noted at day 15. A borderline significant (p=0.09) up-regulation of maspin was observed at treatment failure. (See Table 4a and 4b). No associations between IL-6, IL-8 and Maspin with PSA at treatment failure were observed (data not shown).
Table 4a. Correlative Markers.
4a: Correlative Marker Values at 3 Time Points
| Marker | Median | Range |
|---|---|---|
| IL-6 (pg/ml) | ||
| Day 1 | 5.9 | 1.4–56.9 |
| Day 15 | 8.4 | 1.7–112.4 |
| End of Treatment (TX) | 11.9 | 1.7–62.7 |
| IL-8 (pg/ml) | ||
| Day 1 | 13.8 | 6.9–162.6 |
| Day 15 | 14.3 | 9.0–217.6 |
| End of Treatment | 11.9 | 9.7–20.3 |
| Maspin* (fold change) | ||
| Day 1 | −3.0 | −3.7–14.3 |
| Day 15 | 8.3 | −7.2–11.5 |
| End of Treatment | 26.1 | 0.5–233.0 |
Note values represent a fold change from a normalized measurement of internal control GAPDH.
Table 4b. Correlative Markers.
4b: Correlative Marker Change from Baseline
| Marker | Median | Range | P-value |
|---|---|---|---|
| IL-6 (pg/ml) | |||
| Day 15 change from baseline | 0.4 | −7.7–55.5 | 0.57 |
| End of TX change from baseline | 4.5 | 0.3–16.0 | 0.03 |
| IL-8 (pg/ml) | |||
| Day 15 change from baseline | 3.0 | −49.5–55.0 | 0.73 |
| End of TX change from baseline | −1.8 | −39.2–2.9 | 0.31 |
| Maspin (fold change) | |||
| Day 15 change from baseline | 6.4 | −8.5–15.3 | 0.44 |
| End of TX change from baseline | 28.7 | −13.8–236.0 | 0.09 |
Quality of Life
Of the patients who completed QOL assessments, 6 patients completed questionnaires at baseline and end of study treatment, and 5 patients completed questionnaires through 28 and 56 days (end of cycles 1 and 2) after registration. The median emotional functioning (EF) and physical functioning (PF) scores at baseline were 83.3 and 76.7 points, respectively. At 28 and 56 days after registration, no clear trends in EF scores were observed. The PF scores declined a median of 13.3 points from baseline at both 28 and 56 days.
EF and PF scores declined or remained unchanged for a majority of patients at the end of study treatment. At the end of treatment, EF scores decreased a median of 4.2 points with declines in 3 (50%) patients and no change in 1 (17%) patient. The median PF score dropped 20 at the end of treatment (range: 60 point decline to 6.7 point increase) with 5 out of 6 (83%) patients having a declining PF score.
Three out of the four patients who experienced grade 3 fatigue completed QOL questionnaires during the adverse event. Both EF and PF scores had decreased from baseline values. The PF scores declined from 60 to 40, 93.3 to 67.7, and 60 to 0 points from baseline for three patients. Similarly, EF scores declined from 91.7 to 83.3 for two patients and from 58.3 to 33.3 points from baseline for one patient at the time of the event.
Discussion
Patients with metastatic, hormone-refractory prostate cancer who fail frontline docetaxel-based chemotherapy have no standard treatment options available to them. Newer treatment for this poor-prognosis patient population is urgently needed. Concern surrounding the dysregulation of the AR in patients with metastatic, hormone-refractory disease is substantiated by many preclinical studies. Novel agents that impact on the AR have the potential to affect the biology of the disease.
17-AAG, a benzoquinone ansamycin antiobiotic with antiproliferative activity, acts by binding to the hydrophobic ATP/ADP-binding site on Hsp90. Hsp90 along with other client proteins is integral in activating downstream target genes.
In this phase II trial of 15 eligible patients, the patient population was typical of most studies in this setting; elderly males with good performance status with predominantly bony metastasis and on treatment with zoledronic acid. The median number of cycles given was 2 and only one patient required a dose reduction due to elevated transaminases. Unfortunately, there were no PSA responses seen. Indeed, this was a disappointing result. It is possible that a dose of 300 mg/m2 IV weekly for two cycles of treatment was an insufficient amount of drug and treatment time to produce a meaningful PSA response at this highly elevated PSA level (median 261 ng/ml). The median time from the last chemotherapy treatment was 91 days suggesting that the prostate cancer was in an aggressive or advanced state. The median time to PSA progression was also rapid at 1.8 months and the 6-month overall survival of this group of patients was as expected at 71%.
The AR can also be activated by other factors such as cytokines including interleukin-6 (IL-6). The IL-6 receptor is believed to interact with the HER2 receptor, promoting phosphorylation and activating down-stream pathways, ultimately leading to increased AR activation [26]. Increased serum levels of IL-6 have been reported in patients with hormone-refractory prostate cancer [27, 28]. Preclinical data confirmed that androgen-sensitive prostate cancer cell lines do not constitutively express IL-6 whereas androgen-insensitive prostate cancer cell lines do [29]. One reason IL-6 expression in prostate cancer cell is increased is attributed to deregulated IL-6 promoter activity. Although the number of samples available for correlative studies was small in this study, there was no apparent association of IL-6 or IL-8 activity with PSA.
Maspin is a Hsp90-associated tumor suppressive protein [7]. Maspin expression in prostate cancer is inversely correlated with tumor grade and AR, but positively correlated with disease free survival of patients who received hormonal ablation therapies [8, 9]. Maspin protein has not been directly detected in patients’ sera. However, real-time PCR detection of maspin mRNA was previously shown to be specifically associated with circulating epithelial-derived cancer cells [30]. Although 17-AAG treatment in our study did not lead to significant decrease of serum PSA levels, patients’ tumor burden as judged by PSA was not further increased. Thus, the increase of maspin mRNA in circulation is likely a result of increased expression per circulating prostate tumor cell. Furthermore, since Hsp90 inhibition may lead to increased AR turnover [14, 31], 17-AAG may have increased maspin expression in prostate tumor cells by inhibiting AR, despite the fact that such inhibition on AR did not lower PSA in a clinically significant manner.
The most significant severe toxicity was grade 3 fatigue. In the 4 patients who experienced grade 3 fatigue, quality of life measurements were obtained in 3 patients. The quality of life measurements revealed declining PF and EF scores. Drops in PF scores were more dramatic than EF declines. In addition, these declines in PF were greater than any changes seen in the other 3 patients during cycles 1 and 2 with declines of 20, 26.7 and 60.0. These findings suggest that an increase in fatigue was correlated with patient subjective reports of declining quality of life.
17-AAG in patients with metastatic, hormone-refractory prostate cancer who received at least one prior systemic therapy did not show a significant activity with regards to PSA response. However, correlative studies suggest tumor suppressive maspin might be up-regulated. Since maspin has been shown to further increase prostate tumor cell sensitivity to drug-induced apoptosis [32], it remains a possibility that hormone refractory prostate cancer patients may respond more favorably to the combination of 17-AAG with another agent that can directly activate the apoptotic pathway. Single agent activity at a weekly dose of 300 mg/m2 was minimal and does not warrant further investigation.
Acknowledgments
This study is supported by the UO1-CA062487-12, N01-CM-17104, R01CA84176.
Footnotes
Participating Institutions
This phase II trial is being conducted within the Phase 2 Consortium. Participating institutions include Karmanos Cancer Institute at Wayne State University (Principal Investigator- Elisabeth I. Heath, M.D.), Detroit, MI, Mayo Clinic Cancer Center, Rochester, MN, University of Wisconsin Carbone Comprehensive Cancer Center, Madison, WI, Howard University College of Medicine, Washington, DC, the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, Mayo Clinic Jacksonville, Jacksonville, FL, and Mayo Clinic Scottsdale, Scottsdale, AZ.
References
- 1.Sadi MV, Walsh PC, Barrack ER. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer. 1991;67:3057–64. doi: 10.1002/1097-0142(19910615)67:12<3057::aid-cncr2820671221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay A, Wang L, Lopez-Casillas F, Mendoza V, Yeh IT, Sun L. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. Prostate. 2005;63:81–90. doi: 10.1002/pros.20166. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Wu J, Mick R, et al. Farnesyltransferase inhibitor effects on prostate tumor micro-environment and radiation survival. Prostate. 2005;62:69–82. doi: 10.1002/pros.20122. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AJ. Hsp90’s secrets unfold: new insights from structural and functional studies. Trends Cell Biol. 1999;9:262–8. doi: 10.1016/s0962-8924(99)01580-9. [DOI] [PubMed] [Google Scholar]
- 7.Yin S, Li X, Meng Y, et al. Tumor suppressive maspin regulates cell response to oxidative stress by direct interaction with glutathione S-transferase. J Biol Chem. 2005 doi: 10.1074/jbc.M503522200. [DOI] [PubMed] [Google Scholar]
- 8.Pierson CR, McGowen R, Grignon D, Sakr W, Dey J, Sheng S. Maspin is up-regulated in premalignant prostate epithelia. Prostate. 2002;53:255–62. doi: 10.1002/pros.10107. [DOI] [PubMed] [Google Scholar]
- 9.Zou Z, Zhang W, Young D, et al. Maspin expression profile in human prostate cancer (CaP) and in vitro induction of Maspin expression by androgen ablation. Clin Cancer Res. 2002;8:1172–7. [PubMed] [Google Scholar]
- 10.Meissner A, Petersenn S, Heidemann HT, Osterkamp U, Simon R, Schulte HM. Pharmacokinetics of oral isosorbide-5-mononitrate in patients with ischemic heart failure. Klin Wochenschr. 1991;69:213–9. doi: 10.1007/BF01646943. [DOI] [PubMed] [Google Scholar]
- 11.Grenert JP, Sullivan WP, Fadden P, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–50. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 12.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–50. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 13.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 14.Solit DB, Zheng FF, Drobnjak M, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- 15.Banerji U, O’Donnell A, Scurr M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan RK, Trump DL, Eiseman JL, et al. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–91. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 17.Grem JL, Morrison G, Guo XD, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23:1885–93. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 18.Goetz MP, Toft D, Reid J, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23:1078–87. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 19.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 20.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Yin S, Meng Y, Sakr W, Sheng S. Endogenous inhibition of histone deacetylase 1 by tumor-suppressive maspin. Cancer Res. 2006;66:9323–9. doi: 10.1158/0008-5472.CAN-06-1578. [DOI] [PubMed] [Google Scholar]
- 22.Yin S, Li X, Meng Y, et al. Tumor-suppressive maspin regulates cell response to oxidative stress by direct interaction with glutathione S-transferase. J Biol Chem. 2005;280:34985–96. doi: 10.1074/jbc.M503522200. [DOI] [PubMed] [Google Scholar]
- 23.Qiu Y, Ravi L, Kung HJ. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393:83–5. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 24.Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999;41:127–33. doi: 10.1002/(sici)1097-0045(19991001)41:2<127::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Twillie DA, Eisenberger MA, Carducci MA, Hseih WS, Kim WY, Simons JW. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995;45:542–9. doi: 10.1016/S0090-4295(99)80034-X. [DOI] [PubMed] [Google Scholar]
- 26.Zerbini LF, Wang Y, Cho JY, Libermann TA. Constitutive activation of nuclear factor kappaB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–15. [PubMed] [Google Scholar]
- 27.Mercatali L, Valenti V, Calistri D, et al. RT-PCR determination of maspin and mammaglobin B in peripheral blood of healthy donors and breast cancer patients. Ann Oncol. 2006;17:424–8. doi: 10.1093/annonc/mdj109. [DOI] [PubMed] [Google Scholar]
- 28.Hieronymus H, Lamb J, Ross KN, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–30. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Yin S, Reddy N, Spencer C, Sheng S. Bax mediates the apoptosis-sensitizing effect of maspin. Cancer Res. 2004;64:1703–11. doi: 10.1158/0008-5472.can-03-2568. [DOI] [PubMed] [Google Scholar]