Abstract
With increasing efforts to develop and utilize mouse models of a variety of neuro-developmental diseases, there is an urgent need for sensitive neuroimaging methods that enable in vivo analysis of subtle alterations in brain anatomy and function in mice. Previous studies have shown that the brains of Fibroblast Growth Factor 17 null mutants (Fgf17−/−) have anatomical abnormalities in the inferior colliculus (IC)–the auditory midbrain–and minor foliation defects in the cerebellum. In addition, changes in the expression domains of several cortical patterning genes were detected, without overt changes in forebrain morphology. Recently, it has also been reported that Fgf17−/− mutants have abnormal vocalization and social behaviors, phenotypes that could reflect molecular changes in the cortex and/or altered auditory processing / perception in these mice. We used manganese (Mn)-enhanced magnetic resonance imaging (MEMRI) to analyze the anatomical phenotype of Fgf17−/− mutants in more detail than achieved previously, detecting changes in IC, cerebellum, olfactory bulb, hypothalamus and frontal cortex. We also used MEMRI to characterize sound-evoked activity patterns, demonstrating a significant reduction of the active IC volume in Fgf17−/− mice. Furthermore, tone-specific (16- and 40-kHz) activity patterns in the IC of Fgf17−/− mice were observed to be largely overlapping, in contrast to the normal pattern, separated along the dorsal-ventral axis. These results demonstrate that Fgf17 plays important roles in both the anatomical and functional development of the auditory midbrain, and show the utility of MEMRI for in vivo analyses of mutant mice with subtle brain defects.
Keywords: MEMRI, DBM, IC, activity mapping, auditory, tonotopic map
Introduction
Several secreted proteins in the Fibroblast Growth Factor (Fgf) family are critical for neural patterning during embryogenesis. Among these, Fgf8 has been shown to be the main molecule responsible for “organizer” activity–the ability of a cell or tissue to send signals that instruct the fates of surrounding cells–during development of the mid-hindbrain (MHB) region (Crossley et al., 1996; Lee et al., 1997; Ye et al., 1998; Sato et al., 2001), and is also critical for normal forebrain development (Shimamura & Rubenstein, 1997; Meyers et al., 1998; Ye et al., 1998; Fukuchi-Shimogori & Grove, 2001; Garel et al., 2003; Storm et al., 2006). Fgf17 expression overlaps with Fgf8, and also plays a role in the development of the MHB and cortex (Hoshikawa et al., 1998; Maruoka et al., 1998; Xu et al., 1999; 2000; Reifers et al., 2000; Liu et al., 2003; Fukuchi-Shimogori & Grove, 2003). While Fgf8−/− mice are embryonic lethal, Fgf17−/− mice are viable and fertile, having mild defects in the MHB, including (non-quantified) reduction of the inferior colliculus (IC), and small foliation changes in the cerebellum (Xu et al., 2000). Fgf17−/− mutants also show altered expression domains of genes that mark sub-regions of frontal cortex, without any obvious change in forebrain morphology (Cholfin & Rubenstein, 2007; 2008).
Interestingly, Fgf17−/− mice exhibit abnormal social behaviors, including decreased vocalization in pups and deficits in several adult behaviors involving social interactions, reminiscent of autism in humans (Scearce-Levie et al., 2008). It was suggested that these abnormal behaviors may result from altered forebrain function, based on evidence of reduced activation of the immediate early-gene Fos in Fgf17−/− frontal cortex after exploration of novel environments (Scearce-Levie et al., 2008). However, it is unclear whether altered auditory function, as a result of the known defects in the IC, the auditory midbrain, also contributes to the behavioral phenotypes in Fgf17−/− mice.
We hypothesized that whole brain anatomical MRI analysis would provide a more complete and quantitative characterization of the Fgf17−/− mouse morphological phenotype than previous qualitative studies based on histology. We also sought to investigate the possibility that Fgf17−/− mice have altered auditory function as a result of the reduction in IC. Previously, we have shown that MEMRI can be used to detect and analyze sound-evoked activity patterns in the mouse IC (Yu et al., 2005; 2007; 2008), based on the activity-dependent accumulation of paramagnetic Mn ions in neural cells via uptake though voltage-gated calcium channels (Lin & Koretsky, 1997). In addition to characterizing the normal mouse IC, we also demonstrated the utility of MEMRI to analyze altered IC activity patterns in mice exposed to atypical sound-rearing environments during auditory brain development (Yu et al., 2007). In the current study, we employed similar MEMRI-based functional analysis approaches to characterize differences in IC activity patterns, comparing Fgf17−/− mice and control littermates. Our findings of altered auditory function in Fgf17−/− mutants may help to explain at least some of the behavioral phenotypes that have been reported in these mice.
Materials and Methods
Animals
All mice used in these studies were maintained under protocols approved by the Institutional Animal Care and Use Committee of New York University School of Medicine. Fgf17 mutant mice were obtained from the Ornitz lab (Washington University), and Fgf17+/− × Fgf17+/− breeding pairs were mated to produce littermate Fgf17+/+ wildtype (WT), Fgf17+/− heterozygous and Fgf17−/− homozygous mutants, while Fgf17−/− × Fgf17+/− breeding pairs were mated to produce littermate Fgf17+/− and Fgf17−/− mice. Genotype analysis was performed via polymerase chain reaction from tail DNA, using primers CP3:TTGGCTTCTCTGGGACTCTACC; cre2:CCATGAGTGAACGAACCTGG to detect the mutant allele, and r1:GACAGCAGAGAATCAATAGCTGC; r2:GAAGTTTCTCCAGCGATGGG to detect wild type Fgf17, as described previously (Basson et al., 2008).
For MEMRI, WT, Fgf17+/−, and Fgf17−/− mice were injected intraperitoneally (IP) with an aqueous solution of MnCl2 (0.4 mmol/kg) one day before imaging (Yu et al., 2005; 2007). After injection, mice were placed in a controlled acoustic environment inside an acoustic isolation chamber (Mac-1, Industrial Acoustics) for 24-h. During this time, mice were maintained under normal living conditions, including a 12-h light / 12-h dark cycle and free access to food and water. Neither MnCl2 administration, nor sound stimulation, induced any obvious abnormal behavior during the 24-h time period.
Auditory Brainstem Recordings
Auditory brainstem recordings (ABR) were measured using calibrated equipment and protocols, as described in detail by Willott (2006). Prior to ABR testing, mice were anesthetized with an intramuscular injection of a mixture of ketamine (50-mg/kg) and xylazine (95-mg/kg). ABR testing was performed in a double-walled acoustic chamber (Industrial Acoustics), using an isothermic heating pad (K20, Baxter) to maintain body temperature at 36–38°C. Silver electrodes were inserted subcutaneously at the vertex of the skull, and just inferior to each ear (ispilateral=reference; contralateral=ground). The biological signal, between the vertex and reference electrodes, was amplified (DAM-50E, World Precision Instruments), bandpass filtered from 0.3–3 kHz, 90 dB/octave, (VBF8.04, Stewart Electronics), digitized at a 25-kHz sampling rate and recorded over a 10-ms window (System II, Tucker Davis Technologies). Response was measured to both click and pure tone (8-, 16-, 24- and 32-kHz) stimuli, decreasing the amplitude in 5-dB steps, from 95 to 5 dB peak sound pressure level (SPL). Threshold was determined as the level one step above that where no discernible ABR waveform was detected. Differences in the threshold data for the three genotypes were analyzed statistically with ANOVA (one-way). Note that 32-kHZ was the highest frequency that could be achieved reliably with the existing ABR recording system.
MRI data acquisition
MEMRI imaging was performed as described in detail previously (Yu et al., 2005; 2007). Briefly, T1-weighted brain images were acquired with a 3D gradient echo pulse sequence (echo time, TE= 4-ms; repetition time, TR=50-ms; flip angle = 65°), resulting in a volumetric image set covering the entire brain, with isotropic spatial resolution of 100-μm in a total imaging time of 110 minutes per mouse. MRI was performed on a micro-imaging system (SMIS) consisting of a 7-Tesla horizontal magnet (Magnex Scientific) with actively shielded gradients (Magnex: 250-mT/m gradient strength; 200-μs rise time) and a 25-mm (inner diameter) quadrature Litzcage mouse head coil (Doty Scientific). All mice were imaged at 3 weeks of age (postnatal days 21-24, P21-24), where P0 denotes the day of birth.
Acoustic stimulation protocol
The acoustic environment and sound stimuli were described in detail previously (Yu et al., 2005; 2007). Sound stimulation in the current study consisted of calibrated broadband sound (1-59 kHz) and single tones (16- and 40-kHz) played through a speaker mounted inside the acoustic isolation chamber. The carrier frequencies were amplitude modulated at 4-Hz with a modulation range of 90%. For all experiments, the amplitude-modulated stimuli had peak amplitudes of 89 dB SPL, measured at the calibration point in the geometrical center of the cage, 10-cm above the cage bottom (Yu et al., 2007).
For tonotopic (pure tone) studies, mice were first imaged at P21, after injection of MnCl2 (0.4-mmol/kg, IP) at P20 and 24h of exposure to 40-kHz. The same mice were subsequently kept in a quiet environment for two days, and then injected IP with a second, smaller dose of MnCl2 (0.2-mmol/kg, IP) at P23 for imaging at P24 after 24h of exposure to 16-kHz. For comparison, a separate group of Fgf17−/− mice was imaged at P21, 24h after injection of MnCl2 (0.4-mmol/kg) and exposure to 16-kHz. The 16-kHz activity patterns at age P21 and P24 were compared to determine the effects of mouse age and the longitudinal experimental protocol on the MEMRI enhancement patterns and the resulting statistical maps.
Histology and Immunohistochemistry
Mice were anesthetized via IP injection of a mixture of ketamine (100-mg/kg) and xylazine (10-mg/kg) in phosphate buffered saline (PBS) and perfused transcardially with PBS, followed by 4% paraformaldehyde. For frozen sections, the brains were removed quickly, post-fixed for 24-h in 4% paraformaldehyde at 4°C, and cryoprotected in (4°C) 15% and 30% sucrose solution, successively. After embedding in OCT media (Tissue-Tek, Sakura), frozen 12-μm sections were cut on a cryostat for histological analysis. Immunohistochemistry was performed using standard staining procedures with Neurogranin primary antibody (1:1000; Chemicon), followed by an Alexa-555-conjugated donkey anti-rabbit secondary antibody (Molecular Probes). For cell density analysis, the fixed brains were embedded in paraffin and 20-μm sections were cut on a microtome. To assay the number of cells in the IC, we quantified 3 cresyl violet stained sections of the medial IC in each mouse using NIH ImageJ software, counting cells in an area of 1.65-mm2 located centrally along the dorsal-ventral axis. Cells were chosen for quantification only if the nucleus was fully visible and in focus.
Analysis of Whole Brain and IC Volume
As an initial comparison of anatomical phenotypes, we performed volumetric analyses of segmented brain and IC volumes. Using the propagating active contour filter in Amira software (TGS), whole brains were segmented semi-automatically, using manual editing to refine the contours from each individual Fgf17+/− and Fgf17−/− mouse. Whole brain templates were created using rigid, 12-parameter affine transformation for registration and averaging the groups of Fgf17−/− and Fgf17+/− mice separately. The registered mouse brain images were then normalized, adjusting the whole brain histograms to obtain equal mean and standard deviation (for later intensity-based analyses). ICs were extracted from the processed brain images using 3D IC masks created separately for Fgf17−/− and Fgf17+/− mice. The dorsal-caudal borders of the IC were easily identified by the adjacent cerebral spinal fluid (CSF) and cerebellum. The rostral border was identified by the decrease in Mn-enhancement in the IC compared to the superior colliculus, while the ventral borders were defined by the nucleus of the branchium of the IC and the CSF in the midbrain aqueduct. Using the resulting average masks, the IC was segmented, and the volume computed as the product of the voxel volume with the number of IC voxels, for each mouse. Whole brain and IC volumes were computed as the product of the voxel volume with the humber of brain and IC voxels, respectively. Differences in volume were analyzed statistically using Student’s t-test (two-way), with significance set at p<0.05.
Deformation-Based Morphometry
Fgf17+/− and Fgf17−/− mice were analyzed for anatomical differences with unbiased deformation-based morphometry (DBM), using software obtained from the Mouse Imaging Centre (Toronto, Canada), which is based on image registration tools developed and distributed by the Montreal Neurological Institute (Collins et al., 1994). At completion of the registration process, local volume differences were identified computationally without prospective identification of anatomical structures. DBM methods for anatomical phenotyping in mice have been described previously (Kovacevic et al., 2005; Nieman et al., 2006; 2007; Lerch et al., 2008). The generation of a reference average image began by normalization of the orientation, location and intensity of all component images. Subsequently, an average space was defined that was unbiased to any particular image by averaging registration results of pair-wise image registrations (Woods et al., 1998). After generating an initial average in this orientation by taking the mean of image intensities at each voxel, each image was re-registered to the average iteratively to generate progressively refined averages. At each iteration, the registration was performed at a finer scale, until the final average with full-resolution (100-μm) was achieved. The final set of deformations from the Fgf17+/− and the Fgf17−/− groups to the average were then analyzed for significant anatomical differences. For volumetric differences, the local size changes encoded by the deformations were computed using the determinant of the Jacobian matrix, a multiplicative scale factor that represents voxel volume changes. A voxelwise Student’s t-test of log-Jacobian values was used to identify regions of statistically significant change, correcting for multiple statistical comparisons using the false discovery rate (Benjamini & Hochberg, 1995).
Analysis of IC Activity
The image analysis protocols for analyzing IC activity have been described in detail previously (Yu et al., 2008). Statistical analysis of MEMRI signals in the IC was performed voxel-wise with a two-tailed Student’s t-test to compare the active IC volume between broadband sound stimulated and quiet control mice (Yu et al. 2005; 2008). To study the tonotopic organization within the IC, we analyzed the active IC volume after exposing mice to pure 16- and 40-kHz tones. In these experiments, maps of signal hyperintensity were created by setting a signal intensity (SI) threshold to be SIMean+1.5 SISD, where the mean (SIMean) and standard deviation (SISD) were measured from the histogram for each IC volume. The location of the active IC under pure tone conditions was located within the whole IC volume by measuring the distance between the centroids of each volume. Comparisons of the locations of the 16- and 40-kHz activity patterns in Fgf17−/− and Fgf17+/− mice were then made on the basis of their centroid locations (after averaging the left and right centroid locations for each mouse individually). For visualization of the variability between mice, the standard deviations of the centroid positions on each principal axis were used to define the radii of an ellipsoid, and the ellipsoids for the 16- and 40-kHz activity patterns were overlaid to show both the location and variability of the centroids for each activity pattern. Differences in centroid separation were analyzed statistically using Student’s t-test (two-way), with significance set at p<0.05.
Region-of-interest (ROI) measurements were also made in the cochlear nucleus, as described previously (Yu et al., 2005), to determine whether there were any differences in the auditory input circuit, upstream of the IC, comparing Fgf17−/− and Fgf17+/− mice.
Results
ABR recordings show no peripheral hearing defects in Fgf17 mutant mice
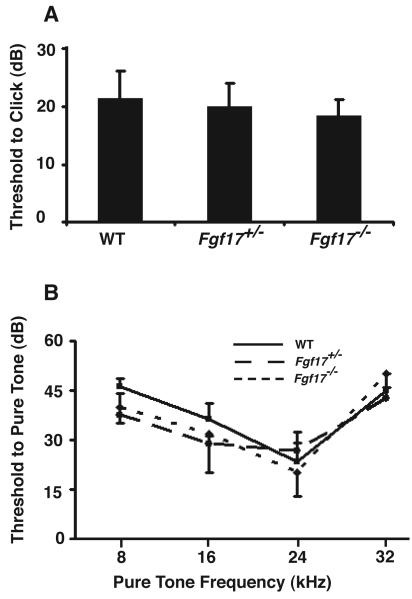
ABR thresholds were measured in 4-week old WT (N=4), Fgf17+/− (N=4) and Fgf17−/− (N=4) littermates (Fig. 1). The 4-week age was chosen as the earliest developmental stage that consistent ABR recordings could be acquired with the existing experimental setup. There were no differences in the threshold responses after click (Fig. 1A) or pure tone (Fig. 1B) stimulation. We concluded that loss of Fgf17 does not result in hearing impairment in these mice.
Fig 1. ABR recordings detected no hearing deficits in Fgf17 mutant mice.
ABR threshold measurements after click stimulation were similar between Fgf17+/+ wild type (WT), Fgf17+/−, and Fgf17−/− mice (N=4 for each genotype), with no significant difference between genotypes (A). During exposure to different pure tones, ABR threshold measurements were also similar among the three groups, with no statistically significant differences (B).
Analysis of anatomical brain phenotypes in Fgf17−/− mice
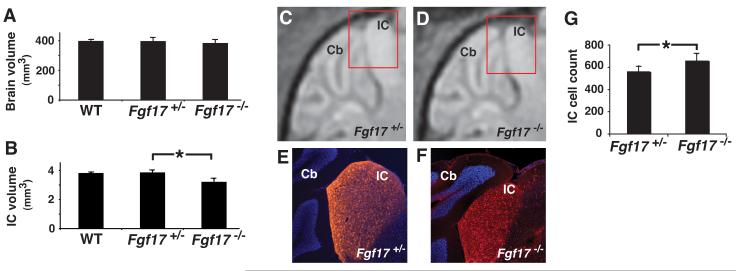
Analysis of whole brain and IC volumes was first performed using MEMRI images of small groups of P21 WT (N=4) and Fgf17+/− (N=4), confirming previous reports that there is no obvious phenotype in heterozygous Fgf17 mutants (Xu et al., 2000). Volumetric and statistical analyses were then performed in larger groups of Fgf17+/− (N=14) and Fgf17−/− (N=14) mice, showing no differences in the whole brain volumes between WT, Fgf17+/− and Fgf17−/− mice (Fig. 2A), or in IC volumes between WT and Fgf17+/− mice, while the IC volume in Fgf17−/− mice was 17 ± 5% (mean ± standard deviation) smaller than in Fgf17+/− mice (p=0.0001; Fig. 2B), confirming the previous (qualitative) histological results (Xu et al., 2000). These results provide the first quantification of the anatomical IC phenotype of Fgf17 knockout mice.
Fig 2. MEMRI and histology confirmed the reduction of IC in Fgf17−/− mice.
3D MEMRI images of the brain and IC allowed quantitative volumetric analysis. Quantitative analysis showed no difference between the whole brain volumes of WT (N=4), Fgf17+/− (N=14) and Fgf17−/− mice (N=14) (A), or between the IC volumes of WT and Fgf17+/− mice, while the IC volume in Fgf17−/− was significantly reduced compared to Fgf17+/− mice (B; *p=0.0001). This reduction in IC size was also evident in sagittal MEMRI images (C,D) and matched histological (E,F), using Neurogranin (red) staining to identify the IC nuclei (A,B). Nuclear DAPI (blue) staining was used as a counterstain, highlighting the adjacent cerebellum (Cb). The red inset boxes (C,D) show the approximate position of the histological sections (E,F) relative to the MEMRI images. Quantitative analysis showed a significant increase in the number of cells per unit area in 3 slices of the central IC, comparing Fgf17+/− (N=3) and Fgf17−/− (N=3) mice (G; *p=0.026).
After MEMRI, brains were sectioned for histological analysis, staining for Neurogranin, a protein kinase C (PKC) substrate expressed by IC neurons (Huang et al., 1993; Neuner-Jehle et al., 1996). There was good qualitative agreement between in vivo MEMRI images (Fig. 2C,D) and Neurogranin staining (Fig. 2E,F). Note that Neurogranin is more highly expressed in the IC compared to adjacent brain regions, allowing identification of the IC nuclei in histological sections (Fig. 2E,F). Quantitative analysis of IC cell density revealed a 17% increase in Fgf17−/− (N=3) compared to Fgf17+/− (N=3) mice (Fig. 2G), closely matching the magnitude of the morphological decrease, and showing that the number of IC cells was not affected by loss of Fgf17.
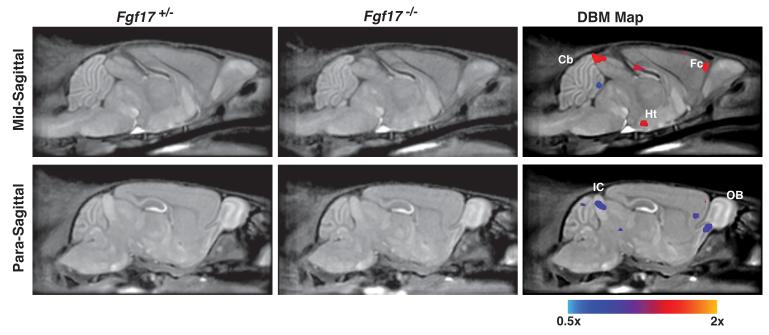
To further explore the anatomical phenotypes in Fgf17 mutant mice, automated and unbiased DBM analysis was performed to compare P21 Fgf17−/− (N=10) and Fgf17+/− (N=15) control mice (Fig. 3, false discovery rate, FDR=0.05; Table 1). The resulting deformation maps showed small but statistically significant regional size differences between the two groups of mice. These included a reduced volume in the IC and anterior cerebellum in Fgf17−/− mice, and an increased volume in the fluid space above the IC, consistent with the IC deletion. In the forebrain, DBM analysis detected an expansion of the anterior-medial rhinal fissure, extending into the more lateral cortical regions suggesting possible alterations in the structure of the frontal cortex, previously undetected by histological analysis. Finally, the DBM maps showed a significant increase in the volume of a medial subregion of hypothalamus, and a decrease in the ventral-caudal olfactory bulb (OB), both previously undetected phenotypes in Fgf17−/− mutants.
Fig 3. DBM analysis revealed morphological differences in Fgf17−/− mice.
Slices through 3D MRI datasets show the average Fgf17+/− and Fgf17−/− images, respectively, in the left and middle column. In the right column, statistically significant voxel size changes are highlighted (5% false discovery rate, FDR). The color scale indicates regions that were significantly larger (red/yellow; scale goes up to 2×) or smaller (blue; scale goes down to 0.5×) in the Fgf17−/− mice compared to the Fgf17+/− control littermates. Labels: cerebellum, Cb; frontal cortex, Fc; hypothalamus, Ht; inferior colliculus, IC; olfactory bulb, OB.
Table 1.
Summary of DBM results comparing Fgf17−/− and Fgf17+/− mice. There were significant volume decreases (−) in the inferior colliculus (IC), cerebellum (Cb) and olfactory bulb (OB), and significant volume increases (+) in the cerebral spinal fluid overlying the IC (IC-CSF), anterior-medial rhinal fissure (AM-RF), and the hypothalamus (Ht). DBM mapping was performed with a false discovery rate (FDR) of 0.05.
| Mid-hindbrain | Forebrain | ||||
|---|---|---|---|---|---|
| IC | IC-CSF | Cb | AM-RF | Ht | OB |
| − | + | − | + | + | − |
MEMRI reveals a significant decrease in the active IC of Fgf17−/− mice
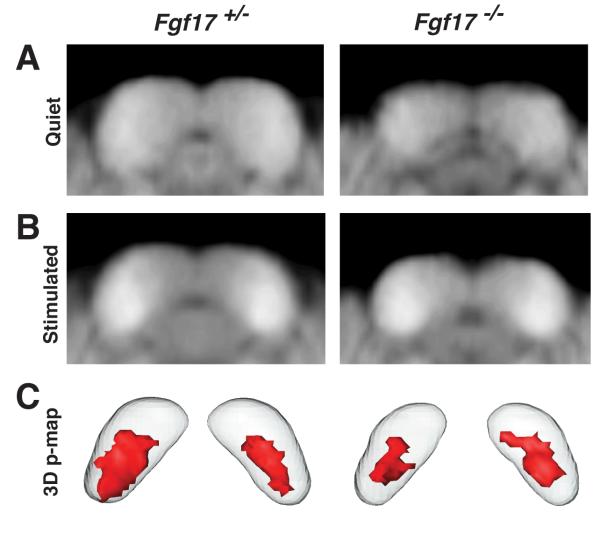
To assess the functional consequences resulting from loss of Fgf17 during midbrain development, we analyzed regions of enhanced MEMRI signal in Fgf17+/− and Fgf17−/− mice after periods of defined sound stimulation. This approach was previously demonstrated to allow analysis of the functional core of the IC (Yu et al., 2005). For this analysis, we compared IC signal intensities between control mice kept in a quiet environment (Fig. 4A; N=7 for each genotype) and mice stimulated with broadband sound, covering the entire audible frequency range for mice up to 59-kHz (Fig. 4B; N=7 for each genotype). Consistent with the ABR data, analysis of signal intensities in the cochlear nucleus showed no differences between Fgf17+/− and Fgf17−/− mice (data not shown), indicating that any differences detected in the IC were not simply due to a global reduced activity in the auditory inputs of Fgf17−/− mice. Active IC regions for each genotype were characterized by 3D p-maps (Yu et al., 2008), consisting of voxels with significantly increased signal intensity compared to quiet controls (p<0.05, Fig. 4C). There was a 36% reduction in the average volume of the active IC, comparing Fgf17−/− (0.38-mm3) and Fgf17+/− (0.60-mm3) mice. When expressed as a ratio of active-to-total IC volume, the ratio in Fgf17−/− mice was still more than 20% lower than in Fgf17+/− littermates (Table 2). These results show that loss of Fgf17 leads to a significant reduction in the volume of the IC region activated by auditory input, beyond that predicted by the morphological deletion.
Fig 4. MEMRI revealed a reduced functional IC in Fgf17−/− mice.
Comparison of averaged coronal IC MEMRI images of mice maintained in a quiet environment (A) to mice exposed to broadband (1-59 kHz) sound stimuli (B) revealed MEMRI enhancement after stimulation in both Fgf17+/− (left) and Fgf17−/− (right) mice. Voxelwise statistical analysis identified the IC voxels with significant signal enhancement, displayed in 3D p-maps (C; red contours, p ≤ 0.05), showing a reduction in the “active IC” of Fgf17−/− compared to Fgf17+/− mice.
Table 2.
Analysis of IC volumes and active-to-total IC volume ratios in Fgf17−/− and Fgf17+/− mice. Results are expressed as mean ± standard deviation for each entry.
| Genotype | IC Volume (mm3) | Active to Total IC Volume Ratio (%) |
|---|---|---|
| Fgf17+/− | 3.85 ± 0.16 | 15.87 ± 0.67 |
| Fgf17−/− | 3.20 ± 0.24 | 12.09 ± 0.90 |
MEMRI reveals a significantly altered IC tonotopic map in Fgf17−/− mice
To further examine the functional organization of the IC in Fgf17 mutants, we compared tone-specific activity patterns in Fgf17+/− and Fgf17−/− mice. Frequency selectivity is a hallmark feature of the central auditory system, mainly attributed to the tonotopic projection of afferents into the IC and other auditory nuclei, which results in spatial patterning of tone-specific activity in each nucleus. In the normal IC, the tonotopic map is relatively simple, with lower frequencies activating more dorsal neurons, while higher frequencies activate more ventral neurons (Romand & Ehret, 1990). Longitudinal MEMRI experiments were performed to compare the positions of 16- and 40-kHz IC activity patterns in both Fgf17+/− (N=7) and Fgf17−/− (N=10) mice. 40-kHz MEMRI data were first acquired in each mouse at P21. Mice were then kept in a quiet environment for two days to allow activity induced Mn accumulation to be cleared from the IC (Yu et al., 2005), after which 16-kHz MEMRI data were acquired in each mouse at P24. Tone-specific activity patterns were determined using thresholds based on the signal intensities in the MEMRI images (Yu et al., 2005).
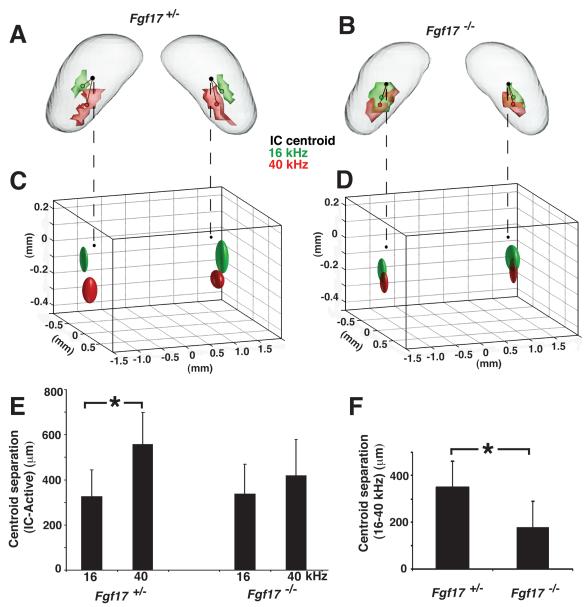
In Fgf17+/− mice, 16- and 40-kHz activity patterns appeared normal (Yu et al., 2007), with well-separated domains aligned tonotopically along the dorsal-ventral axis of the IC (Fig. 5A). In contrast, the 16- and 40-kHz patterns were largely overlapping in Fgf17−/− mice, with only a small dorsal displacement of the 16-kHz region relative to 40-kHz region (Fig. 5B). Analysis of the distribution of tone-specific centroids (Fig. 5C,D) and statistical comparison of the spatial location of the centroids (Fig. 5E,F) both demonstrated and quantified the significantly reduced separation between the 16- and 40-kHz activity patterns in Fgf17−/− mice compared to Fgf17+/− controls. Specifically, the (16-40 kHz) centroid-centroid distance measured in Fgf17−/− mice was 49% smaller than Fgf17+/− controls. Interestingly, this centroid-centroid separation in Fgf17+/− mice (mean ± standard deviation = 351 ± 110 μm) was virtually identical to the distance measured in WT mice in a previous study (352 ± 27 μm; Yu et al., 2007), further confirming the lack of a detectable IC phenotype in Fgf17+/− heterozygous mutants. Taken together, these results indicate that in addition to the reduction in the size of the functional IC, there is also a significant alteration in the tonotopic organization of the IC in Fgf17−/− mutant mice.
Fig 5. MEMRI demonstrated an altered tonotopic map in Fgf17−/− IC.
16- (green) and 40-kHz (red) activity patterns and centroids (small green and red circles) were measured in the IC of Fg17+/− (A) and Fg17−/− (B) mice. The 16- and 40-kHz activity patterns were separated along the dorsal-ventral tonotopic axis in the IC of Fgf17+/− mice, but largely overlapped in Fgf17−/− mice. Likewise, ellipsoids representative of the population variability showed the normal separation in Fgf17+/− mice (C), but not Fgf17−/− mice (D). Quantitative analysis comparing the distance from each activity centroid to the IC centroid (small black circles) showed a significant difference in the two frequencies in Fgf17+/− mice, but not in Fgf17−/− mice (E; *p=0.0057). There was also a 49% reduction in the separation between the 16- and 40-kHz centroids in Fgf17−/− mice compared to Fgf17+/− mice (F; *p= 0.0074).
Finally, to determine whether the longitudinal imaging protocol might affect the second set of 16-kHz measurements, a separate group of Fgf17−/− mice (N=6) was tested once only with 16-kHz stimulation at P21. There were no significant differences between these data and 16-kHz data from P24 Fgf17−/− mice after 40-kHz testing at P21 (Suppl. Fig. 1), showing that the longitudinal protocol used in these investigations was equivalent to testing individual groups of mice, between P21 and P24, at each frequency.
Discussion
This study provides the first in vivo quantitative analysis of morphological and functional defects in the brains of Fgf17 knockout mice. In comparisons of 3D MEMRI images of Fgf17+/− and Fgf17−/− mutants, unbiased whole brain DBM analysis demonstrated significant morphological differences in the IC and cerebellum, regions previously reported to be altered based on histology (Xu et al., 2000). In addition, the olfactory bulbs in Fgf17−/− mice were found to be significantly smaller, while the fissure anterior to the medial frontal cortex, as well as more lateral frontal cortical regions were larger. This last result suggests a possible change in the anatomical structure of the frontal cortex in Fgf17−/− mice, an interesting finding given the altered gene expression in that region (Cholfin & Rubenstein, 2007), and the recent report of subtle cortical white matter abnormalities assessed by diffusion tensor imaging (Moldrich et al., 2010). In the IC there was good qualitative agreement between MEMRI and histology, while 3D MEMRI analysis was used to quantify the reduction in the Fgf17−/− IC volume. Interestingly, quantitative MEMRI activity mapping showed an even greater decrease in the active IC volume of Fgf17−/− mice after broadband sound stimulation. Moreover, the organization of IC neurons receiving auditory afferent projections is likely altered in Fgf17−/− mice, since the tone-specific (16- and 40-kHz) activity patterns were abnormal.
One question we aimed to address with our data was what is the relationship between the functional and anatomical phenotypes in Fgf17−/− mice. Some reduction in the volume of the active IC core might be expected in a smaller IC, without necessarily implying an altered organization of neurons and/or axonal projections in the IC of Fgf17−/− mice. Arguing against the possibility that the functional changes are simply byproducts of the altered morphology are the relative magnitudes of the observed phenotypes. First, the decrease in active IC volume after broadband stimulation (36%) was more than twice the morphological decrease (17%). Furthermore, the distance between the centroids of the 16- and 40-kHz patterns was reduced by 49% in Fgf17−/− mice compared to Fgf17+/− mice, a reduction well beyond that necessary to accommodate either the IC or active-IC volume changes. It should be noted that the quantitative parameters derived from the statistical maps, and their relative magnitudes, should only be interpreted in comparisons between genotypes (Fgf17−/− vs. Fgf17+/−), and not as absolute measures of brain activity, as measured, for example by electrophysiology. As with any statistical test, our choice of the p<0.05 detection threshold, although conventional, is somewhat arbitrary. Nevertheless, the statistical mapping results strongly suggest that the tonotopic organization of the IC is abnormal in Fgf17−/− mutants, leading us to speculate on the underlying cause of these changes. One possibility is that Fgf17 is involved in patterning the tonotopically ordered afferent innervations in the IC, which could be tested in future by performing axon-tracing studies, for example to determine whether defects exist in connectivity between IC and cochlea in Fgf17−/− mutants. Interestingly, there was no difference in the total number of IC cells in Fgf17−/− compared to Fgf17+/− mice, again suggesting that the functional differences are not a simple consequence of the morphological alterations.
It is also interesting to consider the current results in light of the behavioral abnormalities reported in Fgf17−/− mutant mice. The major behavioral phenotypes were decreased vocalizations of the mutant pups after isolation from their mothers, and decreased interaction time between adult Fgf17−/− males and wildtype females, and between Fgf17−/− male-female pairs after extended time in a novel environment, compared to wildtype pairs (Scearce-Levie et al., 2008). Although these behavioral phenotypes were previously attributed to changes in cortical gene expression, it is equally likely that they result from altered patterns of auditory activity implied by our data. Future investigation of auditory function is therefore important for understanding the Fgf17−/− behavioral phenotypes.
In the embryonic MHB, Fgf8 expression is restricted to the anterior hindbrain, whereas Fgf17 is more broadly expressed in the hindbrain as well as the posterior midbrain (anlage of the IC), and persists over a longer developmental window (Xu, et al. 2000; Liu et al., 2003). Based on mutant studies, Fgf17 is thought to play a secondary role to Fgf8 during embryogenesis, particularly during MHB development. Genetic studies in mutant zebrafish embryos (no isthmus, noi = Pax2.1; acerebellar, ace = Fgf8) detected no Fgf17 expression in the MHB, indicating that Pax2.1 and Fgf8 are crucial upstream components in the pathway that activates Fgf17 (Lun & Brand, 1998; Reifers et al., 2000). Similarly, in Fgf8 conditional mutant mice, Fgf17 expression is lost after Fgf8 is ablated (Chi et al., 2003). The separate roles of Fgf17 and two Fgf8 isoforms (Fgf8a and Fgf8b) have also been demonstrated in the mouse MHB. Fgf8b acts as a potent hindbrain organizer molecule, able to induce hindbrain genes and repress midbrain genes (Sato et al., 2001; Liu et al., 2003). In contrast, Fgf17, similar to Fgf8a, primarily induces midbrain structures (Liu et al. 2003). Since Fgf17 expression is lost in Fgf8 mutants, it cannot compensate for loss of Fgf8, likely accounting for part of the phenotypic difference between mice lacking Fgf8 (Sun et al., 1999; Shamim et al., 1998; Chi et al. 2003) or Fgf17 (Xu et al., 2000; Liu et al., 2003).
Our results show that loss of Fgf17 during embryonic brain development has a significant effect on postnatal brain function. Given the interactions between Fgf17 and Fgf8 during embryogenesis described above, it would be interesting in future to assess allelic series of Fgf mutants, comparing activity patterns in the brains of Fgf17, Fgf8 and Fgf8/17 double mutants, as well as conditional alleles of both genes. This would enable detailed analyses of the relationships between embryonic patterning defects due to misregulation of Fgf signaling and postnatal brain function. The abnormal tonotopic maps in the Fgf17−/− mice suggest that Fgf signaling is involved in the guidance of tonotopically organized afferents innervating the IC. However, it is still unclear whether Fgf17 directly regulates axonal targeting, or whether it regulates other guidance molecules indirectly. Thus, the molecular mechanisms of Fgf signaling in the formation of tonotopic maps remain to be uncovered. This study demonstrates the importance of neuroimaging approaches like MEMRI and DBM for identifying and quantifying subtle changes in brain morphology, and for providing results that reveal functional abnormalities beyond the anatomical phenotypes. This study is also the first demonstration that MEMRI approaches can be applied to analyze functional phenotypes in the central auditory system of mutant mice. Importantly, MEMRI results can be used to guide future studies of altered circuitry and behavior in a variety of genetically engineered mice.
Supplementary Material
Suppl Fig 1: Effect of longitudinal MEMRI on the 16-kHz IC activity patterns In the longitudinal MEMRI experiments used to analyze tonotopic maps, the 16-kHz IC activity patterns were acquired from Fgf17−/− and Fgf17+/− mice at P24, after measuring the 40-kHz activity pattern at P21. Mn remaining in 40-kHz IC activity regions might therefore confound the signal enhancement results after 16-kHz stimulation at P24. We therefore measured the 16-kHz activity pattern in a naïve group of Fgf17−/− mice at P21. The 16-kHz activity pattern in naïve P21 mice (blue) was very similar to that in P24 mice (green) (A). Quantitative studies showed similar distributions of the two sets of 16-kHz activity centers (B), including the overlap with the distribution of (P21) 40-kHz activity centers (red). These analyses also showed nearly identical separation between each 16-kHz activity centroid and the geometrical IC centroid, comparing P21 and P24 Fgf17−/− mice (C; N≥6 for each group, p=0.1421). These results confirmed that the overlap of the 16- and 40-kHz activity patterns was part of the Fgf17−/− mutant phenotype, and not simply an artifact of the experimental design.
Acknowledgments
This research was supported in part by grants from the NIH (R01NS038461, R01HD050767). We thank David Ornitz (Washington University) for providing the Fgf17 mutant mice used in these studies, and Dan Sanes (NYU) for insightful discussions during the course of this work. We also thank Anand Mhatre (NYU School of Medicine) for advice on the ABR measurements, and Mark Henkelman, John Sled and Jason Lerch (Mouse Imaging Centre, Toronto Canada) for providing the software used for the DBM analysis.
Abbreviations
- ABR
auditory brainstem recording
- DBM
deformation based morphometry
- Fgf17
fibroblast growth factor 17
- IC
inferior colliculus
- MRI
magnetic resonance imaging
- MEMRI
manganese (Mn)-enhanced MRI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basson MA, Echevarria D, Ahn CP, Sudarov A, Joyner AL, Mason IJ, Martinez S, Martin GR. Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development. 2008;135:889–98. doi: 10.1242/dev.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Ser B-Methodol. 1995;57:289–300. [Google Scholar]
- Chi CL, Martinez S, Wurst W, Martin GR. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130:2633–44. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA. 2007;104:7652–57. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol. 2008;509:144–55. doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–74. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–31. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–14. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Hoshikawa M, Ohbayashi N, Yonamine A, Konishi M, Ozaki K, Fukui S, Itoh N. Structure and expression of a novel fibroblast growth factor, FGF-17, preferentially expressed in the embryonic brain. Biochem Biophys Res Commun. 1998;244:187–91. doi: 10.1006/bbrc.1998.8239. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Chen HC. Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch Biochem Biophys. 1998;305:570–580. doi: 10.1006/abbi.1993.1463. [DOI] [PubMed] [Google Scholar]
- Kovacevic N, Henderson JT, Chan E, Lifshitz N, Bishop J, Evans AC, Henkelman RM, Chen XJ. A three-dimensional MRI atlas of the mouse brain with estimates of the average and variability. Cereb Cortex. 2005;15:639–45. doi: 10.1093/cercor/bhh165. [DOI] [PubMed] [Google Scholar]
- Lee SM, Danielian PS, Fritzsch B, McMahon AP. Evidence that FGF8 signaling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development. 1997;124:959–69. doi: 10.1242/dev.124.5.959. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Carroll JB, Spring S, Bertram LN, Schwab C, Hayden MR, Henkelman RM. Automated deformation analysis in the YAC128 Huntington disease mouse model. Neuroimage. 2008;39:32–39. doi: 10.1016/j.neuroimage.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Koretsky AP. Manganese ion enhances T1-weighted MRI during brain activation: an approach to direct imaging of brain function. Magn Reson Med. 1997;38:378–88. doi: 10.1002/mrm.1910380305. [DOI] [PubMed] [Google Scholar]
- Liu A, Li JY, Bromleigh C, Lao Z, Niswander LA, Joyner AL. FGF17b and FGF18 have different midbrain regulatory properties from FGF8b or activated FGF receptors. Development. 2003;130:6175–85. doi: 10.1242/dev.00845. [DOI] [PubMed] [Google Scholar]
- Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–62. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- Maruoka Y, Ohbayashi N, Hoshikawa M, Itoh N, Hogan BL, Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74:175–77. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–41. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Pannek K, Hoch R, Rubenstein JL, Kurniawan ND, Richards LJ. Comparative mouse brain tractography of diffusion magnetic resonance imaging. Neuroimage. 2010;51:1027–36. doi: 10.1016/j.neuroimage.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner-Jehle M, Denizot JP, Mallet J. Neurogranin is locally concentrated in rat cortical and hippocampal neurons. Brain Res. 1996;733:149–54. [PubMed] [Google Scholar]
- Nieman BJ, Flenniken AM, Adamson SL, Henkelman RM, Sled JG. Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol Genomics. 2006;24:154–62. doi: 10.1152/physiolgenomics.00217.2005. [DOI] [PubMed] [Google Scholar]
- Nieman BJ, Lerch JP, Bock NA, Chen XJ, Sled JG, Henkelman RM. Mouse behavioral mutants have neuroimaging abnormalities. Hum Brain Mapp. 2007;28:567–75. doi: 10.1002/hbm.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifers F, Adams J, Mason IJ, Schulte-Merker S, Brand M. Overlapping and distinct functions provided by fgf17, a new zebrafish member of the Fgf8/17/18 subgroup of Fgfs. Mech Dev. 2000;99:39–49. doi: 10.1016/s0925-4773(00)00475-5. [DOI] [PubMed] [Google Scholar]
- Romand R, Ehret G. Development of tonotopy in the inferior colliculus. I. Electrophysiological mapping in house mice. Brain Res Dev Brain Res. 1990;54:221–34. doi: 10.1016/0165-3806(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Sato T, Araki I, Nakamura H. Inductive signal and tissue responsiveness defining the tectum and the cerebellum. Development. 2001;128:2461–69. doi: 10.1242/dev.128.13.2461. [DOI] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, Rubenstein JL, Mucke L. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7:344–54. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Shamim H, Mahmood R, Logan C, Doherty P, Lumsden A, Mason I. Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development. 1999;126:945–59. doi: 10.1242/dev.126.5.945. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–18. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–44. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–46. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF. Measurement of the auditory brainstem response (ABR) to study auditory sensitivity in mice. Curr Protoc Neurosci. 2006 doi: 10.1002/0471142301.ns0821bs34. Ch 8, Unit 8.21B. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–52. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Xu J, Lawshe A, MacArthur CA, Ornitz DM. Genomic structure, mapping, activity and expression of fibroblast growth factor 17. Mech Dev. 1999;83:165–78. doi: 10.1016/s0925-4773(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu Z, Ornitz DM. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development. 2000;127:1833–43. doi: 10.1242/dev.127.9.1833. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–66. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Yu X, Wadghiri YZ, Sanes DH, Turnbull DH. In vivo auditory brain mapping in mice with Mn-enhanced MRI. Nat Neurosci. 2005;8:961–68. doi: 10.1038/nn1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Sanes DH, Aristizabal O, Wadghiri YZ, Turnbull DH. Large-scale reorganization of the tonotopic map in mouse auditory midbrain revealed by MRI. Proc Natl Acad Sci USA. 2007;104:12193–98. doi: 10.1073/pnas.0700960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zou J, Babb JS, Johnson G, Sanes DH, Turnbull DH. Statistical mapping of sound-evoked activity in the mouse auditory midbrain using Mn-enhanced MRI. Neuroimage. 2008;39:223–30. doi: 10.1016/j.neuroimage.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl Fig 1: Effect of longitudinal MEMRI on the 16-kHz IC activity patterns In the longitudinal MEMRI experiments used to analyze tonotopic maps, the 16-kHz IC activity patterns were acquired from Fgf17−/− and Fgf17+/− mice at P24, after measuring the 40-kHz activity pattern at P21. Mn remaining in 40-kHz IC activity regions might therefore confound the signal enhancement results after 16-kHz stimulation at P24. We therefore measured the 16-kHz activity pattern in a naïve group of Fgf17−/− mice at P21. The 16-kHz activity pattern in naïve P21 mice (blue) was very similar to that in P24 mice (green) (A). Quantitative studies showed similar distributions of the two sets of 16-kHz activity centers (B), including the overlap with the distribution of (P21) 40-kHz activity centers (red). These analyses also showed nearly identical separation between each 16-kHz activity centroid and the geometrical IC centroid, comparing P21 and P24 Fgf17−/− mice (C; N≥6 for each group, p=0.1421). These results confirmed that the overlap of the 16- and 40-kHz activity patterns was part of the Fgf17−/− mutant phenotype, and not simply an artifact of the experimental design.