Abstract
Extensive evidence suggests that the reinforcing effects of cocaine involve inhibition of dopamine transporters (DAT) and subsequent increases in dopamine (DA) levels in the striatum. We have previously reported that cocaine inhibits the DAT within 4–5 sec of intravenous injection, matching the temporal profile of the behavioral and subjective effects of cocaine. Intravenous injection of GBR-12909, a high affinity, long-acting DAT inhibitor, also inhibits DA uptake within 5 sec. Given that high affinity, long-acting drugs are considered to have relatively low abuse potential, we found it intriguing that GBR-12909 had an onset profile similar to that of cocaine. To further explore the onset kinetics of both low and high affinity DAT inhibitors, we examined the effects of intravenous cocaine (1.5 mg/kg), methylphenidate (1.5 mg/kg), nomifensine (1.5 mg/kg), GBR-12909 (1.5 mg/kg), PTT (0.5 mg/kg), and WF23 (0.5 mg/kg) on electrically-evoked DA release and uptake in the nucleus accumbens core. Results indicate that all of the DAT inhibitors significantly inhibited DA uptake within 5 sec of injection. However, the timing of peak uptake inhibition varied greatly between the low and high affinity uptake inhibitors. Uptake inhibition following cocaine, methylphenidate, and nomifensine peaked 30 sec following injection. In contrast, peak effects for GBR-12909, PTT, and WF23 occurred between 20 and 60 min following injection. These observations suggest that the initial onset for intravenous DAT inhibitors is extremely rapid and does not appear to be dictated by a drug’s affinity.
Keywords: nucleus accumbens, cocaine, methylphenidate, nomifensine, voltammetry, rat
Considerable evidence suggests that the mesolimbic dopamine (DA) system plays a critical role in the reinforcing effects of cocaine (Roberts et al., 1977; Ritz et al., 1987; Woolverton and Johnson, 1992; Wise, 1996; Koob and Le Moal, 1997). In particular, it is well accepted that cocaine elevates extracellular DA levels in the nucleus accumbens (NAc) by blocking DA transporter (DAT) activity and that these actions participate in the acute psychostimulant effects of cocaine. Nevertheless, it remains unclear whether there is a one-to-one relationship between DAT inhibition and the rapid subjective affects of cocaine, particularly due to conflicting reports on the time course of cocaine effects (Koob and Bloom, 1988; Kuhar et al., 1991; Wise et al., 1995; Volkow et al., 1997; Wise et al., 2008; Wakazono and Kiyatkin, 2008). For example, microdialysis studies suggest that cocaine increases DA levels approximately 2–5 min following intravenous (i.v.) injection (Pettit and Justice, Jr., 1989; Wise et al., 1995; Hemby et al., 1997; Ahmed et al., 2003). By comparison, electrophysiological studies indicate that DA neurons in the ventral tegmental area (VTA) are inhibited within 1 min of i.v. cocaine, due to increased extracellular DA levels and subsequent activation of DA autoreceptors (Pitts and Marwah, 1987; Einhorn et al., 1988; Batsche et al., 1992; Hinerth et al., 2000). Using fast scan cyclic voltammetry we have previously shown that various doses of cocaine elicit significant DA uptake inhibition within a matter of seconds (Mateo et al., 2004; España et al., 2008). Similar initial effects on DA uptake inhibition were also observed following i.v. injections of various doses of the high affinity, long acting DAT inhibitor 1-(2-bis(4-fluorphenyl)-methoxy)-ethyl-4-(3-phenyl-propyl)piperazine (GBR-12909). These latter results are surprising, considering that high affinity drugs such as GBR-12909 are thought to have a lower abuse potential than cocaine due to slower onset and longer acting effects (Busto and Sellers, 1986; Oldendorf, 1992; Woolverton and Wang, 2004). Therefore, the fact that GBR-12909 increased DAT inhibition within the same time frame as cocaine suggests that all DAT inhibitors may have similar rapid onset when injected intravenously. Given that some putative pharmacotherapies for cocaine abuse rely on long-acting high affinity DAT inhibitors to mitigate the effects of cocaine (Froimowitz et al., 2000; Newman and Kulkarni, 2002; Gardner et al., 2006; Runyon and Carroll, 2006; Froimowitz et al., 2007; Rothman et al., 2008) determining the exact temporal profile for the early effects of both short and long acting DAT inhibitors is critical to our understanding of the addiction processes.
In the present study, we used in vivo fast scan cyclic voltammetry in anesthetized rats to examine the effects of several uptake inhibitors with varying affinities for the DAT. We compared the effects of i.v. cocaine (1.5 mg/kg), methylphenidate (1.5 mg/kg), nomifensine (1.5 mg/kg), GBR-12909 (1.5 mg/kg), 2β-propanoyl-3β-(4-tolyl)-tropane (PTT; 0.5 mg/kg), and 2β-propanoyl-3β-(2-naphthyl)-tropane (WF23; 0.5 mg/kg) on DA uptake inhibition in the NAc core. DA uptake parameters were measured at several time points, including 5, 30, and 60 sec post i.v. injection.
EXPERIMENTAL PROCEDURES
Animals
Adult male Sprague-Dawley rats (325–375g) were housed in pairs on a 12:12 h light:dark cycle with food and water available ad libitum. All protocols and animal care procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University Health Sciences.
Surgery
Urethane-anesthetized rats were used to avoid potential interference from behavioral factors and to avoid alterations in DA uptake kinetics that can occur when using other anesthetics (Greco and Garris, 2003). Rats were anesthetized with urethane (1.5 g/kg, i.p.; Sigma-Aldrich, St. Louis, MO, USA) and implanted with an i.v. catheter into the right jugular vein as previously described (España et al., 2008). Rats were subsequently placed in a stereotaxic apparatus, a stimulating electrode was lowered into the VTA (−5.2 A, +1.1 L, −7.5 V), and a carbon fiber electrode was initially lowered into the caudate putamen (+1.3 A, +1.3 L, −4.5 V), until a 1 sec, 60 Hz monophasic (2 ms; 300 µA) stimulation train elicited a robust DA signal (España et al., 2010a; España et al., 2010b). The caudate putamen displays higher levels of DA release and faster uptake (~ 4 µM) than the NAc core ~ 2.5 µM; (Kuczenski et al., 1991; Jones et al., 1995; Wu et al., 2001) and thus it is a useful region to maximize recording conditions. Once stimulator and carbon fiber electrode locations achieved adequate levels of release in the caudate putamen, the carbon fiber electrode was lowered 2 – 2.5 mm further into the NAc core, which yields lower DA release levels and slower DA uptake.
Data acquisition
The electrode potential was linearly scanned as a triangular waveform from −0.4 V to 1.2 V and back to −0.4 V vs Ag/AgCl during an 8 msec period. Cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms using a scan rate of 400 V/s using a voltammeter/amperometer (Chem-Clamp, Dagan Corporation, MN). The magnitude of electrically-evoked DA release and transporter-mediated uptake kinetics were monitored and DA overflow curves were fitted to a exponential decay model (Yorgason et al., 2011) using locally written Labview-based (National Instruments, Austin, TX) software (Demon Voltammetry and Analysis Software, Wake Forest University Health Sciences 2010) to obtain measures for DA release and uptake. Extracellular concentrations of DA were assessed by comparing the current at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations of known concentrations of DA (1–3 µM).
Drugs and infusion procedures
Once a stable baseline of three consecutive collections was obtained, rats received an experimenter-delivered, 2 sec, ~200 µl i.v. bolus of cocaine (1.5 mg/kg n=8), methylphenidate (1.5 mg/kg n=5), nomifensine (1.5 mg/kg n=5), GBR-12909 (1.5 mg/kg), PTT (0.5 mg/kg n=5) or WF23 (0.5 mg/kg n=6). The selected doses of cocaine and GBR-12909 were based on previous studies indicating their robust effects on DA uptake inhibition at 5 and 30 sec post i.v. injection (Mateo et al., 2004; España et al., 2008). The doses used for methylphenidate and nomifensine were based on observations indicating similar DAT affinities as cocaine (see Table I; John and Jones, 2007). The doses used for PTT and WF23 were similar to those used in rat self-administration studies (Roberts et al., 1999). The 2 sec delivery of DAT inhibitors was chosen to ensure robust changes in DA uptake inhibition at early time points, given that previous observations indicate that the rate of cocaine delivery influences the magnitude of DA uptake inhibition (Samaha et al., 2004; Samaha and Robinson, 2005).
Table I.
Binding affinities for low and high affinity DAT inhibitors
Following completion of the i.v. injection, voltammetry recordings were initiated and the first electrical stimulation of DA efflux occurred 5 sec after the beginning of the collection. DA responses were measured at this 5 sec time-point, and also at 30, 60, and 90 sec post-injection. Additional collections were taken 2, 3, 4, and 5 min post-injection, and every 5 min thereafter for a minimum of 90 min. Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Rockville, MD, USA), methylphenidate and GBR-12909 were obtained from Sigma-Aldrich, nomifensine from Hoechst Roussel (Sommerville, NJ, USA), and PTT and WF23 were donated by Huw M. L. Davies (Emory University, Atlanta, GA, USA). All drugs were dissolved in 0.9% saline. Table I shows the binding affinities for the DAT inhibitors examined.
Data analysis and statistics
DA uptake was fitted to a single exponential curve, and tau values were calculated using Labview software (National Instruments) as previously described (Yorgason et al., 2011). To verify that baseline conditions were stable, a one-way repeated measures ANOVA was conducted for the three baseline collections immediately prior to drug treatment with time as the repeated measures variable. To examine the rapidity of DAT inhibitor-induced alterations in DA uptake, a one-way repeated measures ANOVA was conducted with time (baseline immediately prior to drug injection vs 5 sec after drug injection) as the repeated measures variable. For time course comparisons, a one-way repeated measures ANOVA across all time points was initially conducted, and subsequently, Tukey’s HSD posthoc comparisons were conducted for each post-drug injection time point relative the baseline time point immediately prior to drug injection.
Analyses comparing the magnitude of DA uptake inhibition across DAT blockers used a one-way between subjects ANOVA with treatment (cocaine, methylphenidate, nomifensine, GBR-12909, PTT or WF23) as the between subjects variable at various time points of interest (pre-drug saline, 5, 30 and 60 sec). Where appropriate, simple effect analyses were conducted using one-way ANOVAs. All statistical analyses were conducted using SPSS (SPSS Inc, Chicago, IL).
RESULTS
Low Affinity DAT inhibitors
To examine the onset of DA uptake inhibition following low affinity DAT inhibitors, electrically-evoked DA release and uptake were measured in the NAc core of rats that received a 2 sec, i.v. bolus of cocaine (1.5 mg/kg n=8), methylphenidate (1.5 mg/kg n=5), or nomifensine (1.5 mg/kg n=5).
Cocaine
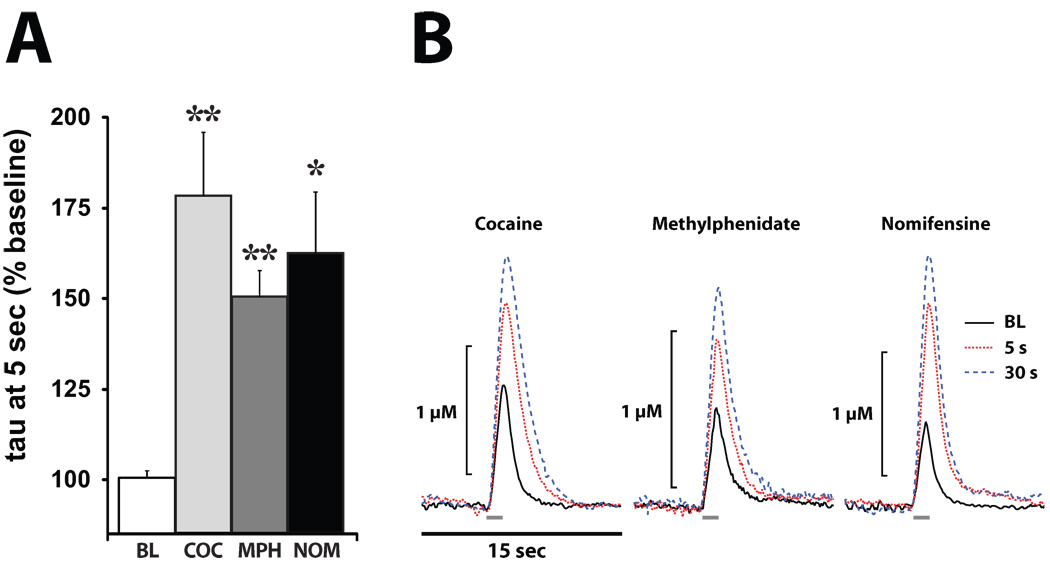
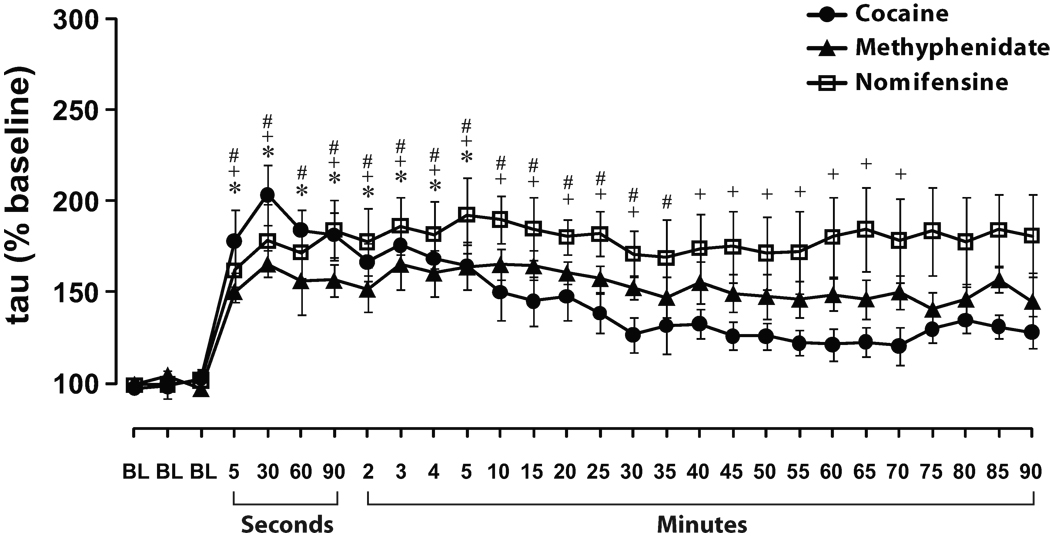
As shown in figures 1 and 2, relative to baseline DA signaling, cocaine significantly inhibited DA uptake 5 sec after i.v. injection (F(1,7) = 18.2, P < 0.01). Examination of the time-course of cocaine effects indicated that maximal levels of uptake inhibition were reached within 30 sec of injection and that DA uptake returned to baseline levels within 1 hr.
Figure 1. Low affinity DAT inhibitors reduce DA uptake within 5 sec of i.v. injection.
(A) Shown are means ± SEMs for exponential decay constants (tau), expressed as a percent of baseline (BL) following 1.5 mg/kg i.v. injections of cocaine (COC), methylphenidate (MPH), and nomifensine (NOM). (B) Shown are representative concentration-time traces of DA responses from representative rats following injections of COC, MPH, and NOM. Electrical stimulation of the VTA (60 Hz for 1 sec; gray bars) rapidly induced DA release in the NAc.*P<0.05; **P<0.01.
Figure 2. Time course of DA uptake inhibition following i.v. injection of low affinity DAT inhibitors.
Shown are means ± SEMs for exponential decay constants (tau) expressed as a percent of baseline (BL), during the 90 min following 1.5 mg/kg i.v. injections of cocaine, methylphenidate, and nomifensine. All low affinity DAT inhibitors significantly inhibited DA uptake within 5 sec and maximal levels of inhibition were observed within 30 sec. Note that the x-axis is divided into second (5–90 sec) and minute (2–90 min) intervals. *P<0.05 for the cocaine time points relative to pre-cocaine baseline conditions. #P<.05 for the methylphenidate time points relative to pre-methylphenidate baseline conditions. +P<0.05 for the nomifensine time points relative to pre-nomifensine baseline conditions.
Methylphenidate
Similar to cocaine, methylphenidate significantly inhibited DA uptake (F(1,4) = 74.5, P < 0.001) 5 sec after the injection and maximal levels of uptake inhibition were reached within 30 sec (Figs. 1 and 2). No statistically significant differences were observed between the effects of methylphenidate and cocaine during the first 5 min following injection. Examination of the time-course of methylphenidate effects indicated that, unlike cocaine, DA uptake inhibition did not return to baseline levels for the duration of the experiment, likely reflecting the slower clearance of this drug (Volkow et al., 1995).
Nomifensine
Similar to cocaine and methylphenidate, nomifensine significantly inhibited DA uptake (F(1,4) = 10.5, P < 0.05) 5 sec after injection and maximal levels of uptake inhibition were reached within 30 sec (Figs. 1 and 2). No statistically significant differences were observed between the effects of nomifensine and cocaine during the first 5 min. Examination of the time-course of nomifensine effects revealed that, similar to methylphenidate, DA uptake inhibition did not return to baseline levels for the duration of the experiment (Zahniser et al., 1999).
High affinity DAT inhibitors
To examine the onset of DA uptake inhibition following high affinity DAT inhibitors, electrically-evoked DA release and uptake were measured in the NAc core of rats that received a 2 sec, i.v. bolus of GBR-12909 (1.5 mg/kg n=7), PTT (0.5 mg/kg n=5), or WF23 (0.5 mg/kg n=5).
GBR-12909
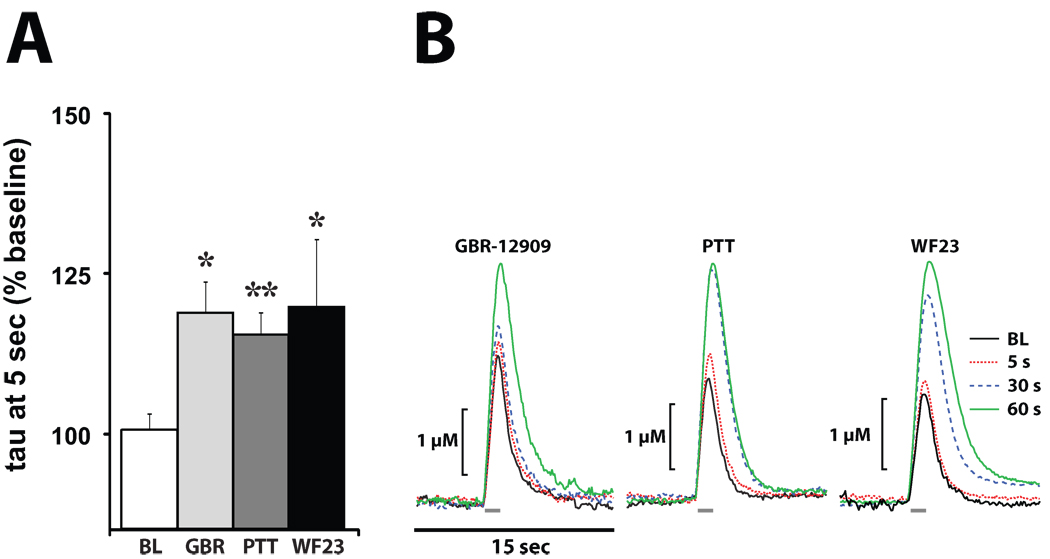
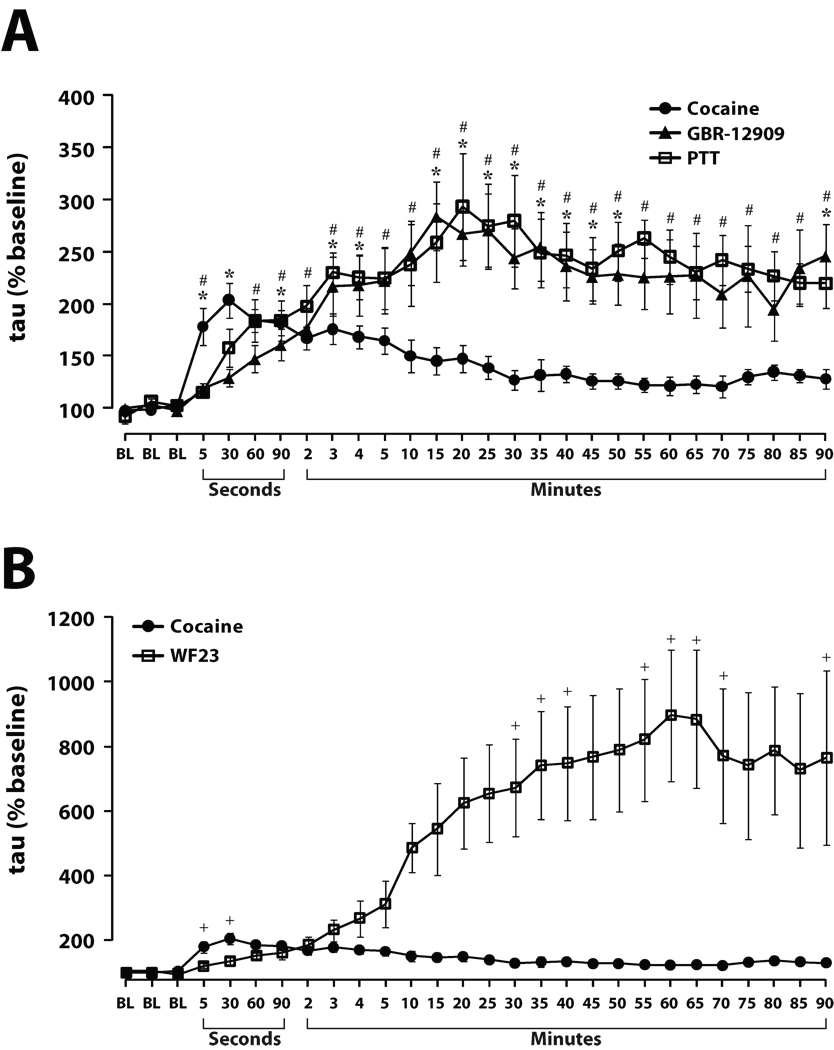
As shown in figures 3 and 4, relative to baseline DA signaling, GBR-12909 inhibited DA uptake 5 sec following injection (F(1,6) = 7.0, P < 0.05). Unlike methylphenidate and nomifensine, the effects of GBR-12909 were significantly less robust at this early time point when compared to cocaine (F(1,14) = 10.6, P < 0.01), however, by the 60 sec time point this difference in uptake inhibition was no longer significant (F(1,14) = 4.3, P = 0.06). Examination of the time course effects of GBR-12909 indicated that DA uptake inhibition did not approach maximal levels until 15 min following injection and remained elevated for the remainder of the experiment.
Figure 3. High affinity DAT inhibitors reduce DA uptake within 5 sec of i.v. injection.
(A) Shown are means ± SEMs for exponential decay constants (tau), expressed as a percent of baseline (BL) following i.v. injections of GBR-12909 (GBR; 1.5 mg/kg), PTT (0.5 mg/kg), and WF23 (0.5 mg/kg). (B) Shown are representative concentration-time traces of DA responses from representative rats following i.v. injections of GBR, PTT, and WF23. Electrical stimulation of the VTA (60 Hz for 1 sec; gray bars) rapidly induced DA release in the NAc. *P<0.05; **P<0.01.
Figure 4. Time course of DA uptake inhibition following i.v. injection of high affinity DAT inhibitors.
Shown are means ± SEMs for exponential decay constants (tau), during the 90 min following i.v. injections of (A) GBR-12909 (1.5 mg/kg) and PTT (0.5 mg/kg), or (B) WF23 (0.5 mg/kg). The effects of cocaine depicted on Figure 2 are shown in both (A) and (B) for comparison. GBR-12909 and PTT significantly inhibited DA uptake within 5 sec, however, unlike the low affinity DAT inhibitors, maximal levels of inhibition were observed in approximately 15–20 min. WF23 also inhibited DA uptake 5 sec after injection, and maximal levels of uptake inhibition were reached after approximately 60 min of injection. Note that the x-axis is divided into second (5–90 sec) and minute (2–90 min) intervals. *P<0.05 for the GBR-12909 time points relative to pre-GBR-12909 baseline conditions. #P<0.05 for the PTT time points relative to pre-PTT baseline conditions. +P<0.05 for the WF23 time points relative to pre-WF23 baseline conditions.
PTT
PTT significantly inhibited DA uptake (F(1,5) = 28.0, P < 0.01) 5 sec after injection (Figs. 3 and 4), and similar to GBR-12909, the effects were significantly less robust at this early time point when compared to cocaine (F(1,12) = 7.8, P < 0.05). At the 30 sec time point this difference in uptake inhibition was no longer significant (F(1,12) = 3.3, P = 0.1). Examination of the time-course effects of PTT indicated that DA uptake inhibition did not reach maximal levels until 20 min and remained elevated for the remainder of the experiment.
WF23
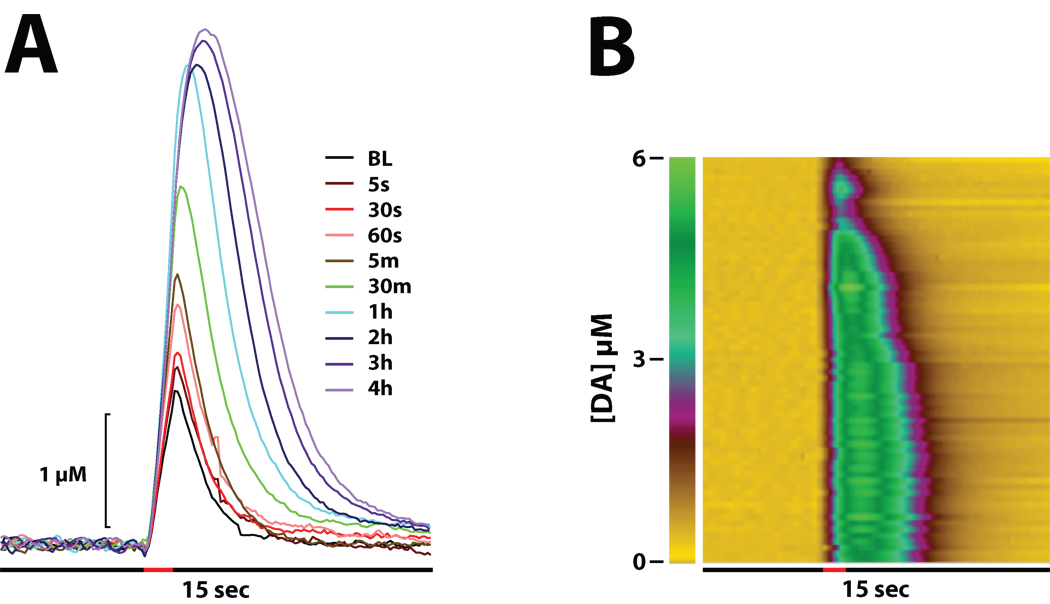
As shown in figures 3–5, WF23 significantly inhibited DA uptake (F(1,5) = 6.8, P < 0.05) 5 sec after injection. Similar to GBR-12909 and PTT, the effects of WF23 were significantly less robust at this early time point compared to cocaine (F(1,13) = 7.5, P < 0.05), however, by the 60 sec time point this difference in uptake inhibition was no longer significant (F(1,13) = 3.7, P = 0.08). Examination of the time course of WF23 effects indicated that DA uptake inhibition did not approach maximal levels until approximately 60 min following injection and remained elevated for over 4 hrs post injection. Figure 5 shows representative data from one experiment showing the effects of WF23 over a 4 hr period.
Figure 5. Time course of DA overflow curves following i.v. injection of WF23.
Shown are representative concentration-time traces (A) and a sequential concentration-trace colormap (B) for DA responses from a representative rat following i.v. injections of WF23 (0.5 mg/kg) over a 4 hr period. Electrical stimulation of the VTA (60 Hz for 1 sec) is denoted by the red bars. Representative concentration traces are shown for collections taken at (A) baseline (BL), and various timepoints (seconds, minutes, and hours) following injection. (B) The colormap shows individual DA concentration traces plotted along the y-axis for collections made at 5, 30, 60, 90 sec, 2, 3, 4, and 5 min and every 5 min thereafter for the 4 hr period depicted in (A). The x-axis denotes time, while the z-axis represents DA concentrations as a pseudocolor.
DISCUSSION
The current studies demonstrate that both low and high affinity DAT blockers inhibit DA uptake within 5 sec of injection. For cocaine, maximal levels of DA uptake inhibition were observed within 30 sec and returned to baseline levels in approximately 1 hr. This effect of cocaine on DA uptake followed a similar time course to that reported previously (Mateo et al., 2004; España et al., 2008). For methylphenidate and nomifensine, peak uptake inhibition also occurred within 30 sec of injection, however, the effects of these drugs on DA uptake were longer lasting, likely reflecting the slower clearance of these agents (Volkow et al., 1995; Zahniser et al., 1999).
Similar to the low affinity DAT inhibitors, the high affinity drugs, GBR-12909, PTT, and WF23 also produced significant uptake inhibition within 5 sec of i.v. injection. Interestingly, however, the initial effects of these agents were significantly less robust when compared to cocaine. Additionally, the peak effects following high affinity inhibitors were protracted. For example, GBR-12909 and PTT maximally inhibited DA uptake between 15 and 20 min post injection, while DAT inhibition by WF23 reached maximal levels 1 hr after injection.
The current results are consistent with previous work indicating rapid central DA uptake inhibitory actions of cocaine. For example, our previous voltammetry observations show robust DA uptake inhibition in NAc within 5 sec of various doses of i.v. cocaine (Mateo et al., 2004; España et al., 2008) and that these effects are related to central, and not to secondary, peripheral actions of this drug (España et al., 2008). Similar to the effects observed following cocaine, we also observed significant DA uptake inhibition following multiple doses of i.v. GBR-12909 within 5 sec of administration, indicating that it is not a unique property of cocaine to enter the brain and inhibit uptake quickly. Importantly, the initial effects of cocaine on DAT inhibition occur on a similar timescale as the most rapid behavioral effects of cocaine in rats (Kiyatkin et al., 2000; Mateo et al., 2004).
The present observations provide additional evidence that both low affinity, short acting and high affinity, long acting DAT inhibitors produced rapid DA uptake inhibition when administered i.v. despite widely different temporal profiles for maximal effects. Based on these observations we speculate that the initial timing of DA uptake inhibition is dictated by route of administration and speed of delivery (Henningfield and Keenan, 1993; Samaha et al., 2002; Samaha and Robinson, 2005). In contrast, the timing of maximal effects may be regulated by pharmacokinetic factors such as affinity for the DAT, lipophilicity, and transport across the blood-brain barrier (Stathis et al., 1995; Xu et al., 1997).
A limited number of studies have investigated the time course of cocaine effects and suggested a potential mismatch between the rapid onset of cocaine’s actions on behavior and the putatively slower effects on DA uptake parameters. For example, microdialysis studies show that cocaine increases DA levels within minutes of i.v. injection (Pettit and Justice, Jr., 1989; Wise et al., 1995; Hemby et al., 1997; Ahmed et al., 2003), while PET imaging studies demonstrate measurable DAT occupancy at the earliest time point sampled (1 min) following i.v. cocaine (Fowler et al., 1998). Additionally, in a rat amperometry study, i.v. cocaine was shown to inhibit DA uptake within 20 sec of injection (Samaha et al., 2004). Similar timing of effects has also been shown using electrophysiological studies which suggest that cocaine-induced increases in DA levels and associated changes in neuronal firing rates occur within minutes of i.v. cocaine (Pitts and Marwah, 1987; Einhorn et al., 1988; Batsche et al., 1992; Hinerth et al., 2000; Wise et al., 2008; Wakazono and Kiyatkin, 2008). Although these data are informative, the relatively low temporal resolution of microdialysis and PET techniques limits the ability to measure the rapid changes in DA uptake observed in the current studies. Furthermore, electrophysiological studies do not offer direct evidence for the effects of cocaine and other DAT blockers on DA uptake inhibition. Rather these studies provide information on the time course of the postsynaptic effects of cocaine-induced increases in extracellular DA levels, which reflects the time required for accumulation of extracellular DA, increased binding of DA to receptors, and the activation of second messenger systems that eventually lead to alterations in firing rates.
Several reports suggest that DAT inhibitors with slow onset kinetics and longer durations of action pose a lower abuse liability than DAT inhibitors like cocaine with fast onset/offset kinetics (Busto and Sellers, 1986; Oldendorf, 1992; Volkow et al., 1995; Woolverton and Wang, 2004). This is thought to be related to the slow onset of action, which has been shown to affect the reinforcing properties of, and sensitization to, cocaine and other psychostimulants (Gossop et al., 1992; Henningfield and Keenan, 1993; Gossop et al., 1994; Hatsukami and Fischman, 1996; Kollins et al., 1998; Abreu et al., 2001; Samaha et al., 2002; Liu et al., 2005; Samaha and Robinson, 2005). A slow rate of onset may be achieved with i.p. or oral delivery, or when administered in a time-release formulation, such as GBR-12909 decanoate ester, a slowly released DAT inhibitor (Glowa et al., 1996). However, the present observations indicate that high affinity drugs do not have slow onset when they are delivered intravenously. Thus, the abuse potential of these drugs when administered i.v. may be similar to those of fast acting drugs such as cocaine. Nevertheless, it is important to note that, relative to cocaine, the high affinity inhibitors GBR-12909, PTT, and WF23 produced significantly less robust effects during the 5–30 sec period following injection. Therefore, it is possible that the reduced initial effects observed with high affinity DAT inhibitors could influence the rewarding and/or reinforcing properties of these drugs.
Several observations indicate that high affinity, long acting drugs, including those examined herein, are self-administered via i.v. routes of delivery (Roberts, 1993; Roberts et al., 1999; Woolverton et al., 2001; Lile et al., 2002). For example, in rats, GBR-12909, PTT, and WF23 produce levels of self-administration that are comparable to those observed with cocaine, although the inter-injection interval is much longer for the high-affinity DAT inhibitors (Roberts, 1993; Roberts et al., 1999). Similarly, non-human primates will also self-administer GBR-12909 at levels observed with cocaine, methylphenidate and nomifensine (Bergman et al., 1989; Howell and Byrd, 1991). Interestingly, PTT and WF23 appear to display a different profile of intake than GBR-12909 or the low affinity DAT inhibitors in monkeys. For instance, PTT and WF23 are capable of maintaining reinforced responding in rhesus monkeys with a history of cocaine intake, but not in cocaine naïve animals (Lile et al., 2002). The mechanisms underlying the differential reinforcing effects of PTT and WF23 observed across cocaine-experienced or cocaine-naïve monkeys are unknown. Nevertheless, it is possible that previous exposure to cocaine produces physiological changes that alter the responsivity of DA systems to these high affinity DAT inhibitors and ultimately alters their reinforcing efficacy. Cocaine-induced plasticity of this type has recently been demonstrated using voltammetry techniques. In those studies, Ferris and colleagues demonstrated that a history of binge cocaine intake produces long lasting changes in DAT function that lead to reduced DA responses to cocaine (Ferris et al., 2011). Whether these changes in DAT sensitivity are responsible for the differential responses to PTT and WF23 between cocaine naïve and cocaine experienced animals remains to be determined.
Conclusion
The euphoric effects of cocaine in humans can occur within seconds of administration (Seecof and Tennant, Jr., 1986; Evans et al., 1996; Zernig et al., 2003). Although it cannot be determined explicitly that there is a direct relationship between alterations in DAT inhibition and the rewarding effects of cocaine, the present observations demonstrate that the initial effects of both low and high affinity DAT inhibitors occur on a similar time scale as the rapid subjective effects of cocaine.
Acknowledgments
We would like to thank Dr. Huw M. L. Davies from Emory University for his generous gift of PTT and WF23. These studies were funded by grant R01 DA021325 and DA030161 (SRJ), T32AA007565 (JTY), and K01 DA025279 (RAE).
Glossary
- DA
Dopamine
- NAc
Nucleus accumbens
- DAT
Dopamine transporter
- i.v.
Intravenous
- VTA
Ventral tegmental area
- GBR-12909
1-(2-bis(4-fluorphenyl)-methoxy)-ethyl)-4-(3-phenyl-propyl)piperazine
- PTT
2β-propanoyl-3β-(4-tolyl)-tropane
- WF23
2β-propanoyl-3β-(2-naphthyl)-tropane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Batsche K, Granoff MI, Wang RY. 5-HT3 receptor antagonists fail to block the suppressant effect of cocaine on the firing rate of A10 dopamine neurons in the rat. Brain Res. 1992;592:273–277. doi: 10.1016/0006-8993(92)91685-8. [DOI] [PubMed] [Google Scholar]
- Bennett BA, Wichems CH, Hollingsworth CK, Davies HM, Thornley C, Sexton T, Childers SR. Novel 2-substituted cocaine analogs: uptake and ligand binding studies at dopamine, serotonin and norepinephrine transport sites in the rat brain. J Pharmacol Exp Ther. 1995;272:1176–1186. [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Busto U, Sellers EM. Pharmacokinetic determinants of drug abuse and dependence. A conceptual perspective. Clin Pharmacokinet. 1986;11:144–153. doi: 10.2165/00003088-198611020-00004. [DOI] [PubMed] [Google Scholar]
- Dutta AK, Coffey LL, Reith ME. Highly selective, novel analogs of 4-[2-(diphenylmethoxy)ethyl]- 1-benzylpiperidine for the dopamine transporter: effect of different aromatic substitutions on their affinity and selectivity. J Med Chem. 1997;40:35–43. doi: 10.1021/jm960638e. [DOI] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010a;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Roberts DCS, Jones SR. Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience. 2008;155:250–257. doi: 10.1016/j.neuroscience.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DCS, Jones SR. Hypocretin 1 / Orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2010b;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Cone EJ, Henningfield JE. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J Pharmacol Exp Ther. 1996;279:1345–1356. [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DC, Jones SR. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry. 2011;69:201–207. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding YS, Wang GJ. Measuring dopamine transporter occupancy by cocaine in vivo: radiotracer considerations. Synapse. 1998;28:111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Froimowitz M, Gu Y, Dakin LA, Nagafuji PM, Kelley CJ, Parrish D, Deschamps JR, Janowsky A. Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter. J Med Chem. 2007;50:219–232. doi: 10.1021/jm0608614. [DOI] [PubMed] [Google Scholar]
- Froimowitz M, Wu KM, Moussa A, Haidar RM, Jurayj J, George C, Gardner EL. Slow-onset, long-duration 3-(3',4'-dichlorophenyl)-1-indanamine monoamine reuptake blockers as potential medications to treat cocaine abuse. J Med Chem. 2000;43:4981–4992. doi: 10.1021/jm000201d. [DOI] [PubMed] [Google Scholar]
- Gardner EL, Liu X, Paredes W, Giordano A, Spector J, Lepore M, Wu KM, Froimowitz M. A slow-onset, long-duration indanamine monoamine reuptake inhibitor as a potential maintenance pharmacotherapy for psychostimulant abuse: effects in laboratory rat models relating to addiction. Neuropharmacology. 2006;51:993–1003. doi: 10.1016/j.neuropharm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Pan D, Chen R, Chaturvedi G, Ding YS. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996;58:231–239. doi: 10.1016/0024-3205(96)00052-5. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Fantegrossi WE, Lewis DB, Matecka D, Rice KC, Rothman RB. Sustained decrease in cocaine-maintained responding in rhesus monkeys with 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-hydroxy-3-phenylpropyl) piperazinyl decanoate, a long-acting ester derivative of GBR 12909. J Med Chem. 1996;39:4689–4691. doi: 10.1021/jm960551t. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Cocaine: patterns of use, route of administration, and severity of dependence. Br J Psychiatry. 1994;164:660–664. doi: 10.1192/bjp.164.5.660. [DOI] [PubMed] [Google Scholar]
- Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA. 1996;276:1580–1588. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hinerth MA, Collins HA, Baniecki M, Hanson RN, Waszczak BL. Novel in vivo electrophysiological assay for the effects of cocaine and putative "cocaine antagonists" on dopamine transporter activity of substantia nigra and ventral tegmental area dopamine neurons. Synapse. 2000;38:305–312. doi: 10.1002/1098-2396(20001201)38:3<305::AID-SYN9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1991;258:178–185. [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J Neurochem. 1995;64:2581–2589. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Terry P. Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology (Berl) 2000;148:90–98. doi: 10.1007/s002130050029. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kiyatkin DE, Rebec GV. Phasic inhibition of dopamine uptake in nucleus accumbens induced by intravenous cocaine in freely behaving rats. Neuroscience. 2000;98:729–741. doi: 10.1016/s0306-4522(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR, Pazzaglia PJ, Ali JA. Comparison of acute behavioral effects of sustained-release and immediate-release methylphenidate. Exp Clin Psychopharmacol. 1998;6:367–374. doi: 10.1037//1064-1297.6.4.367. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, Cocaine, and Fencamfamine - Relationship Between Locomotor and Stereotypy Response Profiles and Caudate and Accumbens-Dopamine Dynamics. Journal of Neuroscience. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Birmingham AM, Wang Z, Woolverton WL, Davies HM, Nader MA. The reinforcing efficacy of the dopamine reuptake inhibitor 2beta-propanoyl-3beta-(4-tolyl)-tropane (PTT) as measured by a progressive-ratio schedule and a choice procedure in rhesus monkeys. J Pharmacol Exp Ther. 2002;303:640–648. doi: 10.1124/jpet.102.039180. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Budygin EA, Morgan D, Roberts DCS, Jones SR. Fast onset of dopamine uptake inhibition by intravenous cocaine. Eur J Neurosci. 2004;20:2838–2842. doi: 10.1111/j.1460-9568.2004.03736.x. [DOI] [PubMed] [Google Scholar]
- Newman AH, Kulkarni S. Probes for the dopamine transporter: new leads toward a cocaine-abuse therapeutic--A focus on analogues of benztropine and rimcazole. Med Res Rev. 2002;22:429–464. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH. Some relationships between addiction and drug delivery to the brain. NIDA Res Monogr. 1992;120:13–25. [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pitts DK, Marwah J. Cocaine modulation of central monoaminergic neurotransmission. Pharmacol Biochem Behav. 1987;26:453–461. doi: 10.1016/0091-3057(87)90147-x. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DCS. Self-administration of GBR 12909 on a fixed ratio and progressive ratio schedule in rats. Psychopharmacology (Berl) 1993;111:202–206. doi: 10.1007/BF02245524. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. Self-administration of cocaine analogs by rats. Psychopharmacology (Berl) 1999;144:389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008;75:2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon SP, Carroll FI. Dopamine transporter ligands: recent developments and therapeutic potential. Curr Top Med Chem. 2006;6:1825–1843. doi: 10.2174/156802606778249775. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Seecof R, Tennant FS., Jr Subjective perceptions to the intravenous "rush" of heroin and cocaine in opioid addicts. Am J Drug Alcohol Abuse. 1986;12:79–87. doi: 10.3109/00952998609083744. [DOI] [PubMed] [Google Scholar]
- Stathis M, Scheffel U, Lever SZ, Boja JW, Carroll FI, Kuhar MJ. Rate of binding of various inhibitors at the dopamine transporter in vivo. Psychopharmacology (Berl) 1995;119:376–384. doi: 10.1007/BF02245852. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, Dewey S, Ashby C, Liebermann J, Hitzemann R. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Wakazono Y, Kiyatkin EA. Electrophysiological evaluation of the time-course of dopamine uptake inhibition induced by intravenous cocaine at a reinforcing dose. Neuroscience. 2008;151:824–835. doi: 10.1016/j.neuroscience.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS ONE. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Hecht GS, Agoston GE, Katz JL, Newman AH. Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology (Berl) 2001;154:375–382. doi: 10.1007/s002130000616. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johnson KM. Neurobiology of cocaine abuse. Trends Pharmacol Sci. 1992;13:193–200. doi: 10.1016/0165-6147(92)90063-c. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Xu L, Kelkar SV, Lomenzo SA, Izenwasser S, Katz JL, Kline RH, Trudell ML. Synthesis, dopamine transporter affinity, dopamine uptake inhibition, and locomotor stimulant activity of 2-substituted 3 beta-phenyltropane derivatives. J Med Chem. 1997;40:858–863. doi: 10.1021/jm960739c. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. J Neurosci Methods. 2011. Demon voltammetry and analysis software: analysis of cocaine-dopamine transporter interactions using several kinetic measurements. doi:10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther. 1999;289:266–277. [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: drug users' self-reports versus researchers' perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]