Abstract
My laboratory has been interested for some time in the influence of iron, a nutrient that is essential for both microbial pathogens and their mammalian hosts, on the course of infectious disease. Our studies indicate that alterations in the expression of host molecules that sequester or transport iron can have direct effects on pathogen growth and can also have an impact on the ability to mount normal immune responses. We have elucidated the mechanistic basis for some of these observations, and have started to apply our findings in strategies to control abnormalities of inflammation and iron metabolism. I will review here what we have learned about the interactions between iron and immunity and discuss the implications of the information that we have acquired.
Keywords: Iron metabolism, Infection, Inflammation, Macrophage, Siderophore
Introduction
Iron is required by most organisms as an essential cofactor in many important biological processes [1]. However, iron can be toxic to cells when present at high concentrations because of its ability to promote the formation of damaging oxidative radicals. Since both iron deficiency and iron excess can compromise cellular function, the levels of iron that cells are exposed to have to be regulated precisely. The molecular mechanisms involved in the regulation of iron homeostasis have become increasingly clear as a result of work carried out in several laboratories over the last few years [2, 3]. I will start by describing these mechanisms since they provide the conceptual framework for our investigations into the role of iron in host–pathogen interactions.
Regulation of iron metabolism
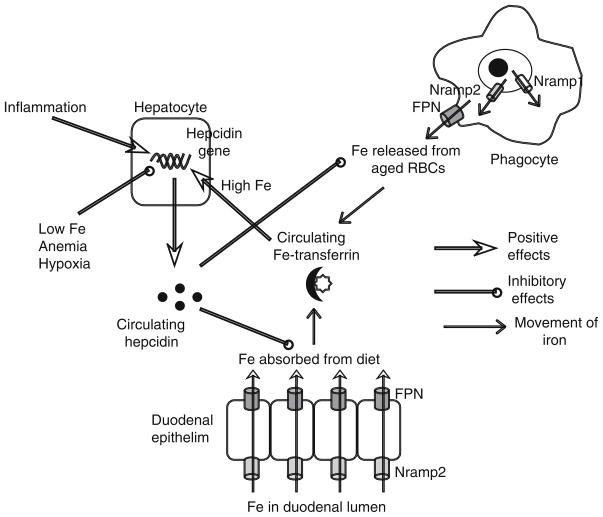
In mammals, the major portion of the iron entering the circulation, amounting to about 24 mg per day, is recycled from aged red blood cells (RBCs) that are destroyed by the macrophages of the reticuloendothelial system. Iron released from the breakdown of RBC hemoglobin is transported from the lumen of the phagosome into the cytosol by the transporter Nramp2, with the related transporter Nramp1 also probably contributing to this process [3–6] (Fig. 1). Some of the iron is utilized by the cell, excess is stored in association with ferritin, and the rest is pumped out of the cell by the exporter ferroportin (FPN). A small amount of iron is also absorbed from the diet—1–2 mg per day. The absorption occurs mainly in the duodenum, where Nramp2 and FPN mediate the movement of iron across the apical and basolateral surfaces, respectively, of the epithelial cell. Iron that is effluxed by macrophages or enterocytes is transported in association with transferrin and is endocytosed into cells following binding to the ubiquitously expressed type I transferrin receptor (TfR1). The internalized iron is released in the acidic environment of endolysosomal compartments and is transported into the cytosol via Nramp2.
Fig. 1.
Systemic iron metabolism in mammals. Hepcidin is the key regulator of iron homeostasis. It controls the amount of iron released into the serum by phagocytes and duodenal enterocytes by modulating FPN expression on these cells. Expression of hepcidin by hepatocytes is regulated by circulating iron-transferrin levels, by iron requirements, and by inflammation
Homeostatic maintenance of serum iron levels involves the sensing of serum iron-transferrin complexes followed by the activation of regulatory mechanisms that appropriately adjust the amount of iron entering the circulation. The liver plays a key role in this process. The iron sensing function is carried out by a hepatocyte surface protein called HFE, which associates with TfR1 [7]. When serum iron levels are high, the binding of iron-transferrin to TfR1 displaces HFE, which then binds to the related, hepatocyte-specific protein, TfR2 [8–10]. The HFE–TfR2 interaction activates signals that function together with those induced by bone morphogenetic protein 6 (BMP6) to transcriptionally up-regulate the expression of a secreted peptide known as hepcidin [11–13]. Hepcidin binds to FPN, inducing its internalization and lysosomal degradation and thus reducing the amount of iron effluxed from duodenal enterocytes and macrophages [14–16]. In addition to being up-regulated by elevated serum iron levels, hepcidin expression is also induced by inflammatory cytokines such as IL-6 [17–19]. Conversely, conditions in which the requirement for iron increases, such as iron deficiency, anemia, and hypoxia, are associated with decreased expression of hepcidin, a corresponding elevation of FPN expression, and increased movement of iron into the circulation [13]. Thus, the HFE-hepcidin-FPN axis plays a central role in ensuring that release of iron from RBC destruction and absorption of iron from the gut are regulated in response to systemic iron status and demands.
Intracellular free iron concentrations are influenced by systemic levels of the element, but are also regulated by alterations in the expression of proteins that transport or store iron. Most of the iron that enters the cell is brought in by TfR1 and Nramp2, while the iron that is not immediately utilized or exported via FPN is stored in high molecular weight complexes with ferritin. The expression of TfR1, Nramp2, FPN and ferritin is modulated in response to changes in intracellular iron via iron response elements (IREs) present in the 5′or 3′untranslated regions of the corresponding mRNAs [20]. The IREs are bound by iron regulatory proteins (IRPs) when intracellular free iron levels are low. The binding of IRPs to the IREs in the 3′untranslated regions of the TfR1 and Nramp2 mRNAs has the effect of stabilizing the transcripts, increasing expression of the proteins and facilitating influx of iron. On the other hand, the binding of IRPs to the IREs in the 5′untranslated regions of ferritin and FPN mRNAs inhibits their translation, decreasing expression of the proteins, and preventing storage and export of iron, respectively. The IRE-IRP system thus senses changes in intracellular iron and brings about coordinated alterations in iron import, efflux, and storage that maintain homeostasis.
Ferroportin and intracellular pathogen growth
As alluded to earlier, iron is essential for the survival and growth of almost all organisms. Furthermore, an important strategy of mammalian antimicrobial defense is based on depriving pathogens of this essential nutrient [21]. One of the best-studied examples of this strategy is Nramp1, the transporter that pumps iron out of the phagosome [22, 23]. Numerous experiments in mouse models and in tissue culture have shown that the ability of Nramp1 to lower phagosomal iron concentrations influences the survival and growth of several intracellular pathogens, including Myocbacterium bovis BCG, Salmonella typhimurium, and Leishmania donovani. Polymorphisms in the NRAMP1 gene have also been linked in some human studies to altered susceptibility to tuberculosis and leprosy [24]. Based on the idea that there may be additional mechanisms that deprive intracellular pathogens of iron, we initiated investigations some years ago to examine the effects of altered FPN expression on the intracellular growth of S. typhimurium. We found that elevation of FPN levels in either HeLa cells or J774 murine macrophages led to a significant inhibition of intracellular Salmonella growth, whereas hepcidin-induced down-regulation of FPN expression had the opposite effect [25]. Using Salmonella strains transformed with iron-regulated transcriptional reporters, we showed that elevated FPN levels were associated with the increased expression of a bacterial gene that is induced by low iron concentrations, suggesting that FPN-mediated iron efflux led ultimately to depletion of the element in the immediate microenvironment of the pathogen. Since these original observations, several other groups have replicated our findings, both with Salmonella and with several other intracellular bacterial pathogens, including M. tuberculosis, Chlamydia psittaci, C. trachomatis, and Legionella pneumophila [26–28]. Studies have also shown that activation of macrophages, either by bacterial infection or by interferon γ, is associated with transcriptional up-regulation of FPN, suggesting that FPN-mediated iron efflux may be an integral part of the phagocyte antimicrobial repertoire [27, 29]. It is important to note, however, that lipopolysaccharide (LPS)-induced hepcidin expression in macrophages may counteract some of the effects of increased transcription of the FPN gene [30–32].
The results of the investigations detailed above suggest that changes in FPN expression or function can influence the growth of pathogens inside macrophages by altering iron availability. They also raise the possibility that variations in macrophage FPN levels, which are seen in inflammatory states and in inherited and acquired disorders of iron metabolism [3, 13], may contribute to the altered susceptibility to infection that is often associated with such conditions [33, 34].
Ferroportin and the inflammatory response
In an extension of our tissue culture studies, we analyzed the effects of altered macrophage FPN expression on the course of S. typhimurium infection in vivo using Hfe knockout mice. In these animals, as in humans with the most common inherited disorder of iron metabolism (type I hemochromatosis), the absence of a functional Hfe protein results in a failure of normal iron sensing, leading to low hepcidin levels, elevated FPN expression on macrophages and the intestinal epithelium, and the development of a progressive systemic iron overload syndrome [7, 13, 35, 36]. We expected to find that the increased levels of macrophage FPN would inhibit the growth of Salmonella in these cells, and, consequently, that the mutant mice would be relatively resistant to the infection. Surprisingly, the results of our experiments indicated that the Hfe-deficient mice had higher numbers of Salmonella in the gut and in systemic tissues [37]. A potential explanation for this unexpected finding was provided by our observation that the knockout animals had a significantly attenuated intestinal inflammatory response to the bacteria. Thus, we reasoned that less robust inflammation would lead to less efficient clearance of the pathogen. At about the same time, other researchers reported that Hfe knockout mice were more susceptible to M. avium infection, although they did not characterize the inflammatory responses in the mutant animals [38]. On further investigation, we found that peritoneal macrophages from the Hfe-deficient mice produced lower levels of pro-inflammatory cytokines such as TNFα and IL-6 than wild-type cells when infected with Salmonella or stimulated with LPS in vitro. In a series of biochemical experiments that analyzed cytokine protein and mRNA levels, and the association of cytokine transcripts with polyribosomes, we showed that the abnormality of TNFα and IL-6 expression in the Hfe-deficient macrophages was at the level of mRNA translation. Moreover, we found that the abnormality could be corrected by iron loading or hepcidin treatment of the mutant macrophages, and could be reproduced in wild-type cells by iron chelation, suggesting that it was the low intracellular iron levels in the former (caused by their elevated FPN expression) that was responsible for the impaired cytokine biosynthesis. Recently, our collaborators demonstrated similar effects of elevated macrophage FPN levels on the translation of inducible nitric oxide synthase (iNOS), indicating that decreased intracellular iron compromised the translation of several, although not all, transcripts involved in inflammation and antimicrobial defense [37, 39].
We continued our experiments with the Hfe knockout macrophages in order to characterize the effects of decreased intracellular iron on TNFα expression more precisely. By analyzing the responses to a number of different Toll-like receptor (TLR) ligands, we were able to determine that the Hfe deficiency only affected responses to TLR4, the major receptor for LPS, whereas responses to TLR2 and TLR3 were intact [40]. Furthermore, only a subset of TLR4-activated responses were impaired in the mutant macrophages, viz., those that depended on the TRAM and TRIF adaptor proteins that are recruited to the TLR4 cytoplasmic domain in response to LPS stimulation [41, 42]. Responses dependent on another pair of adaptors used by TLR4—Mal and MyD88—were found to be intact in the Hfe-deficient cells. Based on the pattern of abnormal responses we uncovered, and on our observations that surface TLR4 expression, as well as TRAM and TRIF mRNA levels, were normal in the knockout macrophages, we now think that the effect of Hfe deficiency and low intracellular iron is at one of the early steps of signal transduction—TLR4 endocytosis or interactions between TLR4 and TRAM or between TRAM and TRIF—required to connect TLR4 selectively to the TRAM/TRIF pathway [43]. We are currently trying to elucidate the exact mechanism involved in the iron-dependent impairment of TLR4 signaling in the Hfe knockout macrophages.
Our investigations have shed light on the abnormalities of innate immunity caused by disordered iron metabolism. Such abnormalities may be an important factor that influences the course of infection in situations where hepcidin and FPN levels are altered. Our studies have also revealed a previously unappreciated role for TRAM- and TRIF-dependent signals in the regulation of cytokine translation. Ongoing experiments are directed at clarifying exactly how the TRAM/TRIF pathway influences the translation of TNFα and IL-6.
Ferroportin and infectious disease
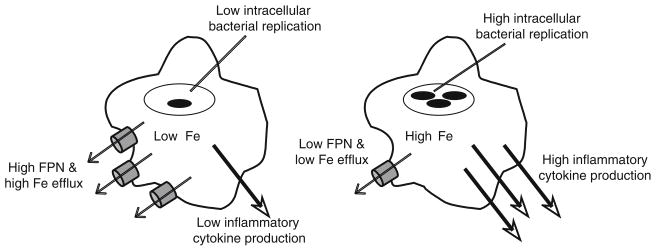
Taken together, the studies described in the preceding sections indicate that FPN can influence the course of infection in 2 ways—a direct effect on bacterial growth resulting from iron deprivation, and an indirect effect secondary to the attenuated pathogen-induced inflammatory response (Fig. 2). In vivo, the latter appears to predominate so that the net outcome is poor control of bacterial multiplication in the Hfe-deficient mice. It remains to be seen whether these observations are generalizable to other pathogens. It is possible that in some cases, the direct effects of FPN on bacterial growth may outweigh the effects on the host response. It is also important to mention that not all investigators have observed the influence of Hfe deficiency on inflammation that we have described. In contrast to our data, Nairz et al. found that Hfe knockout mice were relatively resistant to Salmonella infection in vivo. Macrophages from their mutant animals inhibited the intracellular growth of Salmonella better than wild-type cells because the higher levels of FPN associated with Hfe deficiency impaired iron acquisition by the pathogen [44]. These investigators did not observe any abnormalities of the inflammatory response in the knockout animals, either in vivo or in isolated macrophages. Their experiments are thus indicative of an exclusive effect of FPN on intracellular pathogen growth. We do not yet have a clear explanation for the discordance between the results of Nairz et al. and our own findings. There are a number of potential contributory factors, including differences in the route of in vivo infection (oral in our study, intraperitoneal in theirs), duration of in vitro infection (2–6 h in our experiments, 24 h in theirs), and the nature of the knockout (deletion of exon 4 and part of exon 3 in the mice used in our studies, and deletion of exons 2 and 3 in the mice used by Nairz et al.) [44–46]. To add to the confusion, a third study by De Domenico et al. recently showed that the binding of hepcidin to FPN activated signals that suppressed LPS-induced TNFα and IL-6 mRNA and protein expression and that hepcidin-deficient mice had exaggerated inflammatory responses following injection of LPS [47]. Based on those results, Hfe-deficient mice, which have abnormally low levels of hepcidin, would be expected to have enhanced, not attenuated, inflammatory responses. Clearly, further work will be required to resolve these conflicting findings and to shed light on the interactions between the HFE-hepcidin-FPN system and the inflammatory response.
Fig. 2.
Effects of macrophage FPN levels on intracellular bacterial growth and inflammatory cytokine expression. The diagram on the left depicts the effects of high FPN levels–low intracellular iron, with consequent inhibition of bacterial growth and decreased inflammatory cytokine production. The effects of low FPN are shown on the right–high intracellular iron, with consequent promotion of bacterial growth and increased inflammatory cytokine production
Do our findings have relevance for infectious disease in humans? Patients with type I (HFE mutation-associated) hemochromatosis are known to be susceptible to certain systemic bacterial infections, especially Vibrio vulnificus septicemia and hepatic abscesses caused by Yersinia enterocolitica [33, 34, 48, 49]. Some of this susceptibility may be explained by the effects of elevated serum iron levels on microbial growth, an idea that is supported by experimental evidence in the case of V. vulnificus [48, 50]. However, our data from the Hfe knockout mice raise the possibility that impaired inflammatory responses, with consequent defects in bacterial clearance, may also be important contributing factors. In keeping with this idea, peripheral blood monocytes from individuals with hemochromatosis have been found to secrete reduced amounts of TNFα in response to LPS [51]. Another type of iron overload syndrome, sometimes referred to as type IV hemochromatosis, is particularly common in African and African-American populations and is associated with dominant inactivating mutations in the FPN gene [35, 52–54]. Individuals with this disorder have an increased risk of tuberculosis [55], which might be explained by the observations, made by our group as well as by others, that decreased expression or function of FPN in macrophages favors the growth of bacterial pathogens in these cells because of intracellular accumulation of iron [25, 28].
Inflammation and iron homeostasis
While we have been working to elucidate the abnormalities associated with Hfe deficiency, we have also been trying to develop a practical application of our findings. Because of our long-standing interest in inflammatory bowel disease (IBD), we have focused on iron metabolism in this condition. Like several other chronic inflammatory disorders, IBD is often associated with an iron deficiency anemia that is refractory to oral iron supplements [56, 57]. The pathogenesis of this so-called anemia of chronic disease (ACD) is now known to involve inflammation-induced up-regulation of hepcidin expression, with consequent down-regulation of FPN, iron sequestration in macrophages and enterocytes, and decreased availability of iron for erythropoiesis [17–19, 58, 59]. There are very few investigations into the role of hepcidin in IBD, but one small study has demonstrated increased urinary hepcidin levels in active Crohn’s disease, correlating with elevated serum IL-6, and decreased intestinal iron absorption [60]. These observations are consistent with our own data showing that hepcidin expression is significantly increased in murine models of IBD [40]. Since our work with the Hfe knockout mice indicated that hepcidin contributed to the inflammatory process by raising intra-macrophage iron concentrations and that low hepcidin levels were associated with attenuated inflammatory responses [37, 40, 61], we reasoned that inhibiting hepcidin expression in IBD could have beneficial effects for both the intestinal inflammation and ACD by allowing FPN levels to rise. In order to test this idea, we used 2 structurally and mechanistically distinct inhibitors of BMP-induced hepcidin expression identified by our collaborators—the small organic compound dorsomorphin, which blocks signals activated by multiple BMPs, and the soluble recombinant fusion protein HJV.Fc, which prevents the binding of BMPs 2,4 and 6 to their receptor and coreceptor [62, 63]. To analyze the effects of these reagents, we used the piroxicam/IL-10 knockout murine colitis model of IBD [64]. We found that treatment of the colitic mice with either one of the inhibitors led to the expected decrease in hepcidin expression, and more importantly, to a significant reduction in the severity of intestinal inflammation and an elevation of serum iron levels [40]. These results bolster the idea that hepcidin-induced intracellular iron sequestration contributes not only to the pathogenesis of ACD but also to the inflammatory process. They also suggest that a therapeutic strategy based on inhibition of hepcidin expression could be beneficial in the management of human IBD. We are currently conducting further experiments to evaluate this strategy using additional models of IBD and more specific inhibitors of hepcidin.
Iron sequestration and antimicrobial defense
Since iron is essential for the growth and virulence of most microbial pathogens, it should not be surprising that the mammalian immune system has evolved ways to deprive microorganisms of this vital element. I have alluded earlier to the roles played by Nramp1 and FPN in this process. The increased expression of hepcidin that accompanies inflammation is also generally viewed as part of the strategy of pathogen iron deprivation since it leads to a decrease in serum iron levels. In addition, the host produces secreted molecules that directly compete with the infecting microorganism for iron. One of the first molecules of this type to be studied is lactoferrin, an iron-binding glycoprotein that is abundantly expressed in mucosal secretions of the respiratory, gastrointestinal and mammary epithelia [65]. It is also a component of neutrophil granules and is up-regulated at sites of infection. While exogenously administered lactoferrin has been shown in multiple experiments to inhibit the growth of microorganisms in vitro and in animal models, its in vivo physiologic role is much less clear since lactoferrin-deficient mice do not show any abnormalities in the responses to several bacterial pathogens [33, 66].
Siderocalin (also known as lipocalin 2, neutrophil gelatinase-associated lipocalin, NGAL, and 24p3) is a member of the lipocalin family of secreted proteins that has been shown recently to have antimicrobial functions based on iron deprivation [67]. It is expressed by macrophages, neutrophils, and various epithelial cells and is up-regulated in response to infectious and inflammatory stimuli. A significant advance in understanding the function of siderocalin occurred when structural characterization of the recombinant protein expressed in E. coli revealed that it was bound tightly to a low molecular weight iron-associated molecule of bacterial origin—enterobactin—that functioned as a siderophore or iron scavenger [68]. Many bacteria synthesize and release enterobactin-related siderophores as a way of obtaining iron from their environment, and the ability to produce these molecules is often an important aspect of pathogen virulence [69]. Thus, sequestration of iron-loaded siderophores by siderocalin represents a direct attack on an important microbial iron acquisition mechanism. As would be expected, siderocalin has been shown to inhibit bacterial growth in vitro, and, even more significantly, siderocalin-deficient mice have enhanced susceptibility to infection with pathogenic strains of E. coli and Klebsiella pneumoniae [68, 70–72]. Also, illustrating the importance of siderocalin in antimicrobial defense is the fact that certain bacterial pathogens such as Salmonella have developed a mechanism to evade the action of siderocalin by producing modified enterobactins that are not bound by the protein [73, 74]. Indeed, the increased expression of siderocalin in the gut during Salmonella infection may actually favor colonization by the pathogen by inhibiting growth of the commensal microflora [75].
In addition to its ability to sequester enterobactin-like siderophores, siderocalin has also been shown recently to bind to a different category of siderophores, the carboxymycobactins, produced by M. tuberculosis and other mycobacteria [76]. In keeping with this finding, Saiga et al. demonstrated that siderocalin is involved in inhibiting M. tuberculosis multiplication in vitro and in vivo [77]. In similar studies, we found that the growth of M. tuberculosis, either in bacterial medium or within mouse macrophages, was significantly suppressed by the addition of recombinant siderocalin [78]. Siderocalin may also play a role in protection against tuberculosis in humans. In a study of contacts of patients with active pulmonary tuberculosis, people of African descent were found to be more susceptible to infection, an observation that was correlated with lower circulating neutrophil counts and lower serum siderocalin concentrations [79]. Interestingly, serum siderocalin levels are also low in individuals with human immunodeficiency virus infection, an abnormality that could contribute to decreased resistance to M. tuberculosis [80].
It has generally been assumed that the bacteriostatic action of siderocalin is based on its ability to bind to bacterial siderophores. However, it has been shown very recently that siderocalin can also bind to endogenous mammalian siderophores. Two such siderophores have been identified—the metabolite catechol, and 2,5-dihydroxybenzoic acid (DHBA), both of which are structurally related to enterobactin [81–83]. These discoveries provide support for earlier suggestions that siderocalin may be involved in moving iron into or out of cells by binding a mammalian siderophore [84–87]. They also raise the possibility that one mechanism of siderocalin-mediated bacteriostasis, especially in the case of pathogens that reside within host cells, may involve alterations of intracellular iron levels rather than direct binding of bacterial siderophores. The finding that macrophages from siderocalin-deficient mice have elevated iron content and support the growth of Salmonella better than wild-type cells is consistent with this idea [44].
Iron that is bound to siderophores such as catechol and DHBA is part of the “labile” intracellular pool that is able to catalyze the generation of potentially cytotoxic hydroxyl radicals from oxygen species like peroxide and superoxide [81–83, 88–90]. Infections, inflammatory states, and environmental stresses are associated with the production of multiple reactive oxygen intermediates, and the presence of labile iron under these conditions can promote cell death and tissue damage. By sequestering siderophore-associated iron, siderocalin diminishes the labile iron pool and reduces hydroxyl radical production. Thus, the increase in siderocalin expression that accompanies infection and inflammation can be viewed not only as a component of antimicrobial defense, but also as a strategy to minimize the damage caused by reactive oxygen species. It is worth mentioning in this context that some of the effects of siderocalin deficiency on bacterial infection in vivo [70–72, 77] may in fact be secondary to tissue damage. Given the multiple ways in which siderocalin may influence the course of infection, and since inadequate sequestration of iron has been implicated in the pathogenesis of disorders such as Parkinson’s, Huntington’s, and Alzheimer’s diseases [89, 90], it will be of interest to determine whether abnormalities of siderocalin expression or function are involved in susceptibility to infectious and degenerative diseases in humans. Although a recent pilot study did not find any association between siderocalin gene polymorphisms and susceptibility to tuberculosis in a South African population [91], more comprehensive investigations of this issue are required.
Conclusion
It has long been appreciated that the host’s iron status can have a significant impact on susceptibility to and the course of infectious disease, and conversely, that infection and inflammation can alter iron homeostasis [33, 34, 58, 59]. With the recent advances in knowledge about how iron metabolism is regulated, we now have a better understanding of the molecular basis for this interaction between iron and infection. Work in my laboratory has tried to capitalize on this information in order to elucidate basic aspects of the immune response and to devise novel approaches to correcting abnormalities of immune function or iron metabolism. It is our hope that the results of our experiments in tissue culture and mouse models will lead ultimately to practical applications that will benefit individuals with such abnormalities.
Acknowledgments
Work in the author’s laboratory is supported by grants from the National Institutes of Health (R56AI089700), the Broad Medical Research Program (IBD-0253) and the Crohn’s and Colitis Foundation of America (1754).
References
- 1.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131:568S–80S. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–30. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Biggs TE, Baker ST, Botham MS, et al. Nramp1 modulates iron homeostasis in vivo and in vitro: evidence for a role in cellular iron release involving de-acidification of intracellular vesicles. Eur J Immunol. 2001;31:2060–70. doi: 10.1002/1521-4141(200107)31:7<2060::aid-immu2060>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Soe-Lin S, Sheftel AD, Wasyluk B, et al. Nramp1 equips macrophages for efficient iron recycling. Exp Hematol. 2008;36:929–37. doi: 10.1016/j.exphem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Soe-Lin S, Apte SS, Andriopoulos B, Jr, et al. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc Natl Acad Sci USA. 2009;106:5960–5. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 8.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalin iron sensing. J Biol Chem. 2006;281:28494–8. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt PJ, Toran PT, Giannetti AM, et al. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7:205–14. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Chen J, Kramer M, et al. Interaction of the hereditary hemochromatosis protein, HFE, with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–27. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andriopoulos B, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–7. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meynard D, Kautz L, Darnaud V, et al. Lack of BMP6 induces massive iron overload. Nat Genet. 2009;41:478–81. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:13, 1–4. doi: 10.1146/annurev-med-050109-142444. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 15.De Domenico I, Ward DM, Langelier C, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–78. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Domenico I, Lo E, Ward DM, et al. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA. 2009;106:3800–5. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P, Peng H, Gelbart T, Beutler E. The IL-6 and LPS-induced transcription of hepcidin in Hfe- TfR2-and β2-microglobulin-deficient mice. Proc Natl Acad Sci USA. 2004;101:9263–5. doi: 10.1073/pnas.0403108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee P, Peng H, Gelbart T, et al. Regulation of hepcidin transcription by IL-1 and IL-6. Proc Natl Acad Sci USA. 2005;102:1906–10. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/ iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Cherayil BJ. Ironing out the wrinkles in host defense: interactions between iron homeostasis and innate immunity. J Innate Immun. 2009;1:455–64. doi: 10.1159/000210016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes JR, Gros P. Divalent metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- 23.Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–70. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Moller M, Hoal EG. Current findings, challenges and novel approaches to human genetic susceptibility to tuberculosis. Tuberculosis (Edinb) 2010;90:71–83. doi: 10.1016/j.tube.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Chlosta S, Fishman DS, Harrington L, et al. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun. 2006;74:3065–7. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olakanmi O, Schlesinger LS, Britigan BE. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J Leukoc Biol. 2007;81:195–204. doi: 10.1189/jlb.0606405. [DOI] [PubMed] [Google Scholar]
- 27.Nairz M, Theurl I, Ludwiczek S, et al. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–40. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 28.Paradkar PN, De Domenico I, Durchfort N, et al. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–74. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nairz M, Fritsche G, Brunner P, et al. Interferon γ limits the availability of iron for intra-macrophage Salmonella typhimurium. Eur J Immunol. 2008;38:1923–36. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- 30.Peyssonaux C, Zinkernagel AS, Datta V, et al. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood. 2006;107:3727–32. doi: 10.1182/blood-2005-06-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen NB, Callaghan KD, Ghio AJ, et al. Hepcidin expression and iron transport in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L417–25. doi: 10.1152/ajplung.00484.2005. [DOI] [PubMed] [Google Scholar]
- 32.Theurl I, Theurl M, Seifert M, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111:2392–9. doi: 10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- 33.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–53. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 34.Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–4. doi: 10.1093/jn/137.5.1341. [DOI] [PubMed] [Google Scholar]
- 35.Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139:393–408. doi: 10.1053/j.gastro.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Zhou XY, Tomatsu S, Fleming RE, et al. Hfe gene knock-out produces a mouse model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 1998;95:2492–7. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Johnson EE, Shi HN, et al. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol. 2008;181:2723–31. doi: 10.4049/jimmunol.181.4.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes-Pereira S, Rodrigues PN, Appelberg R, et al. Increased susceptibility to Mycobacterium avium in hemochromatosis protein Hfe-deficient mice. Infect Immun. 2008;76:4713–9. doi: 10.1128/IAI.00612-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson EE, Sandgren A, Cherayil BJ, et al. Role of ferroportin in macrophage-mediated immunity. Infect Immun. 2010;78:5099–106. doi: 10.1128/IAI.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Harrington L, Trebicka E, et al. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest. 2009;119:3322–8. doi: 10.1172/JCI39939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheedy FJ, O’Neill LA. The Troll in Toll: Mal and TRAM as bridges for TLR2 and TLR4 signaling. J Leukoc Biol. 2007;82:196–203. doi: 10.1189/jlb.1206750. [DOI] [PubMed] [Google Scholar]
- 42.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 43.Kagan JC, Su T, Horng T, et al. TRAM couples endocytosis of TLR4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–8. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nairz M, Theurl I, Schroll A, et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009;114:3642–51. doi: 10.1182/blood-2009-05-223354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy JE, Montross LK, Cohen DE, et al. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94:9–11. [PubMed] [Google Scholar]
- 46.Bahram S, Gilfillan S, Kuhn LC, et al. Experimental hemochromatosis due to MHC class I HFE deficiency: immune status and iron metabolism. Proc Natl Acad Sci USA. 1999;96:13312–7. doi: 10.1073/pnas.96.23.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Domenico I, Zhang TY, Branch LW, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120:2395–405. doi: 10.1172/JCI42011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bullen JJ, Spalding PB, Ward CG, et al. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–9. [PubMed] [Google Scholar]
- 49.Bergmann TK, Vinding K, Hey H. Multiple hepatic abscesses due to Yersinia enterocolitica infection secondary to primary hemochromatosis. Scand J Gastroenterol. 2001;36:891–5. doi: 10.1080/003655201750313450. [DOI] [PubMed] [Google Scholar]
- 50.Wright AC, Simpson LM, Oliver JD. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981;34:503–7. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordeuk VR, Ballou S, Lozanski G, et al. Decreased concentrations of TNFα in supernatants of monocytes from homozygotes for hereditary hemochromatosis. Blood. 1992;79:1855–60. [PubMed] [Google Scholar]
- 52.Gordeuk VR, Caleffi A, Corradini E, et al. Iron overload in Africans and African-Americans and a common mutation in the SLC40A1 (ferroportin 1) gene. Blood Cells Mol Dis. 2003;31:299–304. doi: 10.1016/s1079-9796(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 53.Beutler E, Barton JC, Felitti VJ, et al. Ferroportin 1 (SLC40A1) variant associated with iron overload in African-Americans. Blood Cells Mol Dis. 2003;31:305–9. doi: 10.1016/s1079-9796(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 54.McNamara L, Gordeuk VR, MacPhail AP. Ferroportin (Q248H) mutations in African families with dietary iron overload. J Gastroenterol Hepatol. 2005;20:1855–8. doi: 10.1111/j.1440-1746.2005.03930.x. [DOI] [PubMed] [Google Scholar]
- 55.Boelaert JR, Vandecasteele SJ, Appelberg R, et al. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195:1745–53. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- 56.Gomolion F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659–65. doi: 10.3748/wjg.15.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599–610. doi: 10.1038/nrgastro.2010.151. [DOI] [PubMed] [Google Scholar]
- 58.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–93. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–22. doi: 10.1146/annurev.nutr.012809.104804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semrin G, Fishman DS, Bousvaros A, et al. Impaired intestinal iron absorption in Crohn’s disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101–6. doi: 10.1097/01.mib.0000235097.86360.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherayil BJ. Cross-talk between iron homeostasis and intestinal inflammation. Gut Microbes. 2010;1:65–9. doi: 10.4161/gmic.1.1.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babitt JL, Huang FW, Xia Y, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117:1933–9. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berg DJ, Zhang J, Weinstock JV, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–42. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 65.Legrand D, Mazurier J. A critical review of the roles of host lactoferrin in immunity. Biometals. 2010;23:365–76. doi: 10.1007/s10534-010-9297-1. [DOI] [PubMed] [Google Scholar]
- 66.Ward PP, Mendoza-Meneses M, Park PW, et al. Stimulus-dependent impairment of neutrophil oxidative burst response in lactoferrin-deficient mice. Am J Pathol. 2008;172:1019–29. doi: 10.2353/ajpath.2008.061145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clifton MC, Corrent C, Strong RK. Siderocalins: siderophore-binding proteins of the innate immune system. Biometals. 2009;22:557–64. doi: 10.1007/s10534-009-9207-6. [DOI] [PubMed] [Google Scholar]
- 68.Goetz DH, Holmes MA, Borregaard N, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 69.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen contol. Microbiol Mol Biol Rev. 2007;71:413–51. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 71.Berger T, Togawa A, Duncan GS, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103:1834–9. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan YR, Liu JS, Pociask DA, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182:4947–56. doi: 10.4049/jimmunol.0803282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fischbach MA, Lin H, Liu DR, et al. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol. 2006;2:132–8. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 74.Fischbach MA, Lin H, Zhou L, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci USA. 2006;103:16502–7. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raffatellu M, George MD, Akiyama Y, et al. Lipocalin 2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–86. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmes MA, Paulsene W, Jide X, et al. Siderocalin (Lcn2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Saiga H, Nishimura J, Kuwata H, et al. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol. 2008;181:8521–7. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]
- 78.Johnson EE, Srikanth CV, Sandgren A, et al. Siderocalin inhibits the intracellular replication of Mycobacterium tuberculosis in macrophages. FEMS Immunol Med Microbiol. 2010;58:138–45. doi: 10.1111/j.1574-695X.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycbacteria. J Clin Invest. 2007;117:1988–94. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Landro L, Damas JK, Flo TH, et al. Decreased serum lipocalin 2 levels in human immunodeficiency virus-infected patients: increase during highly active anti-retroviral therapy. Clin Exp Immunol. 2008;152:57–63. doi: 10.1111/j.1365-2249.2008.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Devireddy LR, Hart DO, Goetz DH, et al. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–17. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bao G, Clifton M, Hoette TM, et al. Iron traffics in circulation bound to a siderocalin (NGAL)-catechol complex. Nat Chem Biol. 2010;6:602–9. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Philpott C. Bioinorganic chemistry: getting a grip on iron. Nat Chem Biol. 2010;6:568–70. doi: 10.1038/nchembio.411. [DOI] [PubMed] [Google Scholar]
- 84.Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by lipocalin. Mol Cell. 2002;10:1045–56. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 85.Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603–6. doi: 10.1016/s0092-8674(02)01164-9. [DOI] [PubMed] [Google Scholar]
- 86.Devireddy LR, Gazin C, Zhu X, et al. A cell surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 87.Richardson DR. 24p3 and its receptor: dawn of a new iron age? Cell. 2005;123:1175–7. doi: 10.1016/j.cell.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 88.Breuer W, Shvartsman M. Cabantchik ZI: Intracellular labile iron. Int J Biochem Cell Biol. 2008;40:350–4. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 89.Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular, other progressive inflammatory, degenerative diseases. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kell DB. Towards a unifying systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol. 2010;84:825–89. doi: 10.1007/s00204-010-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baker M, Wilson D, Sabeti PC, et al. Host genetic factors involved in iron regulation may influence susceptibility to Mycobacterium tuberculosis: a pilot study. Abstract presented at the Fourth Annual New England TB Symposium; July 2010. [Google Scholar]