Abstract
Purpose
The use of MRI to monitor immune cell infiltration into various pathologies is well established. In an effort to boost the magnetic material within immune cells, this work attempted to label resting monocytes within bone marrow, in mice, by intravenous administration of micron-sized iron oxide particles (MPIOs), similar in fashion to the administration of (U)SPIO.
Procedures
MPIOs were incubated with various immune cells both in culture, and in whole blood. Flow cytometry and histology were used to analyze magnetic cell labeling. Also, MPIOs were injected intravenously into mice. In vivo, high-resolution 3-D MRI was performed on mouse legs, and signal changes were quantified. Flow cytometry and histology were used to analyze magnetic cell labeling of bone marrow resident cells.
Results
It is demonstrated here that monocytes and neutrophils can indeed endocytose MPIOs both in cell culture and ex vivo in whole blood. However, despite rapid accumulation of MPIOs within the bone marrow following injection, MPIOs did not label monocytes or any other hematopoietic cell type in the marrow. Hypotheses are drawn to explain these results in light of recent usage of MPIOs for immune cell tracking.
Conclusions
Systemic administration of various MPIO formulations showed that MPIOs arrive in bone marrow rapidly following injection and remain there for at least 7 days. Data also shows slow clearance of some particles from the tissue over this period. While MPIOs can efficiently label monocytes in culture and in whole blood ex vivo, they were not found to label bone marrow resident monocytes.
Keywords: MRI, Iron oxide, Monocytes, Bone marrow
Introduction
The use of MRI to track monocyte infiltration is well established in a number of pathologies. These include diseases and injuries of the brain such as stroke and MS (reviewed in [1]), diseases of the heart and vessels such as atherosclerosis [2] and myocardial infarction [3], organ transplantation [4], and arthritis [5], among others. The general experimental protocol starts with an injury/disease already present. For instance, a stroke has already been created, or a plaque already exists, or an organ has been transplanted. Thus, there already exists an activated immune system responsive to the malady. Next, iron oxide nano-particles are administered intravenously. In nearly every case, ultrasmall superparamagnetic iron oxides (USPIOs; 30–50 nm) have been administered, rather than SPIO (100–200 nm). Lastly, MRI is performed, commonly 24 h post USPIO delivery. Dark contrast in the MRI at the site of the injury/disease is evidence of infiltrated immune cells harboring iron particles. The most commonly expressed hypothesis for cell labeling is that circulating monocytes can endocytose material from the blood and/or bone marrow-borne monocytes pick up USPIO prior to leaving the marrow. In contrast to other types of MRI-based cell tracking studies, the use of MRI to monitor the migration of monocytes, labeled in vivo with USPIO, has been performed many times in humans [6, 7].
There are at least two variations on this generalized experimental protocol which could benefit medical research. The first is the labeling of resting monocytes prior to activation by disease or injury. This would enable visualization of the earliest phases of a confirmed injury or disease, which, for many diseases, provides a unique window of opportunity for treatment. The second is the use of magnetically labeled cells of the innate immune response (e.g., neutrophils, dendritic cells, or macrophages) to act as sentries, i.e., to survey the body for unknown maladies. This would be especially important in the identification of small tumors or metastases. In both cases, due to the low number of expect cells which migrate to the disease/injury, cells would likely be required to possess enough magnetic material in low numbers of cells as to non-ambiguously identify diseases.
Micron-sized iron oxide particles (MPIOs) have emerged as an alternative contrast agent for cellular MRI [8, 9]. These particles have three major advantages over (ultra) small superparamagnetic iron oxide (U)SPIOs for magnetic cell labeling. First, these particles more efficiently package iron, that is, these particles have higher iron content based on volume. Secondly, MPIOs have higher r2* molar relaxivity than (U)SPIOs due to the physical size of the magnetic entity, forcing relaxation towards the static dephasing regime [10]. Thirdly, MPIOs have been shown to readily and easily label a variety of phagocytic cell types without the need for complexation with transfection agents or potentially harmful electroporation [9]. Indeed, it has been demonstrated that single cells can be detected in vivo using MPIOs [11]. Thus, if MPIOs can label monocytes in vivo, these particles may be enabling in detecting low numbers of homing monocytes in vivo.
MPIOs do have a major disadvantage compared with (U) SPIOs. MPIOs are efficiently cleared from the circulation by the liver and spleen, and by the kidney to some extent. This is challenging in that in order to label bone marrow-borne monocytes, MPIOs need to arrive in the bone marrow. However, work using systemically delivered fluorescent 0.5-μm latex beads has demonstrated robust labeling of monocytes in vivo [12]. Furthermore, Wu et al. [4] and Yang et al. [3] have demonstrated MRI detection of magnetically labeled monocyte infiltration in organ rejection and myocardial infarct models, respectively. Lastly, a recent study demonstrated that resting monocytes can be labeled in vivo with small fluorescent iron oxide nanoparticles prior to injury [13]. Taken together, these studies provided motivation to determine whether systemically delivered MPIOs can in fact reach bone marrow and if they can label resting monocytes.
Materials and Methods
MPIOs were 1.63-μm COOH-functionalized (n=4), 1.63-μm NH2-functionalized (n=3), and 0.86-μm COOH-functionalized (n=3; Bangs Labs). Zeta potential of 1.63-μm COOH and NH2-functionalized were measured on a ZetaPALS in DI water, pH 7.4. All particles were styrene-/divinylbenzene-coated with magnetite content of 43% for 1.63 μm to 46% for 0.86 μm, and green fluorescent.
In vitro Labeling of Neutrophils and Monocytes
Neutrophils and monocytes were labeled with MPIOs both in culture and in whole blood. Rat whole blood was harvested. Blood was then mixed with an equal volume of dextran/saline solution (3% dextran T-500 (Pharmacia) in 0.9% NaCl) and incubated in upright position ∼20 min at room temperature. The upper leukocyte-rich plasma layer was aspirated and cells were pelleted from the plasma by centrifuging at 250×g for 10 min at 5°C. Cells were then resuspended in 0.9% NaCl, layered on top of 10 mL of Ficoll-Hypaque solution, and centrifuged at 400×g for 40 min at 20°C, with no brake. Mononuclear cells were obtained by aspirating the cloudy band of mononuclear cells at the saline/Ficoll-Hypaque interface. The top saline layer and Ficoll-Hypaque layer were then aspirated, leaving the neutrophil/RBC pellet. Residual RBCs were removed by subjecting cells to hypotonic lysis in 0.2% NaCl for exactly 30 s. Isotonicity was restored by adding 1.6% NaCl. 1×106 neutrophils and monocytes were plated separately in 6-well plates in RPMI supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% l-glutamine. MPIOs were added to the cells at a concentration of 20 MPIOs/cell (33 μg/mL Fe) and allowed to incubate overnight. Cells were then spun onto slides via cytospin cytocentrifuge and stained with Wright Giemsa.
For labeling of neutrophils and monocytes in whole blood, prior to cell isolation, MPIOs were added to the blood at a concentration of 20 MPIOs/cell (assuming ∼1.5×106 neutrophils or monocytes/mL; 33 μg/mL Fe) and incubated at 37°C for 3 h. Then, red blood cells were lysed with ACK lysis buffer for 5 min at room temperature, after which all remaining cells were collected by centrifugation. Cells were resuspended in the same growth medium as above and cultured overnight. This was done to isolate macrophages by their affinity to adhere to plastic, with B and T lymphocytes remaining suspended. The next day, non-adherent cells were washed away and adherent cells were scraped from the dish. Following collection by centrifugation, cells were stained with anti-mouse CD11b antibody with direct Cy5 conjugation. Flow cytometry (Becton Dickson LSR II) was performed for quantification of the number of double positive green fluorescent (MPIO-labeled) and Cy5 fluorescent (monocytes/macrophages) cells. Some cell samples at the 3-h time point were spun onto slides via cytospin cytocentrifuge and stained with Wright Giemsa.
In vivo Labeling of Monocytes
Four-week-old CD-1 mice were injected intra-ocularly with various quantities of different MPIO preparations. Injection volumes were 30 (1% solids), 100 (1% solids), and 150 μL (2% solids), delivering 100, 330 or 1,000 μg iron, respectively, to ∼10 g mice. Animals then underwent in vivo MRI immediately after injection, at 6 h post injection, then again at 1, 2, 4, and 7 days post-injection. MRI consisted of 3D gradient echo (TR=100 ms, TE=10 ms, 1 average) of the femur at 50 μm isotropic resolution (384×192×192 over 1.92×0.96×0.96 cm) at 4.0 T (Bruker Biospec) using a homebuilt solenoid coil. Animals were anesthetized with 1% isoflurane delivered by nosecone with respiratory monitoring accomplished with a respiratory pillow and gating system (SA Instruments). MRI data analysis was performed in Amide.
Histology and Flow Cytometry
At either 2 or 7 days post-injection, animals were euthanized. Legs were removed from animals and sterilized with ethanol. Femurs were dissected and the distal end and femoral head were carefully removed with bone scissors. Using a 25-G needle, bone marrow was aspirated from both femurs into 2% fetal bovine serum in phosphate-buffered saline (PBS). Cells were washed once with 2% fetal bovine serum in PBS. Total bone marrow was stained for CD45 (cells of hematopoietic origin) or CD11b (specific for monocytes/macrophages) using Pe-/Cy5-conjugated rat anti-mouse CD45 and Cy5-conjugated rat anti-mouse CD11b, respectively. Flow cytometry was performed at 2 and 7 days post-injection to measure the total number of bone marrow cells double positive for green fluorescent MPIOs and red fluorescent cells of interest. For some animals, femurs were dissected, decalcified, and cryosectioned for histology. Toluidine staining was performed on some sections to reveal cellular architecture. For some animals, liver, spleen, and kidneys were removed and processed for frozen sectioning. Optical and fluorescence microscopy on cell slides and tissue sections were performed with a Leica Fluorescent MZ FLIII stereomicroscope.
Results
In vitro Labeling of Neutrophils and Monocytes
The first set of studies sought to identify whether blood resident phagocytic cells could endocytose MPIOs following isolation and culture. Figure 1a, b shows optical (1a) and fluorescent (1b) microscopic images of monocytes labeled in cell culture with MPIOs following cell isolation. It can be seen that monocytes are nearly completely full of MPIOs. Neutrophils could also be labeled (data not shown). Next, monocytes and neutrophils were tested for labeling with MPIOs in whole blood. Of the cells that were subject to flow cytometry (adherent cells from whole blood), 65.6% were positive for CD11b, meaning that these were monocytes/macrophages, and of those, 30.5% were double positive for MPIO labeling. Figure 1c shows dual fluorescent/optical microscopic images of monocytes (1c) and neutrophils (1d) containing MPIOs following incubation of MPIOs in whole blood. It can be seen that in these experiments, versus labeling of isolated monocytes and neutrophils in culture, cells endocytosed lower numbers of particles.
Fig. 1.
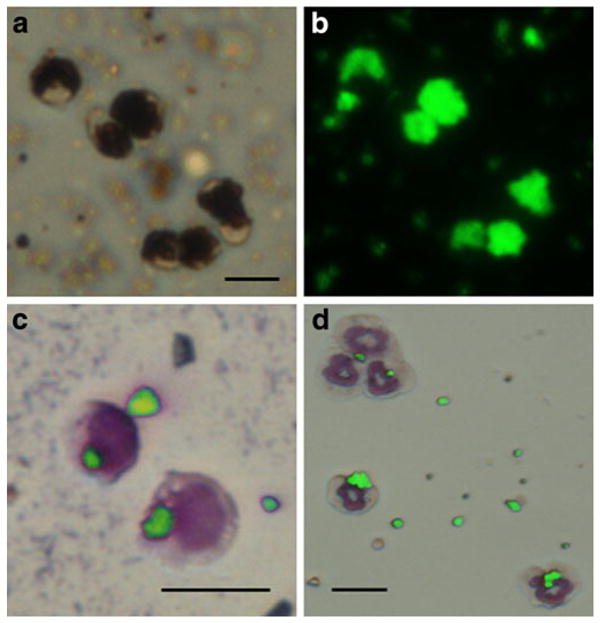
a Optical and b fluorescent microscopic image of monocytes labeled with MPIOs. These cells were labeled in culture following isolation from blood. Dual optical/fluorescent microscopic image of two monocytes (c) and five neutrophils (d) with internalized MPIOs. Some extracellular beads remain. Wright Giemsa-stained cytospun slides. All scale bars are 10 μm.
MRI
Following the success of in vitro labeling of monocytes, attempts were made to label monocytes directly in situ with MPIOs. MRI was used to investigate the contrast evolution in the femur to report on the arrival and location of the particles. In vivo MRI of live mouse femur was accomplished at 50 μm isotropic, with scan times of 61 min, aided by a robust restraint system and a dedicated, tight-fitting solenoid coil.
MRI of femurs prior to injection showed bright contrast, with dark contrast at the ends of the bones due to the trabeculae (Fig. 2a). MRI of femurs as early as 1 h post-injection demonstrated widespread uptake of MPIOs throughout the bone marrow, as evidenced by dark contrast in T2*-weighted gradient-echo MRI (Fig. 2b). The degree of bone marrow contrast was dependent on the amount of particles injected, with 30-μL injections producing ∼50% overall drop on bone marrow signal intensity and 100 μL and higher nearly depleting signal intensity in the marrow. For animals injected with the highest dose of MPIOs, contrast in the marrow continued to increase, even up to 4 days post-injection (Fig. 2c–f). This data is quantified in Fig. 3. Differences in timing of contrast evolution or degree of contrast between the COOH- (zeta potential=−19 mV) and NH2-functionalized particles (zeta potential=−8 mV) were not detected.
Fig. 2.
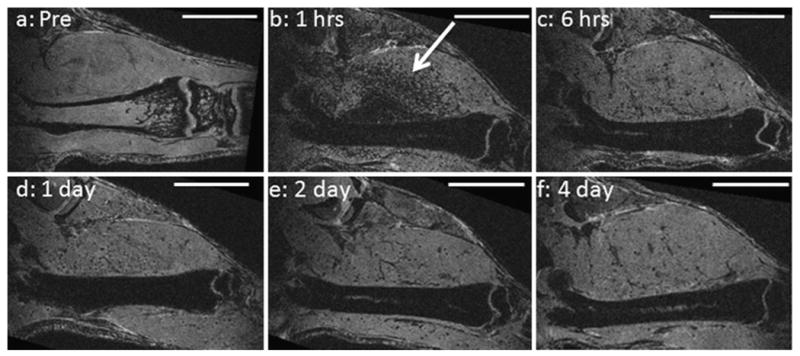
Single MRI slices extracted from 3-D data sets of same mouse femur a prior to injection of MPIOs, b 1 h, c 6 h, d 1 day, e 2 days, and f 4 days after injection of MPIOs. Bone marrow is dark from b–f. Bright streak in center of bone is blood vessel. Arrow points to dark, punctate contrast in muscle. All scale bars are 1 cm.
Fig. 3.
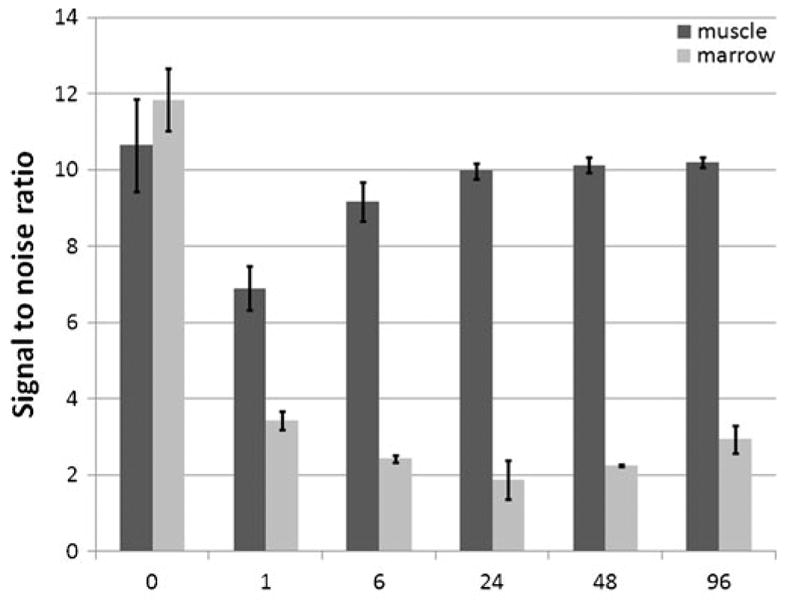
Quantification of signal changes in muscle and bone marrow immediately before MPIO injection (time=0) and several time points following injection of 100-μg iron. Error bars are standard deviations between three animals.
A second finding was that while at 1 h post-injection bone marrow was full of MPIOs, there was also widespread dark, punctate contrast throughout the tissue (arrows, Fig. 2b–f). During the next 7 days, the dark, punctate contrast in the tissue lessened almost to control levels (Fig. 3). Previous data on clearance rate for injected MPIOs revealed that MPIOs clear from the circulation within 5 min [3]. This is confirmed by MRI as the bright streak in the center of the femur is a blood vessel visible even at 1 h post-injection because MPIOs have cleared. Thus, these particles are likely trapped within small capillaries in the tissue or within extracellular matrix components.
Histology
Toluidine staining (Fig. 4a) on decalcified tissue sections of bone marrow reveals the classical cellular makeup of bone marrow, consisting mainly of small, dark stained monocytes, with some lighter stain granulocytes. Larger megakaryocytes are also present. Figure 4b shows an unstained section where many small, brown particles are dispersed throughout the tissue. Fluorescence microscopic analysis of bone marrow aspirates clearly showed that MPIOs were in the aspirate, but in no instance was an MPIO found within a cell; rather, MPIOs were free floating.
Fig. 4.
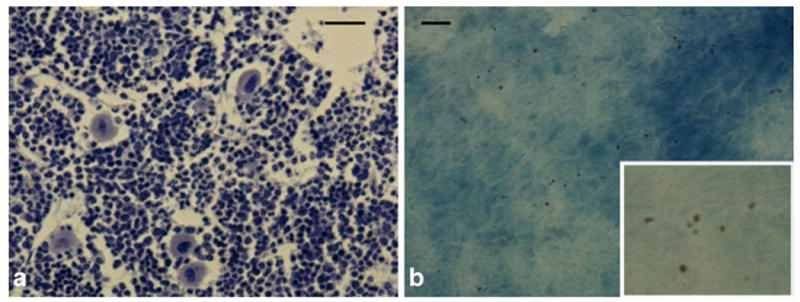
a Toluidine staining on decalcified tissue sections of bone marrow. b Unstained section where many small, brown particles are dispersed throughout the tissue. Inset is 3× magnification of parent image. Scale bars in a and b are 75 and 15 μm, respectively.
Flow Cytometry
Flow cytometry was performed at 2 and 7 days post-injection. At 2 days post-injection, flow cytometry for CD11b (Fig. 5a), a marker for monocytes, and CD45 (Fig. 5b), a marker for cells of the hematopoietic lineage, consistently demonstrated that >95% of bone marrow cells were CD45+ and that ∼70% were CD11b+. However, flow cytometry detected barely any double positive cells (<0.5%), that is, virtually no bone marrow cells contained MPIOs (Fig. 4). This was also the case at 7 days post-injection (Fig. 5c).
Fig. 5.
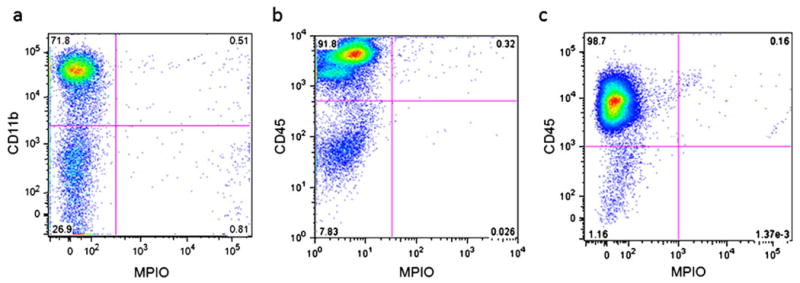
a Flow cytometry for CD11b, a marker for monocytes, and b CD45, a marker for cells of the hematopoietic lineage, at 2 days post-injection. c Flow cytometry for CD45 at 7 days post-injection. All plots are shown with green fluorescence from MPIOs on x-axis.
Discussion
Monocytes and macrophages can be labeled in cell culture with large amounts of magnetic material using either (U) SPIO or MPIO, enough to enable single cell detection in vivo. Due to the long half-life of USPIO in the blood (25–30 h), USPIO have the potential to interact long enough with monocytes in blood or marrow in vivo to enable endocytosis. However, the amount of labeling of monocytes in vivo is much lower than that achieved in vitro, and may preclude robust in vivo single-cell detection. Because MPIOs pack enough iron per particle such that one or a few particles are needed for detection [8], MPIOs are an attractive alternative for in vivo labeling of monocytes for subsequent single-cell detection. However, as opposed to USPIO which have a long blood half-life, MPIOs are cleared from blood within minutes [3], thus leaving endocytosis by monocytes in the marrow as a reasonable hypothesis.
This work shows that indeed, MPIOs rapidly arrive in large quantities in bone marrow following systemic delivery. However, following both microscopic and flow cytometric analysis of bone marrow cells, it was determined that MPIOs were not inside CD11b+ monocytes, nor CD45+ cells of hematopoietic origin. This was the case for all MPIO formulations. As expected, MPIOs were also present in large quantities in the liver, spleen, and kidneys and remained there for the entire 7 days of the studies.
These results are challenging to interpret in light of recent reports on the successful use of MPIOs to prelabel monocytes for MRI-based cell tracking of inflammatory response. For example, Yang et al. delivered MPIOs in much smaller dose to mice 7 days prior to myocardial infarction (MI) and observed a substantial number of iron-laden macrophages in the infarct starting at 3 days post-MI [3]. Similarly, Ye et al. delivered MPIOs 1 day prior to cardiac transplant and detected iron-laden macrophages within rejected organs at 1 week post-transplant [14]. However, neither of these papers investigated when or where the macrophages acquired the label.
The data presented in this work and others suggests three possible hypotheses for MPIO labeling of monocytes. The first hypothesis is that, indeed, monocytes can be labeled directly in bone marrow with MPIOs, but that it requires depletion of the circulating monocyte population by administration of clodronate. Tacke et al. used this approach to label marrow resident monocytes [12]. Activation of the immune system may also be required, resultant from injury or disease, as was the case for Yang et al. [3] and Ye et al. [14].
The second hypothesis is that the majority of MPIO labeling of monocyte occurs in the spleen. This is supported by histology showing widespread distribution of MPIOs in the spleen as long as 7 days post-injection. Indeed, the spleen is a reservoir for monocytes. Wu et al. show convincing evidence that intravenous delivery of USPIO/protamine sulfate complex efficiently labels splenocytes [15].
The third hypothesis is that macrophages which have already responded to the insult endocytose MPIOs from the circulation. This is supported by the observation that, despite extremely fast clearance of MPIOs from the circulation (half-life <5 min) [3], MPIOs continue to accumulate in bone marrow for several days following the injection. This occurs with a concomitant clearance of MPIOs from vessels in the muscle. Thus, there is a slower clearance of MPIOs from the total vasculature and is likely undetectable by the methods previously used to measure clearance rate, i.e., fluorescence signal in blood, or MRI of the blood itself.
A final point is the consequences of the hypotheses drawn above, or more directly, why is it important to determine the mechanism of in vivo magnetic cell labeling of monocytes. A major reason is that if we understand the mechanism, we can devise methodologies for enhancing labeling. A key to lowering detection thresholds for labeled cells is to boost the amount of magnetic material within labeled cells. Strategies have been developed which either functionalize the surface of particles to make them more attractive for monocyte endocytosis [16] or as in the work discussed in this paper, the use of MPIOs. If the marrow presents a physiological or physical barrier to particle labeling, and most magnetic labeling occurs in the spleen, clinical approaches to monocyte tracking are likely to be altered. For example, since MPIOs can label monocytes in whole blood within a 3-h incubation period, perhaps a better mechanism for clinical implementation of MPIO-labeled monocytes is to draw blood, incubate with MPIOs, then reinject the labeled blood back to the patient. Or, as demonstrated by Tacke et al. [12], if it is neutrophils which can endocytose large particles and deliver them to marrow-resident monocytes, then neutrophil targeting might be preferable for in vivo labeling.
Conclusion
MPIOs may have beneficial uses for MRI-based cell tracking of immune cell infiltration into injury or disease. Systemic administration of various MPIO formulations showed that MPIOs arrive in bone marrow rapidly following injection and remain there for at least 7 days. Data also show slow clearance of some particles from the tissue over this period. While MPIOs can efficiently label monocytes in culture and in whole blood ex vivo, they were not found to label bone marrow-resident monocytes. These results were obtained in animals in the absence of injury or disease.
Acknowledgments
Dr. Mark Horowitz, Department of Orthopaedics and Rehabilitation, Yale University School of Medicine, is acknowledged for teaching us many of the biological procedures described herein, as well as for allowing us to perform the bone sectioning and histology in his laboratory. Dr. Menachem Elimelech, Chemical Engineering, Yale University, is acknowledged for providing us access to the Zeta PALS system. Dr. Diane Krause, Department of Laboratory Medicine, Yale University School of Medicine, is thanked for providing us access to a cytospin. Support received by National Institutes of Health grants DP2 OD004362, P30 NS052519, and P30 DK072442, and the Dana Foundation.
Footnotes
Conflict of Interest. The authors declare that they have no conflict of interest.
References
- 1.Stoll G, Bendszus M. New approaches to neuroimaging of central nervous system inflammation. Curr Opin Neurol. 2010;23(3):282–286. doi: 10.1097/WCO.0b013e328337f4b5. [DOI] [PubMed] [Google Scholar]
- 2.Fayad ZA, Razzouk L, Briley-Saebo KC, Mani V. Iron oxide magnetic resonance imaging for atherosclerosis therapeutic evaluation: still “rusty?”. J Am Coll Cardiol. 2009;53:2051–2052. doi: 10.1016/j.jacc.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Yang Y, Yanasak N, Schumacher A, Hu TC. Temporal and noninvasive monitoring of inflammatory-cell infiltration to myocardial infarction sites using micrometer-sized iron oxide particles. Magn Reson Med. 2010;63:33–40. doi: 10.1002/mrm.22175. [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci USA. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dardzinski BJ, Schmithorst VJ, Holland SK, Boivin GP, Imagawa T, Watanabe S, Lewis JM, Hirsch R. MR imaging of murine arthritis using ultrasmall superparamagnetic iron oxide particles. Magn Reson Imaging. 2001;19:1209–1216. doi: 10.1016/s0730-725x(01)00448-9. [DOI] [PubMed] [Google Scholar]
- 6.Saleh A, Schroeter M, Ringelstein A, Hartung HP, Siebler M, Modder U, Jander S. Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke. 2007;38:2733–2737. doi: 10.1161/STROKEAHA.107.481788. [DOI] [PubMed] [Google Scholar]
- 7.Howarth SP, Tang TY, Graves MJ, King-Im JM, Li ZY, Walsh SR, Gaunt ME, Gillard JH. Non-invasive MR imaging of inflammation in a patient with both asymptomatic carotid atheroma and an abdominal aortic aneurysm: a case report. Ann Surg Innov Res. 2007;1:4. doi: 10.1186/1750-1164-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA. 2004;101:10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro EM, Skrtic S, Koretsky AP. Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med. 2005;53:329–338. doi: 10.1002/mrm.20342. [DOI] [PubMed] [Google Scholar]
- 10.Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 12.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montet-Abou K, Daire JL, Hyacinthe JN, Jorge-Costa M, Grosdemange K, Mach F, Petri-Fink A, Hofmann H, Morel DR, Vallee JP, Montet X. In vivo labelling of resting monocytes in the reticuloendothelial system with fluorescent iron oxide nanoparticles prior to injury reveals that they are mobilized to infarcted myocardium. Eur Heart J. 2010;31(11):1410–1420. doi: 10.1093/eurheartj/ehp547. [DOI] [PubMed] [Google Scholar]
- 14.Ye Q, Wu YL, Foley LM, Hitchens TK, Eytan DF, Shirwan H, Ho C. Longitudinal tracking of recipient macrophages in a rat chronic cardiac allograft rejection model with noninvasive magnetic resonance imaging using micrometer-sized paramagnetic iron oxide particles. Circulation. 2008;118:149–156. doi: 10.1161/CIRCULATIONAHA.107.746354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu YJ, Muldoon LL, Varallyay C, Markwardt S, Jones RE, Neuwelt EA. In vivo leukocyte labeling with intravenous ferumoxides/protamine sulfate complex and in vitro characterization for cellular magnetic resonance imaging. Am J Physiol Cell Physiol. 2007;293:C1698–C1708. doi: 10.1152/ajpcell.00215.2007. [DOI] [PubMed] [Google Scholar]
- 16.Yoo MK, Park IY, Kim IY, Park IK, Kwon JS, Jeong HJ, Jeong YY, Cho CS. Superparamagnetic iron oxide nanoparticles coated with mannan for macrophage targeting. J Nanosci Nanotechnol. 2008;8:5196–5202. doi: 10.1166/jnn.2008.1118. [DOI] [PubMed] [Google Scholar]


