Abstract
Background
Whether lower urinary tract symptoms (LUTS), including voiding, storage, and urinary incontinence, are affected by dietary micronutrients is uncertain.
Objective
To test the hypothesis that carotenoid, vitamin C, zinc, and calcium intakes are associated with LUTS and urinary incontinence in women.
Design, setting, and participants
During an observational, cross-sectional, population-based epidemiologic study of 2060 women (30–79 yr of age) in the Boston Area Community Health (BACH) survey (2002–2005), data were collected by validated food frequency questionnaire and in-person interviews and analyzed using multivariate regression.
Measurements
LUTS, storage, and voiding symptoms were assessed using the American Urological Association Symptom Index (AUASI) and a validated severity index for urinary incontinence.
Results and limitations
Women who consumed high-dose vitamin C from diet and supplements were more likely to report storage symptoms, especially combined frequency and urgency (>500 vs <50 mg/d; odds ratio [OR]: 3.42; 95% confidence interval [CI], 1.44–8.12). However, greater consumption of dietary vitamin C or β-cryptoxanthin was inversely associated with voiding symptoms (ptrend < 0.01). Both dietary and supplemental calcium were positively associated with storage symptoms (eg, supplement >1000 mg/d vs none; OR: 2.04; 95% CI, 1.35–3.09; ptrend = 0.0002). No consistent associations were observed for β-carotene, lycopene, or other carotenoids, although smokers using β-carotene supplements were more likely to report storage problems. Whether the observed associations represent direct causes of diet on LUTS is uncertain.
Conclusions
High-dose intakes of vitamin C and calcium were positively associated with urinary storage or incontinence, whereas vitamin C and β-cryptoxanthin from foods and beverages were inversely associated with voiding symptoms. Results indicate that micronutrient intakes may contribute to LUTS in dose-dependent and symptom-specific ways. Further study is needed to confirm these findings and their relevance to clinical treatment decisions.
Keywords: Ascorbic acid, Calcium, dietary, Carotenoids, Diet, Micronutrients, Nutrition, Lower urinary tract symptoms, Urinary bladder, overactive, Urinary incontinence, Urination disorders, Zinc
1. Introduction
Approximately one-fifth of the female population suffers from lower urinary tract symptoms (LUTS), such as urination urgency, frequency, voiding, and leakage [1–3]. Although LUTS in men are often attributable to benign prostatic hyperplasia (BPH), various origins for the pathogenesis of LUTS in women have been suggested, including increased autonomic nervous system activity, detrusor sensitivity, endothelial dysfunction, chronic inflammation, and oxidative damage [4–7]. Numerous studies have provided evidence that decreased abdominal obesity and increased physical activity may improve LUTS—particularly urinary incontinence—in women [2,8]. The role of diet, however, has been relatively under-researched, despite plausible mechanisms of action of nutrients via antioxidant pathways or irritable effects on the bladder.
Of the few previous studies examining dietary micronutrients in relation to LUTS, the majority have focused on BPH in men, generally finding inverse associations with vitamin C, carotenoids, and zinc [9–12]. To date, the only epidemiologic study to have published data on the relationship between dietary micronutrient composition and LUTS in women has been the UK Leicestershire MRC Incontinence Study, which examined 1-yr incidence of overactive bladder (OAB; as defined by urgency symptoms or urge urinary incontinence) or stress-related urinary incontinence [13,14]. Results showed positive associations between zinc and stress urinary incontinence [13] but not OAB [14]; otherwise, there were few consistent associations with micronutrients. However, the multivariate models did not consider potentially important confounders, such as waist circumference or comorbidities [15]. Furthermore, it remains uncertain whether associations differ for other LUTS subtypes, which may involve unique pathophysiologic mechanisms. To further investigate associations between micronutrients and LUTS in women, we used data from a population-based survey focused on urologic symptoms, with extensive data on lifestyle and medical factors: the Boston Area Community Health (BACH) survey. Previously, we had published findings from BACH showing that greater intakes of total energy and saturated fat versus polyunsaturated fat were positively associated with LUTS and urinary incontinence in women [16,17]. Given the possibility that oxidative stress may contribute to LUTS, in this analysis, we examined associations between micronutrients with antioxidant capacity—particularly carotenoids, vitamin C, and zinc—and moderate to severe LUTS in women.
2. Methods
We analyzed cross-sectional, observational, epidemiologic data using case-control methods. Data were from the BACH survey—a population-based, random, stratified sample survey of urologic symptoms and risk factors [18]. From 2002 to 2005, BACH recruited 3202 women 30–79 yr of age from three racial/ethnic groups in Boston, Massachusetts. All participants provided written informed consent. The study was approved by the New England Research Institutes’ institutional review board.
Participants completed an English or Spanish version of the validated Block food frequency questionnaire (FFQ), which assesses usual eating habits over the past year and dietary supplement use [19]. Information about urologic symptoms, comorbidities, lifestyle, and anthropometrics was obtained by in-person, at-home interview. Urologic symptoms (Table 1) were assessed during the in-home interview.
Table 1.
Assessment methods and operational definitions of lower urinary tract symptoms
| Symptom | Assessment method and operational definition |
|---|---|
| Total LUTS | AUASI score >8 (of total possible 35 points) [35,36], indicating moderate to severe symptoms; sensitivity analyses considered |
| AUASI score >15 (indicating high to moderately severe LUTS) [9,10] | |
| Voiding symptoms: | AUASI score >5 (of total possible 20 points) on the four |
| Intermittency | AUASI questions regarding the following voiding symptoms: |
| Weak urinary stream | Stop and start again several times while you urinate |
| Hesitancy | Weak urinary stream |
| Incomplete bladder emptying | Push or strain to begin urination |
| A sensation of not emptying your bladder completely after you finished urinating | |
| Voiding symptoms were defined independent of storage symptoms. | |
| Storage symptoms | AUASI score >4 (of total possible 15 points) on three AUASI questions regarding frequency, urgency, and nocturia |
| Frequency | Frequent urination during the day or having to urinate <2 h after finishing urinating (AUASI question)—“fairly often,” “usually,” or “almost always” in the last month—or urinating >8 times per day in the past 7 d |
| Urgency | Strong urge or pressure to urinate immediately with little or no warning or difficulty postponing urination (AUASI question)—“fairly often,” “usually,” or “almost always” in the last month—or strong urge or pressure to urinate immediately at least several times (>4) in the past 7 d |
| Nocturia | Had to go to the bathroom to empty the bladder during the night after falling asleep on average at least once each night in the past 7 d. |
| Storage symptoms were defined independent of voiding symptoms. | |
| Urinary incontinence | Sandvik Severity Index score >3, indicating moderate to severe urinary incontinence [37]. This definition corresponds to at least weekly leakage or monthly leakage of volumes more than a few drops in the past 12 mo. The validated Sandvik Severity Index assesses frequency (less than once/month, more than once/month, more than once/week, or daily) and amount (drops, small splashes, or more) of urine leakage, resulting in a composite score ranging from 1 to 12. |
| Continuous severity score among the subsample of women with any urine leakage in the past 12 mo |
LUTS = lower urinary tract symptoms; AUASI = American Urological Association Symptom Index.
2.1. Data analysis
The final sample size for this analysis was 2060 women. Women were excluded from analysis if they did not complete the FFQ (n = 615), reported an implausible daily energy intake (outside 600–3500 kcal/d), omitted >60 of the 103 dietary questions (n = 423), or had surgery for urinary incontinence or on the bladder (n = 104). Nutrient intakes were adjusted for total energy intake using residuals. Supplement use of β-carotene, vitamin C, zinc, and calcium in the past year (including doses from multivitamin/mineral combinations and individual supplements) was considered in dose categories, with nonusers of each supplemental nutrient as the reference.
We used logistic regression to calculate odds ratios (OR) and 95% confidence intervals (CI). Continuous symptoms scores (American Urological Association Symptom Index [AUASI] scores among all participants; urinary incontinence severity scores among 597 women reporting any urine leakage in the past year) were analyzed using generalized linear regression. Multivariate logistic regression models were created by manually adding and removing potential confounders associated with the exposure and outcome at hand, retaining those that remained statistically significant (p < 0.10) or affected estimates >10%. Relevant sociodemographic, lifestyle, and medical characteristics included in final models are listed in the Table 3 footnotes. Interactions between β-carotene and current cigarette smoking were tested, because studies have reported harmful properties of high-dose β-carotene when combined with tobacco smoking [12,20]. For sensitivity analyses of high or moderately severe symptoms (eg, AUASI > 15), results are presented only where they notably differed from those for the primary outcome of moderate to severe LUTS. Observations were weighted inversely proportional to their probability of selection. Statistical analyses were conducted using SUDAAN version 10.0 software (RTI International, Research Triangle Park, NC, USA).
Table 3.
Odds ratios for lower urinary tract symptoms and urinary incontinence by dietary micronutrient intakes among women (n = 2060) in the Boston Area Community Health survey (2002–2005)1
| Quartile of dietary intake OR (95% CI) | p for trend | p for trend in symptom score2 | ||||
|---|---|---|---|---|---|---|
| 1 (referent) |
2 | 3 | 4 | |||
| β-carotene, median μg/d | 969 | 1949 | 3318 | 6121 | – | – |
| Total LUTS | 1.00 | 1.08 (0.67–1.74) | 0.91 (0.55–1.49) | 0.88 (0.54–1.42) | 0.39 | 0.83 |
| Voiding symptoms | 1.00 | 0.99 (0.56–1.75) | 0.86 (0.45–1.64) | 0.90 (0.47–1.70) | 0.69 | 0.71 |
| Storage symptoms | 1.00 | 1.13 (0.74–1.73) | 1.11 (0.75–1.63) | 1.04 (0.67–1.60) | 0.94 | 0.87 |
| Urinary incontinence | 1.00 | 1.22 (0.70–2.13) | 0.87 (0.51–1.48) | 1.19 (0.66–2.14) | 0.86 | 0.12 |
| Lycopene, median μg/d | 313 | 724 | 1281 | 2492 | – | – |
| Total LUTS | 1.00 | 1.24 (0.77–1.99) | 0.71 (0.43–1.17) | 0.97 (0.60–1.57) | 0.80 | 0.80 |
| Voiding symptoms | 1.00 | 1.14 (0.65–2.00) | 0.98 (0.53–1.81) | 0.95 (0.53–1.70) | 0.77 | 0.38 |
| Storage symptoms | 1.00 | 1.17 (0.75–1.82) | 1.00 (0.65–1.54) | 1.06 (0.67–1.68) | 0.49 | 0.71 |
| Urinary incontinence | 1.00 | 1.00 (0.53–1.90) | 0.47 ** (0.26–0.82) | 0.69 (0.40–1.19) | 0.52 | 0.89 |
| α-carotene, median μg/d | 62 | 144 | 269 | 519 | – | – |
| Total LUTS | 1.00 | 1.26 (0.79–2.02) | 0.87 (0.52–1.45) | 1.28 (0.81–2.03) | 0.96 | 0.87 |
| Voiding symptoms | 1.00 | 0.82 (0.47–1.43) | 0.78 (0.42–1.44) | 0.83 (0.45–1.51) | 0.49 | 0.65 |
| Storage symptoms | 1.00 | 1.22 (0.83–1.80) | 0.69 (0.46–1.05) | 0.85 (0.58–1.26) | 0.20 | 0.86 |
| Urinary incontinence | 1.00 | 0.80 (0.44–1.46) | 0.89 (0.47–1.66) | 1.11 (0.60–2.06) | 0.38 | 0.22 |
| Lutein, median μg/d | 492 | 1095 | 2189 | 5525 | – | – |
| Total LUTS | 1.00 | 0.94 (0.52–1.70) | 0.99 (0.53–1.83) | 0.90 (0.49–1.62) | 0.13 | 0.58 |
| Voiding symptoms | 1.00 | 1.12 (0.60–2.10) | 0.92 (0.47–1.77) | 0.78 (0.40–1.54) | 0.91 | 0.29 |
| Storage symptoms | 1.00 | 1.08 (0.68–1.72) | 1.30 (0.84–2.00) | 1.41 (0.86–2.31) | 0.27 | 0.88 |
| Urinary incontinence | 1.00 | 0.57 (0.30–1.09) | 1.00 (0.50–2.00) | 0.95 (0.48–1.87) | 0.75 | 0.05 |
| β-cryptoxanthin, median μg/d | 35 | 86 | 153 | 283 | – | – |
| Total LUTS | 1.00 | 1.30 (0.80–2.12) | 0.93 (0.58–1.49) | 0.83 (0.50–1.36) | 0.29 | 0.003 |
| Voiding symptoms | 1.00 | 1.51 (0.81–2.84) | 0.98 (0.49–1.96) | 0.87 (0.50–1.54) | 0.08 | 0.01 |
| Storage symptoms | 1.00 | 0.94 (0.64–1.38) | 0.74 (0.49–1.11) | 0.73 0.49–1.10) | 0.05 | 0.01 |
| Urinary incontinence | 1.00 | 0.82 (0.44–1.56) | 0.67 (0.37–1.21) | 0.72 (0.43–1.21) | 0.30 | 0.52 |
| Total carotenoids, median μg/d | 1108 | 2089 | 3385 | 5766 | – | – |
| Total LUTS | 1.00 | 1.02 (0.64–1.62) | 0.75 (0.47–1.21) | 0.74 (0.44–1.26) | 0.68 | 0.81 |
| Voiding symptoms | 1.00 | 0.67 (0.40–1.13) | 0.55 (0.29–1.05) | 0.62 (0.32–1.19) | 0.49 | 0.67 |
| Storage symptoms | 1.00 | 1.08 (0.70–1.66) | 0.91 (0.61–1.35) | 0.84 (0.54–1.30) | 0.81 | 0.88 |
| Urinary incontinence | 1.00 | 0.85 (0.43–1.67) | 1.07 (0.59–1.92) | 1.00 (0.52–1.93) | 0.70 | 0.13 |
| Vitamin A, median IU/d | 3615 | 5583 | 8098 | 12 917 | – | – |
| Total LUTS | 1.00 | 0.76 (0.45–1.27) | 0.74 (0.46–1.18) | 0.76 (0.47–1.21) | 0.26 | 0.46 |
| Voiding symptoms | 1.00 | 0.54 (0.29–1.02) | 0.54 (0.29–1.02) | 0.65 (0.37–1.14) | 0.47 | 0.56 |
| Storage symptoms | 1.00 | 0.97 (0.64–1.48) | 1.04 (0.69–1.58) | 0.89 (0.57–1.38) | 0.41 | 0.65 |
| Urinary incontinence | 1.00 | 0.92 (0.51–1.68) | 1.01 (0.59–1.75) | 0.91 (0.48–1.72) | 0.93 | 0.08 |
| Vitamin C, median mg/d | 51 | 87 | 141 | 243 | – | – |
| Total LUTS | 1.00 | 1.17 (0.76–1.82) | 1.35 (0.82–2.21) | 0.68 (0.42–1.11) | 0.10 | 0.01 |
| Voiding symptoms | 1.00 | 0.70 (0.40–1.24) | 0.73 (0.38–1.41) | 0.47** (0.27–0.82) | 0.04 | 0.002 |
| Storage symptoms | 1.00 | 0.86 (0.56–1.32) | 1.46 (0.97–2.20) | 0.82 (0.52–1.30) | 0.23 | 0.13 |
| Urinary incontinence | 1.00 | 0.81 (0.43–1.53) | 1.11 (0.63–1.96) | 0.87 (0.49–1.53) | 0.94 | 0.45 |
| Calcium, median mg/d | 482 | 737 | 1026 | 1466 | – | – |
| Total LUTS | 1.00 | 0.84 (0.55–1.29) | 0.79 (0.45–1.39) | 0.89 (0.53–1.52) | 0.87 | 0.71 |
| Voiding symptoms | 1.00 | 1.04 (0.58–1.84) | 0.74 (0.37–1.51) | 1.11 (0.61–2.02) | 0.72 | 0.50 |
| Storage symptoms | 1.00 | 1.18 (0.82–1.71) | 1.10 (0.74–1.62) | 1.38 (0.91–2.09) | 0.31 | 0.20 |
| Urinary incontinence | 1.00 | 2.16** (1.16–4.01) | 1.78* (1.01–3.15) | 2.17* (1.13–4.15) | 0.15 | 0.55 |
| Zinc, median mg/d | 7.6 | 9.3 | 10.9 | 13.1 | – | – |
| Total LUTS | 1.00 | 1.08 (0.68–1.72) | 1.16 (0.75–1.80) | 1.41 (0.89–2.24) | 0.28 | 0.68 |
| Voiding symptoms | 1.00 | 0.65 (0.35–1.21) | 0.94 (0.52–1.71) | 0.93 (0.51–1.70) | 0.89 | 0.95 |
| Storage symptoms | 1.00 | 1.14 (0.75–1.74) | 1.15 (0.75–1.77) | 1.49* (1.05–2.12) | 0.04 | 0.30 |
| Urinary incontinence | 1.00 | 1.26 (0.67–2.39) | 0.94 (0.48–1.82) | 1.32 (0.73–2.37) | 0.73 | 0.97 |
OR = odds ratio; CI = confidence interval; LUTS = lower urinary tract symptoms; UTI = urinary tract infection.
p < 0.05.
p < 0.01.
All models controlled for age (5-yr age groups), race/ethnicity; waist circumference (quintiles); total energy intake (quintiles); use of antispasmodic or anticholinergic medication; and history of arthritis/rheumatism, asthma, or cardiac disease. Models for voiding symptoms additionally adjusted for cigarette smoking (pack-year categories), alcohol consumption (quintiles), physical activity (low, medium, or high), and current depression symptoms. Models for total LUTS and storage symptoms additionally adjusted for cigarette smoking, alcohol consumption, physical activity, current depression symptoms, diabetes, menopausal status, and total fluid intake (quintiles). Models for urinary incontinence additionally adjusted for menopausal status, ever giving birth vaginally, history of UTI, and dietary intake ratio of saturated and polyunsaturated fatty acids (quintiles).
Tests for trends with the continuous score were conducted using generalized linear multivariate models, adjusting for the same factors as in the logistic regression. For urinary incontinence, the continuous severity score was analyzed among the subsample of women (n = 597) who reported any urine leakage in the past year.
3. Results
Of the 2060 women in this analysis, 425 (17.6% weighted) had moderate to severe total LUTS, 8.3% had voiding symptoms, and 35.2% had storage symptoms. Among women with storage symptoms, frequency (66.1%) and nocturia (52.0%) were most prevalent. Urinary incontinence was present in 257 (12.5%) women, most commonly (43.9%) mixed stress and urge types. The voiding symptoms most frequently reported were intermittency (3.5%) and incomplete emptying (4.9%). Overall, women with LUTS were older; less physically active; and had higher waist circumferences, total energy intakes, and more comorbid medical conditions (Table 2).
Table 2.
Weighted characteristics of 2060 women in the Boston Area Community Health survey, overall and by lower urinary track symptom statusa, 2002–2005
| Total (n = 2060) | LUTS (n = 425) | No/mild LUTS (n = 1635) | ||||
|---|---|---|---|---|---|---|
| Mean (SE) | % | Mean (SE) | % | Mean (SE) | % | |
| Age, yr | 48.9 (0.6) | – | 51.4 (1.1) | – | 48.3 (0.6) | – |
| Race/ethnicity: | ||||||
| Black | – | 30.2 | – | 36.1 | – | 28.9 |
| Hispanic | – | 13.0 | – | 10.5 | – | 13.6 |
| White | – | 56.8 | – | 53.5 | – | 57.5 |
| Cigarette smoking status: | ||||||
| Never smoked | – | 50.6 | – | 46.6 | – | 51.4 |
| Former smoker | – | 26.5 | – | 28.0 | – | 26.2 |
| Current smoker | – | 22.9 | 25.5 | – | 22.4 | |
| Alcohol intake, g/d | 5.7 (0.4) | – | 3.8 (0.5) | – | 6.2 (0.5) | – |
| BMIb | 28.9 (0.3) | – | 30.6 (0.6) | – | 28.6 (0.3) | – |
| Waist circumference, cm | 89.6 (0.6) | – | 92.6 (1.4) | – | 89.0 (0.6) | – |
| Physical activity levelc: | ||||||
| Low | – | 26.6 | – | 37.5 | – | 24.3 |
| Medium | – | 53.4 | – | 52.0 | – | 53.7 |
| High | – | 19.9 | – | 10.5 | – | 21.9 |
| Medical history: | ||||||
| Diabetes mellitus | – | 8.2 | – | 13.0 | – | 7.1 |
| Cardiac disease | – | 7.3 | – | 12.3 | – | 6.3 |
| Cancer | – | 9.2 | – | 11.9 | – | 8.6 |
| Arthritis or rheumatism | – | 28.3 | – | 45.7 | – | 24.6 |
| Asthma | – | 18.2 | – | 30.9 | – | 15.4 |
| Depression symptomsd | – | 18.4 | – | 30.8 | – | 15.7 |
| UTI | – | 46.1 | – | 63.4 | – | 42.4 |
| Use of diuretic agents | – | 13.0 | – | 17.1 | – | 12.1 |
| Use of antispasmodic or anticholinergic agents | – | 1.4 | – | 5.9 | – | 0.5 |
| Use of any vitamin/mineral supplements | – | 51.2 | – | 51.2 | – | 51.2 |
| Menopausal/hormone use status: | ||||||
| Premenopausal | – | 24.1 | – | 11.5 | – | 26.8 |
| Perimenopausal | – | 21.1 | – | 22.0 | – | 20.9 |
| Naturally postmenopausal | – | 22.4 | – | 24.5 | – | 22.0 |
| Surgically postmenopausal | – | 14.5 | – | 21.8 | – | 12.9 |
| Hormone use | – | 15.5 | – | 16.7 | – | 15.2 |
| Undetermined | – | 2.5 | – | 3.5 | – | 2.2 |
| Total energy intakee, kcal/d | 1589 (19) | – | 1719 (57) | – | 1561 (21) | – |
LUTS = lower urinary tract symptoms; SE = standard error; BMI = body mass index; UTI = urinary tract infection; AUASI = American Urological Association Symptom Index.
Moderate to severe LUTS defined by AUASI score >8.
Weight (kg)/height (m)2.
Physical activity measured by means of the Physical Activity Scale for the Elderly [38]; scores were classified as follows: <100 = low, 100–249 = medium, >250 = high.
Current depression symptoms, as assessed using the abbreviated version of the Center for Epidemiologic Studies Depression Scale [39].
Total energy intake does not include energy derived from alcohol consumption.
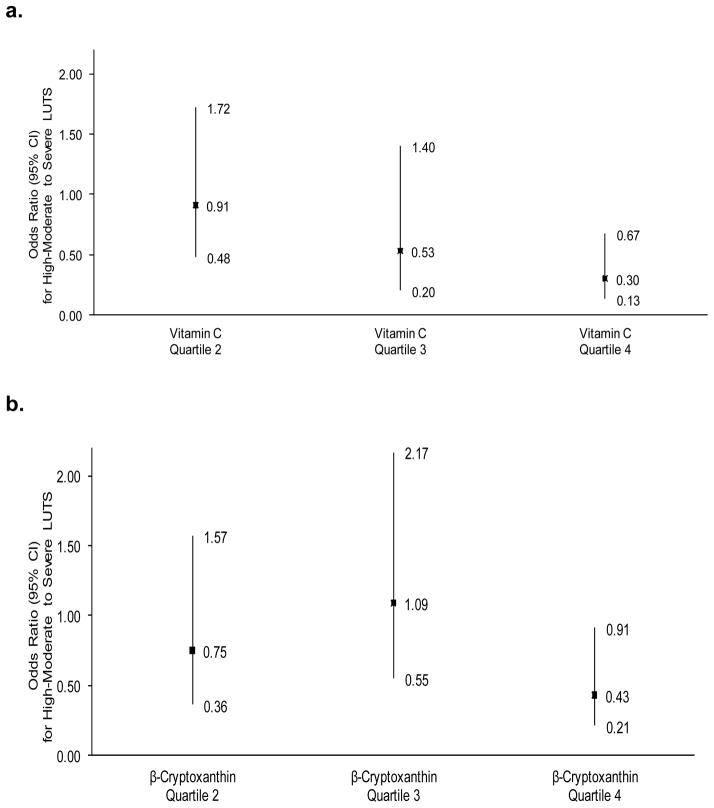
Associations between dietary nutrient intakes and LUTS are presented in Table 3. No consistent associations were observed for dietary β-carotene, lycopene, α-carotene, lutein, total carotenoids, or vitamin A. However, statistically significant inverse associations were observed between β-cryptoxanthin intake and AUASI scores—particularly voiding symptoms. Similar findings were observed for dietary vitamin C, which was highly correlated with β-cryptoxanthin (r = 0.83; p < 0.01). For the outcome of high to moderate or severe symptoms, the inverse associations were statistically significant (Fig. 1). Exploring specific symptoms, all individual voiding symptoms were inversely associated with dietary β-cryptoxanthin or vitamin C, with “hesitancy” most statistically significant (ptrend < 0.01).
Fig. 1.
Odds ratios and 95% confidence intervals for high to moderately severe lower urinary tract symptoms (American Urological Association Symptom Index >15; n = 121 cases) by dietary vitamin C intake or β-cryptoxanthin intake. (a) Dietary vitamin C; ptrend = 0.004; (b) dietary β-cryptoxanthin; ptrend = 0.03.
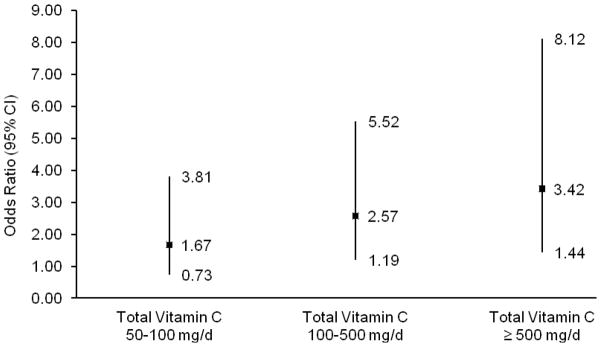
In contrast, use of vitamin C or β-carotene supplements had statistically significant positive associations with storage symptoms (Table 4)—particularly frequent daytime urination and urgency. For β-carotene, the positive association with storage symptoms was specifically among current smokers (>3000 μg/d vs nonuser; OR: 3.67; 95% CI, 1.15–11.7; ptrend = 0.01) compared to former smokers (OR: 2.19; 95% CI, 0.92–5.19) or those who had never smoked (OR: 1.24; 95% CI, 0.58–2.64), with a borderline significant test for interaction (pinteraction = 0.05). No interactions with smoking were observed for vitamin C. Doses of supplemental vitamin C were generally higher than dietary intake levels; thus, to better understand the observed discrepancies, we then examined total (dietary plus supplemental) vitamin C. Similar to dietary vitamin C, total vitamin C was inversely associated with voiding symptoms (ptrend = 0.02). Meanwhile, greater total vitamin C intake was positively associated with storage symptoms of frequent daytime urination (eg, > 400 vs <100 mg/d; OR: 1.88; 95% CI, 1.19–2.98; ptrend = 0.04) and urgency (OR: 1.87; 95% CI, 1.01–3.46; ptrend = 0.17). The magnitude of this association increased for women who reported a combination of both urgency and frequency symptoms (Fig. 2). There were no associations with nocturia.
Table 4.
Associations between supplemental use of β-carotene, vitamin C, calcium, or zinc and lower urinary tract symptoms among women in the Boston Area Community Health survey (n = 2060)1
| Dose category of intake | p for trend | p for trend in the symptom score | |||
|---|---|---|---|---|---|
| Nonuser | Low–medium | High | |||
| Nutrient | 1 | 2 | 3 | ||
| β-carotene | 0 μg | <3000 μg | >3000 μg | – | – |
|
|
|||||
| No. | 1141 | 765 | 154 | – | – |
| Total LUTS | 1.0 | 1.50* (1.01–2.24) | 1.34 (0.67–2.67) | 0.23 | 0.18 |
| Voiding symptoms | 1.0 | 1.45 (0.89–2.36) | 1.70 (0.71–4.04) | 0.15 | 0.52 |
| Storage symptoms2 | 1.0 | 1.60** (1.15–2.22) | 1.80* (1.07–3.02) | 0.008 | 0.11 |
| Urinary incontinence | 1.0 | 1.56 (0.98–2.47) | 0.96 (0.46–1.99) | 0.64 | 0.62 |
| Vitamin C | 0 mg | <250 mg | >250 mg | – | – |
|
|
|||||
| No. | 1026 | 722 | 312 | – | – |
| Total LUTS | 1.0 | 1.47 (0.97–2.21) | 1.07 (0.62–1.85) | 0.83 | 0.18 |
| Voiding symptoms | 1.0 | 1.24 (0.76–2.00) | 1.69 (0.91–3.15) | 0.11 | 0.42 |
| Storage symptoms | 1.0 | 1.59** (1.14–2.21) | 1.60* (1.08–2.38) | 0.08 | 0.10 |
| Urinary incontinence | – | 1.50 (0.91–2.47) | 1.22 (0.71–2.11) | 0.83 | 0.57 |
| Zinc | 0 mg | <15 mg | >15 mg | – | – |
|
|
|||||
| No. | 1216 | 720 | 124 | – | – |
| Total LUTS | 1.0 | 1.28 (0.86–1.89) | 1.40 (0.67–2.95) | 0.25 | 0.29 |
| Voiding symptoms | 1.0 | 1.12 (0.70–1.81) | 1.84 (0.84–4.02) | 0.17 | 0.56 |
| Storage symptoms | 1.0 | 1.54** (1.13–2.10) | 1.34 (0.75–2.38) | 0.09 | 0.27 |
| Urinary incontinence | – | 1.68* (1.09–2.59) | 1.19 (0.53–2.64) | 0.64 | 0.30 |
| Calcium | 0 mg | <1000 mg | >1000 mg | – | – |
|
|
|||||
| No. | 1054 | 686 | 320 | – | – |
| Total LUTS | 1.0 | 1.19 (0.78–1.82) | 1.85* (1.09–3.15) | 0.03 | 0.001 |
| Voiding symptoms | 1.0 | 1.00 (0.59–1.69) | 1.90 (0.98–3.68) | 0.13 | 0.14 |
| Storage symptoms | 1.0 | 1.42* (1.02–1.98) | 2.04*** (1.35–3.09) | 0.0002 | 0.0001 |
| Urinary incontinence | 1.0 | 1.24 (0.75–2.05) | 1.51 (0.87–2.63) | 0.35 | 0.85 |
LUTS = lower urinary tract symptoms; OR = odds ratio; CI = confidence interval.
From multivariate models, adjusting for the factors listed in Table 3 footnotes. Trend tests were conducted using the median dose of each category of intake as a continuous variable and testing the statistical significance of its coefficient by the Wald test. For urinary incontinence, the continuous severity score was analyzed among the subsample of women (n = 597) who reported any urine leakage in the past year.
pinteraction = 0.0498 between β-carotene supplement use and current smoking status. Comparing >3000 μg/d β-carotene to nonuser: among current smokers, storage symptoms OR: 3.67 (95% CI, 1.15–11.7), ptrend = 0.01; among former smokers (OR: 2.19; 95% CI, 0.92–5.19), among those who had never smoked (OR: 1.24; 95% CI, 0.58–2.64).
p < 0.05.
p < 0.01.
p < 0.001.
Fig. 2. Odds ratios and 95% confidence intervals for daytime frequency with urgency symptoms (n = 255 cases) by total (dietary plus supplemental) vitamin C intake (p = 0.02; ptrend = 0.10).
CI = confidence interval.
Total calcium intake was associated with increased odds of storage symptoms (ptrend = 0.0004), urinary incontinence (ptrend = 0.04), and overall AUASI score (ptrend = 0.006; data not shown). The associations were of highest magnitude for urinary incontinence (total calcium quartile 4 vs 1; OR: 4.31; 95% CI, 2.42–7.67). Comparing dietary and supplemental sources, dietary calcium was associated with urinary incontinence (Table 3), whereas supplemental calcium was significant for other storage symptoms (Table 4).
Zinc consumed from foods was positively associated with storage symptoms (ptrend = 0.04) but not urinary incontinence or voiding symptoms (Table 3). There were no consistent associations with supplemental zinc.
4. Discussion
In this population-based, cross-sectional study of women, we observed associations between intakes of certain micronutrients and LUTS that varied by symptom subtype. For example, women consuming high-dose vitamin C from diet and supplements were more likely to report storage symptoms, particularly frequency and urgency. However, dietary vitamin C and β-cryptoxanthin were inversely associated with voiding symptoms. No consistent associations were observed for dietary β-carotene, lycopene, or other carotenoids, although smokers using β-carotene supplements were more likely to report storage problems. Additional research, including clinical trials, is needed to confirm these findings.
Given the scarcity of prior data on associations between micronutrients and LUTS in women, we selected nutrients to include in this analysis by considering biologic plausibility of mechanisms of action as well as the results of studies conducted in men. Thus, we analyzed individual carotenoids, vitamin C, and zinc, which have been found to be inversely associated with LUTS as symptomatic BPH in men [9–11], and calcium because of its associations with urinary tract health [21]. We further limited our analysis of supplements to those for which a sufficient number of women reported taking doses in the range of individual supplements to allow analyses of dose trends.
Of the micronutrients included in this analysis, only vitamin C and β-cryptoxanthin from foods and beverages were inversely associated with symptom scores—particularly voiding symptoms. Similarities between vitamin C and β-cryptoxanthin are likely the result of their correlation and shared common sources, primarily orange juice. These results agree with studies of men with high to moderately severe LUTS [10] as well as men with moderate to severe voiding symptoms in BACH [12]. Oxidative damage may contribute to LUTS [6,7], and both vitamin C and β-cryptoxanthin have been shown to protect against damage from oxidative stress as well as enhance DNA repair [22,23]. However, it is possible that other unmeasured or unidentified components of foods high in vitamin C and β-cryptoxanthin contributed to the observed associations—particularly given the lack of similar inverse associations for vitamin C from supplements.
Women using high-dose vitamin C supplements or who had high total (dietary plus supplemental) intake of vitamin C were more likely to report storage symptoms of frequency and urgency. Similar findings for high-dose vitamin C were observed among BACH men [12]. For the average adult, body stores of vitamin C are adequately maintained with 75 mg/d ascorbic acid, and doses above 200 mg are mostly excreted through urine [22]. Highly acidic urine, such as could result from high-dose vitamin C [24], plausibly could affect the urothelium and contribute to LUTS. The urothelium responds to changes in its environment, including urine composition and pH, by releasing diffusible agents and modulating the activity of afferent nerves and underlying smooth muscles [25]. A study of the effects of urinary pH on bladder sensitivity in asymptomatic women found that increased urine acidity led to increased micturition desire [26]. Ascorbic acid has also been found to activate mast cells, which are present in the urothelium, thereby possibly contributing to LUTS [27,28]. These hypothesized pathways are congruent with the null results regarding dietary vitamin C, because vitamin C from diet alone was below the maximum absorbable dose and therefore unlikely to be rapidly excreted through urine.
We observed an interaction between β-carotene and smoking, whereby women who smoked and took β-carotene supplements were more than three times as likely to report storage symptoms compared to smokers who did not take β-carotene. Among nonsmokers, β-carotene supplement use was not associated with LUTS. These findings are consistent with those for men [12] and with prior knowledge on harmful properties of supplemental β-carotene for individuals exposed to environmental carcinogens. At high oxygen pressures and in smoke-exposed animals, β-carotene acts as a pro-oxidant, diminishes retinoid signaling, and enhances cell proliferation [20].
The positive findings regarding calcium intake and LUTS are novel and worthy of further investigation. The Leicestershire MRC Incontinence Study found no associations between dietary calcium and 1-yr incidence of urgency or urinary incontinence in women; however, women who consumed >2.4 cups (568 ml) of milk per day were significantly more likely to develop urgency and OAB symptoms [13,14,29]. Of relevance, calcium supplement use and calcium ions may increase bacterial adherence to uroepithelial cells and urinary tract infection (UTI) risk [21,30]. We confirmed a positive association between total calcium intake and UTI in our data (not shown), but a separate case-control study of dietary calcium did not [31]. Whether asymptomatic UTIs or bacteriuria have a mediating role in our results is uncertain.
Given the noninterventional design of BACH, we cannot be certain that the observed associations were causal. It is possible that unknown factors associated with both dietary intake levels and LUTS affected these results. For example, the positive association between dietary zinc and storage symptoms may be the result of the high correlation between dietary zinc and protein, which was associated with storage symptoms in BACH [17,32]. Also, it is possible that combinations of nutrients from foods are more influential than individual nutrients. A limitation of the cross-sectional design is that women with LUTS may have decreased their intakes of acidic foods in an attempt to relieve symptoms, given popular beliefs on dietary bladder irritants. However, such systematically lower vitamin C intake among cases would have resulted in an inverse or null association between vitamin C and storage symptoms rather than explain the reported positive findings. Furthermore, qualitative data from a subset of symptomatic BACH participants indicates that <0.1% of women considered changing dietary constituents to help cope with LUTS (unpublished data). Recent evidence that LUTS are dynamic and often remit over time among women [33,34] provides additional support for our use of proximate measures of diet and LUTS, assuming relatively immediate mechanisms of action of nutrients on the bladder.
5. Conclusions
Strengths of this analysis are the use of a population-based, racially/ethnically diverse sample of women and validated measures for diet and LUTS. Overall, these findings suggest that for some women, OAB symptoms of frequency and urgency could possibly be ameliorated by modifying high-dose supplements of vitamin C and—among smokers—β-carotene. Above moderate absorbable doses, very high doses of vitamin C may be less likely to offer health benefits while possibly irritating the bladder. If future research confirms the hypothesized pathway of urine acidity, then treatments to directly normalize urine acidity might be beneficial for LUTS. In contrast, foods rich in vitamin C and β-cryptoxanthin have well-known health benefits that may further extend to voiding symptoms, and their consumption is encouraged. Additional research, including randomized trials where appropriate, is needed to confirm these novel findings, which identify possible noninvasive management strategies for LUTS.
Acknowledgments
Funding/Support and role of the sponsor: This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, Grant No. R21DK081844. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Author contributions: Nancy N. Maserejian had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Maserejian, McKinlay.
Acquisition of data: McKinlay.
Analysis and interpretation of data: Maserejian, Giovannucci, McVary.
Drafting of the manuscript: Maserejian.
Critical revision of the manuscript for important intellectual content: Maserejian, Giovannucci, McVary.
Statistical analysis: Maserejian.
Obtaining funding: Maserejian, McKinlay.
Administrative, technical, or material support: Maserejian.
Supervision: McKinlay.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Dr McVary has received speaking fees from Eli Lilly, Sanofi-Aventis, and Advanced Health Media. He has also received grant and research support from GlaxoSmithKline and Allergan as well as consulted for Eli Lilly and Allergan.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) survey. Arch Intern Med. 2006;166:2381–7. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 2.Tennstedt SL, Link CL, Steers WD, McKinlay JB. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: the Boston Area Community Health (BACH) survey. Am J Epidemiol. 2008;167:390–9. doi: 10.1093/aje/kwm356. [DOI] [PubMed] [Google Scholar]
- 3.Wennberg AL, Molander U, Fall M, Edlund C, Peeker R, Milsom I. Lower urinary tract symptoms: lack of change in prevalence and help-seeking behaviour in two population-based surveys of women in 1991 and 2007. BJU Int. 2009;104:954–9. doi: 10.1111/j.1464-410X.2009.08534.x. [DOI] [PubMed] [Google Scholar]
- 4.Hubeaux K, Deffieux X, Ismael SS, Raibaut P, Amarenco G. Autonomic nervous system activity during bladder filling assessed by heart rate variability analysis in women with idiopathic overactive bladder syndrome or stress urinary incontinence. J Urol. 2007;178:2483–7. doi: 10.1016/j.juro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002;4(Suppl 4):S7–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Roosen A, Chapple CR, Dmochowski RR, et al. A refocus on the bladder as the originator of storage lower urinary tract symptoms: a systematic review of the latest literature. Eur Urol. 2009;56:810–9. doi: 10.1016/j.eururo.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Kupelian V, McVary KT, Barry MJ, et al. Association of C-reactive protein and lower urinary tract symptoms in men and women: results from Boston Area Community Health survey. Urology. 2009;73:950–7. doi: 10.1016/j.urology.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–90. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristal AR, Arnold KB, Schenk JM, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167:925–34. doi: 10.1093/aje/kwm389. [DOI] [PubMed] [Google Scholar]
- 10.Rohrmann S, Giovannucci E, Willett WC, Platz EA. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr. 2007;85:523–9. doi: 10.1093/ajcn/85.2.523. [DOI] [PubMed] [Google Scholar]
- 11.Tavani A, Longoni E, Bosetti C, et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: a case-control study from Italy. Eur Urol. 2006;50:549–54. doi: 10.1016/j.eururo.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Maserejian NN, Giovannucci E, McVary KT, McKinlay JB. Dietary, but not supplemental, intake of carotenoids and vitamin C are associated with decreased odds of lower urinary tract symptoms in men. J Nutr. 2011;141:267–73. doi: 10.3945/jn.110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallosso H, Matthews R, McGrother C, Donaldson M. Diet as a risk factor for the development of stress urinary incontinence: a longitudinal study in women. Eur J Clin Nutr. 2004;58:920–6. doi: 10.1038/sj.ejcn.1601913. [DOI] [PubMed] [Google Scholar]
- 14.Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM. Nutrient composition of the diet and the development of overactive bladder: a longitudinal study in women. Neurourol Urodyn. 2004;23:204–10. doi: 10.1002/nau.20028. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald MP, Link CL, Litman HJ, Travison TG, McKinlay JB. Beyond the lower urinary tract: the association of urologic and sexual symptoms with common illnesses. Eur Urol. 2007;52:407–15. doi: 10.1016/j.eururo.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maserejian NN, Giovannucci EL, McVary KT, McGrother C, McKinlay JB. Dietary macronutrient and energy intake and urinary incontinence in women. Am J Epidemiol. 2010;171:1116–25. doi: 10.1093/aje/kwq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maserejian NN, McVary KT, Giovannucci EL, McKinlay JB. Dietary macronutrient intake and lower urinary tract symptoms in women. Ann Epidemiol. doi: 10.1016/j.annepidem.2010.11.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) survey. Eur Urol. 2007;52:389–96. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 20.Paolini M, Abdel-Rahman SZ, Sapone A, et al. Beta-carotene: a cancer chemopreventive agent or a co-carcinogen? Mutat Res. 2003;543:195–200. doi: 10.1016/s1383-5742(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 21.Apicella LL, Sobota AE. Increased risk of urinary tract infection associated with the use of calcium supplements. Urol Res. 1990;18:213–7. doi: 10.1007/BF00295850. [DOI] [PubMed] [Google Scholar]
- 22.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo Y, Azqueta A, Luna L, Bonilla F, Dominguez G, Collins AR. The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis. 2009;30:308–14. doi: 10.1093/carcin/bgn270. [DOI] [PubMed] [Google Scholar]
- 24.Axelrod DR. Ascorbic acid and urinary pH. JAMA. 1985;254:1310–1. doi: 10.1001/jama.1985.03360100058010. [DOI] [PubMed] [Google Scholar]
- 25.Birder LA, Kanai AJ, Cruz F, Moore K, Fry CH. Is the urothelium intelligent? Neurourol Urodyn. 2010;29:598–602. doi: 10.1002/nau.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavin JM, Hosker GL, Smith AR. Does urinary pH influence micturition desire? Neurourol Urodyn. 1997;16:396–7. [Google Scholar]
- 27.Batler RA, Sengupta S, Forrestal SG, Schaeffer AJ, Klumpp DJ. Mast cell activation triggers a urothelial inflammatory response mediated by tumor necrosis factor-alpha. J Urol. 2002;168:819–25. [PubMed] [Google Scholar]
- 28.Dillon PF, Root-Bernstein RS, Lieder CM. Ascorbate enhancement of H1 histamine receptor sensitivity coincides with ascorbate oxidation inhibition by histamine receptors. Am J Physiol Cell Physiol. 2006;291:C977–84. doi: 10.1152/ajpcell.00613.2005. [DOI] [PubMed] [Google Scholar]
- 29.Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int. 2003;92:69–77. doi: 10.1046/j.1464-410x.2003.04271.x. [DOI] [PubMed] [Google Scholar]
- 30.Geesey GG, Wigglesworth-Cooksey B, Cooksey KE. Influence of calcium and other cations on surface adhesion of bacteria and diatoms: a review. Biofouling. 2000;15:195–205. doi: 10.1080/08927010009386310. [DOI] [PubMed] [Google Scholar]
- 31.Kontiokari T, Nuutinen M, Uhari M. Dietary factors affecting susceptibility to urinary tract infection. Pediatr Nephrol. 2004;19:378–83. doi: 10.1007/s00467-003-1410-z. [DOI] [PubMed] [Google Scholar]
- 32.Maserejian NN, Giovannucci EL, McKinlay JB. Dietary macronutrients, cholesterol, and sodium and lower urinary tract symptoms in men. Eur Urol. 2009;55:1179–89. doi: 10.1016/j.eururo.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidler S, Deveza C, Temml C, et al. The natural history of lower urinary tract symptoms in females: analysis of a health screening project. Eur Urol. 2007;52:1744–50. doi: 10.1016/j.eururo.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Wennberg AL, Molander U, Fall M, Edlund C, Peeker R, Milsom I. A longitudinal population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in women. Eur Urol. 2009;55:783–91. doi: 10.1016/j.eururo.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 36.Scarpero HM, Fiske J, Xue X, Nitti VW. American Urological Association Symptom Index for lower urinary tract symptoms in women: correlation with degree of bother and impact on quality of life. Urology. 2003;61:1118–22. doi: 10.1016/s0090-4295(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 37.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:520–4. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 38.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 39.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]