Abstract
Background
The role of malnutrition has not been well studied in patients undergoing surgery for renal cell carcinoma (RCC).
Objective
Our aim was to evaluate whether nutritional deficiency (ND) is an important determinant of survival following surgery for RCC.
Design, setting, and participants
A total of 369 consecutive patients underwent surgery for locoregional RCC from 2003 to 2008. ND was defined as meeting one of the following criteria: body mass index <18.5 kg/m2, albumin <3.5 g/dl, or preoperative weight loss ≥5% of body weight.
Intervention
All patients underwent radical or partial nephrectomy.
Measurements
Primary outcomes were overall and disease-specific mortality. Covariates included age, Charlson comorbidity index (CCI), preoperative anemia, tumor stage, Fuhrman grade, and lymph node status. Multivariate analysis was performed using a Cox proportional hazards model. Mortality rates were estimated using the Kaplan-Meier product-limit method.
Results and limitations
Eighty-five patients (23%) were categorized as ND. Three-year overall and disease-specific survival was 58.5% and 80.4% in the ND cohort compared with 85.4% and 94.7% in controls, respectively (p < 0.001). ND remained a significant predictor of overall mortality (hazard ratio [HR]: 2.41, 95% confidence interval [CI], 1.40–4.18) and disease-specific mortality (HR: 2.76; 95% CI, 1.17–6.50) after correcting for age, CCI, preoperative anemia, stage, grade, and nodal status. This study is limited by its retrospective nature.
Conclusions
ND is associated with higher mortality in patients undergoing surgery for locoregional RCC, independent of key clinical and pathologic factors. Given this mortality risk, it may be important to address nutritional status preoperatively and counsel patients appropriately.
Keywords: Hypoalbuminemia, Malnutrition, Nephrectomy, Renal cell carcinoma
1. Introduction
Renal cell carcinoma (RCC) is one of the most lethal urologic cancers, and the death rate has been rising over time despite an increase in early detection and surgical intervention [1]. Although several studies have defined predictors of mortality after nephrectomy to assist with decision making before surgery, the evaluated factors have generally been nonmodifiable (eg, age, race, gender, stage, and tumor size) [2–4]. Despite evidence from other malignancies that preoperative nutritional status may affect patient morbidity and mortality, the impact of nutritional status on patient outcomes after surgical treatment of RCC has not been specifically addressed.
Although the precise definition of malnutrition differs widely, multiple studies have demonstrated a deleterious impact of poor nutritional status on survival after surgery for gastrointestinal malignancies. Preoperative weight loss, low body mass index (BMI), and hypoalbuminemia have all been associated with worse outcomes after cancer surgery [5–8], and nutritional status also predicts higher mortality and length of stay in medical inpatients [9]. The role of nutritional status in patients undergoing surgery for genitourinary malignancies has not been well evaluated; however, we have recently shown that nutritional status is closely associated with survival in patients undergoing radical cystectomy for bladder cancer [10].
Given the prognostic importance of preoperative malnutrition in surgical patients with other malignancies and its potentially modifiable nature, the significance of nutritional deficiency (ND) in patients with RCC remains a key question. We hypothesized that nutritional status would be associated with worse survival in RCC patients undergoing radical or partial nephrectomy.
2. Methods
We performed a retrospective cohort study of 369 consecutive patients who underwent radical or partial nephrectomy for locoregional RCC from 2003 to 2008. Patients with distant metastases were excluded; however, patients were not excluded for the presence of direct adrenal invasion, regional lymph node metastases, or tumor thrombus. All surgeries were performed at Vanderbilt University Medical Center (Nashville, TN<USA). The treating physicians determined the follow-up regimen, which included chest/abdominal imaging as well as laboratory testing at defined intervals. Cause of death was determined by the treating physicians, death certificate, and/or chart review. Pathologic specimens were evaluated by a surgical pathologist, with stage and grade determined according to the 2010 American Joint Committee on Cancer guidelines and Fuhrman grading system, respectively. Clinical, pathologic, and outcome data were collected prospectively and supplemented by medical record review. Institutional review board approval was obtained for the creation of a prospective database and retrospective analysis of this patient population.
The primary outcomes were overall and disease-specific survival. Duration of follow-up was the time from surgery to the date of death or last clinic visit. Patients alive at last follow-up were censored for overall and disease-specific survival.
We recorded and analyzed clinicopathologic variables including age, gender, race (white vs nonwhite), Charlson comorbidity index (CCI), preoperative anemia (hematocrit <41 for men and <36 for women), Fuhrman grade (I–II vs III–IV), pathologic T stage, lymph node status, tumor histology (clear cell vs non–clear cell), and procedure performed (radical vs partial nephrectomy). Patients without radiographic or palpable evidence of lymphadenopathy generally did not undergo lymphadenectomy (Nx) and were grouped with pathologic N0 patients for analysis. Nutritional status information was gathered through medical record review. Patients were classified into two groups: nutritionally replete (NR) and ND. Although there is no standard definition for ND, we have previously defined ND as the presence of one or more of the following factors: preoperative albumin lower than the institutional-specific normal values (3.5–5 g/dl), unintentional preoperative weight loss ≥5% within 6 mo, or preoperative BMI <18.5 kg/m2 [10]. When change in weight could not be verified by chart records, patient-reported weight change was used.
2.1. Statistical analysis
The relationship between nutritional status and clinicopathologic variables was assessed using chi-square or Fisher exact tests for categorical variables and t test for continuous variables. Univariate survival analyses were performed using the Kaplan-Meier and log-rank methods. For the multivariate survival analyses, Cox proportional hazards models for overall and disease-specific survival were constructed. Those variables found to have a significant association with overall or disease-specific survival on univariate analysis were included in the multivariate analysis: age, stage, grade, nodal status, preoperative anemia, and nutritional status. CCI was also included to control for comorbid conditions. In addition to assessing ND as a composite variable, a separate analysis of overall survival was performed using each of the three ND variables. A total of 357 patients (97%) had complete information for all variables, and these patients were included in the multivariate survival analyses. All analyses were conducted with Stata v.11 data analysis software (StataCorp, College Station, TX, USA).
3. Results
The median age of the cohort was 61 yr (interquartile range [IQR]: 52–69 yr), and median follow-up was 22 mo (IQR: 14–37 mo). The median follow-up for surviving patients was 24 mo (IQR: 16–38 mo). Table 1 shows the distribution of clinicopathologic variables. By univariate analysis, anemia, stage, and grade were significantly associated with ND. Only 11 patients (3%) in the overall cohort had lymph node metastases; however, the association between lymph node status and ND approached statistical significance. Additionally, tumor diameter was greater in ND patients (6.17 vs 5.15 cm; t test p = 0.009).
Table 1.
Patient demographics
| All | Nutritionally deficient | ||||||
|---|---|---|---|---|---|---|---|
| n | % | No | % | Yes | % | P* | |
| All | 369 | 100 | 284 | 77 | 85 | 23 | |
| Age, yr | |||||||
| ≤50 | 79 | 21 | 71 | 25 | 8 | 9 | |
| 51–60 | 100 | 27 | 75 | 26 | 25 | 29 | |
| 61–70 | 110 | 30 | 81 | 29 | 29 | 34 | |
| 71–80 | 66 | 18 | 46 | 16 | 20 | 24 | |
| >80 | 14 | 4 | 11 | 4 | 3 | 4 | 0.035 |
| Sex | |||||||
| Female | 141 | 38 | 107 | 38 | 34 | 40 | |
| Male | 228 | 62 | 177 | 62% | 51 | 60 | 0.7 |
| Race | |||||||
| White | 337 | 91 | 261 | 92 | 76 | 89 | |
| Nonwhite | 30 | 8 | 22 | 8 | 8 | 9 | 0.61 |
| Charlson comorbidity index | |||||||
| 0 | 182 | 49 | 143 | 50 | 39 | 46 | |
| 1 | 92 | 25 | 70 | 25 | 22 | 26 | |
| 2 | 40 | 11 | 29 | 10 | 11 | 13 | |
| 3 | 37 | 10 | 28 | 10 | 9 | 11 | |
| ≥4 | 18 | 5 | 14 | 5 | 4 | 5 | 0.96 |
| Anemia | |||||||
| No | 276 | 75 | 227 | 80 | 49 | 58 | |
| Yes | 92 | 25 | 57 | 20 | 35 | 41 | <0.001 |
| pT stage | |||||||
| T1 | 236 | 64 | 190 | 67 | 46 | 54 | |
| T2 | 42 | 11 | 32 | 11 | 10 | 12 | |
| T3 | 84 | 23 | 59 | 21 | 25 | 29 | |
| T4 | 7 | 2 | 3 | 1 | 4 | 5 | 0.039* |
| N stage | |||||||
| N0 | 358 | 97 | 278 | 98 | 80 | 94 | |
| N+ | 11 | 3 | 6 | 2 | 5 | 6 | 0.073 |
| Grade | |||||||
| I | 50 | 14 | 39 | 14 | 11 | 13 | |
| II | 188 | 51 | 156 | 55 | 32 | 38 | |
| III | 92 | 25 | 65 | 23 | 27 | 32 | |
| IV | 29 | 8 | 17 | 6 | 12 | 14 | 0.009 |
| Histology | |||||||
| Clear cell | 267 | 72 | 205 | 72 | 62 | 73 | |
| Non–clear cell | 94 | 25 | 73 | 26 | 21 | 25 | 0.86 |
| Nephrectomy | |||||||
| Radical | 234 | 63 | 173 | 61 | 61 | 72 | |
| Partial | 135 | 37 | 111 | 39 | 24 | 28 | 0.068 |
All p values from chi-square test, except as noted.
The p value from the Fisher exact test.
Note: Percentages may not add up to 100 due to missing data.
Of the 369 patients in the cohort, 85 (23%) met the criteria for ND by having at least one of the following: preoperative albumin <3.5 g/dl (5.1%), BMI <18.5 kg/m2 (1.5%), and weight loss ≥5% (17.0%) (Table 2). Seven patients (2%) had two ND factors, and no patients had three.
Table 2.
Distribution of patients by nutritional status variables
| Characteristic | Strata | No. (%) |
|---|---|---|
| Albumin | <3.5 g/l | 20 (5.1) |
| ≥3.5 g/l | 339 (87.1) | |
| Body mass index | <18.5 | 6 (1.5) |
| ≥18.5 and <25 | 91 (23.4) | |
| ≥25 and <30 | 119 (30.6) | |
| ≥30 and <40 | 152 (39.1) | |
| Weight loss | >10% | 40 (10.3) |
| 5–10% | 26 (6.7) | |
| None; <5% | 299 (76.9) | |
| Nutritionally deficient | – | 85 (23.0) |
| Nutritionally replete | – | 284 (77.0) |
There were 61 (16.5%) all-cause mortalities (31 [36.5%] in ND vs 30 [10.5%] in controls) and 26 (7.3%) disease-specific deaths (16 [19.8%] in ND vs 26 [7.3%] in controls). Three-year overall survival was 58.5% (95% confidence interval [CI], 43.8–70.5%) for ND patients and 85.5% (95% CI, 78.8–90.2%) for NR patients (Fig. 1; p < 0.001). Three-year disease-specific survival was 80.4% (95% CI, 68.8–88.1%) for ND patients and 94.7% (95% CI, 93.5–98.3%) for nutritionally replete patients (Fig. 2; p < 0.001). On univariate analysis, predictors of overall mortality were age, stage (overall p < 0.001), grade, anemia, and ND (Table 3). Predictors of disease-specific mortality by univariate analysis were anemia, stage (overall p < 0.001), grade, nodal status, and ND (Table 4). On multivariate analysis, ND was an independent predictor of overall mortality (hazard ratio [HR]: 2.41; 95% CI, 1.40–4.18) and disease-specific mortality (HR: 2.76; 95% CI, 1.17–6.50) after correcting for age, CCI, anemia, stage, grade, and nodal status.
Fig. 1.
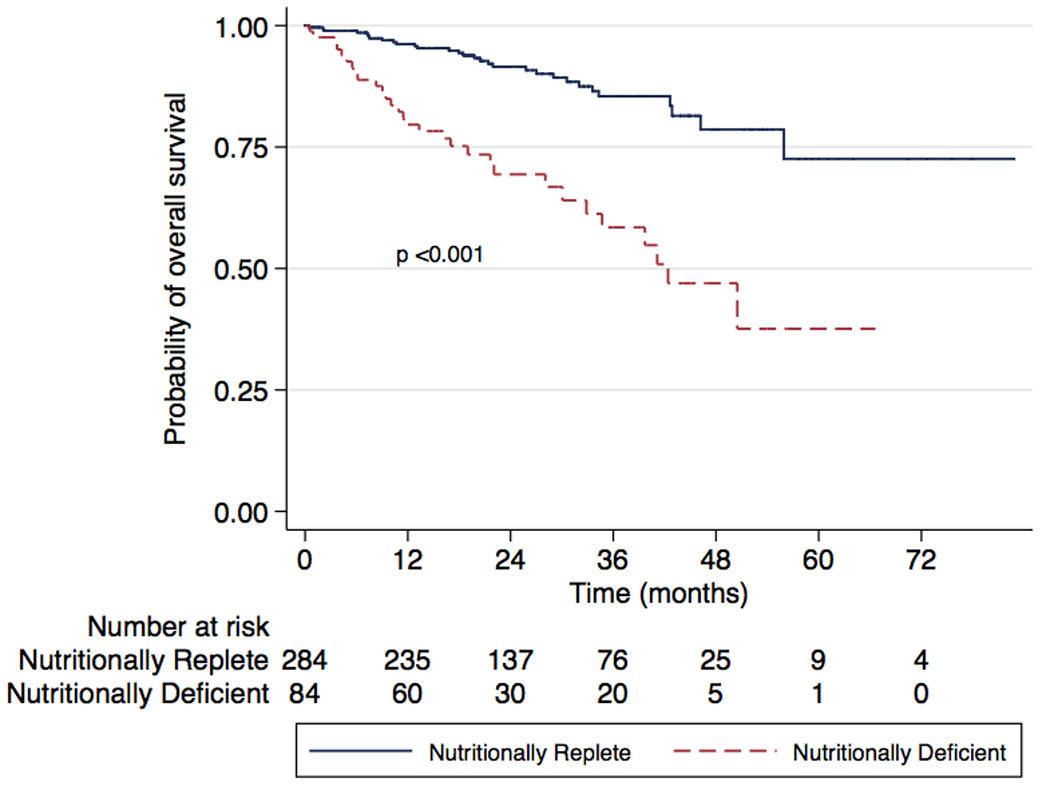
Kaplan-Meier analysis of overall survival in nutritionally deficient and nutritionally replete patients (log rank p < 0.001).
Fig. 2.
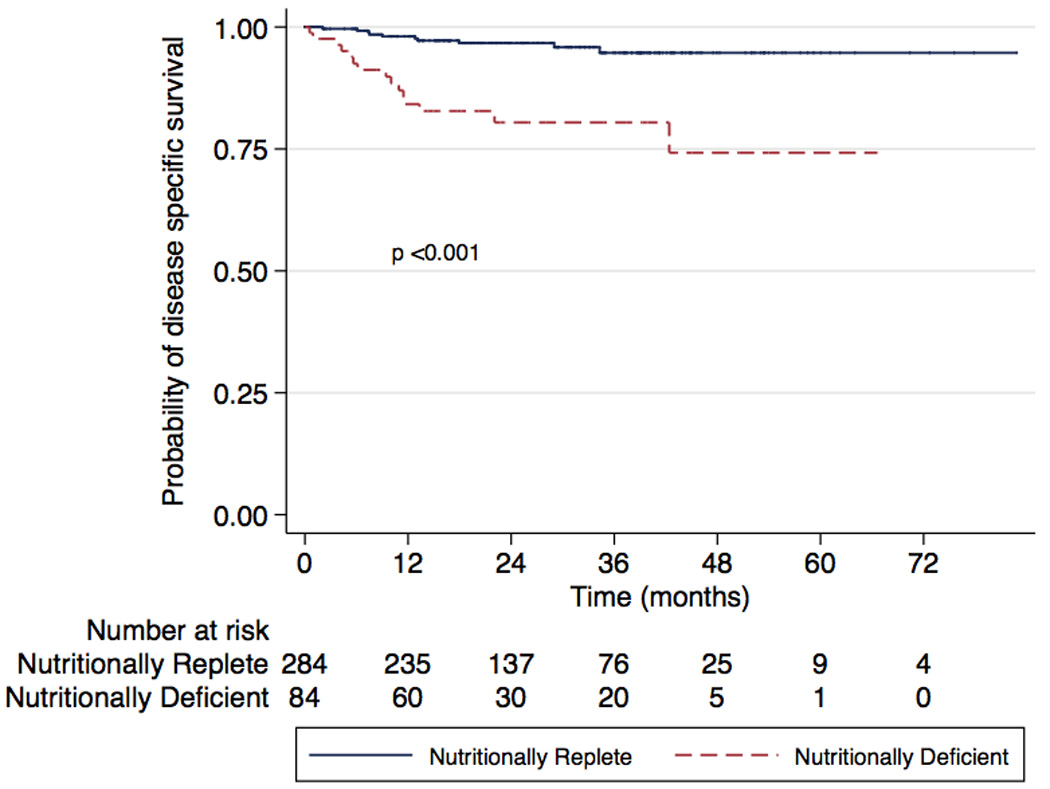
Kaplan-Meier analysis of disease-specific survival in nutritionally deficient and nutritionally replete patients (log rank p < 0.001).
Table 3.
Cox univariate and multivariate regression analysis for overall survival
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | CI | P | HR | CI | p | |
| Age | 1.04 | 1.01–1.06 | 0.001 | 1.02 | 0.99–1.05 | 0.08 |
| Charlson comorbidity index | 1.07 | 0.87–1.31 | 0.51 | 1.07 | 0.85–1.35 | 0.57 |
| pT stage | ||||||
| T1 | Referent | Referent | ||||
| T2 | 0.33 | 0.08–1.38 | 0.13 | 0.21 | 0.03–1.53 | 0.12 |
| T3 | 2.04 | 1.19–3.52 | 0.01 | 1.06 | 0.57–1.97 | 0.86 |
| T4 | 29.30 | 9.32–92.16 | <0.001 | 4.41 | 1.04–18.66 | 0.044 |
| Node positive | 8.67 | 4.08–18.43 | <0.001 | 5.68 | 1.86–17.38 | 0.002 |
| Grade | ||||||
| I–II | Referent | Referent | ||||
| III–IV | 3.67 | 2.15–6.28 | <0.001 | 2.23 | 1.25–3.98 | 0.007 |
| Anemia | 3.44 | 2.06–5.75 | <0.001 | 2.11 | 1.18–3.79 | 0.012 |
| Nutritionally deficient | 3.84 | 2.31–6.38 | <0.001 | 2.41 | 1.40–4.18 | 0.002 |
CI = confidence interval; HR = hazard ratio.
Table 4.
Cox univariate and multivariate regression analysis for disease-specific survival
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | CI | P | HR | CI | p | |
| Age | 1.02 | 0.98–1.05 | 0.32 | 0.99 | 0.96–1.03 | 0.76 |
| Charlson comorbidity index | 0.65 | 0.42–1.02 | 0.063 | 0.73 | 0.42–1.29 | 0.28 |
| pT stage | ||||||
| T1 | Referent | Referent | ||||
| T2 | 3.58 | 0.60–21.45 | 0.16 | 1.77 | 0.18–17.52 | 0.63 |
| T3 | 16.75 | 4.90–57.17 | <0.001 | 6.85 | 1.84–25.52 | 0.004 |
| T4 | 148.91 | 27.45–807.75 | <0.001 | 38.61 | 5.36–277.97 | <0.001 |
| Node positive | 18.87 | 7.80–45.66 | <0.001 | 2.22 | 0.66–7.38 | 0.20 |
| Grade | ||||||
| I–II | Referent | Referent | ||||
| III–IV | 17.29 | 5.13–58.20 | <0.001 | 7.67 | 2.06–28.65 | 0.002 |
| Anemia | 5.54 | 2.42–12.68 | <0.001 | 2.04 | 0.79–5.25 | 0.14 |
| Nutritionally deficient | 5.58 | 2.50–12.41 | <0.001 | 2.76 | 1.17–6.50 | 0.02 |
CI = confidence interval; HR = hazard ratio.
We also assessed the three ND criteria without dichotomization. In addition to the previously listed covariates, albumin and BMI were included as continuous variables, and weight loss was categorized as none, >5%, or >10%. Weight loss (HR: 1.53; 95% CI, 1.09–2.15) and albumin (HR: 2.56; 95% CI, 1.30–5.00) were each independent predictors of overall survival. BMI as a continuous variable was not an independent predictor of overall survival (HR: 0.97; 95% CI, 0.92–1.03; p = 0.33), although only six patients were underweight.
4. Discussion
Although the association between nutritional status and mortality after surgery has been evaluated in other malignancies, few data exist on the impact of nutritional status in patients undergoing surgery for genitourinary cancers. We showed that ND is independently associated with both overall and disease-specific survival in patients undergoing radical or partial nephrectomy for RCC. The magnitude of the association was particularly notable, with HRs of 2.41 and 2.76 for overall and disease-specific survival, respectively.
These findings are supported by evidence from patients undergoing procedures for gastrointestinal malignancies, with a number of studies finding an association between preoperative nutritional status and mortality. A randomized study of neoadjuvant chemotherapy in patients undergoing surgery for esophageal cancer found weight loss >10% to be a significant predictor of mortality [5]. In a prospective study of >1400 patients undergoing colorectal surgery, most for cancer, patients with preoperative weight loss >10% had a nearly four-fold increased risk of postoperative mortality [6].
Although a number of scoring systems have been proposed for standardized assessment of nutritional status, we sought to use objective and easily measurable criteria: albumin, weight loss, and BMI. Many scoring systems include one or more of these objective measures; however, most include subjective measures as well. In addition, these scoring systems have generally not yet been validated, and none have gained widespread consensus. The Nutritional Risk Screening (NRS) tool, for example, consists of assessments of weight loss, BMI, food intake, and disease severity [11]. The NRS was prospectively studied in 456 patients admitted for treatment of a urologic malignancy, and 24% were classified at severe risk of malnutrition based on NRS score, a number comparable with the 23% in our study [12]. Another tool, the Nutritional Risk Index (NRI), is based on serum albumin and change in weight [13]. Both NRS and NRI correlate with the rate and severity of perioperative complications in patients undergoing gastrointestinal surgery [14].
Hypoalbuminemia is an imperfect marker of nutritional status given its long half-life and the potential impact of systemic factors such as inflammation and stress on serum albumin; however, it is an easily obtainable measure and correlates well with other markers of nutritional status [15,16]. Additionally, multiple studies have demonstrated that serum albumin is an important predictor of postoperative morbidity and mortality [13,17]. As a marker of protein-energy malnutrition, albumin provides important information that supplements both BMI and change in body weight. Protein malnutrition can result in edema, impaired organ function, and immunosuppression, but BMI and weight change may remain within normal limits and not accurately reflect nutritional status [18,19].
The potential association between nutritional status and survival in patients undergoing surgery for RCC has not been specifically addressed in prior studies and is not currently used in any RCC predictive models [20]. There is, however, prior evidence that some aspects of nutritional status may be important predictors of outcomes in patients with RCC. In a study aiming to characterize the impact of paraneoplastic effects of RCC, Kim et al [21] assessed a large number of presenting signs and symptoms in patients undergoing nephrectomy for localized or metastatic RCC. They found that hypoalbuminemia, weight loss, anorexia, and malaise were independently associated with disease-specific survival. Termed cachexia-related findings, these four variables were believed to be indicative of paraneoplastic tumor activity. In an analysis of just those patients with localized T1 disease, individuals with at least one of these findings had an increased likelihood of disease-specific death [22].
In contrast to these studies, to our knowledge ours is the first designed to evaluate the impact of nutritional status on survival in patients with RCC. In addition to serum albumin, we used percentage weight loss and BMI to assess preoperative ND more directly. Furthermore, these criteria carry the important advantage of being objective, easily measured values. We found that 23% of patients undergoing surgery for locoregional RCC met at least one of these criteria, with weight loss ≥5% the most frequent indicator of ND. Even after correcting for other clinicopathologic factors, the magnitude of the association between ND and survival was large, suggesting this potentially modifiable variable has a substantial impact on patient outcome. Although both albumin and weight loss were independent predictors of survival on their own, use of a combined definition of ND that includes all three variables is a more practical and comprehensive approach to identifying patient risk based on nutritional status. In addition, anemia was associated with nutritional status, and it was also an independent predictor of mortality, further supporting the need for a complete clinical and laboratory evaluation of these patients before surgery.
Although the relationship between nutritional status and mortality after cancer surgery has been recognized in other malignancies, there are mixed data regarding whether a targeted intervention such as preoperative nutritional supplementation can improve outcomes in these patients. A set of European guidelines for surgical patients supports the use of preoperative nutritional support in the severely malnourished even if it delays surgery [23]. However, although a number of studies in general surgery patients have found nutritional supplementation to decrease morbidity in malnourished patients, few have shown a survival benefit [24–26]. In patients requiring surgery for RCC who meet the criteria for ND, consideration of nutritional intervention may be appropriate. Although our data suggest a potential avenue for improving outcomes in patients with RCC, it remains possible that nutritional status may be a marker of disease status and not be modifiable. Specifically, nutritional status may be a systemic inflammatory response marker because a number of studies have demonstrated a relationship between acute-phase reactants and survival in patients with RCC. In a meta-analysis, increased C-reactive protein, platelet, and erythrocyte sedimentation rate levels were all correlated with increased mortality in RCC patients [27]. Other inflammatory indicators associated with RCC outcomes include interleukin-6, vascular endothelial growth factor, and serum amyloid [27,28]. Further research is needed to determine the effect of these factors on nutrition and whether they may mitigate any benefit of nutritional intervention. Only a controlled prospective trial will be able to assess these questions adequately.
As a single-center retrospective evaluation, this study has important limitations. Median follow-up was only 22 mo, and complete data were not available from all patients in the initial cohort. However, we did have complete information on 97% of patients for all variables, limiting the effect of any potential bias from excluded patients. Additionally, although we controlled for all available clinically and statistically significant confounders in our database, unmeasured confounders may have affected the results. In particular, performance status and patient symptoms at diagnosis were not routinely recorded in this cohort and therefore could not be included in the statistical model. Finally, there are no established criteria to evaluate preoperative nutritional status before radical nephrectomy, and we were limited to a specific set of nutritional parameters. Still, the parameters used here are common ones in nutritional scoring systems, and they offer the benefit of being objective and easily obtainable.
5. Conclusions
We found an independent association between preoperative nutritional status and survival after partial or radical nephrectomy for locoregional RCC. These data suggest a need for increased awareness of preoperative nutritional status and offer a readily assessable tool for improving patient counseling before surgery. Prospective studies are needed to confirm and further understand the impact of nutritional status and potential nutritional intervention in patients with RCC.
Take-home message
We evaluated 369 patients who underwent surgery for renal cell carcinoma and found a significant association between preoperative nutritional deficiency and overall and disease-specific survival. Nutritional intervention may offer a potential avenue for improving the outcomes of patients undergoing nephrectomy.
Acknowledgments
Funding/Support and role of the sponsor: The design and conduct of the project described was supported in part by Award Number K08 CA113452 (PEC) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Todd M. Morgan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Morgan, Barocas, Clark.
Acquisition of data: Morgan, Tang, Stratton, Anderson, Gregg, Clark.
Analysis and interpretation of data: Morgan, Tang, Barocas, Cookson, Chang, Herrell, Smith, Clark.
Drafting of the manuscript: Morgan, Tang, Barocas, Clark.
Critical revision of the manuscript for important intellectual content: Morgan, Tang, Stratton, Barocas, Anderson, Gregg, Chang, Cookson, Herrell, Smith, Clark.
Statistical analysis: Morgan, Barocas, Clark.
Obtaining funding: Clark.
Administrative, technical, or material support: Clark.
Supervision: Barocas, Chang, Cookson, Herrell, Smith, Clark.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Michael S. Cookson is a financially affiliated consultant for Watson and Endo, a financially affiliated lecturer for Firmagon and Sanofi-Aventis, is compensated financially for a scientific study/trial by GlaxoSmith Kline, and is noncompensated for scientific research at Astra Zeneca. S. Duke Herrell is a financially affiliated consultant for Aesculap Inc, Covidien Surgical Devices, and Wilex. He is a financially affiliated investigator for Wilex and a financially compensated stockholder at Veran Medical Tech. Sam S. Chang is a compensated consultant for Sanofi-Aventis, Endo, Allergan, and Centocor Orth Biotech. Peter E. Clark is a compensated consultant for Galil Medical. All other authors have nothing to disclose.
References
- 1.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 2.Karakiewicz PI, Suardi N, Capitanio U, et al. A preoperative prognostic model for patients treated with nephrectomy for renal cell carcinoma. Eur Urol. 2009;55:287–295. doi: 10.1016/j.eururo.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 3.Cindolo L, de la Taille A, Messina G, et al. A preoperative clinical prognostic model for non-metastatic renal cell carcinoma. BJU Int. 2003;92:901–905. doi: 10.1111/j.1464-410x.2003.04505.x. [DOI] [PubMed] [Google Scholar]
- 4.Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010;28:311–317. doi: 10.1200/JCO.2009.22.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 6.Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278–283. doi: 10.1001/archsurg.140.3.278. [DOI] [PubMed] [Google Scholar]
- 7.Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23:393–401. doi: 10.1111/j.1365-277X.2010.01058.x. [DOI] [PubMed] [Google Scholar]
- 8.Mullen JT, Davenport DL, Hutter MM, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. 2008;15:2164–2172. doi: 10.1245/s10434-008-9990-2. [DOI] [PubMed] [Google Scholar]
- 9.de Luis D, Lopez Guzman A. Nutritional status of adult patients admitted to internal medicine departments in public hospitals in Castilla y Leon, Spain—a multi-center study. Eur J Intern Med. 2006;17:556–560. doi: 10.1016/j.ejim.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Gregg JR, Cookson MS, Phillips S, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. doi: 10.1016/j.juro.2010.09.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 12.Karl A, Rittler P, Buchner A, et al. Prospective assessment of malnutrition in urologic patients. Urology. 2009;73:1072–1076. doi: 10.1016/j.urology.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 14.Schiesser M, Kirchhoff P, Müller MK, Schäfer M, Clavien P-A. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery. 2009;145:519–526. doi: 10.1016/j.surg.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Baker JP, Detsky AS, Wesson DE, et al. Nutritional assessment: a comparison of clinical judgement and objective measurements. N Engl J Med. 1982;306:969–972. doi: 10.1056/NEJM198204223061606. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 17.Detsky AS, Baker JP, O’Rourke K, et al. Predicting nutrition-associated complications for patients undergoing gastrointestinal surgery. JPEN J Parenter Enteral Nutr. 1987;11:440–446. doi: 10.1177/0148607187011005440. [DOI] [PubMed] [Google Scholar]
- 18.Lipschitz DA. Protein-energy malnutrition. Hosp Pract (Off Ed) 1988;23:87–99. doi: 10.1080/21548331.1988.11703582. [DOI] [PubMed] [Google Scholar]
- 19.Llop JM, Munoz C, Badia MB, et al. Serum albumin as indicator of clinical evolution in patients on parenteral nutrition. Multivariate study. Clin Nutr. 2001;20:77–81. doi: 10.1054/clnu.2000.0159. [DOI] [PubMed] [Google Scholar]
- 20.Lane BR, Kattan MW. Prognostic models and algorithms in renal cell carcinoma. Urol Clin North Am. 2008;35:613–625. doi: 10.1016/j.ucl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Kim HL, Belldegrun AS, Freitas DG, et al. Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol. 2003;170:1742–1746. doi: 10.1097/01.ju.0000092764.81308.6a. [DOI] [PubMed] [Google Scholar]
- 22.Kim HL, Han K-r, Zisman A, Figlin RA, Belldegrun AS. Cachexia-like symptoms predict a worse prognosis in localized T1 renal cell carcinoma. J Urol. 2004;171:1810–1813. doi: 10.1097/01.ju.0000121440.82581.d3. [DOI] [PubMed] [Google Scholar]
- 23.Weimann A, Braga M, Harsanyi L, et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr. 2006;25:224–244. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 25.Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24:7–14. doi: 10.1177/014860710002400107. [DOI] [PubMed] [Google Scholar]
- 26.Muller JM, Brenner U, Dienst C, Pichlmaier H. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet. 1982;1:68–71. doi: 10.1016/s0140-6736(82)90212-4. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Fu X, Zhu X, et al. Prognostic role of systemic inflammatory response in renal cell carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2011 doi: 10.1007/s00432-010-0951-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero AJ, Diaz-Montero CM, Millikan RE, et al. Cytokines and angiogenic factors in patients with metastatic renal cell carcinoma treated with interferon-alpha: association of pretreatment serum levels with survival. Ann Oncol. 2009;20:1682–1687. doi: 10.1093/annonc/mdp054. [DOI] [PMC free article] [PubMed] [Google Scholar]


