Abstract
Metformin, the first-line drug for treating diabetes, selectively kills the chemotherapy-resistant, sub-population of cancer stem cells in genetically distinct types of breast cancer cell lines. In mouse xenografts, injection of metformin and the chemotherapeutic drug doxorubicin near the tumor is more effective than either drug alone in blocking tumor growth and preventing relapse. Here, we show that metformin is equally effective when given orally together with paclitaxel, carboplatin, and doxorubicin indicating that metformin works together with a variety of standard chemotherapeutic agents. In addition, metformin has comparable effects on tumor regression and preventing relapse when metformin combined with a 4-fold reduced dose of doxorubicin that is not effective as a monotherapy. Lastly, the combination of metformin and doxorubicin prevents relapse in xenografts generated with prostate and lung cancer cell lines. These observations provide further evidence for the cancer stem cell hypothesis for cancer relapse, as well as an experimental rationale for using metformin as part of combinatorial therapy in a variety of clinical settings and for reducing the chemotherapy dose in cancer patients.
Keywords: metformin, chemotherapy, xenografts, cancer stem cells
INTRODUCTION
Although chemotherapeutic regimens often suppress tumor growth, cancer patients show high variability in their responses and commonly develop resistance to these drugs and recurrence of the tumor. To explain this phenomenon, the cancer stem cell hypothesis suggests that tumors contain a small number of tumor-forming, self-renewing, cancer stem cells (CSCs) within a population of non-tumor-forming cancer cells (1, 2). Unlike most cells within the tumor, CSCs are resistant to chemotherapy, and after treatment, they can regenerate all the cell types in the tumor through their stem cell-like behavior. A prediction of this CSC hypothesis is that treatments that selectively inhibit cancer stem cells should function together with chemotherapeutic drugs to delay relapse. Strong support for the CSC hypothesis comes from our observations that metformin, the first-line drug for treating diabetes, and miR-200 selectively kill CSCs and act together with doxorubicin to reduce tumor growth and prolong remission (3, 4).
Another major problem that cancer patients face is the high toxicity of the chemotherapeutic drugs. These side effects including anemia, appetite changes, fatigue, hair loss, nausea, vomiting and fertility changes (5). On the other hand, lower doses of these chemotherapeutic agents are not effective in suppressing tumor growth. Thus, the identification of agents that can be combined with lower doses of the existing chemotherapeutic drugs is of high clinical relevance.
Epidemiological studies have revealed that diabetes is correlated with increased cancer risk (6, 7), and that diabetics treated with metformin have reduced risk of developing various types of cancer (8, 9). In addition, mRNA profiling of two isogenic models of cellular transformation defined a transcriptional signature that links multiple types of cancer with diabetes and other metabolic diseases (10). Metformin is an extensively used and well-tolerated drug for treating individuals with type 2 diabetes, obesity, and polycystic ovarian syndrome. Metformin inhibits growth of breast cancer cell lines (11–13), blocks cellular transformation in an inducible model system (3, 10), and has anti-tumor effects in mouse xenografts (3, 10, 14, 15). As mentioned above, we showed that metformin selectively inhibits the growth of CSCs in genetically distinct breast cancer cells lines. Furthermore, in mouse xenografts involving a human breast cancer cell line, co-injection of metformin and doxorubicin intra-peritoneally near the tumor increases the rate of tumor regression compared to treatment with doxorubicin alone, and this combinatorial therapy prevents relapse for at least several months (3).
Our previous studies of metformin-based combinatorial therapy in mouse xenografts were performed by intra-peritoneal injection, and they involved a single concentration of one chemotherapeutic agent and tumors generated by a single breast cancer cell line. Here, we extend these studies to address 1) the efficacy of metformin administered orally as compared with intra-peritoneal injection, 2) the ability of metformin to act together with chemotherapeutic agents other than doxorubicin, 3) the effect of metformin on the concentration of chemotherapeutic drugs needed for a prolonging remission, and 4) whether combinatorial therapy involving metformin is effective on other cancer cell types. Our results show that oral administration of metformin is effective with multiple chemotherapeutic agents and multiple cancer cell types, and that metformin can reduce the dose of chemotherapy necessary to prolong remission. These preclinical observations strongly support the use of metformin-based combinatorial chemotherapy in multiple cancer contexts, and they provide further evidence for the cancer stem cell hypothesis for relapse.
MATERIALS AND METHODS
Cell lines
MCF-10A cells are mammary epithelial cells derived from fibrocystic breast tissue from woman with no family history of breast cancer and no evidence of disease; these cells do not express estrogen receptor (ER). The experiments here use a derivative of MCF-10A containing an integrated fusion of the v-Src oncoprotein with the ligand-binding domain of ER (3). BT-474 cells are human breast carcinoma cells that overexpress HER2 receptor, while MDA-MB-231 cells are human mammary adenocarcinoma cells with mutation in p53 and overexpression of EGF receptor and are highly metastatic. PC3 cells are highly metastatic grade IV prostate adenocarcinoma cells, and A549 cells are human lung epithelial carcinoma cells.
Cell culture
BT-474, MDA-MB-231, and PC3 cells were grown in DMEM media (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), and penicillin/streptomycin (Invitrogen) at 37°C with 5% CO2. A549 cells were grown in RPMI1640 media (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), and penicillin/streptomycin (Invitrogen) at 37°C with 5% CO2. MCF10A ER-Src cells were cultured as described previously (Iliopoulos et al., Cell, 2009) and induced to transform with 1 uM 4OH-tamoxifen dissolved (Sigma) in ethanol. Transformation occur 36h post tamoxifen treatment.
Chemicals
Metformin was dissolved in water and was typically added to 0.1 mmol/L. Doxorubicin and paclitaxel were dissolved in DMSO, and carboplatin was dissolved in water. All chemicals were obtained from Sigma-Aldrich.
Mammosphere culture
Mammospheres were cultured in suspension (1,000 cells/mL) in serum-free DMEM/F12 media, supplemented with B27 (1:50; Invitrogen), 0.4% bovine serum albumin, 20 ng/mL EGF (Preprotech) and 4 ug/mL insulin (Sigma) as described previously (16). Mammosphere growth was tested by placing purified CD44high/CD24low cells from 3 human mammary adenocarcinoma tissues in suspension and adding metformin to 6-d-old mammospheres and counting the number of mammospheres 2 days after treatment.
Human breast tissues
Three snap-frozen ductal carcinoma tissues were purchased from AMS Biotechnology and Biochain Inc. The purification process of CD44high and CD24high derived from human breast tumors has been previously described (17–19), and it is detailed in the supplemental methods.
Xenograft experiments
Cancer cells (5×106) were injected into the right flank of female nu/nu mice (Charles River Laboratories), all of which developed tumors in 10 d with size of ~55 mm3. For each experiment, mice were randomly distributed into equal groups (3–4 mice per group) that were untreated (NT), or treated by intra-tumoral injections every 5 d (four cycles) with 1 mg/kg or 4 mg/kg doxorubicin, 10 mg/kg paclitaxel, 20 mg/kg carboplatin 200 μg/ml metformin (diluted in the drinking water and present throughout the experiment starting at day 10), or combinations that include metformin. Tumor volume (mean ± SD) (mm3) was measured at various times after the initial injection. All the mouse experiments were performed in accordance with Institutional Animal Care and Use Committee procedures and guidelines of Tufts University.
RESULTS
Oral administration of metformin is equally effective as i.p. injection near the tumor
In our previous experiments showing that the combination of metformin and doxorubicin accelerates tumor regression and prolongs remission in mouse xenografts, the drugs were administered by intraperitoneal (i.p.) injection near the tumors (3). However, clinical use of metformin in diabetes is typically performed by oral administration, and this would clearly be a preferred treatment option. To test the effectiveness of oral administration of metformin, mice bearing tumors 10 days after injection of the ER-Src cells were treated with 4 mg/kg doxorubicin (4 cycles of intra-tumoral injection on days 10, 15, 20, 25), 200 μg/ml metformin (in drinking water, which corresponds to 15 mg/kg) or the combination. This protocol involves continuous presence of metformin starting at day 10, which differs from the original procedure in which metformin treatment ceased after the last injection at 20 days.
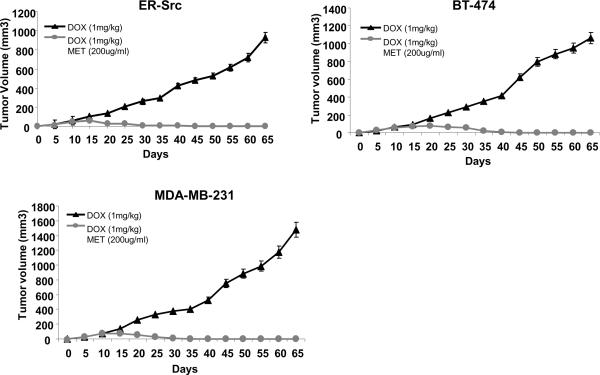
In mice treated with doxorubicin alone, tumor growth was suppressed until day 40, after which time the tumor growth resumed at rates near that observed for untreated control mice (Fig 1A). Mice treated with metformin alone had reduced tumor growth in comparison with the untreated mice, and after day 45 there was a mild regression of the tumor. This level of regression is comparable to that achieved by i.p injection of metformin at 20 mg/kg (10); injection of metformin alone at 2.5 mg/kg had very little effect on tumor formation (3). However, the combination of doxorubicin and metformin not only suppressed tumor growth, but it prolonged remission with no detectable tumor for at least 65 days. Similar results were obtained in xenografts generated by two genetically distinct breast cancer cell lines, BT-474 and MDA-MB-231 (Fig. 1A; BT-474 was more sensitive to doxorubicin alone, and metformin did not accelerate tumor regression in MDA-MB-231). Thus, oral administration of metformin is effective in combination with doxorubicin to accelerate tumor regression (except in tumors derived from MDA-MB-231) and prolong remission in xenografts involving a variety of breast cancer cell lines.
Figure 1.
Oral administration of metformin together with doxorubicin suppress tumor growth and relapse in breast xenografts. A, Tumor volume (mean ± SD) in mice injected (on day 0) with transformed ER-Src cells, BT-474 cancer cells, and MDA-MB-231 cancer cells that were untreated (NT), treated by intra-tumoral injections (days 10, 15, 20, 25) with 4 mg/kg doxorubicin, treated continuously with metformin in drinking water (200 μg/ml) starting at day 10 or both. Tumor volume was monitored 65 days post injection. B, Percent (mean ± SD) of CD44high/CD24low cells (CSCs) derived from tumors derived from the indicated cell lines (day 25) in mice that were untreated, treated with doxorubicin (1 or 4 mg/kg), metformin (200 μg/ml) or both. C, CD44high/CD24low cells (CSCs) and CD44low/CD24high cells (NSCCs) purified from human mammary adenocarcinomas were treated with metformin (0.1 mmol/L) for 48h or left untreated for the same time. Data is presented as percent cell growth, which is determined by the number of metformin-treated cells divided by the number of non-treated cells at the 48h time point. D, Number of mammospheres per 1000 cells derived from human mammary adenocarcinomas after treatment with metformin (0.1 mmol/L) for 48h.
Metformin selectively kills CSCs in human breast tumors
Metformin selectively kills CD44high/CD24low cells (3), which are commonly termed cancer stem cells (CSCs) as defined by their ability to form self-renewing mammospheres and cause tumor formation at high efficiency (3, 4, 16). We will use the term CSC to refer to CD44high/CD24low cells throughout this work, even though such cells can be generated by IL6 treatment of cancer cells and hence do not behave as classic stem cells (19). In accord with our previous experiments involving i.p injection, oral administration of metformin causes a decrease in the percent of CSCs derived from tumors at day 25, whereas doxorubicin alone has no effect (Fig. 1B). Importantly, the combination of metformin and doxorubicin further reduces the percent of CSCs, indicating that therapeutic advantage of oral administration of metformin is linked to its ability to inhibit CSC growth.
As is often the case for CSCs (1, 2), the CSCs examined here are largely resistant to doxorubicin treatment (3). However, the observation that the percent of CSCs in the doxorubicin-treated mice is similar to that of untreated mice suggests that doxorubicin effectively kills CSCs during tumor regression. We suggest that this apparent paradox is explained by the dynamic equilibrium between CSCs and non-stem cancer cells (NSCCs) that occurs in cell culture and mouse xenografts and is mediated by IL6 secretion (19). Specifically, the percent of CSCs is relatively constant, because differentiation of CSCs into NSCCs is balanced by IL6-mediated conversion of NSCCs to CSCs. Thus, doxorubicin directly kills NSCCs, and this indirectly reduces the CSC population by preventing the IL6-mediated conversion.
As our previous results involved CSCs isolated from cancer cell lines, we examined whether metformin selectively inhibits the growth of CSCs from human breast tumors. CSCs (CD44high/CD24low) and non-stem cancer cells (NSCCs; CD44low/CD24high) cells (NSCCs) were purified from human mammary adenocarcinomas, and treated with doxorubicin and/or metformin. As expected, metformin preferentially kills CSCs over NSCCs derived from these human breast tumors (Fig. 1C), and it inhibits growth of mammospheres derived from these tumors (Fig. 1D). Thus, metformin selectively kills breast CSCs obtained from human cancer patients. As shown previously (3), selective killing of CSCs vs. NSCCs is not absolute and depends on the metformin concentration. However, selective killing of CSCs clearly occurs at the metformin concentration used in xenograft experiments, and this concentration is roughly comparable to what is used to treat human patients with type II diabetes.
Metformin works in combination with multiple chemotherapeutic agents
According to the cancer stem cell hypothesis, combinatorial chemotherapy should be effective as long as one agent preferentially kills CSCs whereas the other agent preferentially kills NSCCs. In this regard, metformin and miR-200 selectively inhibit CSCs, and each of these agents prolongs remission in combination with doxorubicin (3, 4). To address this issue in a reciprocal fashion, we examined the effectiveness of metformin in combination with paclitaxel or carboplatin, standard chemotherapeutic drugs for treating breast cancer patients as monotherapies or in combination with other biological therapies such as trastuzumab, a HER2 monoclonal antibody (20, 21). As monotherapies, treatment of ER-Src and MDAMB-231 xenograft tumors with paclitaxel (Fig. 2A) or carboplatin (Fig. 2B) suppressed tumor growth, but did not prevent relapse. At the concentrations used, these drugs were somewhat more effective than doxorubicin, as relapse occurred after 55–60 days as opposed to 50 days. Importantly, the combination of metformin with either paclitaxel or carboplatin prolonged relapse, similarly to that observed with doxorubicin. Thus, metformin-based combinatorial therapy is effective with at least three different chemotherapeutic drugs, suggesting that metformin would be useful in combination with other chemotherapeutic agents that primarily target non-stem cancer cells.
Figure 2.
Combination of metformin with paclitaxel or carboplatin suppresses tumor growth and prolongs remission. A, Tumor volume (mean ± SD) in mice injected on day 0 with transformed ER-Src or MDA-MB-231 cancer cells that were untreated (NT), treated by intratumoral injections (days 10, 15, 20, 25) with 10 mg/kg paclitaxel (PTX) or treated with paclitaxel together with metformin diluted in drinking water (200 μg/ml). B, Tumor volume in mice injected with transformed ER-Src or MDA-MB-231 cancer cells that were untreated (NT), treated by intra-tumoral injections (days 10, 15, 20, 25) with 20 mg/kg carboplatin (CAR) or treated with carboplatin together with metformin diluted in drinking water (200ug/ml).
Metformin can reduce the dose of doxorubicin necessary to prolong remission
As chemotherapy is toxic and causes unwanted, and often serious, side effects in cancer patients (5), a major challenge is to lower the doses of chemotherapeutic drugs without decreasing their effectiveness. We reasoned that the combinatorial effect of metformin might permit the lowering of the doxorubicin dose. In addition, as CSCs can differentiate into NSCCs, metformin might indirectly lower the NSCC burden by inhibiting CSCs. To test this idea, we performed oral, metformin-based combinatorial therapy in xenografts involving three different breast cancer cell lines using a 4-fold reduced concentration of doxorubicin from previous experiments (1 mg/kg instead of 4 mg/kg). As expected, the reduced dose of doxorubicin alone is less effective, as tumor growth was slowed compared to untreated control mice, but tumor regression was not observed (Figure 3). Nevertheless, the combination of metformin with this reduced dose of chemotherapy resulted in complete tumor regression and no detectable relapse for at least 65 days. Indeed, in the presence of metformin, the lowered dose of chemotherapy was equally as effective as the higher dose. These preclinical observations suggest the possibility of using metformin to lower the chemotherapy does in breast cancer patients.
Figure 3.
Metformin is effective when combined with lower doses of doxorubicin. Tumor volume (mean ± SD) in mice injected on day 0 with the indicated cancer cells that were treated by intra-tumoral injections (days 10, 15, 20, 25) with 1 mg/kg doxorubicin, metformin diluted in drinking water (200 µg/ml) or both.
Metformin-based combinatorial therapy is effective in xenografts generated with breast, prostate, and lung cancer cell lines
As our results have been confined to breast cancer cells, we examined whether metformin-based combinatorial therapy would be effective in other cancer types. Indeed, combinatorial treatment of metformin and doxorubicin suppresses growth of prostate (PC3) and lung adenocarcinoma (A549) xenografts tumors and inhibit their relapse (Fig. 4A, B). Tumor regression upon treatment is modestly accelerated by the combination of metformin and doxorubicin, with a slightly more pronounced effect observed with the higher doxorubicin dose than the lower dose. However, the absence of relapse due to metformin treatment was comparable at both doses of doxorubicin for the time frame of the experiment.
Figure 4.
Metformin together with doxorubicin inhibit prostate and lung tumor growth. A, Tumor volume (mean ± SD) in mice injected on day 0 with PC3 prostate cancer cells or B, A549 lung cancer cells that were untreated (NT), treated by intra-tumoral injections (days 10, 15, 20, 25) with 1 or 4 mg/kg doxorubicin (DOX) together with metformin diluted in drinking water (200 μg/ml).
DISCUSSION
Our pre-clinical studies in mouse xenografts indicate that oral administration of metformin together with widely used chemotherapeutic drugs such as doxorubicin, paclitaxel and carboplatin is highly effective in blocking tumor growth and preventing relapse in a variety of cancer cell types. In addition, metformin is effective in combination with reduced dosages of doxorubicin (and presumably other standard chemotherpeutic drugs), and hence appears to increase the effectiveness of standard chemotherapy. As metformin functions in the context of breast cancer cell xenografts by selectively killing breast CSCs, our results suggest the possibility that it might preferentially inhibit highly tumorigenic (CSC-like) cells in other developmental lineages. Our observations indicate that metformin has broad anti-cancer effects of potential utility in a wide variety of clinical contexts both for cancer treatment and for lowering the toxicity associated with standard chemotherapy. Metformin is a long-approved drug with excellent safety record, so clinical tests of these pre-clinical observations are practical and of potential medical significances for some (and perhaps many) types of cancer.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Guannan Wang for help with the CSC experiments. This work was supported by grants to K.S. from the Alexander and Margaret Stewart Trust and the National Institutes of Health (CA 107486).
REFERENCES
- 1.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cells traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells and acts together with chemotherapy to blocks tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliopoulos D, Lindahl-Allen M, Polytarchou C, H.A. H, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–72. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drews RE, Shulman LN. Update in hematology and oncology. Annals of internal medicine. 2010;152(10):655–62. doi: 10.7326/0003-4819-152-10-201005180-00244. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 7.Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007;86:867–71. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 8.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch HA, Iliopoulos D, Joshi A, Zhang Y, Jaeger SA, Bulyk M, et al. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell. 2010;17:348–61. doi: 10.1016/j.ccr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell cycle (Georgetown, Tex. 2009;8:909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell cycle (Georgetown, Tex. 2009;8:2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 13.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 14.Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, et al. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell cycle (Georgetown, Tex. 2010;9(1):188–97. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- 15.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 16.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kB, lin 28, let-7 microRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA. 2011;108:1397–402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang HR, Glaspy J, Allison MA, Kass FC, Elashoff R, Chung DU, et al. Differential response of triple-negative breast cancer to a docetaxel and carboplatin-based neoadjuvant treatment. Cancer. 2010;116:4227–37. doi: 10.1002/cncr.25309. [DOI] [PubMed] [Google Scholar]
- 21.Qi M, Li JF, Xie YT, Lu AP, Lin BY, Ouyang T. Weekly paclitaxel improved pathologic response of primary chemotherapy compared with standard 3 weeks schedule in primary breast cancer. Breast Cancer Res Treat. 2010;123(1):197–202. doi: 10.1007/s10549-010-1000-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.