Abstract
Background
Adolescent offspring of schizophrenia patients (SCZ-Off) are vulnerable to psychiatric disorders. Assessing relationships between clinical and biological measures (such as fMRI) may elucidate pathways of vulnerability in this group. Here we assessed the relationship between clinically assessed premorbid function, and cortico-striatal activity during sustained attention in controls (HC: with no family history of psychosis) and SCZ-Off.
Methods
Subjects (n=39) were assessed using the Schedule Interview for Prodromal Symptoms and the Scale of Prodromal Symptoms. Based on the GAF score, SCZ-Off were cleaved into “high” or “low” clinically functioning sub-groups (SCZ-OffHF, SCZ-OffLF respectively). During fMRI, subjects participated in a modified continuous performance task (CPT-IP). fMRI was conducted on a Bruker MedSpec 4T system (345 EPI scans; TR=2s; 24 slices; 3.8×3.8×4mm).
Results
SCZ-OffLF evinced less activation than both HC and SCZ-OffHF in the executive core of the brain’s attentional system (anterior cingulate, dorsal prefrontal cortex and caudate), but not visuo-spatial regions such as primary visual or superior parietal cortex. Differences were independent of behavioral performance, and reduction in activity was related to GAF score in a dose-dependent manner.
Discussion
Assessing the relationship between clinical measures and brain activity in domains such as attention provides a window into mechanisms of vulnerability in the developing adolescent brain.
Keywords: Schizophrenia, Development, Vulnerability, fMRI, Attention
1. Introduction
Sustained attention, or the ability to remain consistently (as opposed to transiently) focused on an ongoing task, is one of the most basic of cognitive domains and has been the subject of intense research scrutiny (Posner and Rothbart, 1998). In vivo imaging studies suggest that higher order mechanisms in sustained attention, relating to control and vigilance are particularly dependent on the brain’s fronto-striatal resources (Kelley et al., 2008). Sustained attention generally underlies many basic cognitive processes, and itself depends on the development of fronto-striatal regions including the prefrontal cortex, the anterior cingulate cortex and the basal ganglia, regions that lie within the “executive core” of the brain’s attention system (Rueda et al., 2005) and that rapidly connect during adolescence (Barnea-Goraly et al., 2005).
1.1 Adolescence, sustained attention and relevance for schizophrenia offspring
In the schizophrenia spectrum, attention deficits have been hypothesized not only as a marker of the illness itself, but also of risk for schizophrenia (Chen and Faraone, 2000; Cornblatt et al., 1988; Rutschmann et al., 1977). Thus, consistent with neurodevelopmental models of schizophrenia (Rapoport et al., 2005), deficits in sustained attention have been documented in larger cohorts of child and adolescent offspring of schizophrenia parents (SCZ-Off), presumably resulting from developmentally-mediated impairments in essential fronto-striatal neuro-circuitry (Keshavan et al., 2009). The abnormalities associated with risk may in fact result from altered development of critical neuro-circuitry during adolescence. In general, rapid changes in functional development during adolescence relate to rapid changes in the functional organization of cortical and sub-cortical structures. As networks rapidly evolve under a combination of genetic and environmental influences, their susceptibility to developmental deviations in both pre-natal and post-natal periods becomes increasingly acute (Rakic, 1999). The combination of developmental brain changes and psychosocial stressors, together, increases adolescent susceptibility to psychiatric disease resulting in estimated peak onsets of any lifetime mental health disorder as early as fourteen years of age (Kessler et al., 1994). Therefore, the altered trajectory of neurodevelopment hypothesized in the schizophrenia diathesis may significantly impact vulnerable groups such as SCZ-Off. Through adolescence, the expansion of functional competence is particularly marked in the domain of sustained attention, which is relatively immature early in childhood, but rapidly develops through adolescence (Rubia et al., 2006). This ascent in attention-related competence appears closely related to the development of well orchestrated interactions in fronto-parietal and basal-ganglia circuitry (Haber and Calzavara, 2009).
Very early assessments of SCZ-Off recognized the increased incidence of cognitive and behavioral impairments (Marcus, 1974), and several decades of subsequent research have pointed to increasingly reliable deficits in brain structure, behavior and function. Behavioral deficits are generally observed in domains including attention (Cornblatt and Malhotra, 2001; Rosenberg et al., 1997), executive function (Erlenmeyer-Kimling, 2000) and working memory (Diwadkar et al., 2001) and appear to be broadly associated with impairments in fronto-striatal structure and neurochemistry (Diwadkar et al., 2006; Keshavan et al., 2009). Specifically of these, deficits in attention appear to be the centerpiece of impairment in adolescence and the expansion in attention-related competence that is generally observed in controls is absent or attenuated in SCZ-Off (Cornblatt et al., 1999; Cornblatt and Keilp, 1994).
1.2 Attention and clinical measures in SCZ-Off
Only recently have studies used fMRI to investigate the correlates of sustained attention in groups at risk for schizophrenia. Adult siblings of schizophrenia patients (Sepede et al., 2010) showed hypo-activation (or less activation) in frontal circuitry (in the absence of behavioral deficits); differences from controls are amplified under conditions of increased attention demand. These results of impaired frontal recruitment suggest a relationship between vulnerability to schizophrenia and cortical recruitment. The study of child and adolescent SCZ-Off offers particularly unique advantages. This is a sub-group in whom the interactive effects of genetic and developmental vulnerabilities (Lewis and Levitt, 2002) can be assessed. Furthermore it is also possible to observe potential premorbid precursors of the illness in terms of fMRI measures of brain function. Finally, SCZ-Off also offer the opportunity of studying the vulnerable adolescent brain in a medication naïve state, thus providing potentially valuable insights into how the developing, yet vulnerable brain may respond to attention challenges.
In addition to assessing sustained attention-related processing in the brain, we were particularly interesting in investigating the relationship between the fMRI response and measures of premorbid clinical function in SCZ-Off using structured interviews such as the Structure Interview for Prodromal Symptoms (SIPS)(Miller et al., 2003). In SCZ-Off, the degree of risk and vulnerability for the onset of disorders is highly variable suggesting significant heterogeneity in vulnerability markers. Understanding the relationship between clinical assessments and the fMRI response may help in better understanding of the relationship between clinical symptoms and brain function. Characterizing these brain-behavior correlates of vulnerability in SCZ-Off, may significantly assist in the objective parcellation of vulnerability in this heterogeneous sample (Diwadkar et al., 2006).
Here we used fMRI to study mechanisms of sustained attention using a modified continuous performance (Cornblatt et al., 1989; Salgado-Pineda et al., 2004) in a group of controls (with no family history of psychosis to the 2nd degree) and adolescent offspring of schizophrenia patients. Independent from the fMRI analyses, subjects were administered the structured interview for the assessment of prodromal symptoms (Miller et al., 2003; Miller et al., 2002). The assessment of clinical function in SCZ-Off was based on the Global Assessment of Function (GAF) sub-scale of the SIPS. The GAF is an index of the psychological, social, and occupational functioning of individuals providing a measure of the extent of clinical impairment in individuals. This sub-scale is valuable as it allows for the categorical division of subjects into low- (scores < 80) and high- (scores > 80) functioning sub-groups. Based on this approach, we attempted to: a) separately evaluate each of the sub-groups (relative to controls) for differences in activation and, b) investigate the continuous relationship between this clinical measure and fMRI measures across the entire SCZ-Off sample. We hypothesized that if general clinical function impacts the engagement of sustained attention-related regions, subjects with greater clinical impairments would show hypo-responsivity of the fMRI response. The regional specificity of the response can provide evidence of the specificity of the impact of clinical impairments on brain function.
The analyses of fMRI data focused on the regions that collectively conform to the brain’s attention network. Among these were regions such the prefrontal cortex (BA 9/46), the dorsal anterior cingulate cortex and the caudate (Corbetta et al., 1998; Gregoriou et al., 2009; Reynolds et al., 2009) all implicated in higher order supervisory processes in attention, and the superior parietal and primary visual cortices (Offen et al., 2009; Thakral and Slotnick, 2009), implicated in sensory and spatial orienting mechanisms in sustained attention.
2. Methods
2.1 Subjects
Twenty-one healthy controls (HC), with no family history of psychosis to the 2nd degree, and eighteen adolescent offspring of schizophrenia patients (SCZ-Off) participated. The study was approved by the Human Subjects Investigation Committee at Wayne State University, and all subjects (and/or their legal guardians) provided informed consent/assent. Demographic information is provided in Table 1.
Table 1.
Characteristics of the HC and the SCZ-Off including age (with standard deviation), age range and mean GAF score (separated for the SCZ-Off sub-groups). SCZ-Off were drawn from 14 unique families. Thirteen of the ill parents were female.
| HC (n = 21) | SCZ-Off (n = 18) | |
|---|---|---|
| Gender (f/m) | 8/13 | 5/13 |
| Mean age (s.d.) | 14.6 yrs (2.7) | 14.0 yrs (3.1) |
| Age range (years) | 10 – 19 | 8 – 19 |
| Mean GAF score | 86 | SCZ-OffHF (n=6) = 88 SCZ-OffLF (n=12) = 70 |
2.2 Clinical assessment
Subjects were recruited from the greater Detroit area through advertisements and clinical services at the Wayne State University School of Medicine. Rule outs were achieved through telephone and personal interview, and screening questionnaires, to ascertain if subjects had a history of psychotic illness in first-degree relatives. The relationship between parents and offspring was verbally assured and not confirmed with genetic testing. Diagnoses for parents of offspring were reached using the Structured Clinical Interview for DSM-IV schizophrenia (First et al., 1997). All subjects younger than 15 years were clinically evaluated using the Schedule for Affective Disorders and Schizophrenia -Child Version (Kaufman et al., 1997); those aged 15 years or above were assessed using the SCID. In addition, experienced clinical raters assessed participants using the SIPS/SOPS scales (Miller et al., 2003). Within these instruments, the General Assessment of Function (GAF-M) subscale provides classification into subgroups characterized by minimal symptoms (Scores: 81–100) or transient to severe symptoms (Scores: 0–80) with lower scores indicating lower clinical function. In initial fMRI analyses, GAF score was used to cleave the SCZ-Off into clinically high (Minimal symptoms: SCZ-OffHF; n=6, 4 males, age: 14 yrs) - and low (Transient to Severe Symptoms: SCZ-OffLF; n=12, 9 males, age: 13.9 yrs) functioning sub-groups.
2.3 fMRI and task
Functional data were acquired over an 11.5 minute scan using a full body Bruker MedSpec 4.0 Tesla system running the Siemens Syngo console. Gradient echo planar images (EPI) were collected using an 8-channel head coil and the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; matrix size = 64 × 64; field of view (FOV) = 240 mm; voxel size = 3.75 × 3.75 × 4 mm. Images were axially localized, had automatic 3D adjustment, in twenty-four continuous 4 mm slices per brain volume positioned parallel to the anterior commissure/posterior commissure (AC-PC) line.
All subjects performed a modified continuous performance task (identical pairs version) during which three digit numbers were presented in rapid sequence (50 ms, 250 ms SOA) with subjects required to detect the repeated instance of a number. To preempt attention gains based on maximal contrast between figure (numbers) and background (screen)(Dresp and Grossberg, 1999), numbers (RGB:255,255,255, i.e.: white) were presented on background (RGB:225,225,225) that muted figure-ground contrast. Further, to preempt judgments based on low-level features such as an absence of flicker between repeated numbers, fonts alternated between successive digits in a sequence. These manipulations were induced to enhance the demand for attention processing, and reduce the reliance on low level visual features. Control epochs (120 s) involved the presentation of “000” or “111” for passive viewing, providing a measure of visual control. Extended block times (120 s) were used to induce an extended (as opposed to a transient) state of sustained attention. During attention blocks, a total of 480 stimuli were presented (25% targets). Block order was randomized and stimuli within each block were presented in the same pseudo-random order across subjects. Rest epochs (20 s) were interspersed to allow subjects to rest while viewing a fixation marker. Subjects signaled responses with a two-button response box.
2.4 Behavioral data analyses
Sensitivity to the task for both groups was compared using d’ (Macmillan and Creelman, 2005). d’ is the standard measure of discrimination in signal detection theory, and is a metric that includes all four types of behavioral responses (hits, false alarms, correct rejections and misses), thereby providing a comprehensive behavioral measure of how well subjects perform a signal detection task (Wickens, 2001).
2.5 fMRI data analyses
Data were processed with SPM5. Realignment was performed to correct for head motion artifact during the scan. Realigned images were normalized to the Montreal Neurological Institute (MNI) EPI template and voxels resliced to 2 × 2 × 2 mm. Normalized images were smoothed using an 8 mm FWHM Gaussian kernel. Images where estimated motion exceeded 4 mm were discarded from the analyses (<1% of all images).
In first level analyses, rest, control and attention epochs were modeled as separate regressors by convolving with the canonical hemodynamic response function. Serial correlations were corrected for using an auto-regression (AR(1)) filter, and an expanded high-pass filter (256 s) was applied to remove low frequency fluctuations associated with noise. First level contrasts (Attention > Control) were computed for each individual subject to identify responses associated with attention (as opposed simply to visual) related processing.
A second level analyses of covariance (ANCOVA) was employed (age, gender, and IQ as covariates) to assess the main effect of group (HC, SCZ-OffHF, SCZ-OffLF) and differences between groups in the a priori hypothesized regions of interest. Analyses were spatially thresholded using region of interest masks in stereotactic space using previously published methods (Maldjian et al., 2003). To identify inter-group differences, a hierarchical approach to analyses was employed, wherein individual contrasts were inclusively masked with significant clusters under the overall main effect of group (Friston et al., 1995). To assess the continuous relationship between the GAF score and BOLD measured activation in the entire offspring sample, extracted BOLD (normalized to a zero mean) from the clusters of significance based on the overall main effect were submitted to separate linear regressions (with raw GAF score as the single regressor).
To optimize sensitivity to detect clusters with significant voxels (pu<.001), cluster extent (kE) thresholds (pc<.05, corrected) were derived based on 104 Monte Carlo simulations from voxels across the individual regions of interest (Ward, 2000).
3. Results
3.1 Behavioral results
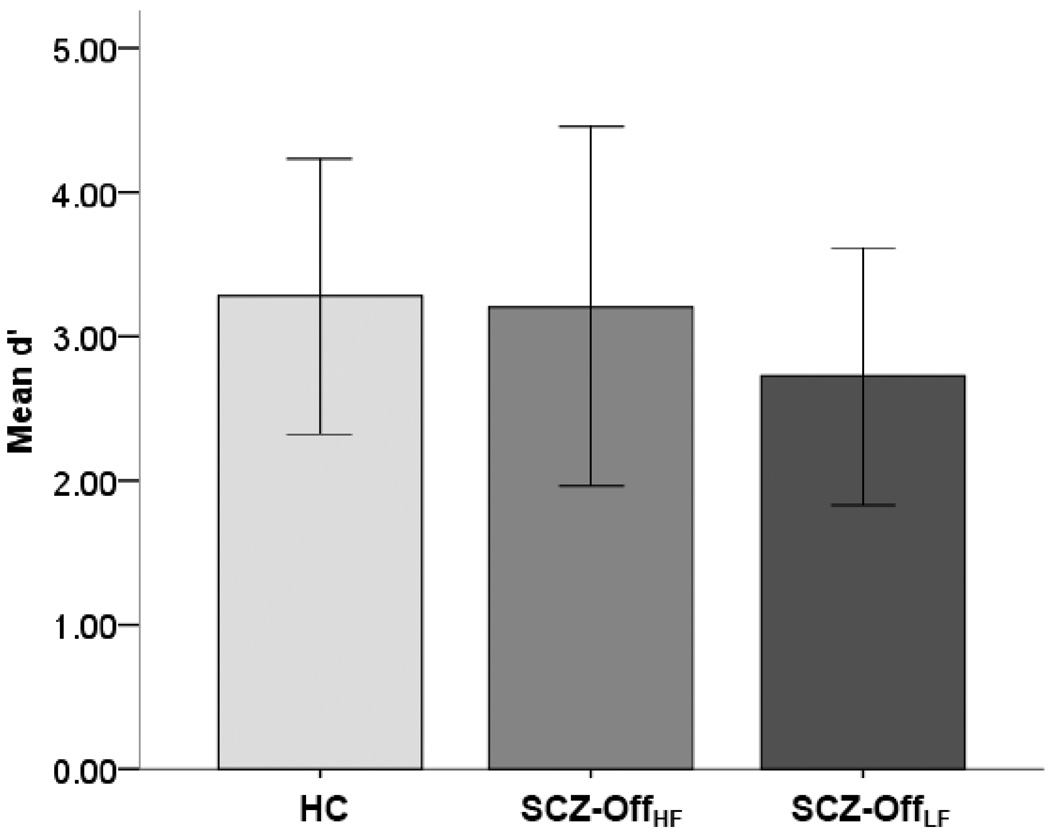
As noted, sensitivity to the task for both groups was compared using d’ (Macmillan and Creelman, 2005). These behavioral data were submitted to an analysis of covariance with group (HC, SCZ-OffHF, SCZ-OffLF), as single factor and age and gender as covariates. Figure 1, depicts performance, demonstrating no significant differences between groups (F2,37<1.2, p>.3). While performance was positively associated with age across the entire sample (n=39), F1,37=15.72, p<.001, separate regression analyses within the SCZ-Off group indicated no relationship between GAF score and d’, F1,16=.05, p>.75. These results indicate that behavioral performance across groups was not compromised, and did not confound any observed inter-group differences in fMRI.
Figure 1.
Mean Performance (d’ ± SD) during the continuous performance task is depicted, revealing equivalent discrimination sensitivity for each of the three groups.
3.2 fMRI results
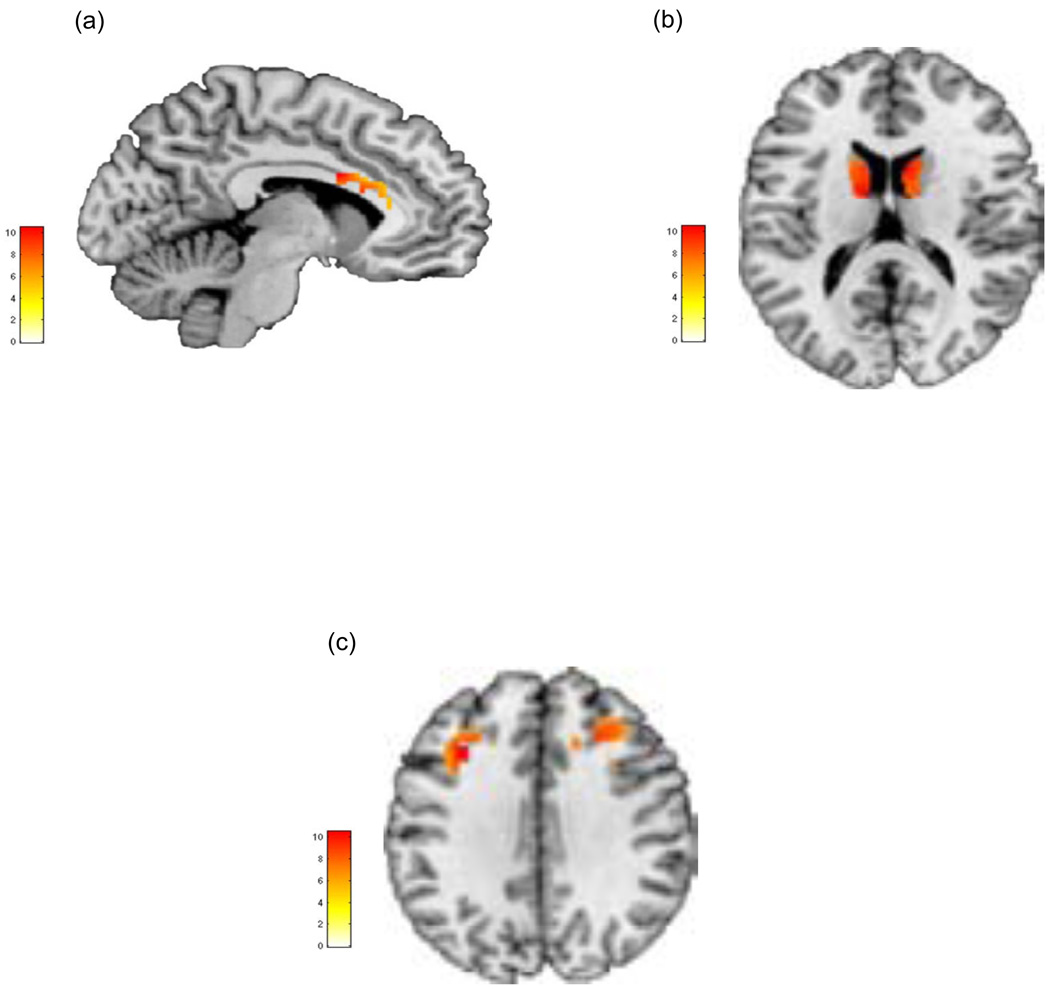
Figure 2 depicts significant clusters under the overall group main effect in the regions of interest for the study (with cluster peaks and statistical values presented in Table 2). As seen, significant main effects of group were observed in each of the anterior cingulate, caudate nucleus and the dorsal prefrontal cortex, with no significant differences observed in either the superior parietal or primary visual cortices.
Figure 2.
Clusters of significance under the main effect of group (HC, SCZ-OffHF, SCZ-OffLF) depicting significant effects are visualized in each of a) anterior cingulate cortex (sagittal view), b) bilateral caudate nucleus (axial view) and c) bilateral dorsal prefrontal cortex (axial view). Clusters are visualized at p<.05 (significance details are detailed in Table 2).
Table 2.
Peaks for the SPM (Statistical Parametric Mapping) analyses are indicated for each contrast of interest (Figure 2). All clusters were corrected for extent (pc<.05, corrected) within the region of, and for the contrast of interest (Ward, 2000). Voxel peaks (Talairach)(Lancaster et al., 2007) are listed at pu<.001. kE=observed voxel extent.
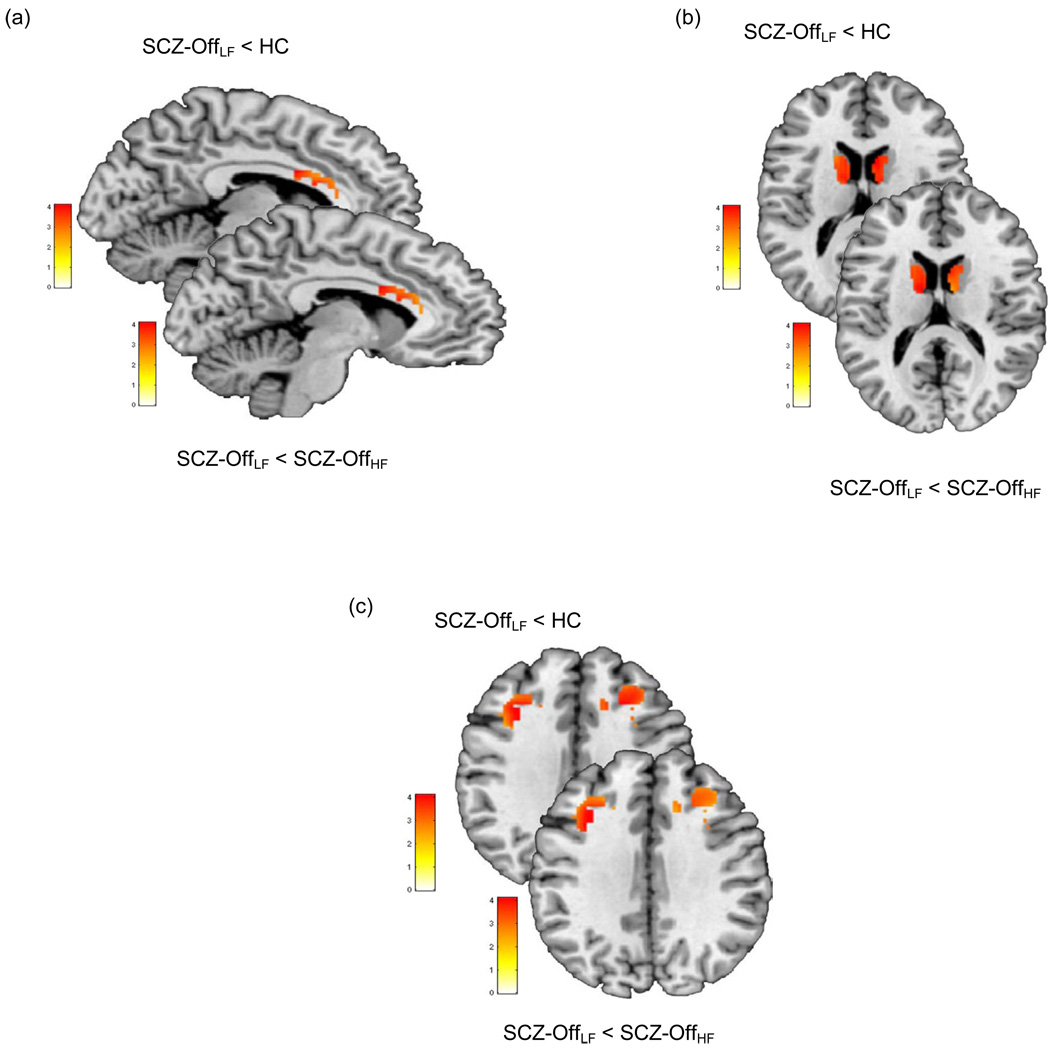
Inclusively masking for the overall main effect (p<.05), pair-wise directional contrasts were employed to compare SCZ-OffLF with each of the HC and the SCZ-OffHF groups, in each of the anterior cingulate, caudate and dorsal prefrontal clusters under the overall main effect. Figure 3 and Table 3 depict significant clusters using pair-wise directional contrasts. As seen, significant hypo-activation in the ACC, dPFC and caudate was observed in SCZ-OffLF compared to healthy controls (pc<.05, corrected). Next, intra-group (within SCZ-Off) comparisons were conducted to compare low- to high-functioning SCZ-Off groups. Again, decreased activation in the ACC, dPFC and caudate was observed for SCZ-OffLF < SCZ-OffHF (pc<.05, corrected).
Figure 3.
Pair wise contrasts under the clusters of significance under the overall main effect (Figure 2) were employed to assess whether activation in SCZ-OffLF was significantly lower than in each of the HC and the SCZ-OffHF groups. As seen in each of the a) anterior cingulate, b) caudate nucleus and c) dorsal prefrontal cortex SCZ-OffLF evinced significantly lower activity compare to the other groups (see Table 3 for details).
Table 3.
Peaks for the SPM analyses are indicated for each contrast of interest (Figure 3 a–c). All contrasts were based on t-statistics of the same ANOVA with the identical effective residual degrees of freedom (df = 33), using the Satterthwaite approximation of the Greenhouse–Geisser correction for violations of the sphericity assumption. All clusters were corrected for extent (pc<.05, corrected) within the region of, and for the contrast of interest (Ward, 2000). Voxel peaks are listed at pu<.001. kE=observed voxel extent.
| Contrast | Region | Peak Statistic (t33) |
kE | Peak (Talairach) | |
|---|---|---|---|---|---|
| Figure 3a | SCZ-OffLF < HC | Ant Cingulate | 4.14 | 612 | x=−7, y=4, z=27 |
| SCZ-OffLF < SCZ-OffHF | Ant Cingulate | 3.81 | 610 | x=−7, y=4, z=27 | |
| Figure 3b | SCZ-OffLF < HC | Caudate | 3.87 | 315 | x=8, y=6, z=18 |
| SCZ-OffLF < SCZ-OffHF | Caudate | 3.92 | 228 | x=−10, y=−4, z=17 | |
| Figure 3c | SCZ-OffLF < HC | dPFC | 3.85 | 212 | x=−29, y=17, z=32 |
| SCZ-OffLF < SCZ-OffHF | dPFC | 3.75 | 208 | x=−30, y=13, z=32 | |
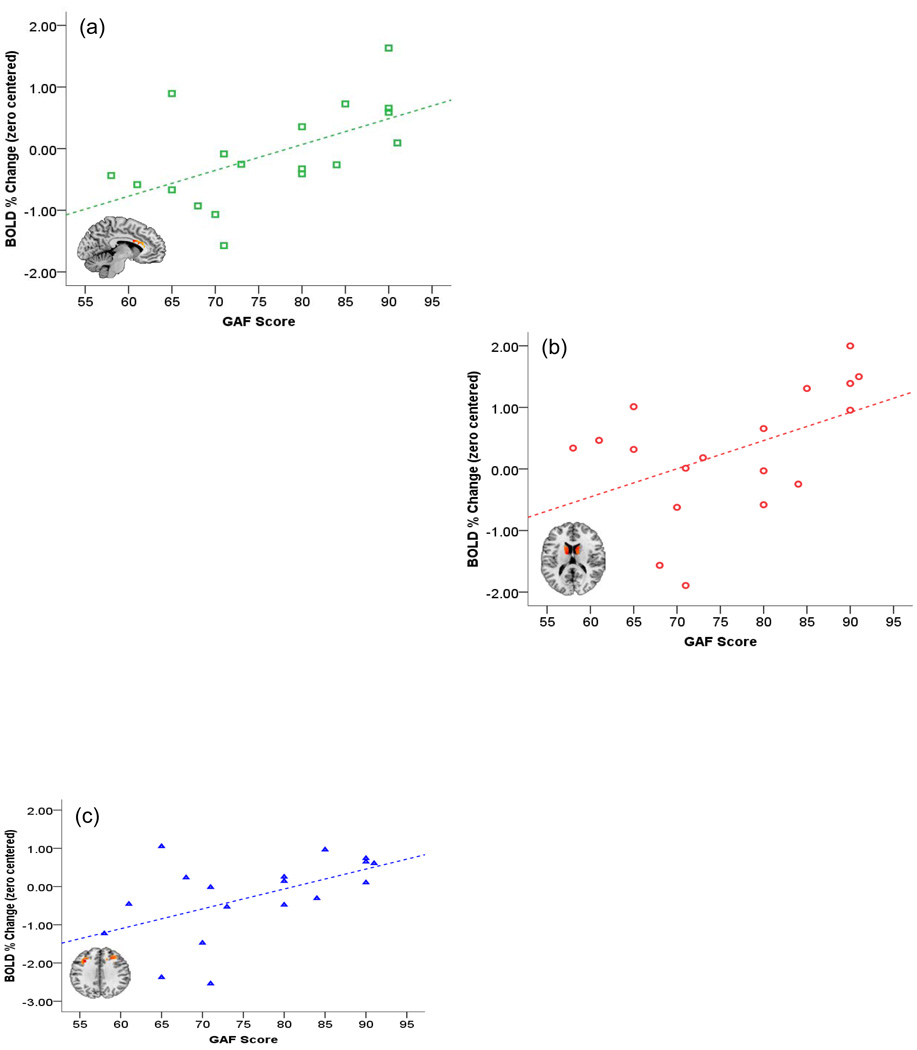
Separate linear regressions between BOLD and raw GAF scre (as the single regressor) on data from each of the caudate, anterior cingulate and dorsal prefrontal cortex are plotted in Figure 4. Each point in each scatter plot refers to a subject in the SCZ-Off group. The graphs show clear positive linear relationships with GAF score (across all SCZ-Off) in each of the frontal regions of interest (i.e., increased GAF or increased function leading to increased observed BOLD). Linear regressions were significant for the anterior cingulate, F1,16=7.66, p<.01, β=.57, the caudate, F1,16=4.71, p<.05, β=.48, and the dorsal prefrontal cortex, F1,16=6.23, p<.02, β=.53.
Figure 4.
In the SCZ-Off group, beta values under the overall main effect (Figure 2) in each of the frontal regions (anterior cingulate, caudate and dPFC) were submitted to regression analyses to assess the relationship between GAF score and activation. As seen, in each of the regions of interest, a dose-response relationship between GAF score and activation was observed across the entire SCZ-Off group. See text for details regarding significance.
4. Discussion
Using fMRI and a sustained attention task, we provide evidence that attention-related activity in the executive core of the attention system is modulated by the presence of clinically assessed functional deficits in SCZ-Off. In particular, SCZ-Off with moderate to severe clinical symptoms showed hypo-responsivity of the dPFC, the ACC and the caudate nucleus, relative to both controls and to their higher functioning SCZ-Off counterparts (Figure 3). Furthermore, a linear (and positive) dose-response relationship between GAF score and frontal activation was observed across the entire SCZ-Off group, with lower GAF scores predicting lower frontal activation during sustained attention (Figure 4). These effects were confined to the executive core of the sustained attention network, and not observed either in the primary or secondary visual areas. Finally, the fMRI results were independent of behavioral performance which within both SCZ-Off sub-groups was within the normal range (Figure 1). This independence suggests that fMRI measures of brain response may be more sensitive to latent vulnerabilities in the developing brain than behavioral measures of performance.
4.1 Sustained attention and fMRI: biology and clinical measures
Sustained attention deficits have been associated with syndromal clinical measures such as schizotypy (Lenzenweger et al., 1991) though the relationship between the brain’s attention response and clinical measures in vulnerable groups such as SCZ-Off have never been assessed before. It is therefore notable, that in our study, premorbid clinical function predicts the attention response in the executive core of the attention system, but not in its more basic visuo-spatial sub-systems. Specialized frontal sub-regions in the brain’s sustained network play significant functional and integrative roles in the organization of cognition, behavior, attention and action (Case, 1992). The dPFC, essential to executive function and working memory (Fuster, 1989; Fuster, 1999), and the dorsal ACC, essential for executive processing and control (Carter et al., 1999) integrate their functions through topographically mapped efferent and afferent connections to regions of the dorsal striatum including the caudate nucleus of the basal ganglia (Calzavara et al., 2007; Paus, 2001). These collective connections, part of the cortico-striatal control “loop” (Haber and Calzavara, 2009) explain the overlapping role of these regions in a wide range of higher order function including attention and working memory. Sustained attention and in particular activity in the fronto-striatal sub-circuit is essential for cognitive control (Coull, 1998; Posner and Rothbart, 1998), and mechanisms of noise filtering, error monitoring and target detection (Sarter et al., 2001). It is therefore unsurprising that sub-threshold presentations of impaired clinical function would correlate with the response of these regions. This specificity points to a potentially critical convergent pathway between emerging clinical symptoms, objectively quantifiable brain function and vulnerability in adolescence that specifically affects critical regions within the neuro-circuitry of a domain.
4.2 Activation and performance proficiency
A notable aspect of these results was a significant decrease in BOLD in relationship to clinical symptoms, without an observable behavioral deficit in either of the SCZ-Off sub-groups. In general, the literature on how activation relates to performance is characterized by some inconsistency, particularly with respect to clinical populations. In schizophrenia per se, hypo-activation of frontal regions during more demanding cognitive tasks such as working memory had lead to an early hypothesis of “hypo-frontality”, or the failure to engage pre-frontal cortical resources in the requisite manner. However, at least in the context of working memory, this picture is considerably more complex when behavioral performance is controlled. Thus, analyses of fMRI signals under conditions of specific behavioral equivalence suggests that clinical populations need increased resources to sub-serve the same level of behavioral performance as controls (Callicott et al., 2000; Jansma et al., 2004; Manoach, 2003), leading to the notion of inefficient cortical processing in schizophrenia (Karlsgodt et al., 2007). Such analyses has not been undertaken during sustained attention tasks, in part because such tasks unfold at a substantially more rapid rate, and because mechanisms of sustained attention do not involve the more effortful sub-processes of maintenance and manipulation of memory tokens that characterize verbal working memory tasks (Tan et al., 2005). However, in healthy subjects investigations of performance measures during sustained attention on brain activation have also lead to complex results. Areas that we assessed (including the dorsal prefrontal cortex, the caudate and the midline frontal structures such as the anterior cingulate) have been shown to be active during rapid sustained attention tasks, but the relationship of this activity to performance is variable. For instance, activity in the midline frontal structures is negatively correlated with response latency, whereas activation in parietal regions is positively correlated with the number of correct responses (Lawrence et al., 2003). Furthermore, during divided attention tasks with differences in parametric load (i.e., the number of visual tokens to track in a moving display), repeated practice served to decrease activation in regions including the dorsal prefrontal cortex, with no difference in task-related accuracy (Tomasi et al., 2004). In general, these studies in healthy subjects suggest that the relationship between behavioral measures and brain activation measured with fMRI is as yet incompletely understood, and that fMRI-changes are observable in the absence of changes in measured task proficiency. In the context of clinical or vulnerable populations, experimental paradigms that involve rapid or automatic processing may help to engage task-relevant regions; selective hypo-activation (as we observed) may then reflect a latent deficiency in the degree of this engagement, in turn providing evidence of a more general overall deficit (Uhlhaas and Singer, 2010). Whereas floor effects may preempt the detection of behavioral differences, the task itself serves as a window into the brain’s response.
4.3 Relationship to previous work in vulnerable and prodromal populations
These results provide a specific focus both continuous with, and extending beyond previous studies. In general, unaffected relatives and/or offspring of schizophrenia patients show an increased vulnerability to impairments in brain structure and function related to the subsequent development of clinically assessed symptoms. For example, brain volume abnormalities in first degree, nonpsychotic relatives of patients are predictive of the subsequent emergence of prodromal symptoms (Ho, 2007), with the prodromal phase associated with neurobiological alterations qualitatively similar to those observed in schizophrenia (Crossley et al., 2009; Fusar-Poli et al., 2007), including cognitive deficits (Keefe et al., 2006; Wood et al., 2003), reductions in frontal and temporal gray matter volume (Borgwardt et al., 2007; Meisenzahl et al., 2008; Pantelis et al., 2003), and disordered front-temporal coupling during demanding executive function tasks (Allen et al., 2010; Crossley et al., 2009). Such studies have by definition employed subjects in advanced stages of clinically defined risk for the onset of psychosis, and typically beyond the adolescent stages of development. We suggest that the present study by virtue of the population assessed, and the age-range, addressed some of these limitations in the extant literature.
4.4. Limitations and Conclusions
The study of vulnerable groups such as SCZ-Off is challenged by conceptual difficulties. Among these is the fact that while these subjects are at significantly increased risk for illness (compared to the general population), a majority of them will not convert to the narrowly defined phenotype of schizophrenia or more generally, psychiatric illnesses of any kind (Erlenmeyer-Kimling et al., 1997). This is not surprising given that developmental processes of plasticity and adaptation in neural network development may confer resilience in some risk groups (Lewis and Levitt, 2002). In the face of developmental heterogeneity and environmental conditions that escape experimental control, understanding the relationship between sub-threshold “clinical” symptoms and measures of brain function and structure can prove valuable (Diwadkar et al., 2006).
The present study is also constrained by its cross-sectional design, which presents a significant restriction on the predictive value of these results. Longitudinal studies may help further clarify the bases of these “bio-psychiatric” relationships between clinical symptoms and executive processing during attention, as well as providing evidence if this correlation presents evidence of a clearly differentiable high-risk sub-group of SCZ-Off. Furthermore, large samples may also permit an assessment of the reliability of these results, as well as the general role of clinical symptoms, and developmental stages (childhood vs. adolescence) on the observed data.
How development unfolds or goes awry (Luna, 2009), and how developmental deviations contribute to the emergence of full disorders such as schizophrenia or bipolar disorder (Andreasen, 1999; Gogtay et al., 2007) are questions that will be central to clinical neuroscience in the coming years. The answers in part are likely to lie in understanding the functions of specific brain circuits associated with behavioral domains such as attention and working memory that have thus far, served as endo- or intermediate-phenotypes or markers of disorders (Gottesman and Gould, 2003). Here we present a motivated framework examining the relationship between clinically assessed function and neuroimaging measures of brain function in adolescence. The systematic nature in which clinical symptoms were related to specific circuits of the brain’s attention network indicate that such approaches may greatly advance the intermediate-phenotype concept in psychiatry and help in unraveling the relationship between clinical symptoms and brain functions in adolescents vulnerable to psychiatric disorders.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (MH68680) and the Children’s Research of Michigan (CRCM) to VAD and the Joseph Young, Sr. Fund to the Dept of Psychiatry & Behavioral Neuroscience. We thank R. Rajarathinem, A. Amirsadri, L. Haddad and A. Jenrow for help in subject characterization. We also thank Jeffrey Stanley and Debra Montrose for helpful discussions, and Serguei Fedorov, Valentina Gumenyuk and Mark Benton for assistance in experimental design and programming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen P, Stephan KE, Mechelli A, Day F, Ward N, Dalton J, Williams SC, McGuire P. Cingulate activity and fronto-temporal connectivity in people with prodromal signs of psychosis. NeuroImage. 49:947–955. doi: 10.1016/j.neuroimage.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia: Bleuler's "fragmented phrene" as schizencephaly. Archives of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U, Pfluger M, D'Souza M, Radue EW, Riecher-Rossler A. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. British Journal of Psychiatry. 2007;51 Suppl:s69–s75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. European Journal of Neuroscience. 2007;26:2005–2024. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Case R. The role of the frontal lobes in the regulation of cognitive development. Brain and Cognition. 1992;20:51–73. doi: 10.1016/0278-2626(92)90061-p. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. American Journal of Medical Genetics. 2000;97:52–57. doi: 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Lenzenweger MF, Erlenmeyer-Kimling L. The Continuous Performance Test, Identical Pairs Version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Research. 1989;29:65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Developmental Psychopathology. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. American Journal of Medical Genetics. 2001;105:11–15. [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Fusar-Poli P, Broome MR, Matthiasson P, Johns LC, Bramon E, Valmaggia L, Williams SC, McGuire PK. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Human Brain Mapping. 2009;30:4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Progress in neuro-psychopharmacology & Biological Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Sweeney JA, Boarts D, Montrose DM, Keshavan MS. Oculomotor delayed response abnormalities in young offspring and siblings at risk for schizophrenia. CNS Spectrums. 2001;6:899–903. doi: 10.1017/s109285290000095x. [DOI] [PubMed] [Google Scholar]
- Dresp B, Grossberg S. Spatial facilitation by color and luminance edges: boundary, surface, and attentional factors. Vision Research. 1999;39:3431–3443. doi: 10.1016/s0042-6989(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. American Journal of Medical Genetics. 2000;97:65–71. doi: 10.1002/(sici)1096-8628(200021)97:1<65::aid-ajmg9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Hilldoff-Adamo UH, Rock D, Roberts SA, Bassett AS, Squires-Wheeler E, Cornblatt BA, Endicott JJ, Pape S, Gottesman II. The New York High-Risk Project. Prevalence and comorbidity of axis I disorders in offspring of schizophrenic parents at 25-year follow-up. Archives of General Psychiatry. 1997;54:1096–1102. doi: 10.1001/archpsyc.1997.01830240052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MD, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Biometrics Research Department. New York: NYSPI; 1997. Structured clinical interview for DSM-IV Axis II personality disorders. [Google Scholar]
- Friston JK, Holmes AP, Worsely KJ, Poline JB, Frith CD, J FRS. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F, McGuire P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex : anatomy, physiology, and neuropsychology of the frontal lobe. 2nd edition. New York: Raven Press; 1989. [Google Scholar]
- Fuster JM. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatrica Scandinavia. 1999;395 Suppl:51–57. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF, 3rd, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. Journal of Child Psychology and Pychiatry, and Alied Dsciplines. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. The American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. Long-range neural coupling through synchronization with attention. Progress in Brain Research. 2009;176:35–45. doi: 10.1016/S0079-6123(09)17603-3. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Research Bulletin. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC. MRI brain volume abnormalities in young, nonpsychotic relatives of schizophrenia probands are associated with subsequent prodromal symptoms. Schizophrenia Research. 2007;96:1–13. doi: 10.1016/j.schres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophrenia Research. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lonnqvist J, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia Research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Poe M, Walker TM, Harvey PD. The relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to functional capacity and real-world functional outcome. Journal of Clinical and Experimental Neuropsychology. 2006;28:260–269. doi: 10.1080/13803390500360539. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex. 2008;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a (1)H spectroscopy study. Schizophrenia Research. 2009;115:88–93. doi: 10.1016/j.schres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. Journal of Cognitive Neuroscience. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Cornblatt BA, Putnick M. Schizotypy and sustained attention. Journal of Abnormal Psychology. 1991;100:84–89. doi: 10.1037//0021-843x.100.1.84. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annual Review of Neuroscience. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. Advances in Child Development and Behavior. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A Users Guide. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Marcus J. Cerebral functioning in offspring of schizophrenics: A possible genetic factor. International Journal of Mental Health. 1974;3:57–73. [Google Scholar]
- Meisenzahl EM, Koutsouleris N, Gaser C, Bottlender R, Schmitt GJ, McGuire P, Decker P, Burgermeister B, Born C, Reiser M, Moller HJ. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophrenia Research. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. The American Journal of Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Offen S, Schluppeck D, Heeger DJ. The role of early visual cortex in visual short-term memory and visual attention. Vision Research. 2009;49:1352–1362. doi: 10.1016/j.visres.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical transactions of the Royal Society of London. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic PT. The importance of being well placed and having the right connections. Annals of the New York Academy of Science. 1999;882:90–106. doi: 10.1111/j.1749-6632.1999.tb08536.x. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Molecular Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex. 2009;19:1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L. Eye-tracking dysfunction in offspring from the New York High-Risk Project: diagnostic specificity and the role of attention. Psychiatry Research. 1997;66:121–130. doi: 10.1016/s0165-1781(96)02975-7. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Report on a continuous performance test. Archives of General Psychiatry. 1977;34:571–575. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, Bernardo M. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. NeuroImage. 2004;21:840–847. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Research & Brain Research Reviews. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sepede G, Ferretti A, Perrucci MG, Gambi F, Di Donato F, Nuccetelli F, Del Gratta C, Tartaro A, Salerno RM, Ferro FM, Romani GL. Altered brain response without behavioral attention deficits in healthy siblings of schizophrenic patients: an event-related fMRI study. NeuroImage. 2010;49:1080–1090. doi: 10.1016/j.neuroimage.2009.07.053. [DOI] [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. fMRI study of maintenance and manipulation processes within working memory in first-episode schizophrenia. The American Journal of Psychiatry. 2005;162:1849–1858. doi: 10.1176/appi.ajp.162.10.1849. [DOI] [PubMed] [Google Scholar]
- Thakral PP, Slotnick SD. The role of parietal cortex during sustained visual spatial attention. Brain Research. 2009;1302:157–166. doi: 10.1016/j.brainres.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. NeuroImage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Milwaukee, WI: Medical College of Wisconsin; 2000. [Google Scholar]
- Wickens TD. Elementary Signal Detection Theory. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- Wood SJ, Pantelis C, Proffitt T, Phillips LJ, Stuart GW, Buchanan JA, Mahony K, Brewer W, Smith DJ, McGorry PD. Spatial working memory ability is a marker of risk-for-psychosis. Psychological Medicine. 2003;33:1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]