Abstract
Proton magnetic resonance spectroscopy (MRS) studies of schizophrenic patients generally reveal reduced levels of N-acetyl aspartate (NAA) when compared with healthy controls. Whether this reduction is due to the disease or to the drugs used for treatment remains an open question. Numerous human and animal studies have attempted to determine the effects of antipsychotics on NAA levels with mixed results. The majority of the animal studies were ex vivo, which may not accurately reflect the in vivo situation, and limitations of the human studies include previous or concomitant medications or other confounds. To overcome these limitations, we dosed 10 rats/group for six months via drinking water with 0.2 or 2 mg/kg/day haloperidol or 10 or 30 mg/kg/day clozapine. Control rats received unadulterated water. Proton MRS data were collected longitudinally over the six month period from a 64 μL voxel containing primarily the right striatum prior to and monthly during drug administration and used to estimate the concentrations of NAA, creatine, and choline. Ratios of NAA, choline, inositol and glutamate+glutamine to creatine were also calculated. Only the Cho/Cr ratio showed a significant time-by-treatment effect (p=0.0285). These results are in agreement with previous studies of the striatum. However, regional and disease-specific effects remain unresolved.
Keywords: Magnetic resonance spectroscopy, N-acetyl aspartate, antipsychotics, schizophrenia, brain
1. Introduction
Proton magnetic resonance spectroscopy (1H-MRS) studies of patients with schizophrenia generally report reduced levels of the metabolites N-acetyl aspartate (NAA), choline (Cho), creatine (Cr), myoinositol (Ins), glutamate (Glu), and glutamine (Gln) in various brain regions (Brugger et al. 2010; Chang et al. 2007; Steen et al. 2005; Theberge et al. 2004; Theberge et al. 2007). These metabolites are easily detected and quantified in proton MRS spectra from human brain and reflect aspects of underlying metabolic processes. NAA is generally considered a marker of neuronal health, Cr energy metabolism, and Cho membrane synthesis or turnover (Ross and Sachdev 2004). NAA levels tend to be decreased in the frontal and temporal lobes, thalamus, and cerebellum of schizophrenic patients (see, for example, the meta analyses by Steen et al. 2005 and Brugger et al 2010). Reductions in Cr and Cho have been reported in prefrontal cortex and thalamus (Yoo et al. 2009). Olbrich et al. (2008) report elevated glutamate+glutamine (GLX) levels, while others report increased Glu/Gln ratios (Bustillo et al. 2009; Shirayama et al. 2010). However, Tayoshi et al. (2009) reported decreased Glu.
Schizophrenic patients usually are stabilized on antipsychotics when they participate in MRS studies. Therefore, changes in metabolite concentrations could result from the disease and/or the antipsychotics. Different medications target different receptor systems and might have different effects on brain metabolism. Studies of the effects of antipsychotics on MRS measures of metabolite levels are problematical because patients are often switched from one medication to another to optimize their treatments, have been on other antipsychotics for some time preceding a variable washout period, or are on concurrent medications to treat other symptoms. Other confounds include diet, socioeconomic status, and substance abuse. This variability in patient subjects makes it difficult to identify the effects of any specific drug.
Animal studies are useful in attempting to differentiate medication effects because there are no confounds associated with exposure to other medications, lifestyle, or the clinical need to adjust medications. The reported results from studies on the effects of antipsychotics in rat brain are often contradictory, which may be due to the methods employed, such as ex vivo techniques that may not accurately reflect in vivo MRS measurements. For example, lactate concentrations rise rapidly post-mortem, so the increased lactate reported by McLoughlin et al. (2009) for some drugs may be more related to the post-mortem interval than the medication. Drug doses were not standardized in these studies, several doses were above clinical standards, and none examined dose-level effects. Only two of the previous studies examined the effects of chronic treatment and none of the treatments examined multiple time points.
Here we report the results of a six-month in vivo MRS study of metabolite levels in the right striatum of normal rats given one of two doses of either clozapine or haloperidol. Time constraints limited us to one brain region, so we chose striatum for comparison with our previous results (Lindquist et al. 2000) and because Harte et al. (2005) reported an NAA increase only in striatum.
2. Materials and Methods
2.1 Animals
The Institutional Animal Care and Use Committee approved these experiments, which were performed according to National Institutes of Health guidelines. Four groups of 6-week-old male Sprague-Dawley rats (n=10/group) were given haloperidol (0.2 mg/kg/day or 2 mg/kg/day) or clozapine (10 mg/kg/day or 30 mg/kg/day) via drinking water for 6 months. Control rats (n=10) were given water. Haloperidol was obtained from Aldrich. Clozapine was obtained from the NIMH chemical synthesis and drug supply program. Stock drug solutions were made by dissolving each drug in dilute HCl and adjusting the pH to 5 using dilute NaOH. The stock solution was diluted as needed to make 500 ml drinking water at the desired concentration for each rat.
Animals were housed singly so the volume of water consumed per animal could be measured. Animals were placed on a 25% restricted diet to reduce growth and ensure the animals would fit in the radiofrequency coil used for the MR experiments for the duration of the experiments. Animals were weighed weekly and the drug concentration in the water adjusted to maintain the desired dose-by-weight. Water was changed twice weekly.
Due to limitations on scanning time, the animals were divided into 3 cohorts of 8, 25, and 17 animals containing approximately equal numbers of each treatment group.
2.2 MRS Data Acquisition
Animals were anesthetized with 5% isoflurane in air and positioned prone on a custom-built holder with their teeth fixed in a bite bar. The rat and holder were then positioned in the center of a 38 mm Litz coil (Doty Scientific, Inc., Columbia, SC). Animals were maintained with 1.5% isoflurane in air during scans. Respiration rate and air temperature were monitored using equipment from Small Animal Imaging, Inc (SAI, Inc., Stony Brook, NY). The air temperature was automatically maintained at 30 °C by a flow of warm air around the animal. Respiration was maintained between 30–60 breaths per minute by adjusting the amount of isoflurane.
Data were acquired from each rat prior to and monthly during antipsychotic dosing using a 7T Bruker BioSpec system (Bruker, Ettlingen, Germany). Axial and sagittal localizer images were acquired for use in positioning the voxel. Spectra were acquired from a 64 μL cubic voxel primarily in the right striatum (Figure 1A). After shimming on the voxel, a double spin echo sequence was used to acquire spectra with (128 averages) and without (4 averages) water suppression at an echo time of 20 ms and a repetition time of 6000 ms. Additional unsuppressed water spectra were acquired at 12 different echo times to calculate the fraction of CSF in the voxel. Eddy currents and phase were corrected with Bruker post-processing routines. Total scan time was approximately 2 hours.
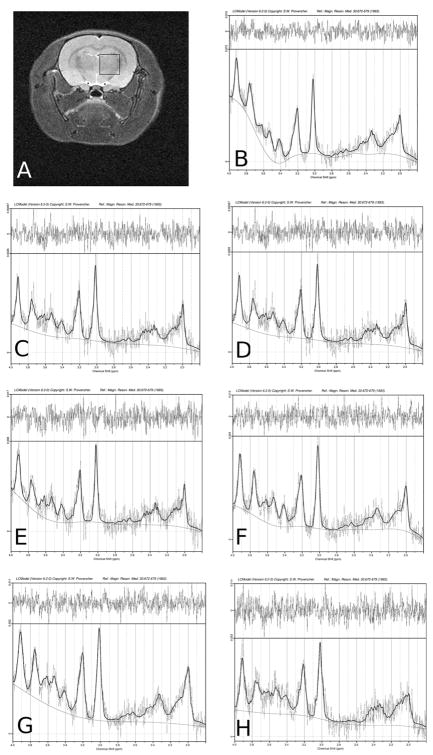
Figure 1.
Representative MR data from a control rat. A) Voxel location in right striatum. LCModel fits to the spectra from months B) 0, C) 1, D) 2, E) 3, F) 4, G) 5, and H) 6.
2.3 Metabolite concentration analysis
Data were imported into LCModel (Provencher 1993) to estimate concentrations of NAA, Cr, Cho, glutamate+glutamine (GLX) and Ins referenced to brain water (assumed to be 43.3 mM/g). Results were used if the Cramer-Rao lower bounds were less than 20% for all metabolites except Ins, where a cut-off of 30% was used. The increased cut-off was used because the coupling pattern of Ins becomes significantly more complex at 7T than at lower field strengths, making fitting this metabolite more difficult. Relaxing the Cramer-Rao lower bounds ensured that there would be sufficient Ins estimates for statistical analysis. LCModel concentration estimates were corrected for the gains and number of averages used to acquire the suppressed and reference data. No corrections were made for relaxation times; results are reported in institutional units.
2.4 Statistics
R (www.r-project.org) was used for all statistical analyses. Weights and water consumption at study start were analyzed using one-way ANOVA. Changes with time or treatment for weight, water consumption, and metabolite levels were analyzed using linear mixed models with the lme package in R. Time, treatment, and cohort were factors. If the lme analysis indicated an effect of time or treatment, the estimable function was to determine the source of the effect. Reported p-values are not corrected for multiple comparisons.
3. Results
There were no significant differences in the initial weights, nor were there significant treatment, cohort, or cohort interactions. There were significant day (p<0.0001) and day-by-treatment effects (p=0.0124). Individual comparisons revealed that the rate of weight gain for the low-dose clozapine group was significantly greater than that of controls (p=0.0020).
There were no significant differences between treatment groups in water intake at the study start. There was a significant day-by-treatment effect (p=0.0465). Further analysis revealed that the rate of water intake for the low-dose clozapine group differed from controls (p=0.045).
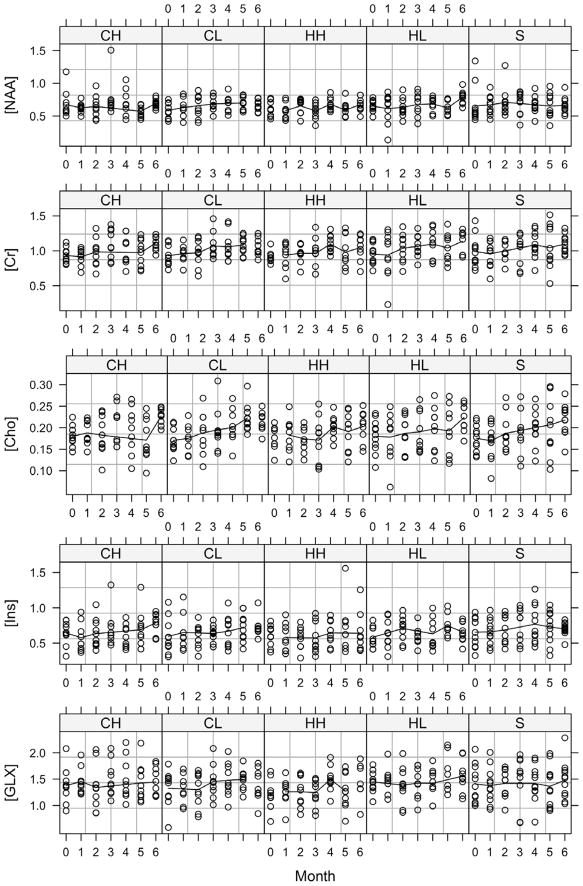
Typical LCModel fits for one rat over the entire treatment period are shown in Figure 1. Approximately 20% of the data were discarded due to instrument malfunctions or high Cramer-Rao lower bounds in the fitted data. Plots of the monthly concentration data are shown in Figure 2. Linear mixed models analysis of the concentration data indicated a significant effect of time for all measured metabolites, a treatment effect only for GLX (p=0.029), and no time-by-treatment effects. Monthly concentration estimates for each of the treatment groups are given in Table 1.
Figure 2.
Monthly concentration estimates in institutional units for each treatment group. CH: 30 mg/kg/day clozapine; CL: 10 mg/kg/day clozapine; HH: 2 mg/kg/day haloperidol; HL: 0.2 mg/kg/day haloperidol; S: control.
Table 1.
Monthly Concentration Estimates1
| Group | Month | N | NAA | Cr | Cho | GLX | Ins |
|---|---|---|---|---|---|---|---|
| Control | 0 | 9 | 0.70 ± 0.29 | 1.05 ± 0.20 | 0.18 ± 0.03 | 1.50 ± 0.34 | 0.72 ± 0.17 |
| 1 | 9 | 0.67 ± 0.16 | 0.93 ± 0.19 | 0.17 ± 0.05 | 1.37 ± 0.38 | 0.66 ± 0.20 | |
| 2 | 9 | 0.72 ± 0.22 | 1.00 ± 0.14 | 0.19 ± 0.05 | 1.44 ± 0.28 | 0.67 ± 0.20 | |
| 3 | 8 | 0.77 ± 0.11 | 1.12 ± 0.14 | 0.21 ± 0.04 | 1.69 ± 0.20 | 0.79 ± 0.22 | |
| 4 | 9 | 0.70 ± 0.11 | 1.12 ± 0.16 | 0.21 ± 0.04 | 1.53 ± 0.24 | 0.82 ± 0.28 | |
| 5 | 7 | 0.71 ± 0.18 | 1.18 ± 0.26 | 0.24 ± 0.06 | 1.55 ± 0.36 | 0.80 ± 0.21 | |
| 6 | 6 | 0.64 ± 0.09 | 1.11 ± 0.13 | 0.22 ± 0.04 | 1.70 ± 0.31 | 0.68 ± 0.11 | |
| High-dose clozapine | 0 | 9 | 0.70 ± 0.20 | 0.94 ± 0.10 | 0.18 ± 0.03 | 1.43 ± 0.29 | 0.68 ± 0.12 |
| 1 | 9 | 0.62 ± 0.08 | 0.92 ± 0.12 | 0.19 ± 0.03 | 1.46 ± 0.25 | 0.58 ± 0.20 | |
| 2 | 8 | 0.69 ± 0.15 | 1.01 ± 0.20 | 0.19 ± 0.05 | 1.44 ± 0.40 | 0.68 ± 0.20 | |
| 3 | 7 | 0.79 ± 0.32 | 1.12 ± 0.22 | 0.20 ± 0.05 | 1.59 ± 0.33 | 0.72 ± 0.29 | |
| 4 | 9 | 0.71 ± 0.19 | 1.02 ± 0.21 | 0.19 ± 0.05 | 1.50 ± 0.40 | 0.60 ± 0.16 | |
| 5 | 10 | 0.57 ± 0.09 | 0.97 ± 0.19 | 0.17 ± 0.05 | 1.43 ± 0.35 | 0.69 ± 0.26 | |
| 6 | 8 | 0.69 ± 0.06 | 1.10 ± 0.11 | 0.22 ± 0.02 | 1.51 ± 0.26 | 0.78 ± 0.15 | |
| High-dose haloperidol | 0 | 7 | 0.63 ± 0.12 | 0.93 ± 0.08 | 0.18 ± 0.03 | 1.30 ± 0.24 | 0.60 ± 0.13 |
| 1 | 8 | 0.60 ± 0.11 | 0.98 ± 0.13 | 0.19 ± 0.03 | 1.32 ± 0.16 | 0.61 ± 0.19 | |
| 2 | 8 | 0.67 ± 0.12 | 0.97 ± 0.14 | 0.18 ± 0.03 | 1.30 ± 0.29 | 0.59 ± 0.16 | |
| 3 | 7 | 0.61 ± 0.09 | 0.99 ± 0.12 | 0.18 ± 0.05 | 1.37 ± 0.15 | 0.59 ± 0.23 | |
| 4 | 9 | 0.69 ± 0.09 | 1.12 ± 0.11 | 0.21 ± 0.02 | 1.54 ± 0.19 | 0.65 ± 0.18 | |
| 5 | 6 | 0.62 ± 0.13 | 1.06 ± 0.27 | 0.20 ± 0.05 | 1.45 ± 0.25 | 0.82 ± 0.39 | |
| 6 | 7 | 0.71 ± 0.09 | 1.08 ± 0.14 | 0.20 ± 0.03 | 1.53 ± 0.24 | 0.59 ± 0.17 | |
| Low-dose clozapine | 0 | 9 | 0.60 ± 0.11 | 0.95 ± 0.13 | 0.18 ± 0.02 | 1.41 ± 0.23 | 0.63 ± 0.24 |
| 1 | 8 | 0.63 ± 0.14 | 0.96 ± 0.13 | 0.18 ± 0.03 | 1.31 ± 0.28 | 0.65 ± 0.26 | |
| 2 | 6 | 0.77 ± 0.10 | 1.08 ± 0.10 | 0.21 ± 0.04 | 1.52 ± 0.15 | 0.65 ± 0.15 | |
| 3 | 9 | 0.71 ± 0.09 | 1.08 ± 0.18 | 0.20 ± 0.05 | 1.50 ± 0.28 | 0.64 ± 0.12 | |
| 4 | 10 | 0.69 ± 0.11 | 1.06 ± 0.20 | 0.20 ± 0.04 | 1.48 ± 0.31 | 0.67 ± 0.22 | |
| 5 | 10 | 0.70 ± 0.09 | 1.08 ± 0.13 | 0.22 ± 0.03 | 1.50 ± 0.19 | 0.72 ± 0.20 | |
| 6 | 6 | 0.68 ± 0.09 | 1.03 ± 0.13 | 0.21 ± 0.03 | 1.54 ± 0.23 | 0.69 ± 0.07 | |
| Low-dose haloperidol | 0 | 11 | 0.65 ± 0.09 | 0.95 ± 0.15 | 0.18 ± 0.04 | 1.45 ± 0.19 | 0.58 ± 0.14 |
| 1 | 9 | 0.67 ± 0.16 | 1.02 ± 0.23 | 0.19 ± 0.04 | 1.47 ± 0.24 | 0.63 ± 0.21 | |
| 2 | 9 | 0.65 ± 0.14 | 1.05 ± 0.21 | 0.19 ± 0.04 | 1.44 ± 0.31 | 0.70 ± 0.18 | |
| 3 | 7 | 0.72 ± 0.18 | 1.11 ± 0.14 | 0.20 ± 0.05 | 1.53 ± 0.23 | 0.63 ± 0.11 | |
| 4 | 9 | 0.69 ± 0.11 | 1.10 ± 0.22 | 0.20 ± 0.05 | 1.42 ± 0.29 | 0.63 ± 0.22 | |
| 5 | 8 | 0.64 ± 0.11 | 1.06 ± 0.22 | 0.19 ± 0.06 | 1.59 ± 0.37 | 0.75 ± 0.16 | |
| 6 | 8 | 0.78 ± 0.11 | 1.12 ± 0.17 | 0.22 ± 0.03 | 1.57 ± 0.32 | 0.63 ± 0.13 |
Results are reported in institutional units as mean ± SD.
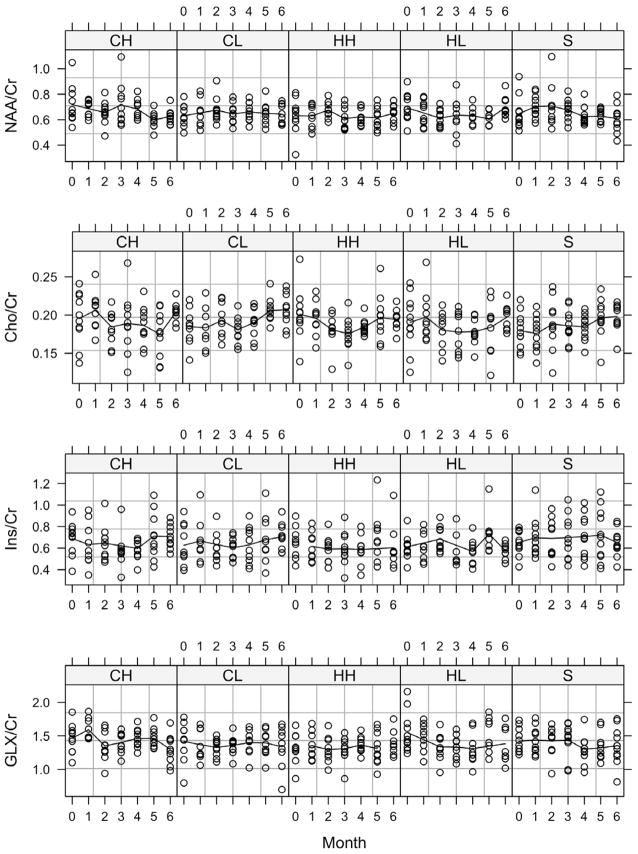
Metabolite ratios to Cr were calculated from the retained LCModel fits; plots of these data are shown in Figure 3. Linear mixed models analysis of the ratio data indicated no significant treatment effects and significant time effects for NAA/Cr (p=0.007) and GLX/Cr (p=0.034). Only Cho/Cr (p=0.0285) showed a significant time-by-treatment interaction.
Figure 3.
Monthly ratios for each of the treatment groups. CH: 30 mg/kg/day clozapine; CL: 10 mg/kg/day clozapine; HH: 2 mg/kg/day haloperidol; HL: 0.2 mg/kg/day haloperidol; S: control.
4. Discussion
Multiple studies of the frontal lobe report no changes in metabolite levels that correlate with medication status (Bustillo et al. 2008; Bustillo et al. 2009; Pae et al. 2004; Szulc et al. 2005; Szulc et al. 2007). Bustillo et al. (2002) reported reductions in frontal NAA levels in patients after a year of haloperidol or quetiapine treatment, but did not differentiate between the antipsychotics or between treatment effects and disease progression. Conversely, Choe et al. (1996) observed increases in NAA/Cr in 13/34 patients following treatment with any antipsychotic. Bertolino et al. (2001) reported increased NAA/Cr in the dorsolateral prefrontal cortex in chronically ill patients following treatment regardless of antipsychotic. In one of the few studies where medication changes did not occur during the study, the NAA/Cr ratio increased after 8 weeks of clozapine therapy (Ertugrul et al. 2009). However, the patients in this study were refractory to other medications, so differences due to disease severity or lingering effects from the previous medications cannot be excluded.
In basal ganglia and thalamus, the majority of papers also find no significant effect of medication on metabolite levels. Of the five studies we found reporting measurements in the basal ganglia (Bertolino et al. 2001; Bustillo et al. 2001; Bustillo et al. 2008; Fannon et al. 2003; Heimberg et al. 1998) only Heimberg et al. reported any change, a decrease in Cho/Cr. In the thalamus, three of five papers found no effects of medication (Bertolino et al. 2001; Bustillo et al. 2009; Heimberg et al. 1998). However, Szulc et al. (2007) reported decreased NAA/Cr in patients treated with typical antipsychotics compared with normal controls, but no differences in NAA/Cr between patients treated with typical or atypical antipsychotics. This may be due to insufficient power, since the data they report show that patients taking atypical antipsychotics had NAA/Cr ratios intermediate between controls and patients on typical medications. The same group reports an increase in NAA/Cr and myoinositol/Cr in the thalamus following at least 4 weeks of stable risperidone therapy (Szulc et al. 2005).
We found no effects of medication dose or duration in the right striatum of rats treated with haloperidol or clozapine compared with vehicle over 6 months. These findings are in agreement with our previous study (Lindquist et al. 2000), where with the same (low) doses of these drugs we found no significant changes in NAA/Cr or Cho/Cr at either 24 hours or after 1 week. We are aware of no other in vivo measurements of these metabolite concentrations in rat brain. Of the other animal studies examining metabolic changes following antipsychotic treatment, only the 6-month ex vivo study by Bustillo, et al. (2006) is readily comparable to these studies due to the similar techniques (high-resolution magic angle spinning 1H MRS of tissue samples vs. in vivo 1H MRS) and similar haloperidol dose (38 mg/kg/month, approximately 1.3 mg/kg/day); they report no changes in any metabolite in any region studied. The remaining studies utilize tissue extracts. The extraction process might free bound metabolite pools, which would be MRS-invisible either in vivo or in tissue samples. Bustillo, et al. (2004) used high performance liquid chromatography to examine NAA concentrations in extracts of various brain regions from rats treated with either haloperidol (6 mg/kg/day) or clozapine (70 mg/kg/day) for 6 weeks and found no change in NAA concentration in any region with treatment. In contrast, Harte et al. (2005), using a similar ex vivo analytical method but lower overall dose of haloperidol (28.5 mk/kg/3 weeks), found increased NAA only in the striatum following a 6-month exposure. McLoughlin et al. (2009), using high resolution proton NMR, report multiple changes in metabolite levels in extracts of prefrontal cortex, dorsal striatum, and hippocampus following a 21-day exposure to haloperidol (1 mg/kg/day) or clozapine (20 mg/kg/day). NAA was increased in frontal cortex and hippocampus for both drugs, but no changes in NAA levels were reported in the striatum. Thus, four of five previous studies also found no evidence that haloperidol or clozapine alter striatal NAA levels. Findings from these studies are summarized in Table 2.
Table 2.
Results of Previous Animal Studies1
| Author | Drug | Dose | Duration | Methods | Findings |
|---|---|---|---|---|---|
| Lindquist, 2000 | Haloperidol | 0.2 mg/kg/day IP | 7 days | In vivo 1H MRS | No effect on NAA/Cr or Cho/Cr |
| Clozapine | 10 mg/kg/day IP | ||||
| Olanzapine | 1 mg/kg/day IP | ||||
| Bustillo, 2004 | Haloperidol | 6 mg/kg/day by gavage twice daily | 40 days | HPLC of brain regions | No effect on NAA |
| Clozapine | 70 mg/kg/day | ||||
| Harte, 2004 | Haloperidol decanoate | 28.5 mg/kg IM every 3 weeks | 24 weeks | HPLC of brain regions | NAA increase only in striatum |
| Bustillo, 2004 | Haloperidol depo | 28 mg/kg/month | 6 months | HR-MAS 1H-MRS of biopsies from various brain regions | No effect on any metabolite in any region |
| McLoughlin, 2009 | Haloperidol | 1 mg/kg/day by gavage | 21 days | HR 1H-MRS of brain extracts | FC: NAA, Lac ↑ Gln, Cr ↓ H: NAA ↑ Glu, Gln, Lac, Cr, mI ↓ S: Lac, Cho ↑ Cr, mI ↓ |
| Clozapine | 20 mg/kg/day by gavage | FC: Cr, Lac ↑ Glu, Gln ↓ H: NAA, Lac ↑ mI ↓ S: Lac, Cr, Cho ↑ |
|||
| Olanzapine | 2 mg/kg/day by gavage | FC: Lac, Cho ↑ Cr, Gln, Glu ↓ H: No effect S: No effect |
|||
| Risperidone | 1 mg/kg/day by gavage | FC: NAA, Cho ↑ Lac, Cr,mI ↓ H: No effect S: No effect |
|||
| Aripiprazole | 40 mg/kg/day by gavage | FC: NAA, Cho ↑ Cr ↓ H: No effect S: NAA, Cho ↑ Cr ↓ |
|||
| Present Study | Haloperidol | 0.2 mg/kg/day in drinking water 2 mg/kg/day |
Monthly over 6 months | In vivo 1H MRS of striatum | No effect on any metabolite |
| Clozapine | 10 mg/kg/day 30 mg/kg/day |
||||
Key to abbreviations: FC: Frontal cortex; H: hippocampus; S: striatum
Several studies have suggested that brain volumes are altered upon antipsychotic treatment (see, for example, Ho et al., (2011). In a study analogous to this one, Vernon et al. (2010) found that striatal and hippocampal volumes of rats treated with haloperidol or olanzapine for 8 weeks did not differ from control animals, although striatal volume increased for all animals, which suggests that the striatal volume may have increased in our study. Changes in striatal volume may not have a significant effect on metabolite concentrations. If a volume increase resulted from an increased cell density, then both NAA and Cho potentially would be elevated. If a volume increase resulted from an increased cell size, but not number, then perhaps NAA and Cho would decrease; however, one might also expect increases in Ins levels due to its role as an osmolyte. No such changes were seen in this study.
The significant time-by-treatment effect for Cho/Cr could be related to brain volume or metabolic changes. Antipsychotics may alter lipid metabolism (Thomas and Yao 2007), increase the number of dendritic spines in the hippocampus (Crichtlow et al. 2006), and affect myelin content (Bartzokis et al. 2009), all of which could be involved in brain volume changes and be reflected by alterations in choline levels. On the other hand, disturbances in energy metabolism might result in changes in creatine levels. Several treatment-dependent effects of antipsychotics on energy metabolism have been reported, including reduced activity of creatine kinase (Assis et al. 2007) and succinate dehydrogenase (Streck et al. 2007). This evidence suggests that choline and creatine levels could change with antipsychotic treatment. While such changes may not be detected separately, they could possibly be detected through ratio data, as occurred here. Although the time-by-treatment effect for Cho/Cr would not survive corrections for multiple comparisons, it is worthy of further study.
The three studies reporting no effect of antipsychotics on metabolite levels used male Sprague-Dawley rats of similar ages, as did the current study. The two studies that reported that metabolite levels were affected by drug treatment used either Long-Evans rats (gender not reported, Harte et al. 2005) or male Wistar rats (McLoughlin et al. 2009). Hence, differences in treatment response due to rat strain cannot be excluded.
The lower drug doses used in this study were chosen to give dopamine D2 receptor occupancies of about 60% (Zhang and Bymaster 1999), since D2 occupancy levels may be better correlates of response than plasma levels (Kapur et al. 2003; Pani et al. 2007). Khan et al. (2003) found that a 2 mg/kg/day haloperidol dose, as used here, produces clinically relevant plasma levels. For clozapine, both doses used in this study produce clinically relevant occupancy and plasma levels (Kapur et al. 2003; Khan et al. 2003). Two dose levels of each drug were used in this study to allow us to examine possible dose-dependent effects on NAA or other metabolite levels. No such dose-dependent effects were observed.
According to Kapur et al. (2000), studies examining the chronic effects of antipsychotics need to adjust the dose or dosing schedule to better mimic the duration of D2 occupancy in humans. The half-lives of haloperidol and clozapine in rats are about 1.5 hours, as opposed to 24 and 12 hours, respectively, in humans (Kapur et al. 2003; Kapur et al. 2000). Kapur et al. (2000) suggest using a large single dose or multiple doses to minimize the fluctuations in D2 occupancy to better mimic human dosing. Of the previous studies, only the 6-week study by Bustillo et al. (2004) used multiple daily (high) doses, which would be expected to minimize fluctuations in receptor occupancy. We chose to use drinking water to attempt to maintain a constant level of D2 occupancy. However, Perez-Costas et al. (2008) have recently shown that although haloperidol in drinking water achieved the desired D2 receptor occupancy, clozapine at either 20 mg/kg or 40 mg/kg did not. D2 receptor occupancy, however, is probably not the only mediator of antipsychotic action (Richtand et al. 2007). Hence, the influence of the different routes of administration and the role of D2 receptor occupancy on metabolite levels remains unclear, but could explain the lack of a dose-dependent response in this study.
There are several limitations to this study. The antipsychotic dose levels were fixed, which is not representative of the typical clinical population. No measurements of plasma levels or receptor occupancy were made, so drug underexposure cannot be excluded. Time constraints permitted only one region of normal brain to be studied, so regional effects of these medications were not investigated. Multiple studies indicate that these drugs may have regionally specific effects (Fannon et al. 2003; McLoughlin et al. 2009; Szulc et al. 2005) and there is evidence that there are regionally specific, and possibly opposing, differences in metabolite levels in patients with schizophrenia (Ende et al. 2003), Finally, the negative findings could be due to insufficient statistical power given the high interindividual variation that we observed in this study. While calculating an effect size for a linear mixed models analysis is difficult due to the interplay of the modeled effects, using the 6-month data in a t-test analysis shows that for NAA, detectable changes would be about 25% of the control value.
The absence of disease in this animal model is another limitation of this study. Lithium has no mood-altering properties when given to normal subjects, despite its usefulness in treating patients with bipolar disease (Stone et al. 2009). Fannon et al. (2003) found that NAA/Cr ratios in the hippocampus differed at baseline between drug-naïve and previously medicated patients or controls, but that no group differences existed following 3 months of treatment, which suggests that treatment normalized NAA levels. It is conceivable that antipsychotic drugs normalize aberrant metabolic levels in specific regions of diseased brain, but have insignificant effects in normal brain. Future studies in multiple brain regions using animal models would address these issues.
In conclusion, our results are in agreement with the majority of reports suggesting that antipsychotic drugs do not significantly alter the concentrations of any MRS-measurable metabolite in the striatum. Regional and/or disease-related changes may still occur, however, and should be the focus of further study.
Acknowledgments
Funding
Funding for this study was provided by NIMH Grant MH074645 (DML); the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributors
DML designed the study, analyzed the data, and wrote the first draft of the manuscript. RSD and DML collected data. KMC assisted with manuscript design, data interpretation, editing and revision for important intellectual content. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assis LC, Scaini G, Di-Pietro PB, Castro AA, Comim CM, Streck EL, Quevedo J. Effect of antipsychotics on creatine kinase activity in rat brain. Basic Clin Pharmacol Toxicol. 2007;101(5):315–319. doi: 10.1111/j.1742-7835.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Stewart SB, Oluwadara B, Lucas AJ, Pantages J, Pratt E, Sherin JE, Altshuler LL, Mintz J, Gitlin MJ, Subotnik KL, Nuechterlein KH. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2–3):322–331. doi: 10.1016/j.schres.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, Weinberger DR. The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry. 2001;49(1):39–46. doi: 10.1016/s0006-3223(00)00997-5. [DOI] [PubMed] [Google Scholar]
- Brugger S, Davis JM, Leucht S, Stone JM. Proton Magnetic Resonance Spectroscopy and Illness Stage in Schizophrenia-A Systematic Review and Meta-Analysis. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Bustillo J, Barrow R, Paz R, Tang J, Seraji-Bozorgzad N, Moore GJ, Bolognani F, Lauriello J, Perrone-Bizzozero N, Galloway MP. Long-term treatment of rats with haloperidol: lack of an effect on brain N-acetyl aspartate levels. Neuropsychopharmacology. 2006;31(4):751–756. doi: 10.1038/sj.npp.1300874. [DOI] [PubMed] [Google Scholar]
- Bustillo J, Wolff C, Myers-y-Gutierrez A, Dettmer TS, Cooper TB, Allan A, Lauriello J, Valenzuela CF. Treatment of rats with antipsychotic drugs: lack of an effect on brain N-acetyl aspartate levels. Schizophr Res. 2004;66(1):31–39. doi: 10.1016/s0920-9964(02)00528-5. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Lauriello J, Rowland LM, Jung RE, Petropoulos H, Hart BL, Blanchard J, Keith SJ, Brooks WM. Effects of chronic haloperidol and clozapine treatments on frontal and caudate neurochemistry in schizophrenia. Psychiatry Res. 2001;107(3):135–149. doi: 10.1016/s0925-4927(01)00102-0. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Lauriello J, Rowland LM, Thomson LM, Petropoulos H, Hammond R, Hart B, Brooks WM. Longitudinal follow-up of neurochemical changes during the first year of antipsychotic treatment in schizophrenia patients with minimal previous medication exposure. Schizophr Res. 2002;58(2–3):313–321. doi: 10.1016/s0920-9964(02)00210-4. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Jung R, Brooks WM, Qualls C, Hammond R, Hart B, Lauriello J. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33(10):2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, Hammond R, Brooks WM, Lauriello J. (1)H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62(12):1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe BY, Suh TS, Shinn KS, Lee CW, Lee C, Paik IH. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Invest Radiol. 1996;31(6):345–352. doi: 10.1097/00004424-199606000-00006. [DOI] [PubMed] [Google Scholar]
- Crichtlow HM, Maycox PR, Skepper JN, Krylova O. Clozapine and haloperidol differentially regulate dendritic spine formation and synaptogenesis in rat hippocampal neurons. Mol Cell Neurosci. 2006;32(4):356–365. doi: 10.1016/j.mcn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. Multiregional 1H-MRSI of the hippocampus, thalamus, and basal ganglia in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2003;253(1):9–15. doi: 10.1007/s00406-003-0398-5. [DOI] [PubMed] [Google Scholar]
- Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, Senturk S, Erbas B, Cila A, Ulug B. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res. 2009;174(2):121–129. doi: 10.1016/j.pscychresns.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Fannon D, Simmons A, Tennakoon L, O’Ceallaigh S, Sumich A, Doku V, Shew C, Sharma T. Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophrenia. Biol Psychiatry. 2003;54(6):587–598. doi: 10.1016/s0006-3223(03)00185-9. [DOI] [PubMed] [Google Scholar]
- Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr Res. 2005;75(2–3):303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Heimberg C, Komoroski RA, Lawson WB, Cardwell D, Karson CN. Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect. Psychiatry Res. 1998;83(2):105–115. doi: 10.1016/s0925-4927(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term Antipsychotic Treatment and Brain Volumes: A Longitudinal Study of First-Episode Schizophrenia. Arch Gen Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305(2):625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kapur S, Wadenberg ML, Remington G. Are animal studies of antipsychotics appropriately dosed? Lessons from the bedside to the bench. Can J Psychiatry. 2000;45(3):241–246. doi: 10.1177/070674370004500302. [DOI] [PubMed] [Google Scholar]
- Khan MM, Parikh VV, Mahadik SP. Antipsychotic drugs differentially modulate apolipoprotein D in rat brain. J Neurochem. 2003;86(5):1089–1100. doi: 10.1046/j.1471-4159.2003.01866.x. [DOI] [PubMed] [Google Scholar]
- Lindquist DM, Hawk RM, Karson CN, Komoroski RA. Effects of antipsychotic drugs on metabolite ratios in rat brain in vivo. Magn Reson Med. 2000;43(3):355–358. doi: 10.1002/(sici)1522-2594(200003)43:3<355::aid-mrm6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- McLoughlin GA, Ma D, Tsang TM, Jones DN, Cilia J, Hill MD, Robbins MJ, Benzel IM, Maycox PR, Holmes E, Bahn S. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. J Proteome Res. 2009;8(4):1943–1952. doi: 10.1021/pr800892u. [DOI] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Rusch N, Buchert M, Thiel T, Hennig J, Ebert D, Van Elst LT. Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry. 2008;9(1):59–63. doi: 10.1080/15622970701227811. [DOI] [PubMed] [Google Scholar]
- Pae CU, Choe BY, Joo RH, Lim HK, Kim TS, Yoo SS, Choi BG, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Neuronal dysfunction of the frontal lobe in schizophrenia. Neuropsychobiology. 2004;50(3):211–215. doi: 10.1159/000079972. [DOI] [PubMed] [Google Scholar]
- Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267–275. doi: 10.1016/j.eurpsy.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm. 2008;115(5):745–753. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology. 2007;32(8):1715–1726. doi: 10.1038/sj.npp.1301305. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Res Brain Res Rev. 2004;44(2–3):83–102. doi: 10.1016/j.brainresrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E, Ikehira H, Hashimoto K, Iyo M. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage. 2010;49(3):2783–2790. doi: 10.1016/j.neuroimage.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30(11):1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, O’Gorman RL, Barker GJ, McGuire PK. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66(6):533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Streck EL, Rezin GT, Barbosa LM, Assis LC, Grandi E, Quevedo J. Effect of antipsychotics on succinate dehydrogenase and cytochrome oxidase activities in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2007;376(1–2):127–133. doi: 10.1007/s00210-007-0178-2. [DOI] [PubMed] [Google Scholar]
- Szulc A, Galinska B, Tarasow E, Dzienis W, Kubas B, Konarzewska B, Walecki J, Alathiaki AS, Czernikiewicz A. The effect of risperidone on metabolite measures in the frontal lobe, temporal lobe, and thalamus in schizophrenic patients. A proton magnetic resonance spectroscopy (1H MRS) Pharmacopsychiatry. 2005;38(5):214–219. doi: 10.1055/s-2005-873156. [DOI] [PubMed] [Google Scholar]
- Szulc A, Galinska B, Tarasow E, Kubas B, Dzienis W, Konarzewska B, Poplawska R, Tomczak AA, Czernikiewicz A, Walecki J. N-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) study. Med Sci Monit. 2007;13(Suppl 1):17–22. [PubMed] [Google Scholar]
- Tayoshi S, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Nakataki M, Ueno S, Harada M, Ohmori T. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophr Res. 2009;108(1–3):69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Theberge J, Al-Semaan Y, Drost DJ, Malla AK, Neufeld RW, Bartha R, Manchanda R, Menon R, Densmore M, Schaefer B, Williamson PC. Duration of untreated psychosis vs. N-acetylaspartate and choline in first episode schizophrenia: a 1H magnetic resonance spectroscopy study at 4.0 Tesla. Psychiatry Res. 2004;131(2):107–114. doi: 10.1016/j.pscychresns.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, Northcott S, Menon RS, Neufeld RW, Rajakumar N, Pavlosky W, Densmore M, Schaefer B, Williamson PC. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Yao JK. Clozapine specifically alters the arachidonic acid pathway in mice lacking apolipoprotein D. Schizophr Res. 2007;89(1–3):147–153. doi: 10.1016/j.schres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Vernon AC, Natesan S, Modo M, Kapur S. Effect of Chronic Antipsychotic Treatment on Brain Structure: A Serial Magnetic Resonance Imaging Study with Ex Vivo and Postmortem Confirmation. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Yeon S, Choi CH, Kang DH, Lee JM, Shin NY, Jung WH, Choi JS, Jang DP, Kwon JS. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res. 2009;111(1–3):86–93. doi: 10.1016/j.schres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology (Berl) 1999;141(3):267–278. doi: 10.1007/s002130050834. [DOI] [PubMed] [Google Scholar]