Abstract
Bovine pericardium is widely used in surgery and is commonly used for a patch after arteriotomy during cardiovascular surgery. Bovine pericardial patches have several advantages compared to prosthetic patches, including superior biocompatability, easy handling, less suture line bleeding and possibly reduced rates of infection. These advantages of bovine pericardium have led to its common use during carotid endarterectomy. However, long-term clinical results reported after carotid endarterectomy have suggested several issues that may be related to the patch including restenosis, pseudoaneurysm formation, infection, fibrosis, calcification and thrombosis. These complications may diminish the long-term efficacy of carotid endarterectomy and suggest potential areas for improvement of surgical patches. Understanding the mechanisms by which bovine pericardium heals after patch angioplasty may lead to next generation tissue engineered patches.
Bovine pericardium (BP) has come into common clinical use during the past 20 years, especially when used as a patch for arterial closure during vascular and cardiac surgery. Bovine pericardial patches possess many technical merits that have led to their widespread adoption in the operating room, including easy handling, less suture bleeding and the ability to immediately perform arterial duplex examination at the site of angioplasty. However, long-term results of this biomaterial are poorly documented and need cautious interpretation as to whether its long term performance is related to the material itself or to the operation in which it is used. For example, it is unclear whether restenosis after carotid endarterectomy is directly related to the patch itself or whether restenosis is an inevitable consequence of the arterial procedure. In addition, there are sporadic reports of unusual complications with BP patches, including patch rupture and cartilaginous metaplasia. Although these reports are unusual, careful examination of these potential areas of improvement may lead to future generations of BP patches with superior performance.
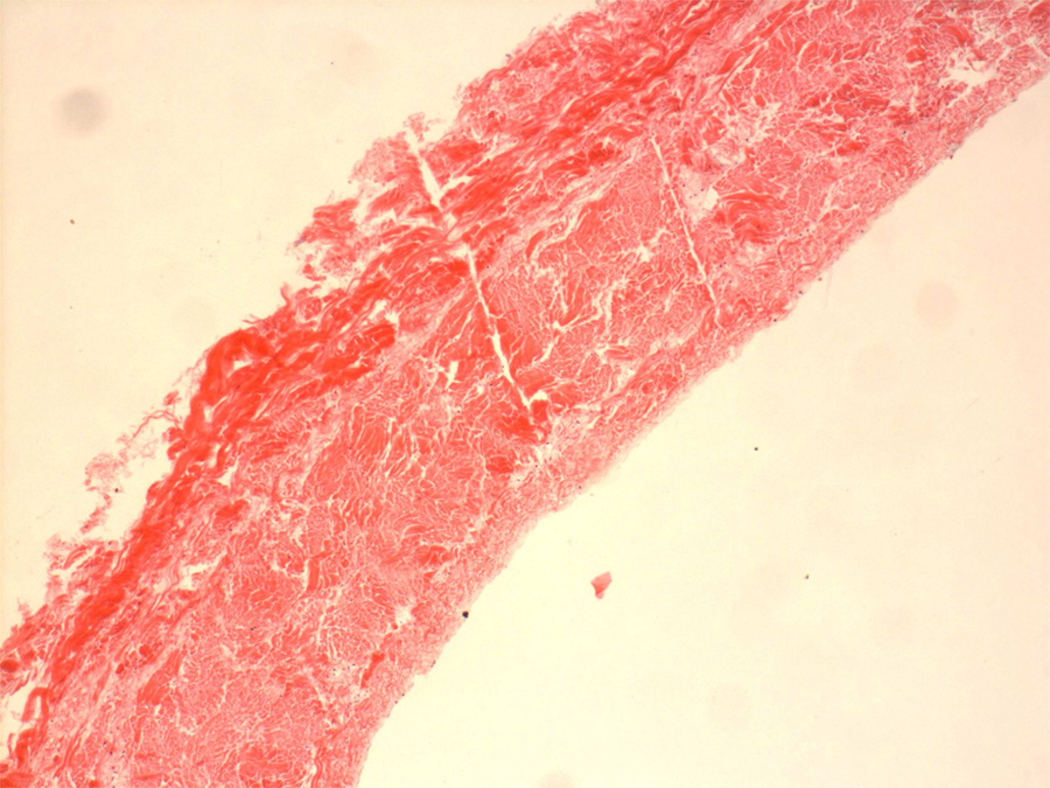
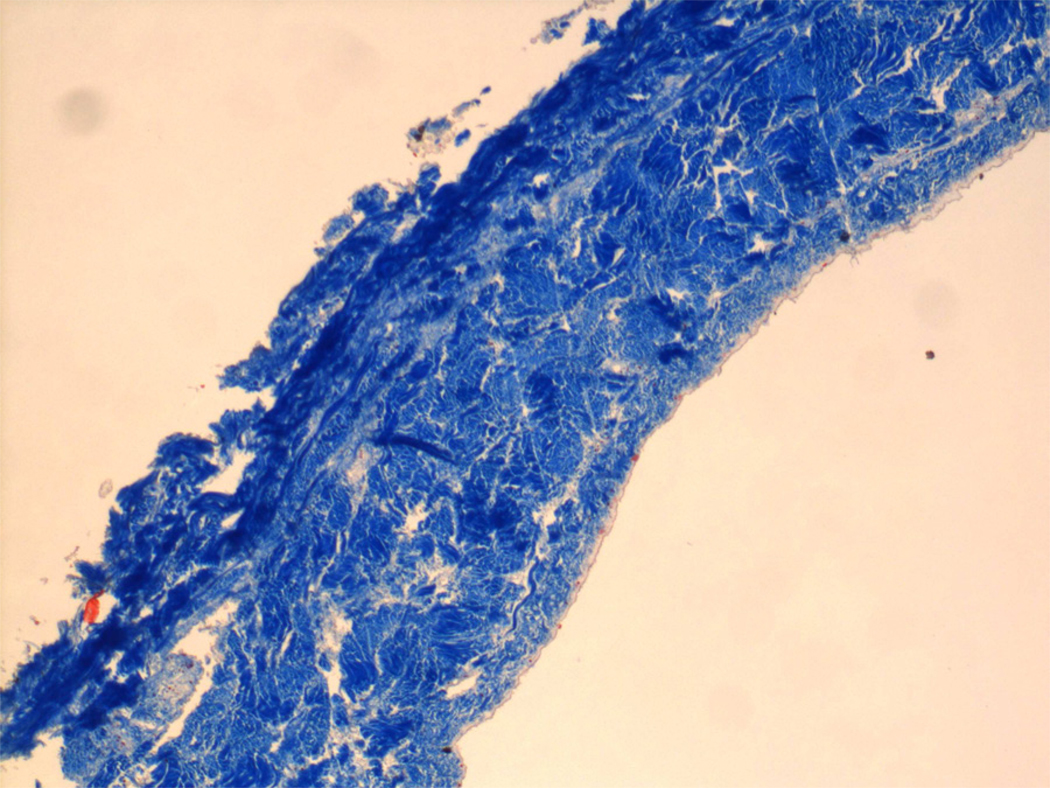
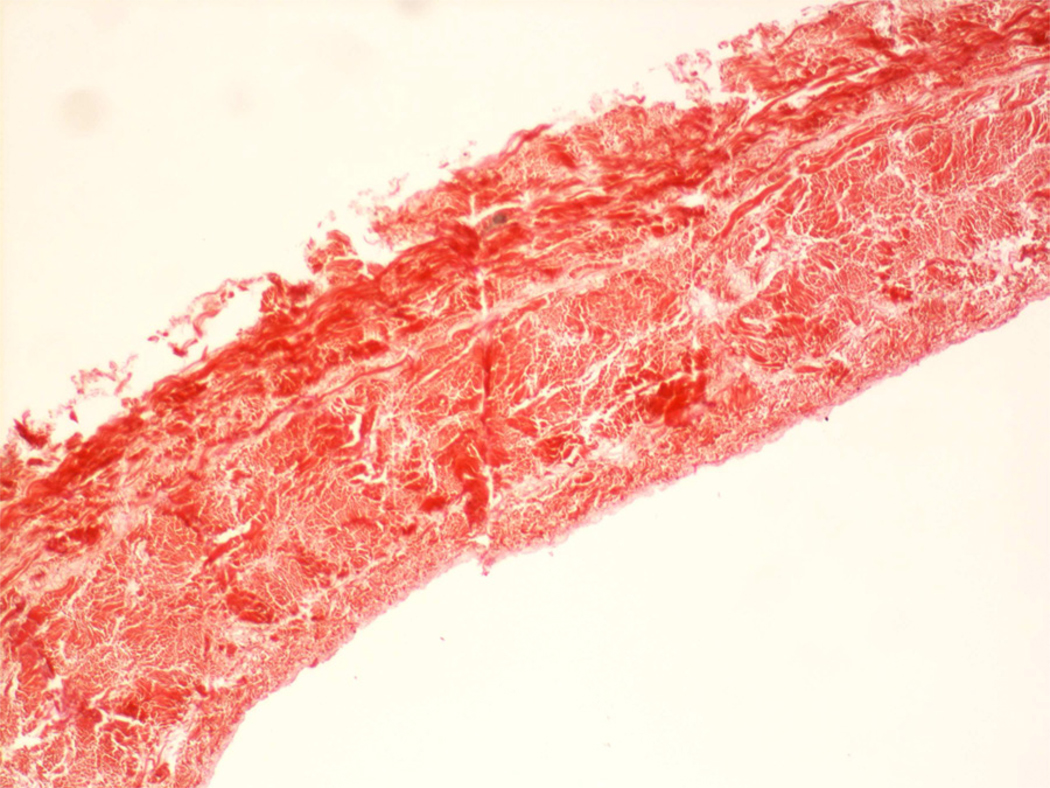
The native structure of bovine pericardium has three layers: 1) the serosa, the inner thin layer consisting of mesothelial cells; 2) the fibrosa, the thicker layer formed by diversely oriented, wavy bundles of collagen and elastin; and 3) the epipericardial connective tissue layer, the outer layer that is partly continuous with the pericardiosternal ligaments. Commercially available patches are processed to be acellular, preventing transplantation of bovine proteins or DNA into the host.(1) Gluteraldehyde is a typical processing agent, crosslinking −NH2 groups of lysine, hydroxylysine, or the N-terminus of amino acids, to form amine linkages with the elimination of water; these amine linkages form covalent bonds between adjacent proteins that are stable at physiological temperature and pH. The resultant cross linking process increases tissue strength to inhibit biodegradation, as well as reduces antigenicity to sterilize the tissue. Figures 1–3 show a commercial grade BP prior to implantation, demonstrating its lack of cells or elastin; only collagen is easily detected in the patch. Elimination of residual gluteraldehyde, prior to patch implantation, is important to prevent late patch calcification in vivo.
Figure 1.
Bovine pericardial patch, stained with H&E. Light red staining is indicative of collagen. There are no cells visible within the patch.
Figure 3.
Bovine pericardial patch, stained with Trichome staining. Dark blue staining is indicative of collagen.
Advantages
Bovine pericardium has several advantages in its use as a cardiovascular patch. These advantages can be divided into two groups, i.e. benefits that have been clearly observed and documented, and benefits that are noticed and likely but as of yet not well documented (Table 1). There are several well-known features of bovine pericardium. First is its reliable consistency; bovine pericardium is able to be manufactured and processed to a consistent nominal 0.5 mm thickness, providing dependable suture retention(2) as well as ideal operative handling characteristics. As such, BP has little suture line bleeding after implantation, similar to autologous vein patches and significantly less than other prosthetic patch materials. Bovine pericardium is also greatly biocompatible; this material is derived from a biological material that is fixed in glutaraldehyde, increasing the strength and stability of the material, while simultaneously reducing antigenicity and potential infections; the increased material strength has been observed to correlate with long-term durability when used clinically. Interestingly, the compliance of BP is more similar to that of the native artery and much greater than several other clinically used prosthetic materials, allowing a tight fitting closure of the angioplasty site, possibly contributing to the minimal suture line bleeding. Since BP is fixed tissue, it also offers the benefit of off-the-shelf availability.(3) Finally, since bovine pericardium is a solid tissue, without air spaces, insonation with ultrasound is possible immediately after implantation.
Table 1.
Advantages of bovine pericardium
| Known benefits: |
| Reliable consistency |
| Ease of handling |
| Durability |
| Strength |
| Biocompatibility |
| Lack of suture line bleeding |
| Off the shelf availability |
| Immediate insonation |
| Possible benefits: |
| Anticalcification |
| Reduced restenosis |
| Reduced infections |
| Supports cellular ingrowth |
There are additional benefits of BP, as an arterial patch, that have been observed but that are not as well documented. Because BP is an acellular material of essentially pure collagen, it may provide a natural microenviroment for host cell migration and proliferation, accelerating endothelialization and tissue regeneration.(1) As such, it has been observed that the restenosis rate after CEA using BP compares favorably with autologous vein and may be slightly lower compared to the rate of restenosis after CEA using a prosthetic patch.(4,5) Some manufacturers claim that their BP possess anticalcification technology that can significantly reduce calcification and support endothelialization.(6,7) There have been several case reports that have reported the use of BP to close an arteriotomy in the presence of an infected field;(8,9) the authors’ personal experience with the material agrees with these observations. However, large series of these cases as well as scientific evidence that BP is either bacteriostatic or bacteriocidal is not currently available, nor have any animal models been described. Whether any of these potential “benefits” actually improve clinical outcomes will require additional reports from long-term observations and studies.
Clinical uses
Vascular surgery
Bovine pericardium is commonly used in vascular surgery, typically as a material used for patch closure of a longitudinal arteriotomy (“patch angioplasty”). Since primary closure of a longitudinal arteriotomy may result in early restenosis, due to neointimal hyperplasia, patch closure is advocated to prevent this technical problem; transverse arteriotomy is typically closed primarily as it is not associated with this type of lumen narrowing, stricturing and restenosis. Most commonly BP patch angioplasty is used after CEA, although it is commonly performed in other medium sized vessels such as the femoral or popliteal arteries, often after thrombectomy or embolectomy. Patch closure after CEA was routinely used by Imparato as early as 1965.(10) Patch angioplasty has been shown to reduce the incidence of both early and late complications after CEA, including reduction of restenosis.(11) Meta-analysis provides strong evidence that carotid patching provides both perioperative and long-term benefits after CEA and supports the standard use of patching during conventional CEA.(12)
The first choice of patch material would logically be, and historically was, the autogenous saphenous vein. Vein patch angioplasty should have the lowest incidence of postoperative thrombosis due to the presence of an intact endothelial surface of the harvested vein, an inherent suppleness and natural compliance that allows for exact approximation to the arterial edge without kinking, and immediate sealing of suture holes thereby decreasing the incidence of suture line bleeding and postoperative cervical wound hematoma.(3,12) Despite these advantages, a vein patch requires a separate harvest procedure, increasing the operative time and possibly risk of infection; patients rarely prefer to have a separate incision on their leg for an operation elsewhere in the body. In addition, early reports of vein patch blowout, with catastrophic results, may have biased some surgeons against using vein patches, despite the association of this complication only with thin or small veins.(13,14) Therefore, some surgeons and patients prefer the use of prosthetic materials that are available “off-the-shelf” to the surgeon, not requiring a separate harvest. Several authors have reported the outcome of using different patches after CEA, with the majority of studies reporting little differences in outcome regardless of the material used, including no significant difference in the rate of recurrent stenosis. Controversially, Neuhauser and Oldenburg have reported that BP patching during CEA may have a lower rate of restenosis compared to the rate after knitted polyester patching.(15) Marien et al (16) concluded that BP patches demonstrated a statistically significant decreased amount of intraoperative suture line bleeding compared with Dacron patches. A recent review of studies comparing patch materials has shown that there is little reliable evidence to guide surgeons as to which material is optimal.(3) More data is still needed to establish standard guidelines, as the differences found between compared materials have typically been very small.(17)
Cardiac and thoracic surgery
BP is widely used in cardiac and thoracic surgery, including use as bioprosthetic valve leaflets, for repair of intracardiac defects, and for repair of diaphragmatic defects. The cardiac surgery literature contains comparisons of BP to other materials, such as the ePTFE patch, for closure of ventricular septal defects.(18) The authors reported that although there were no significant differences between the two materials in outcome after ventricular septal defect closure, the surgeons had a preference for bovine pericardium due to its handling characteristics, elasticity and its lower risk of post-operative endocarditis. BP has been reported to be used in thoracic surgery, particularly for suture line reinforcement during lung volume reduction procedures. Potential complications may include erosion into bronchi and an inflammatory reaction.(19)
Other fields
Bovine pericardium has also been used in other fields of surgery, including general surgery, urologic surgery and ophthalmology. The use of BP to repair abdominal hernias is now well established; some authors have concluded that BP can be used safely and efficiently for the reconstruction of incisional hernias not suitable to direct repair.(20) BP has also been used in the repair of extrahepatic bile duct strictures.(21) Urologists have reported the use of BP as a urethral patch in laboratory animal studies; successful urethral reconstruction was possible in only 20% of the animals; infection and leakage was hypothesized as the cause of the urethrocutaneous fistulas present in 80% of cases. Further studies are necessary to determine whether such fistulas are avoidable, potentially allowing this option for human use. In ophthalmology, a case report describes the use of BP in the treatment of a large corneal perforation secondary to alkali injury. Follow-up at 9 months showed a well-incorporated graft without dehiscence and with minimal inflammation.(22) In the setting of iatrogenic injury of the trachea, the use of BP in reconstruction has been proved to be safe and effective.(23)
Future Directions
In an early report, Araujo et al found no evidence of endothelialization, infection, thrombosis, or aneurysm formation associated with the use of BP for patch angioplasty.(24) However, since then, additional cases and series have reported intimal hyperplasia, restenosis, fibrosis and calcification occasionally associated with BP. Although the response to vascular injury leads to intimal hyperplasia in native vessels, vascular bypass grafts, and bovine pericardial patches, it is not clear whether the underlying fundamental molecular mechanisms are similar in these diverse situations, or whether the initial molecular responses that determine BP healing are fundamentally different from those present in native vessels, yet may still lead to similar gross pathophysiology.
Restenosis
The rate of restenosis after angioplasty – both endovascular angioplasty as well as open patch angioplasty – is a major topic of research. There are a large number of reports that compare the rate of restenosis after placement of different types of patches used in conjunction with CEA; however, most of these series report no substantial differences in outcome between BP and other prosthetic patch materials.(17) It is believed that the main cause of restenosis after CEA performed with patch angioplasty is intimal hyperplasia in the area at or near the patch. However, despite the morphological similarity of this intimal hyperplasia to that seen in a vein bypass graft, a dialysis access conduit, or a prosthetic graft, it is not clear whether the fundamental mechanisms – on a molecular and cellular level – that stimulate intimal hyperplasia under all these clinical circumstances are actually similar. For example, we have recently shown that intimal hyperplasia that occurs in vein grafts, despite its morphological similarity to that which occurs in injured arteries, is associated with loss of the venous identity marker Eph-B4, a molecular pathway that is not known to be active in arteries.(25) Similarly, the molecular and cellular mechanisms that govern BP healing after arterial patch angioplasty are not currently well described; however, it is likely that some mechanisms are similar to, and some mechanisms are different from, those described for other prosthetics such as Dacron or ePTFE. Since the biological signaling pathways that activate vascular healing after patch angioplasty must necessarily depend on the cells that infiltrate the patch, we believe that a characterization of the types of cells that differentially infiltrate these different types of patches will help clarify some of the differences by which different patches may have different biological responses and therefore may need different types of treatment, despite morphologically similar intimal hyperplasia and restenosis.
Pseudoaneurysm
Although unusual, pseudoaneurysm formation is a known complication of angioplasty and bovine vascular graft placement.(26) The pathogenesis of these pseudoaneurysms is related to either graft deterioration or infection, fundamentally distinct processes that result in morphologically similar pathology, analogous to the potential for different mechanisms that lead to morphologically similar intimal hyperplasia. The fundamental strength of the gluteraldehyde-fixed BP patch may be responsible for its low rate of degeneration and possibly its resistance to infection, either of which may be responsible for the in turn for the low rate of pseudoaneurysm formation. Management of pseudoaneurysms is usually challenging, especially when they are present in the carotid artery; pseudoaneurysm rupture may cause fatal hemorrhage and, in the carotid artery, stroke. Correction by open surgery may necessitate the interruption of the carotid artery flow, which can precipitate a stroke, potentially requiring use of a shunt during the procedure. In addition, the rate of cranial nerve injury is likely to be higher in the redo operative field. Recent trends in management of these complex situations have begun to explore the use of endovascular techniques such as deployment of a covered stent-graft.(27)
Thrombosis
Thrombosis of BP patches has not been reported to be a major problem, either acutely or chronically; nevertheless, as applications of BP continue to expand, the fundamental issues of patch resistance to thrombus formation are likely to be encountered.
The fundamental factors that predispose to thrombus formation, e.g. endothelial injury, stasis, and hypercoagulability, remain true since they were first described by Virchow, and these factors may describe both acute and chronic thrombus formation. During CEA, the raw surface of the post-endarterectomy arterial media may be a nidus of thrombus formation; similarly, the collagen surface of the BP patch may be site of initiation of thrombus formation. We routinely administer low molecular weight dextran-40 during the first postoperative day after arterial endarterectomy with patch angioplasty to prevent thrombus formation and propagation at the site of the endarterectomy. Heparin is given during the procedure to prevent thrombus formation in the static artery distally to the clamp, especially if a shunt is not used. Care must be taken when suturing the BP patch in order to prevent injury to the endothelium of the surrounding artery, if an endarterectomy was not performed. A single case of hyperacute rejection with fibrin accumulation has been reported on an aortic valve prosthesis.(28)
During followup of BP patches placed for patch angioplasty, late thrombosis is rarely reported, likely due to the high flow in the carotid artery. It is not currently known whether BP patches heal in human patients by endothelialization. Nevertheless, it is currently believed that endothelialization is one of the most promising solutions in reducing the thrombogenicity of cardiovascular implants.(29) The only clinical data comes from the reported superior long term patency in small series of ePTFE grafts that were seeded with autologous endothelial cells, compared to conventional implants.(30,31) It is likely that future strategies to promote optimal vascular healing of BP patches are likely to involve endothelialization of the patch, either prior to implantation, or to promote it rapidly in situ.
Calcification
Vascular calcification is common in the elderly and is thought to be a major risk factor for cardiovascular morbidity and mortality. BP patches are often used in elderly patients with calcified vessels; as such it might be expected that the underlying process leading to calcium deposition in native vessels may also lead to deposition of calcium in the vessel patch. Historically, it is likely that residual gluteraldehyde that was used in the manufacturing of BP patches increased patch calcification,(32) and thus it is not surprising that more early reports of patch calcification have not be described as has been for aortic valves.(33) However, it is likely that modern manufacturing processes eliminate both the low amount of residual gluteraldehyde as well as any residual cellularity, both of which may increase patch calcification; in addition, the bovine source of pericardium may also play a role in decreased calcification.(34)
Calcification of atherosclerotic plaques occurs when cells that have undergone osteoblastic differentiation in the neointima synthesize and secrete a mineralized matrix containing type I collagen in a process similar to bone formation.(35,36) Medial calcification results in vascular stiffness and increases pulse wave velocity that, in turn, may induce clinical vascular dysfunction including systemic effects such as hypertension and cardiac failure. The mineralization of elastin is different from osteoblastic bone formation, but the pathogenesis of medial calcification is less clearly understood than that which occurs as part of atherosclerosis.(37) Many authors have reported that cells derived from the arterial media, including smooth muscle cells, adventitial fibroblasts, and pericytes, undergo osteochondrogenic differentiation and matrix mineralization under the appropriate conditions in vitro.(38–41) These studies suggest that cell-mediated processes tightly control procalcific and anticalcific mediators in the artery so that ectopic calcification is normally avoided. As such it is possible that if smooth muscle cells and/or fibroblasts migrate into the BP patch, the patch may provide an environment that promotes subsequent calcification and degradation.(42) Hruska et al (37) summarized the currently accepted major theories regarding the mechanism and regulation of vascular calcification: (a) failure of anticalcific mediators; (b) induction of osteochondrogenesis; (c) apoptosis; (d) abnormal calcium and phosphate homeostasis; (e) circulating nucleatic complexes/paracrine factors derived from bone and (f) matrix degradation. As these etiologies become more understood, it is possible that preventive strategies may be able to be incorporated into future generations of patches, i.e. incorporation of anticalcific mediators. These next generation patches might be particularly useful for patients with chronic kidney disease, type 2 diabetes mellitus, and elderly, who might be at increased risk of patch calcification and degradation.
Infection
Infection is always an issue when dealing with the implantation of artificial materials; the rate of prosthetic patch infection after CEA has been estimated to be approximately 0.4% (43). Reports of infection definitively related to BP patches are exceedingly rare and difficult to differentiate from overlying wound infection. Derksen. et al (44) have studied the infection rate after common femoral artery endarterectomy, comparing bovine patches, autologous vein patches and synthetic patches. They hypothesized that bovine pericardium has a similar risk of postoperative infection, comparable to autologous vein.(44,45) The authors found that two independent risk factors for postoperative surgical-site infection: vascular reoperation in the ipsilateral groin and presence of a wound drain. The authors could not prove a significant difference between synthetic and bovine patches in relation to the incidence of postoperative surgical-site infection.
The low rate of definitive infection linked to BP patches has suggested to some surgeons that BP patches may be resistant to infection, and therefore might be an appropriate material to use in the presence of infection.(8,9,46) As this thought relies on the lack of verified cases of BP patch infection, a negative argument, we believe that until an underlying mechanism of resistance to infection is reported for BP patches, such as contact cytotoxicity,(47) there is no reason to believe that BP is immune from becoming infected.
Fibrosis
Fibrosis of patches is unusual and reports of BP patch fibrosis are distinctly rare (48,49). It is likely that the mechanisms that induce patch calcification are similar to those that induce patch fibrosis, although with subtle differences. Further research may help identify fibrocyte-specific signaling pathways as potential therapeutic targets to prevent BP fibrosis.(50)
Future directions
Can seeding mesenchymal stromal cells on BP avoid intimal hyperplasia? In order to provide a base for uniform cardiac tissue regeneration, a mechanism that seeds a spatially uniform distribution of adherent cells onto a scaffold has been described, using BP as the scaffold for the seeded multilayered mesenchymal stromal cells.(51) The results demonstrate that this novel bioengineered tissue graft can serve as a useful cardiac patch to restore the dilated ventricle and stabilize cardiac functions after myocardial infarction.(52) Interestingly, the authors reported increased density of neomicrovessels in the tissue engineered patches compared to control patches, suggesting that tissue regeneration occurs within the porous bovine pericardium through a process involving cell recruitment and tissue-specific differentiation. This exciting research shows the prospect for delivering cell therapy, in a site-specific manner, with bovine pericardial patches. Similarly, other noncellular agents may be delivered.(53)
Figure 2.
Bovine pericardial patch, stained for elastin. No elastin is seen. Dark red staining is indicative of collagen.
Acknowledgments
Grant support: This review was supported in part by the National Institute of Health grant R01-HL095498-01, the American Vascular Association William J. von Liebig Award, as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, Conn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang Y, Liang HC, Wei HJ, et al. Tissue regeneration patterns in acellular bovine pericardia implanted in a canine model as a vascular patch. J Biomed Mater Res A. 2004;69(2):323–333. doi: 10.1002/jbm.a.30003. [DOI] [PubMed] [Google Scholar]
- 2.Obermiller JF, Hodde JP, McAlexander CS, et al. A comparison of suture retention strengths of three biomaterials. Med Sci Monit. 2004;10(1):PI1–PI5. [PubMed] [Google Scholar]
- 3.Muto A, Nishibe T, Dardik H, et al. Patches for carotid artery endarterectomy: Current materials and prospects. J Vasc Surg. 2009;50(1):206–213. doi: 10.1016/j.jvs.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biasi GM, Sternjakob S, Mingazzini PM, et al. Nine-year experience of bovine pericardium patch angioplasty during carotid endarterectomy. J Vasc Surg. 2002;36(2):271–277. doi: 10.1067/mva.2002.123685. [DOI] [PubMed] [Google Scholar]
- 5.Kim GF, Kwon TW, Cho YP, et al. Carotid endarterectomy with bovine patch angioplasty: a preliminary report. Cardiovascular Surgery. 2001;9(5):458–462. doi: 10.1016/s0967-2109(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 6.Cunanan CM, Cubbling CM, Dinh TT, et al. Tissue characterization and calcification potential of commercial bioprosthetic heart valve. Ann Thorac Surg. 2001;71 5 Suppl:S417–S421. doi: 10.1016/s0003-4975(01)02493-6. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed Nov 15, 2010]; http://www.edwards.com/products/porcinevalves/patch.htm. [Google Scholar]
- 8.David TE. The surgical treatment of patients with prosthetic valve endocarditis. Semin Thorac Cardiovasc Surg. 1995;7:47–53. [PubMed] [Google Scholar]
- 9.Jones JM, Sarsam MA. Partial mitral valve replacement for acute endocarditis. Ann Thorac Surg. 2001;72:255–257. doi: 10.1016/s0003-4975(00)02582-0. [DOI] [PubMed] [Google Scholar]
- 10.Imparato AM. The role of patch angioplasty after carotid endarterectomy. J Vasc Surg. 1988;7(5):715–716. [PubMed] [Google Scholar]
- 11.Grimsley BR, Wells JK, Pearl GJ, et al. Bovine pericardial patch angioplasty in carotid endarterectomy. Am Surg. 2001;67(9):890–895. [PubMed] [Google Scholar]
- 12.Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2009 Oct 7;4 doi: 10.1002/14651858.CD000160.pub3. CD000160. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riles TS, Lamparello PJ, Giangola G, Imparato AM. Rupture of the vein patch: a rare complication of carotid endarterectomy. Surgery. 1990;107:10–12. [PubMed] [Google Scholar]
- 14.Archie JP. Carotid endarterectomy saphenous vein patch rupture revisited: Selective use on the basis of vein diameter. J Vasc Surg. 1996;24:346–352. doi: 10.1016/s0741-5214(96)70190-8. [DOI] [PubMed] [Google Scholar]
- 15.Neuhauser B, Oldenburg WA. Polyester vs. bovine peri-cardial patching during carotid endarterectomy: early neurologic events and incidence of restenosis. Cardiovasc Surg. 2003;11(6):465–470. doi: 10.1016/S0967-2109(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 16.Marien BJ, Raffeto JD, Seidman CS, et al. Bovine pericardium vs Dacron for patch angioplasty after carotid endarterectomy. Arch Surg. 2002;137(7):785–788. doi: 10.1001/archsurg.137.7.785. [DOI] [PubMed] [Google Scholar]
- 17.Byrne J, Feustel P, Darling RC., 3rd Primary closure, routine patching, and eversion endarterectomy: what is the current state of the literature supporting use of these techniques? Semin Vasc Surg. 2007;20(4):226–235. doi: 10.1053/j.semvascsurg.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Us MH, Sungun M, Pocan S, et al. A retrospective comparison of bovine pericardium and polytetrafluoroethylene patch for closure of ventricular septal defects. J Int Med Res. 2004;32(2):218–221. doi: 10.1177/147323000403200216. [DOI] [PubMed] [Google Scholar]
- 19.Provencher S, Deslauriers J. Late complication of bovine pericardium patches used for lung volume reduction surgery. Eur J Cardiothorac Surg. 2003;23(6):1059–1061. doi: 10.1016/s1010-7940(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 20.Szerafin T, Leny A, Palotas L, et al. Abdominal hernia repair with no-react treated bovine pericardial patch. Magy Seb. 2008;61 Suppl:61–65. doi: 10.1556/MaSeb.61.2008.Suppl.15. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Xu YD, Li JS. Cholangioplasty by using a patch of bovine pericardium in treatment of stricture of extrahepatic bile duct. Zhonghua Wai Ke Za Zhi. 1994;32(5):269–270. [PubMed] [Google Scholar]
- 22.Khanna RK, Mokhtar E. Bovine pericardium in treating large corneal perforation secondary to alkali injury: a case report. Indian J Ophthalmol. 2008;56(5):429–430. doi: 10.4103/0301-4738.42426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbetakis N, Samanidis G, Paliouras D, et al. Intraoperative tracheal reconstruction with bovine pericardial patch following iatrogenic rupture. Patient Saf Surg. 2008;2:4. doi: 10.1186/1754-9493-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araujo JD, Braile DM, Azenha Filho JO, et al. The use of bovine pericardium as an arterial graft. A five year followup. J Cardiovasc Surg(Torino) 1987;28:434–439. [PubMed] [Google Scholar]
- 25.Kudo FA, Muto A, Maloney SP, et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27(7):1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 26.Brems J, Castaneda M, Garvin PJ. A five year experience with the bovine heterograft for vascular access. Arch Surg. 1986;121(8):941–944. doi: 10.1001/archsurg.1986.01400080087016. [DOI] [PubMed] [Google Scholar]
- 27.Lin PH, Bush RL, Lumsden AB. Successful stent-graft exclusion of a bovine patch related carotid artery pseudoaneurysm. J Vasc Surg. 2003;38(2):396. doi: 10.1016/s0741-5214(03)00428-2. [DOI] [PubMed] [Google Scholar]
- 28.Puckett FA, Stahlfeld KR, Dimarco RF. Hyperacute rejection of a bovine pericardial prosthesis. Tex. Heart Inst J. 2006;33(2):260–261. [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider PA, Hanson SR, Price TM, et al. Confluent durable endothelialization of endarterectomized baboon aorta by early attachment of cultured endothelial cells. J Vasc Surg. 1990;11(3):365–372. [PubMed] [Google Scholar]
- 30.Deutsch M, Meinhart J, Zilla P, et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009 Feb;49(2):352–362. doi: 10.1016/j.jvs.2008.08.101. [DOI] [PubMed] [Google Scholar]
- 31.Bordenave L, Femandez P, Remy-Zolghadri M, et al. In vitro endothelialized ePTFE prostheses: clinical update 20 years after the first realization. Clin Hemorheol Microcirc. 2005;33(3):227–234. [PubMed] [Google Scholar]
- 32.Neethling WM, Hodge AJ, Clode P, Glancy R. A multi-step approach in anti-calcification of glutaraldehyde-preserved bovine pericardium. J Cardiovasc Surg (Torino) 2006 Dec;47(6):711–718. [PubMed] [Google Scholar]
- 33.Rao KP, Shanthi C. Reduction of calcification by various treatments in cardiac valves. J Biomater Appl. 1999 Jan;13(3):238–268. doi: 10.1177/088532829901300305. [DOI] [PubMed] [Google Scholar]
- 34.Glasmacher B, Reul H, Schneppershoff S, Schreck S, Rau G. In vitro calcification of pericardial bioprostheses. J Heart Valve Dis. 1998 Jul;7(4):415–418. [PubMed] [Google Scholar]
- 35.Al-Aly Z, Shao JS, Lai CF, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/−mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 36.Mathew S, Tustison KS, Sugatani T, et al. The mechanism of phosphorus as a cardiovascular risk factor in chronic kidney disease. J Am Soc Nephrol. 2008;19(6):1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hruska KA. Vascular smooth muscle cells in the pathogenesis of vascular calcification. Circ Res. 2009;104(6):710–711. doi: 10.1161/CIRCRESAHA.109.195487. [DOI] [PubMed] [Google Scholar]
- 38.Wada T, McKee MD, Steitz S, et al. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Cir Res. 1999;84(2):166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 39.Jono S, Peinado C, Giachelli CM, et al. Phosphorylation osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275(26):20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 40.Tintut Y, Alfonso Z, Saini T, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108(20):2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 41.Doherty MJ, Ashton BA, Walsh S, et al. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13(5):828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 42.Jorge-Herrero E, Turnay J, Calero P, Olmo N, López De Silanes I, Martín Maestro M, Lizarbe MA, Castillo-Olivares JL. Calcification and identification of metalloproteinases in bovine pericardium after subcutaneous implantation in rats. J Mater Sci Mater Med. 2001 Oct–Dec;12(10–12):1013–1017. doi: 10.1023/a:1012833720931. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan S, Clowes AW. Daron patch infection after carotid endarterectomy: case report and review of literature. Ann Vasc Surg. 2006;6:672–677. doi: 10.1007/s10016-006-9064-5. [DOI] [PubMed] [Google Scholar]
- 44.Derksen WJ, Verhoeven BA, van de Mortel RH, et al. Risk factors for surgical-site infection following common femoral artery endarterectomy. Vascular and Endovasc Surg. 2009;43(1):69–75. doi: 10.1177/1538574408323502. [DOI] [PubMed] [Google Scholar]
- 45.Matsagas MI, Bali C, Arnaoutoglou E, et al. Carotid endarterectomy with bovine pericardium patch angioplasty: mid-term results. Ann Vasc Surg. 2006;20(5):614–619. doi: 10.1007/s10016-006-9102-3. [DOI] [PubMed] [Google Scholar]
- 46.Kunitomo R, Hara M, Utoh J, Sakaguchi H, Uemura S, Kitamura N. Patch reconstruction of the mitral annulus for active infective endocarditis with annular abscess. Ann Thorac Cardiovasc Surg. 2001 Feb;7(1):52–55. [PubMed] [Google Scholar]
- 47.Oswal D, Korossis S, Mirsadraee S, Wilcox H, Watterson K, Fisher J, Ingham E. Biomechanical characterization of decellularized and cross-linked bovine pericardium. J Heart Valve Dis. 2007 Mar;16(2):165–174. [PubMed] [Google Scholar]
- 48.Smith VC, Knauf DG, Alexander JA, et al. Bovine pericardial patch fibrosis requiring reoperation. J of Inves Surg. 1988;1(4):289–290. doi: 10.3109/08941938809141094. [DOI] [PubMed] [Google Scholar]
- 49.Kacowak MH, Levett JM, Majoney DL, et al. Comparative studies of prosthetic materials in the left atrium of the dog. Virchows Arch[A] 1987;411(2):173–177. doi: 10.1007/BF00712741. [DOI] [PubMed] [Google Scholar]
- 50.Bellini A, Mattoli S. The role of fibrocyte, a bone marrow derived mesenchymal progenitor, in reactive and reparative fibroses. Labor Invest. 2007;87(9):858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 51.Chen CH, Wei HJ, Lin WW, et al. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc Res. 2008;1:80(1):88–95. doi: 10.1093/cvr/cvn149. [DOI] [PubMed] [Google Scholar]
- 52.Chang Y, Lai PH, Wei HJ, et al. Tissue regeneration observed in a basic fibroblast growth factor-loaded porous acellular bovine pericardium populated with mesenchymal stem cells. J Thorac Cardiovasc Surg. 2007;134(1):65–73. doi: 10.1016/j.jtcvs.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Chang Y, Lai PH, Wang CC, Chen SC, Chang WC, Sung HW. Mesothelium regeneration on acellular bovine pericardia loaded with an angiogenic agent (ginsenoside Rg1) successfully reduces postsurgical pericardial adhesions. J Thorac Cardiovasc Surg. 2006 Oct;132(4):867–874. doi: 10.1016/j.jtcvs.2006.06.029. [DOI] [PubMed] [Google Scholar]