Abstract
The chemokine CXCL12 and its receptors, CXCR4 and CXCR7, are involved in tumor progression, metastasis, and survival. We investigated the expression of CXCR4, CXCL12 and CXCR7 in malignant pleural mesothelioma to determine if they are possible biomarkers and potential therapeutic targets. Forty-one mesothelioma tumor tissues, 10 normal human pleural tissues and 2 mesothelioma cell lines were stained with anti-CXCR4, anti-CXCL12, anti-CXCR7 and anti-p-Akt antibodies. RT-PCR was performed to determine the expression of CXCR4, CXCL12 and CXCR7 in 6 human mesothelioma cell lines (H28, 211H, H2052, ms-1, H290, and H513) and 1 human normal mesothelial cell line LP9. These seven cell lines were also stained with anti-CXCR7. We found that CXCR4 and CXCL12 were expressed in 97.6% and 78.0% mesothelioma tissue samples, concurrent with strong expression of p-Akt (R2 = 0.739 and 0.620 respectively). In addition, CXCR7 expression was weaker than CXCR4 expression in mesothelioma tissues. Furthermore, RT-PCR showed that CXCR4 and CXCL12 were over-expressed in 5/6 mesothelioma cell lines (211H, H2052, ms-1, H290, and H513), whereas CXCR7 was over-expressed in only 2/6 (H513 and H2052). Moreover, we found that the CXCR4 antagonist AMD3100 inhibited the growth of all 5 mesothelioma cell lines that over-express CXCR4 and CXCL12. Our results suggest that the Akt-mTOR pathway is involved during the interruption of the CXCL12/CXCR4 axis in these five mesothelioma cell lines. In conclusion, CXCR4 and CXCL12 are highly expressed in most mesothelioma cell lines and tumor tissues, suggesting that CXCR4 and CXCL12 may be used as biomarkers for patients with mesothelioma. The CXCL12-CXCR4 interaction may be a potential therapeutic target for mesothelioma.
Keywords: Mesothelioma, CXCL12, CXCR4, CXCR7, Akt
INTRODUCTION
Malignant pleural mesothelioma is an aggressive cancer that originates mostly from the pleura of the lung. Mesothelioma is associated with occupational exposure to asbestos, and about 3000 patients are diagnosed annually in the United States [1]. Most patients are diagnosed at a relatively late stage, which makes curative resection difficult. Despite aggressive treatment with radiation therapy or chemotherapy, the prognosis of mesothelioma has remained poor for decades, with a median survival time between 8–18 months [2]. Therefore, identification of molecular targets in mesothelioma and development of new treatments for this fatal disease are needed.
Chemokines are a family of small cytokines that mainly act as chemo-attractants to guide cell migration. Some chemokines control immune cells and are associated with angiogenesis and cellular maturation. Chemokines exert their biological effects by interacting with G protein-linked transmembrane receptors called chemokine receptors [3]. The chemokine CXCL12, also known as stromal derived factor 1, is expressed by stromal cells such as fibroblasts and endothelial cells [4, 5]. CXCL12 is also expressed in breast cancer, brain tumors, and in colorectal cancer [6–8].
Chemokine receptors are 7- transmembrane G-coupled proteins [9–11]. To date, 20 chemokine receptors (CCR1-11, CXCR1-7, XCR1, and CX3CR1) have been identified. Among these receptors, CXCR4 is of particular importance in tumor biology, especially in tumor metastasis. CXCR4 is the specific chemokine receptor for CXCL12 and is expressed by immune cells and various types of cancer cells, among others [12–14]. In colorectal cancer, CXCR4 is significantly associated with advanced tumors and with lymphatic or distant metastasis, and high expression of CXCR4 is reportedly a strong and independent predictor of early distant relapse [6, 15–17]. These findings suggest that CXCL12 and CXCR4 play important roles in local progression, dissemination, and immune evasion of cancer cells.
Recently, a novel receptor for CXCL12 has been identified, termed CXCR7/RDC1 [18, 19]. In humans, CXCR7 is expressed on tumor -associated blood vessels and on distinct malignant cells, including breast, lung, and prostate cancer cells [19–22]. Numerous authors have reported CXCR4 expression in tumors and demonstrated that the CXCR4/CXCL12 axis can trigger cell adhesion, directional migration, and proliferation in tumor cells. In addition, recent data suggest that CXCR7 has key functions in promoting tumor development and progression [20]. However, little is known about the involvement of CXCL12 and CXCR4 in mesothelioma, and no reports have described the expression of CXCR7 in mesothelioma. Here we show that CXCR4 and CXCL12 are over-expressed in human mesothelioma tissue when compared to normal pleura. We also detected CXCR4 and CXCL12 expression in human mesothelioma cell lines. Simultaneously, CXCR7 was weakly expressed in a subset of mesothelioma tissues and cell lines.
METHODS
Reagents
Anti-CXCR4 clone 12G5 and anti-CXCL12 clone 79018 were purchased from R&D Systems (Minneapolis, MN, USA). Anti-CXCR7 clone was purchased from Abcam (Cambridge, MA, USA). Anti-phospho Akt (Ser473) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Human CXCL12 was purchased from Upstate (Lake Placid, NY, USA). AMD3100 was purchased from Sigma (St. Louis, MO, USA)
Cell lines and cell culture
Human mesothelioma cell lines H28, 211H, H2052, ms-1, H290, H513 and human normal mesothelial cell line LP9 were purchased from the Cell Culture Core Facility at Harvard University (Boston, MA). The mesothelioma cell lines were maintained in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated FBS, penicillin (100IU/ml) and streptomycin (100µg/ml). LP9 was maintained in M199 supplemented with 10% (v/v) heat-inactivated FBS, 10ng/ml EGF and 0.4µg/ml hydrocortisone, penicillin (100IU/ml), and streptomycin (100µg/ml).
Tissue samples and immunohistochemistry
Fresh mesothelioma and adjacent normal pleural tissues were obtained from patients with malignant plural mesothelioma who were undergoing surgical resection of the primary tumor. Primary human mesothelioma samples from 41 patients were fixed in formalin and embedded in paraffin in 4-µm tissue microarray sections. In seven of these patients, a small amount of normal pleura tissue had been obtained simultaneously to serve as controls. All human tissue samples were obtained and analyzed in accordance with procedures approved by the institutional review board of the University of California, San Francisco (IRB H8714-22942-01).
The tissue microarray sections contained normal pleura tissue samples from another 3 patients and samples of the human mesothelioma cell lines H290 and 211H. The sections were immunostained using a biotin -streptavidin -peroxidase method [23]. Sections underwent routine deparaffinization and rehydration. Slides were then immersed in 10 mM sodium citrate buffer (pH 6.0), boiled for 10 min on a hot plate, and then allowed to cool for 20 min. Sections were then incubated for 10 min in 3% hydrogen peroxide in distilled water, washed in PBS three times for 5 min and incubated with 10% normal horse serum in PBS for 30 min. After three washes in PBS buffer, the sections were incubated overnight at 4°C with 2 µg/ml of primary anti-CXCR4 antibody, clone 12G5 (R&D system), anti-CXCL12, clone 79018 (R&D system), anti-CXCR7 (Abcam) and anti-phospho Akt (Ser473). The sections were then incubated with biotin -labeled secondary antibodies respectively and streptavidin-peroxidase (1:30) for 20 min each. Slides were stained for 5 min with 0.05% 3,3′-diaminobenzidine tetrahydrochloride freshly prepared in 0.05 M Tris–HCl buffer (pH 7.6) containing 0.024% hydrogen peroxidase, and then counterstained with hematoxylin, dehydrated, and mounted in Diatex. Mesothelioma cell lines H28, H2052, ms-1, 211H, H513 and H290 and normal mesothelial cell line LP9 were immunostained using primary anti-CXCR7 antibody by the same procedure.
The followinf scoring system was employed: −, no stain; +, weak staining (30% or above stained cellularity considered as positive); ++, moderate staining (10% or above stained cellularity considered as positive); +++, strong staining (positive). All scoring systems are under low magnification (10−).
Reverse transcription-PCR (RT-PCR)
Total RNA from the human mesothelioma cell lines H28, 211H, H2052, ms-1, H290, H513 and from the human normal mesothelial cell line LP9 were obtained using an RNA extraction kit (Qiagen, Valencia, CA). The cultured cells from all cell lines were harvested once the cells reached confluence. Total RNA was extracted from the cells according to the manufacturer’s instructions. Concentration and quality of the RNA solutions were measured and the solutions were then stored at −80° C. RT-PCR was carried out using the One -Step RT-PCR kit (Invitrogen, Carlsbad, CA) and the RNA as a template. According to the RT-PCR kit protocol, after the reaction, cocktails (2X Reaction Mix, Template RNA F and R primers, RT/Platinum Taq Mix and Autoclave d distilled water) were incubated at 50° C for 30 min and then at 94° C for 2 min. DNA was amplified under the following cycling conditions: denaturation at 94° C for 15 s, annealing at 55° C for 30 s, and extension at 72° C for 1 min. Thirty cycles were used for glyceraldehyde -3-phosphate dehydrogenase (GAPDH, used as an internal control to monitor if RNA isolation and RT-PCR were reliable), and 35 cycles were used for the other primer pairs. Cycling was followed by a final extension step at 72° C for 10 min. For human CXCR4, the forward primer 5’-CAGCAGGTAGCAAAGTGACG-3’ and the reverse primer 5’-GTAGATGGTGGGCAGGAAGA-3’ yield a 208-bp product. For human CXCL12, the forward primer 5’-TCAGCCTGAGCTACAGATGC-3’ and the reverse primer 5’-CTTTAGCTTCGGGTCAATGC-3’ yield a 269-bp product. For human CXCR7, the forward primer 5’-GCAGAGCTCACAGTTGTTGC-3’ and the reverse primer 5’-GCTGATGTCCGAGAAGTTCC-3’ yield a 360-bp product. The primers used for GAPDH were 5’-GAA GGT CGG AGT CAA CGG ATT T-3’ and 5’-ATG GGT GGA ATC ATA TTG GAA C-3’, yielding a 212-bp product. The PCR products were analyzed by agarose gel electrophoresis.
Cell proliferation and viability assay
Cells (3×103) from mesothelioma lines H28, 211H, H2052, ms-1, H290, H513 and normal mesothelial cell line LP9 were cultured in 96-well flat-bottom plates and allowed to adhere overnight. The media was then replaced with DMED supplemented with 0.1% BSA. Twelve hours later, 100 ng/ml CXCL12 was added for stimulation. The cells without CXCL12 stimulation were cultured as control. The proliferative and viability activity was determined by the CellTiter-Glo Luciferase Assay System (Promega, Madison, WI, USA) using a GLOMAX 96 Microplate Luminometer (Promega) at 450 nm 24 and 72 hours after stimulation. The background control (blank) was obtained by adding the same volume of culture medium (100 µl) and CellTiter-Glo Luciferase Assay Substrate (100 µl) as used in the experimental wells. AMD3100 (Sigma) was added to the mesothelioma cell lines in concentrations of 0, 0.1, 0.3, 1, 3, 10, 30 and 100µM respectively during the stimulation.
Western blot analysis
Half a million cells from the mesothelioma lines H28, 211H, H2052, ms -1, H290 and H513 were seeded in each well of 6 well culture plates and allowed to adhere overnight. Subsequently, cells were serum-starved in DMEM containing 0.1% BSA for 12 h. Cells were then incubated with recombinant human CXCL12 (100 ng/ml), with or without CXCR4 inhibitor AMD3100 (0.01, 0.1, 1 and 10µM respectively) in DMEM/0.1% BSA.
Cells were treated with CXCL12 for 0, 2, 5, 10 or 30 minutes without AMD3100. The other cells that were incubated with both CXCL12 and different concentrations of AMD3100 were treated for 5 min. M-PER Mammalian Protein Extraction Reagent (Pierce, Rockford, IL, USA) was used to prepare the cell lysates. Crude protein extracts were used directly for western blot analysis. Protein levels in the extracts were quantified using the Bradford assay. Equal amounts of total protein (30µg per lane) were resolved on 4~15% polyacrylamide gels using standard sodium dodecyl sulfate polyacrylamide gel electrophoresis techniques, and then transferred onto a nitrocellulose membrane (0.45 mm, Millipore Millex, USA) in 25 mM Tris-base, 190 mM glycine, and 20% methanol using a semidry blotter. Membranes were blocked for 1 h at room temperature in TBST (TBS containing 0.5% Tween-20) with 4% non-fat dry milk, then incubated overnight at 4° C with the primary antibodies diluted 1:1000 in TBST containing 4% non-fat dry milk. The membranes were washed in TBST three times and then incubated for 1 h with secondary antibody, either peroxidase-conjugated anti-mouse or anti-rabbit (Santa Cluz Biotechnology, Santa Cluz, CA, USA). GAPDH (1:5000) was used as a loading control. All primary antibodies were from Cell Signaling (Danvers, MA, USA). The blots were washed in TBST three times and the proteins were detected by using the SuperSignal west Femto (Thermo, Rockford, IL, USA) and Chemilmager 5500 (Alpha Innotech, Santa Clara, CA, USA).
Statistical analysis
Correlation analyses were performed using SPSS 13.0 (SPSS Inc, Chicago, USA). We used 0, 1, 2 and 3 as representatives of −, +, ++ and +++ respectively during the calculation.
RESULTS
Immunohistochemistry of CXCR4, CXCL12, p-Akt and CXCR7
The positive or negative results of CXCL12, CXCR4, CXCR7 and p-Akt staining in mesothelioma samples of the tissue microarray sections are shown in Figures 1–3 and Tables 1–3. The results of CXCR7 staining in mesothelioma cell lines H28, H2052, ms-1, 211H, H513 and H290 and normal mesothelial cell line LP9 are shown in Fig. 2. In the mesothelioma samples, the strong positive (+++) ratio was high (39.1%) in CXCR4 staining. The strong positive ratios of CXCL12, and p-Akt staining were lower than that of CXCR4 staining. The strong positive ratio of CXCR7 was the lowest and the negative ratio of CXCR7 was the highest. However, the positive ratio of CXCR7 staining in the mesothelioma samples was still higher than that of normal tissue samples (71.9% vs 11.1%) and the negative ratio was lower than that of normal tissue samples (28.1% vs 88.9%). In some of the mesothelioma samples, the positive regions were similar in CXCL12, CXCR4, CXCR7, and p-Akt staining.
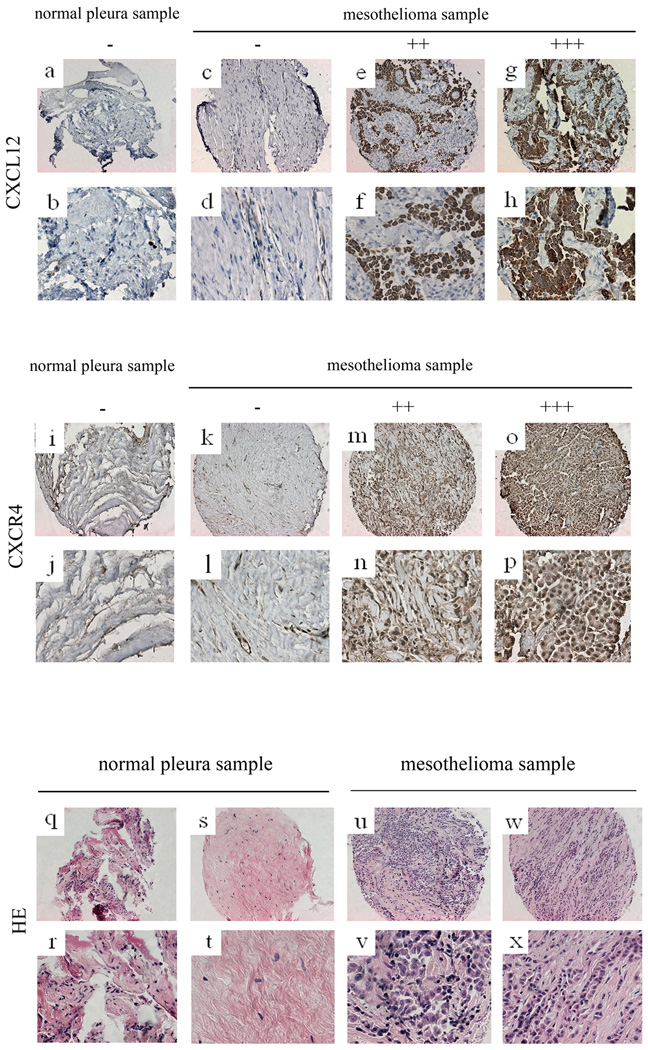
FIG. 1.
Immunohistochemistry of CXCL12, CXCR4 and HE staining of mesotheliome and normal pleura samples. a~h: immunohistochemistry of CXCL12. a, b: normal pleura sample. c~h: mesothelioma samples. c, d: negative. e, f: moderate stain. g, h: strong stain. i~p: immunohistochemistry of CXCR4. i, j: normal pleura sample. k~p: mesothelioma samples. k, l: negative. m, n: moderate stain. o, p: strong stain. q~x: HE staining. q~t: normal pleura samples. u~x: mesothelioma samples.
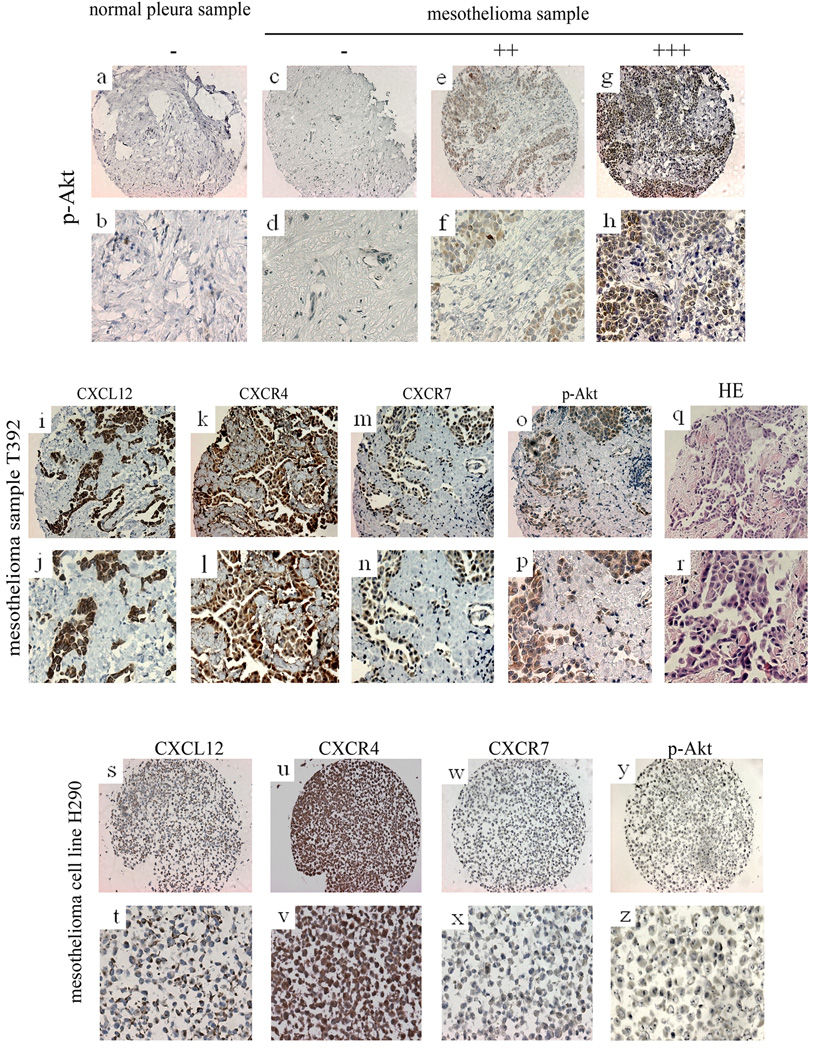
FIG. 3.
Immunohistochemistry of p-Akt, immunohistochemistry of CXCL12, CXCR4, CXCR7 and p-Akt and HE staining in one mesothelioma sample and one mesothelioma cell line (H290). a~h: immunohistochemistry of p-Akt. a, b: normal pleura sample. c~h: mesothelioma samples. c, d: negative. e, f: moderate stain. g, h: strong stain. i~r: immunohistochemistry of CXCL12, CXCR4, CXCR7 and p-Akt and HE staining in one mesothelioma sample. i, j: immunohistochemistry of CXCL12. k, l: immunohistochemistry of CXCR4. m, n: immunohistochemistry of CXCR7. o, p: immunohistochemistry of p-Akt. q, r: HE staining. s~z: immunohistochemistry of CXCL12, CXCR4, CXCR7 and p-Akt in one mesotheliomacell line (H290). s, t: immunohistochemistry of CXCL12. u, v: immunohistochemistry of CXCR4. w, x: immunohistochemistry of CXCR7. y, z: immunohistochemistry of p-Akt.
Table 1.
Immunohistochemistry findings from primary human mesothelioma samples
| Number of the sample |
IHC of anti- CXCL12 |
IHC of anti- CXCR4 |
IHC of anti- CXCR7 |
IHC of anti- pAKT |
|---|---|---|---|---|
| T370 | +++ | +++ | +++ | +++ |
| T410 | ++ | +++ | ++ | +++ |
| T453 | ++ | +++ | ++ | ++ |
| T473 | ++ | +++ | + | +++ |
| T484 | ++ | +++ | + | ++ |
| T501 | ++ | +++ | ++ | ++ |
| T392 | ++ | ++ | ++ | + |
| T394 | ++ | +++ | + | ++ |
| T246 | ++ | +++ | + | ++ |
| T514 | ++ | +++ | + | ++ |
| T713 | +++ | +++ | + | +++ |
| T775 | ++ | +++ | +++ | ++ |
| T809 | + | +++ | + | +++ |
| T869 | + | ++ | ++ | ++ |
| T862 | + | ++ | + | + |
| T241 | + | + | + | + |
| T264 | + | + | + | + |
| T237 | + | + | + | + |
| T737 | + | + | + | + |
| T829 | + | + | + | + |
| N244 | + | + | + | + |
| N237 | + | ++ | − | + |
| T416 | + | ++ | − | + |
| T777 | + | ++ | − | + |
| T858 | + | ++ | − | + |
| T936 | + | ++ | − | + |
| N472 | + | + | − | + |
| T795 | + | + | − | − |
| T396 | + | + | ND | + |
| T439 | − | + | − | + |
| N242 | − | + | − | + |
| T839 | − | − | + | + |
| T472 | + | + | 27− | − |
| N331 | + | + | − | − |
| T331 | − | + | + | − |
| T773 | − | + | − | + |
| T374 | ++ | +++ | ND | ND |
| T660 | ++ | +++ | ND | ND |
| T696 | +++ | +++ | ND | ND |
| T242 | ++ | +++ | ND | ND |
| T300 | + | + | ND | ND |
| T813 | − | + | ND | ND |
| T383 | − | + | ND | ND |
| N745 | − | + | ND | ND |
| T401 | − | + | ND | ND |
| N777 | − | + | − | − |
| T324 | − | + | − | − |
| T905 | − | + | − | − |
| N233 | − | − | − | − |
| N775 | − | − | − | − |
| N869 | − | − | − | − |
N, normal tissue; T, tumor tissue; IHC, immunohistochemistry; −, no stain; +, weak stain; ++, moderate stain; +++, strong stain, ND not done.
Table 3.
Positive and negative number and ratio of CXCL12, CXCR4, CXCR7 and p-Akt in normal pleura samples
| − Number(ratio) |
+ Number(ratio) |
++ Number(ratio) |
+++ Number(ratio) |
|
|---|---|---|---|---|
| CXCL12 | 6(60.0%) | 3(30.0%) | 1(10.0%) | 0(0%) |
| CXCR4 | 3(30.0%) | 6(60.0%) | 1(10.0%) | 0(0%) |
| CXCR7 | 8(88.9%) | 1(11.1%) | 0(0%) | 0(0%) |
| p-Akt | 6(66.7%) | 3(33.3%) | 0(0%) | 0(0%) |
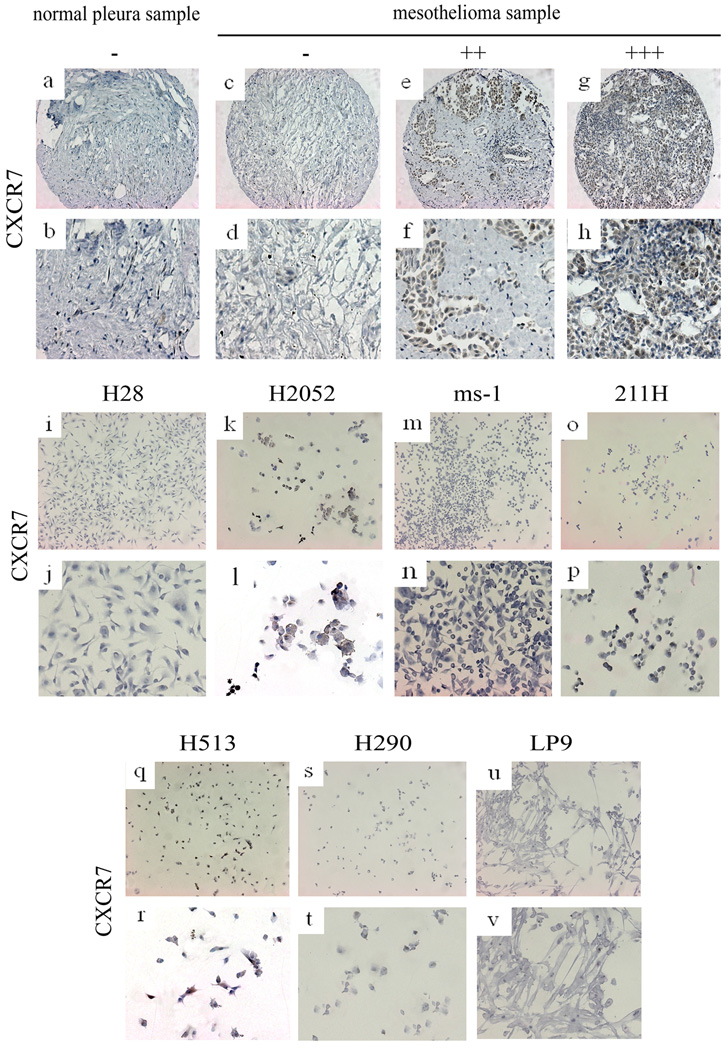
FIG. 2.
Immunohistochemistry of CXCR7. a, b: normal pleura sample. c~h: mesothelioma samples. i~t: mesothelioma cell lines. i, j: H28, negative. k, l: H2052, positive. m, n: ms-1, negative. o, p: 211H, hegative. q, r: H513, positive. s, t: H290, negative. u, v: normal mesothelial cell line LP9, negative.
In specimens of normal pleural tissue, CXCL12, CXCR4, CXCR7, and p-Akt staining was negative (−) or weak (+) at the cell membrane or in the region of cytoplasm near the membrane. Only one sample was moderate positive (++) in CXCL12 staining and 1 was moderate positive (++) in CXCR4 staining.
The overall positive ratios of CXCL12, CXCR4, CXCR7and p-Akt staining in mesothelioma samples were 78.0%, 97.6%, 71.9% and 84.8%, respectively. The overall positive ratios in normal pleura samples were 40%, 70%, 11.1% and 33.3%, respectively. CXCR4 was correlated with both CXCL12 (R2 = 0.794) and p-Akt (R2 = 0.739). The correlations were weaker between CXCL12 and p-Akt, CXCR7 and CXCL12, and CXCR7 and p-Akt (R2 = 0.620, 0.514 and 0.508, respectively). CXCR4 was not correlated with CXCR7 (R2 = 0.408). After being stained with anti-CXCR7 antibody, the mesothelioma cell lines H2052 and H513 showed moderate positive staining; 211H and H290 showed weak staining. The mesothelioma cell lines H28 and ms-1, and the human normal mesothelial cell line LP9 showed negative staining (Fig. 2, i~v). In the tissue microarray sections, the staining of mesothelioma cell lines H290 and 211H was the same (Fig. 3, s~z). The CXCL12 staining was moderate positive, CXCR4 staining was strongly positive, CXCR7 and p-Akt staining were weakly positive.
Expression of CXCR4, CXCL12 and CXCR7 in cell lines
The results of RT-PCR of mesothelioma cell lines H28, H2052, ms-1, 211H, H513 and H290 and normal mesothelial cell line LP9 indicated that CXCR4 was over-expressed in mesothelioma cell lines H2052, ms-1, 211H, H513 and H290, but was weakly expressed in H28 and normal mesothelial cell line LP9. CXCL12 was over-expressed in H2052, ms-1, 211H, H513 and H290, but was weakly expressed in LP9 and was not detectable in H28. CXCR7 was expressed in H2052 and H513, but only minimally expressed in H28, 211H, H290 and LP9, and was not detectable in ms-1. GAPDH was detected in all of the 7 cell lines as expected (Fig. 4A). The immunostaining of CXCR7 in the cell lines H2052 and H513 was positive. Simultaneously, the results of RT-PCR showed that the CXCR7 was expressed in these 2 cell lines (Fig. 2I–V).
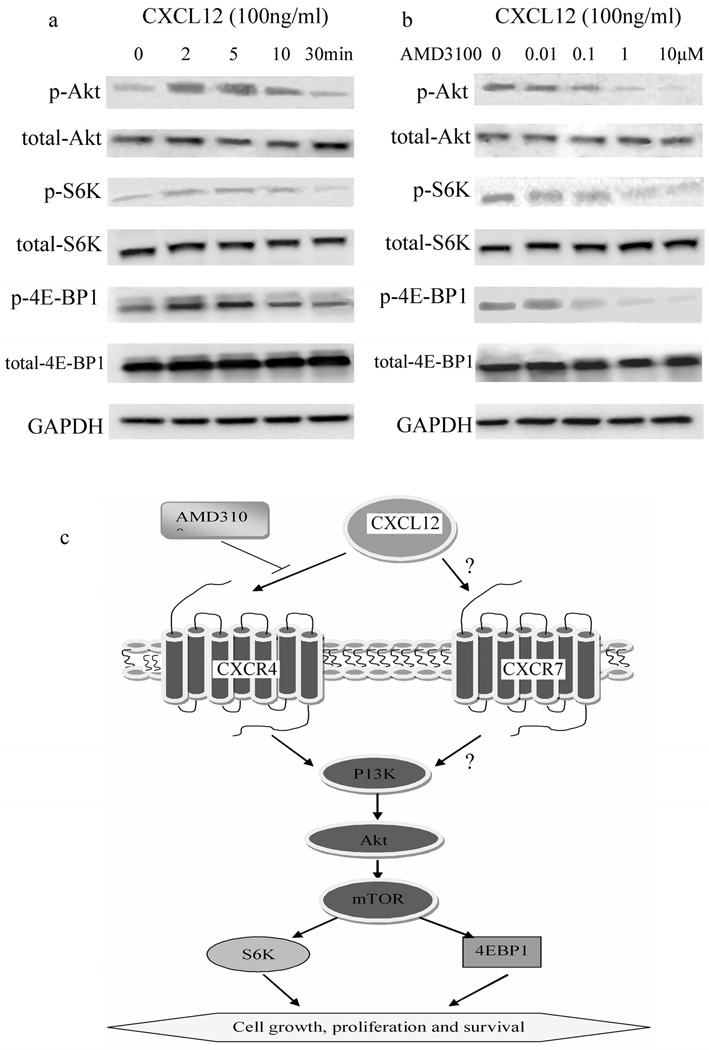
FIG. 4.
Expression of CXCR4, CXCL12 and CXCR7 in cell lines and cell viability assay. a: RT-PCR result for CXCR4, CXCL12, CXCR7 and GAPDH (as control) in 6 mesothelioma cell lines (H28, H2052, ms -1, 211H, H513 and H290) and 1 normal mesothelial cell line LP9. b: cell viability assay of 6 mesothelioma cell lines (H28, H2052, ms-1, 211H, H513 and H290) and 1 normal mesothelial cell line LP9.
Cell proliferation and viability assay
After mesothelioma cell lines H28, H2052, ms-1, 211H, H513 and H290 and normal mesothelial cell line LP9 were starved for 12 h, CXCL12 treatment induced cell proliferation in several mesothelioma cell lines in 24 h. This effect was blocked by the CXCR4 inhibitor AMD3100 in mesothelioma cell lines H2052, ms -1, 211H, H513 and H290 in a dose-dependent manner (Fig. 4B). Inhibition of mesothelioma cell growth by AMD3100 resulted in lower proliferation and viability in these 5 lines. AMD3100 did not inhibit the cell growth in H28 and LP9. The dose response curve indicated that the higher dose of AMD3100, the lower the viability of mesothelioma, and the IC50 values show that the inhibiting effects differed among the mesothelioma cell lines (Table 4).
Table 4.
IC 50 values of AMD3100 in the mesothelioma cell lines
| H28 | H2052 | ms-1 | 211H | H513 | H290 | LP9 | |
|---|---|---|---|---|---|---|---|
| IC50 values (µM) | none | 1.11 | 2.54 | 6.65 | 1.36 | 1.27 | none |
Inhibition of Akt-mTOR signaling by AMD3100 in mesothelioma cells
CXCL12 induced rapid and intense activation of the Akt pathway in mesothelioma cell lines H28, H2052, ms-1, 211H, H513 and H290 (The results for H290 are shown in Fig. 5A). Enhanced phosphorylation of Akt, S6K and 4E -BP1 peaked at 5 and 10 min after stimulation with CXCL12. CXCL12-induced activation of Akt, S6K and 4E-BP1 in mesothelioma cells lines H2052, ms -1, 211H, H513 and H290 was inhibited selectively and dose-dependently by a CXCR4 inhibitor, AMD3100 (The result for H290 is shown in Fig. 5B).
FIG. 5.
Western blot result and CXCL12, CXCR4, CXCR7 and Akt pathway. a, b: Western blot result. c: proposed outline of CXCL12/CXCR4/CXCR7/Akt-mTOR pathway in mesothelioma.
DISCUSSION
Chemokines are considered to play a significant part in cancer migration, production and growth [24]. Previous studies have shown a significant correlation between chemokine receptor status in human cancers, and prognosis and/or metastases in a variety of malignant tumors such as T-cell leukemia (CCR4) [25], hepatocellular carcinoma (CCR6) [26], gastric carcinoma (CCR7) [27], renal cell carcinoma (CXCR3) [28], ovarian cancer (CXCR4) [29], osteosarcoma (CXCR4) [30], colorectal cancer (CCR7 and CXCR4) [31,32], and malignant melanoma (CXCR3 and CXCR4) [33, 34]. In our study, we investigated the expression of CXCL12, CXCR4 and CXCR7, which are the most important chemokines and chemokine receptors, in human mesothelioma. We found that CXCR4 is over-expressed in most of the human mesothelioma samples tested, and that it is expressed at low levels in most normal pleural tissue. CXCL12 is also expressed in mesothelioma, but at somewhat lower levels than CXCR4, though still at higher levels than in normal pleura. Originally characterized as a pre-B-cell stimulatory factor, CXCL12 is a chemotactic factor for T cells, monocytes, pre-B cells, dendritic cells, and hematopoietic progenitor cells.
Because cancer cell migration and invasion share many similarities with leukocyte trafficking [24], chemokines are thought to be very important in organ-selective cancer metastasis and recurrence. Receptor CXCR4 immunoreactivity is found not only in cancer cells, but also in lymphocytes in the tumor stroma [6]. CXCL12 and its receptor, CXCR4, were recently found to influence the dissemination, immune rejection, and neoangiogenesis of human gastrointestinal cancer [6]. Furthermore, a mechanism for CXCR4 activation during tumor-cell evolution has been described in patients with the von Hippel-Lindau (VHL) syndrome. In these patients, inactivation of the VHL tumor suppressor gene in incipient tumor cells results in over-expression of CXCR4, which confers a selective survival advantage and the tendency to home to selected organs [35]. It is interesting that CXCL12 is normally produced by the stromal cells of lymph nodes, lung, liver, and bone marrow, the organs that are the most frequent sites for metastasis [36]. Accordingly, the high frequency of mesothelioma recurrence and metastasis is, at least in part, due to the high expression of CXCR4 and CXCL12.
CXCR7 was absent in most normal pleural tissues and expressed in low levels in most mesothelioma samples. The expression and biological importance of CXCR7 are thought to depend on the cell types investigated. CXCR7 was recently shown to be highly expressed in human glioma cells and non-small-cell lung cancer [37, 38]. In breast cancer cells, CXCR7 activation fails to cause Ca2+ mobilization or cell migration, but provides a growth advantage [19], whereas in prostate cancer cells, CXCR7 expression is associated with enhanced adhesive and invasive activities in addition to increased survival [21]. In healthy mice, CXCR7 is expressed in cardiomyocytes, osteocytes, and brain cells [39–41]. CXCR7-deficient mice die prenatally with severe heart defects that are also reported for CXCL12 and CXCR4 knockout animals [40].
Our finding that CXCR4 and CXCL12 were both highly expressed in five (H2052, ms -1, 211H, H513 and H290) of the six mesothelioma cell lines studied, whereas CXCR7 was expressed in only two (H2052 and H513) and minimally expressed in another three (H28, 211H and H290), suggests that the expression of chemokines and their receptors varies in mesothelioma cells. Interestingly, the two mesothelioma cell lines that express CXCR7 also showed the highest expression level of CXCR4 among all mesothelioma cell lines tested. These data suggest that CXCR7 may be involved in a subset of mesotheliomas that have more aggressive metastatic behavior and CXCR4 may positively alter the expression levels of CXCR7. This suggestion is not in agreement with the possible CXCR4-CXCR7 reciprocal regulation in prostate cancer [19]. Wang et al. reported that signaling by CXCR7 activates the Akt pathway in prostate cancer [21]. However, the relationship between CXCR7 and the Akt pathway in mesothelioma is still not clear (Fig. 5c). Our data also suggest that a CXCR4 antagonist such as AMD3100, without simultaneous blockage of CXCR7, may be an inefficient strategy for a therapeutic approach in the subset of patients with CXCR7 expression.
Our results suggest that CXCL12 can induce proliferation in most mesothelioma cell lines. At the same time, ADM3100, the CXCR4 antagonist, can inhibit proliferation and viability in most mesothelioma cell lines. These results suggest that the CXCL12/CXCR4 axis is important for most mesothelioma cells and that blocking it impairs their viability. Therefore, inhibiting the chemokines and their receptors may have clinical applicability for mesothelioma treatment. However, CXCL12 did not induce proliferation and AMD3100 did not inhibit viability in mesothelioma cell line H28, probably because CXCR4, CXCL12 and CXCR7 expression was very low in H28. This result is in agreement with our RT-PCR findings. If the inhibitor of chemokines and their receptors is used in the treatment of mesothelioma, the expression level of chemokines and their receptors should be detected first. In short, our data suggest that CXCL12/CXCR4 is a potential therapeutic target for patients with mesothelioma.
Akt was rapidly and strongly phosphorylated by the chemokine CXCL12 (Fig. 4a), leading us to hypothesize that the Akt pathway can be activated by the chemokines and their receptors. The PI3K-dependent signals, including Akt, mTOR, S6K and 4E-BP1, can promote the occurrence, proliferation and survival of tumor cells [42–46]. Therefore, our western blot results suggest that the chemokines and receptors play an important role in human malignant mesothelioma. AMD3100 is a CXCR4 antagonist. A concentration of 1 µM has been shown to inhibit HIV entry into cells in vitro [47]. The p70 ribosomal S6 kinase (p70S6K, S6K) and eIF4E-binding protein 1 (4E-BP1) are two major downstream components of Akt and mTOR [48, 49]. Phosphorylation of p70S6K allows the translation of ribosomal proteins [50]. Phosphorylation of 4E-BP1 regulates cap-dependent translation by enabling the formation of an active eIF4E complex [51]. We found that CXCL12 induced the activation of p70S6K and 4E-BP1, which were downstream of Akt-mTOR pathways, and this activation was dose-dependently blocked by CXCR4 inhibitor AMD3100.
In summary, our study shows that CXCL12 and CXCR4 are over-expressed in 5 of 6 human mesothelioma cell lines and in 22/41 and 31/41 mesothelioma tissues, respectively. CXCL12 is also a functional activation factor of Akt downstream pathways and the CXCR4 antagonist AMD3100, which suggests that CXCL12/CXCR4 interaction is a potential therapeutic target for mesothelioma. Our data also indicate that CXCR7 may play a role in a subset of mesotheliomas, and that the CXCR7 factor should be considered when blocking the CXCL12-CXCR4 interaction. The role of CXCR7 in human mesothelioma, and whether the CXCL12-CXCR4 interaction may be a potential therapeutic target, requires further investigation.
Table 2.
Positive and negative number and ratio of CXCL12, CXCR4, CXCR7 and p-Akt in mesothelioma samples
| − Number(ratio) |
+ Number(ratio) |
++ Number(ratio) |
+++ Number(ratio) |
|
|---|---|---|---|---|
| CXCL12 | 9(22.0%) | 16(39.0%) | 13(31.7%) | 3(7.3%) |
| CXCR4 | 1(2.4%) | 18(43.9%) | 6(14.6%) | 16(39.1%) |
| CXCR7 | 9(28.1%) | 15(46.9%) | 6(18.8%) | 2(6.3%) |
| p-Akt | 5(15.2%) | 14(42.4%) | 8(24.2%) | 6(18.2%) |
ACKNOWLEDGMENTS
The present work was supported by the NIH grant R01 CA140654-01A1 (L. Y.). We are grateful for support from the Kazan, McClain, Abrams, Fernandez, Lyons, Greenwood, Harley & Oberman Foundation, Inc., the Estate of Robert Griffiths, the Jeffrey and Karen Peterson Family Foundation, Paul and Michelle Zygielbaum, the Estate of Norman Mancini, and the Barbara Isackson Lung Cancer Research Fund. We thank Lorretta Chan and Rick Baehnev for their important directions in staining. We also thank Pamela Derish in the UCSF Department of Surgery for editorial assistance with the manuscript.
Footnotes
CONFLICT OF INTEREST STATEMENT:
The authors have declared that no competing interests exist.
STATEMENT OF AUTHOR CONTRIBUTIONS Conceived and designed the experiments: TL HL DJ LY. Performed the experiments: TL YCW LY AA. Analyzed the data: HL CH JLT. Contributed reagents/materials/analysis tools: CH ZX. Wrote the paper: TL HL DJ LY. Reviewed and revised the manuscript: JLT AA. Gave important directions to the study and revised the manuscript: DJ.
REFERENCES
- 1.Bueno R. Mesothelioma clinical presentation. Chest. 1999;116:444S–445S. doi: 10.1378/chest.116.suppl_3.444s. [DOI] [PubMed] [Google Scholar]
- 2.Nowak R, Mrozek S, Miecznikowski W. The radiometric analysis of the chest and spine deformity following surgical treatment of idiopathic scoliosis by the C−D method. Ortop Traumatol Rehabil. 2002;4:559–566. [PubMed] [Google Scholar]
- 3.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 4.Salvucci O, Yao L, Villalba S, et al. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 5.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 6.Yoshitake N, Fukui H, Yamagishi H, et al. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer. 2008;98:1682–1689. doi: 10.1038/sj.bjc.6604363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajetto A, Barbieri F, Pattarozzi A, et al. CXCR4 and SDF1 expression in human meningiomas: a proliferative role in tumoral meningothelial cells in vitro. Neuro Oncol. 2007;9:3–11. doi: 10.1215/15228517-2006-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewan MZ, Ahmed S, Iwasaki Y, et al. Stromal cell-derived factor -1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60:273–276. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 10.Luster AD. Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 11.Zlotnik A, Yoshie O. Chemokines. a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 12.Schimanski CC, Galle PR, Moehler M. Chemokine receptor CXCR4-prognostic factor for gastrointestinal tumors. World J Gastroenterol. 2008;14:4721–4724. doi: 10.3748/wjg.14.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koizumi K, Hojo S, Akashi T, et al. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–213. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 16.Matsusue R, Kubo H, Hisamori S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. doi: 10.1245/s10434-009-0599-x. [DOI] [PubMed] [Google Scholar]
- 17.Ottaiano A, Franco R, Aiello Talamanca A, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II–III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–2803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- 18.Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 19.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao Z, Luker KE, Summers BC, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumorassociated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Shiozawa Y, Wang J, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 22.Meijer J, Ogink J, Roos E. Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo. Br J Cancer. 2008;99:1493–1501. doi: 10.1038/sj.bjc.6604727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Li Q, Bavikatty N, Michael CW. The role of immunohistochemistry in distinguishing squamous cell carcinoma from mesothelioma and adenocarcinoma in pleural effusion. Semin Diagn Pathol. 2006 Feb;23(1):15–19. doi: 10.1053/j.semdp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi K, Hojo S, Akashi T, et al. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9:3625–3634. [PubMed] [Google Scholar]
- 26.Uchida H, Iwashita Y, Sasaki A, et al. Chemokine receptor CCR6 as a prognostic factor after hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:161–168. doi: 10.1111/j.1440-1746.2005.04157.x. [DOI] [PubMed] [Google Scholar]
- 27.Mashino K, Sadanaga N, Yamaguchi H, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 28.Klatte T, Seligson DB, Leppert JT, et al. The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J Urol. 2008;179:61–66. doi: 10.1016/j.juro.2007.08.148. [DOI] [PubMed] [Google Scholar]
- 29.Jiang YP, Wu XH, Shi B, et al. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol. 2006;103:226–233. doi: 10.1016/j.ygyno.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Laverdiere C, Hoang BH, Yang R, et al. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res. 2005;11:2561–2567. doi: 10.1158/1078-0432.CCR-04-1089. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Ta keuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 32.Schimanski CC, Schwald S, Simiantonaki N, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 33.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 34.Monteagudo C, Martin JM, Jorda E, et al. CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factors. J Clin Pathol. 2007;60:596–599. doi: 10.1136/jcp.2005.032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staller P, Sulitkova J, Lisztwan J, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–313. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 36.Rollins BJ. Chemokines. Blood. 1997;3:909–928. [PubMed] [Google Scholar]
- 37.Hattermann K, Held-Feindt J, Lucius R, et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 2010;70(8):3299–3308. doi: 10.1158/0008-5472.CAN-09-3642. [DOI] [PubMed] [Google Scholar]
- 38.Iwakiri S, Mino N, Takahashi T, et al. Higher expression of chemokine receptor CXCR7 is linked to early and metastatic recurrence in pathological stage I nonsmall cell lung cancer. Cancer. 2009 Jun 1;:2580–2593. doi: 10.1002/cncr.24281. [DOI] [PubMed] [Google Scholar]
- 39.Sierro F, Biben C, Martínez-Muñoz L, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerrits H, van Ingen Schenau DS, Bakker NE, et al. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46:235–245. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 41.Schönemeier B, Kolodziej A, Schulz S, et al. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510:207–220. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]
- 42.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signallng controls tumour cell growth[J] Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 43.Davies MA, Stemke-Hale K, Lin E, et al. Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma. Clin Cancer Res. 2009;15(24):7538–7546. doi: 10.1158/1078-0432.CCR-09-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Yoshizawa A, Fukuoka J, Shimizu S, et al. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin Cancer Res. 2010;16(1):240–248. doi: 10.1158/1078-0432.CCR-09-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarbassov DD, Guetin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 47.Schols D, Struyf S, Van Damme J, et al. Henson and E. De Clercq, Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown EJ, Beal PA, Keith CT, et al. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 49.Brunn GJ, Hudson CC, Sekulic A, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 50.Grove JR, Banerjee P, Balasubramanyam A, et al. Cloning and expression of two human p70 S6 kinase polypeptides differing only at their amino termini. Mol Cell Biol. 1991;11:5541–5550. doi: 10.1128/mcb.11.11.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pause A, Belsham GJ, Gingras AC, et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 50-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]