Abstract
Induced pluripotent stem cells (iPSCs) have been considered as the major component for personalized regenerative medicine. However, the potential of iPSCs in constructing tissue-engineered (TE) blood vessels has not been exploited. In the present study, we generated mouse iPSCs with the combination of over-expression of 4 iPS factors and knock-down of p53 gene. The established iPSCs were then directed to differentiate into smooth muscle cells (SMCs) with the treatment of 10−5M all-trans retinoid acid (RA). The vehicle dimethyl sulfoxide (DMSO) treatment served as a spontaneous differentiation control. The differentiated cells were then cultured on three-dimensional (3D) macro-porous nanofibrous (NF) poly(l-lactide) (PLLA) scaffolds in vitro. Our data showed that the expression of SMC specific marker genes, including myocardin, smoothelin, SM22α and SMMHC, were higher for the group induced by RA than for the group treated by DMSO, while pluripotent marker gene expression was repressed by the RA treatment. Upon subcutaneous implantation, the implanted cells maintained the SMC phenotype. In conclusion, the data suggest that iPSCs derived SMCs can be an important cell source for personalized vascular tissue engineering applications.
Keywords: Induced pluripotent stem cells, Smooth muscle cells, Scaffolds, Vascular tissue engineering
Introduction
Vascular smooth muscle cells (SMCs), embedded in collagen rich extracellular matrix (ECM), play an important role in maintaining the viability and activity of endothelium, and regulating blood pressure in response to various stimuli [1]. Therefore, the tissue layer containing functional SMCs (tunica media) is critical to the successful regeneration of damaged vascular tissues. Mature vascular SMCs, isolated from donor tissues, have been used for the construction of tissue-engineered (TE) blood vessels [2–5]. However, mature vascular SMCs have limited ability of proliferation and expansion, making it necessary to explore alternative SMC source. The beauty of the therapeutic potential of embryonic stem cells (ESCs) lies in the ability of ESCs to recover or replace damaged tissues through its pluripotency [6]. However, as a practical and potential candidate for regenerative medicine, ESCs have to be synchronized with the patient’s immune system. Recently developed induced pluripotent stem cells (iPSCs), either from human or mouse fibroblasts [7, 8], have successfully overcome the serious immunological limitations imposed by other technologies, such as somatic cell nuclear transfer and cell fusion. Thus, iPSC technology is expected to advance the development of patient-specific cell therapy by providing abundant, pluripotent patient-specific stem cells. We and others have shown the capability of iPSCs to differentiate into SMCs [9, 10]. On the other hand, recently three-dimensional (3D) nano-fibrous (NF) poly-L-lactide (PLLA) scaffolds, mimicking the architecture of ECM, have been applied to support the growth of human aortic SMCs [4]. Here, we combined these two techniques and investigated the three-dimensional growth of mouse iPSCs-derived SMCs on NF scaffolds, aiming for future patient-specific blood vessel regeneration.
Materials and methods
Establishment of mouse iPSCs
iPSC 4-in-1 lentiviral vectors encoding Oct4, Klf4, Sox2 and c-Myc (OKSM) were constructed by engineering 2A-fusion of the four open reading frames behind human EF1a enhancer/promoter in pTYF backbone [11]. The short-hairpin RNA (shRNA) sequences against p53 (target site -gcagtcacagcacatgacg-, 85% knockdown efficiency) [12] were inserted into pTYF-EF lentiviral vectors carrying a puromycin selection marker [13]. VSV-G pseudotyped lentiviral vectors were produced in HEK293T cells and concentrated as previously described [14]. Briefly, for pMX-based and pMSCV-based retroviruses, vectors were transfected using Lipofectamine following the manufacturers’ directions. One day after transfection, the medium was replaced with fresh culture medium. pTYF-based lentivirus vectors [15] were transfected by Superfect (Qiagene) according to the manufacturer’s protocol. Supernatant containing viruses was collected and filtered through a 0.45μm filter after transfection for two days. Mouse iPSCs were induced as previously described [16, 17]. Briefly, mouse embryonic fibroblasts (mEF, passage 3 to 5) were infected (day 0) with pMX-based retroviruses together with pTYF-EF-based lentivirus for p53 shRNAs. On day 2, cells were passaged onto another gelatin-coated plate. Medium was changed every 2 days. On day 6 to 7, cells were fixed for alkaline phosphatase activity study and colonies that displayed strong staining and showed mouse ESC-like morphology were scored positive. To assess the reprogramming efficiency, cells were trypsinized 3 days after retroviral infection and 104 cells were plated onto 6-well plates on top of irradiated mEFs with mouse ESCs culture medium. Medium was changed every other day. After 2 weeks, the dishes were analyzed for stem cell specific marker analysis with stem cell characterization kit (Millipore, CA), following the manufacturer’s manual. Fluorescence images were photographed under an Olympus BX51 fluorescence microscope.
Induced SMCs differentiation from iPSCs
Established iPSCs were routinely expanded. The cells were induced to differentiate into SMCs in vitro when treated with 10−5M of all-trans retinoid acid (RA) following the protocol described in our previous report [9], or spontaneously differentiated with the treatment of the vehicle dimethyl sulfoxide (DMSO).
Fabrication of 3D NF PLLA scaffolds
3D NF PLLA scaffolds were prepared and fabricated following our previous report [4]. Briefly, PLLA with an inherent viscosity of approximately 1.6 dL/g was purchased from Boehringer Ingelheim (Ingelheim, Germany). PLLA in tetrahydrofuran (THF) (10% wt/v) solution was cast into an assembled sugar template (formed from bound sugar spheres, 125–250 µm in diameter) under a mild vacuum. The polymer-sugar composite was phase separated at −20°C overnight and then immersed into cyclohexane to exchange THF for 2 days. The resulting composites were freeze-dried and the sugar spheres were leached out in distilled water and freeze-dried again to obtain highly porous scaffolds. The scaffolds were cut into circular disks with dimensions of 3.6 mm in diameter and 1 mm in thickness. For cell culture and implantation studies, the scaffolds were sterilized with ethylene oxide before use.
Cell culture on 3D NF PLLA scaffolds
The 3D NF PLLA scaffolds were pre-wetted in 70% ethanol for 30 minutes, washed three times with PBS for 30 minutes each, and twice in the cell culture medium for 2 hours each. 2.5×105 cells were seeded into each scaffold. The cell-seeded scaffolds were then transferred to 6-well plates on an orbital shaker. The culture medium was changed twice a week.
Scanning electron microscopy (SEM) observation
Samples were first rinsed in PBS, fixed in 2.5% glutaraldehyde and 2% paraformaldehyde overnight, and post-fixed in 1% osmium tetroxide for 1 hour. Samples were dehydrated in increasing concentrations of ethanol and hexamethyldisilizane. The samples were then sputter-coated with gold and observed under a scanning electron microscope (Philips XL30 FEG).
Gene expression analysis with quantitative real-time polymerase chain reaction (PCR)
Total cellular RNA from each group designated by culture/implantation times was extracted using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions and treated with DNase I (Qiagen). cDNA was synthesized with superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA). PCR amplification was performed with primers indicated in Supplemental Table S1 using SYBR Green supermix kit (Bio-rad, Hercules, CA) following the instructions. All RNA samples were adjusted to yield equal amplification of 18S RNA as an internal standard.
Histological and immunofluorescence analysis
Constructs were washed in PBS, fixed with 3.7% formaldehyde in PBS overnight, dehydrated through a graded series of ethanol, embedded in paraffin, and sectioned at a thickness of 5 µm. For immunofluorescence analysis, after deparaffinization and block with 1% serum, the slides were incubated with primary antibodies to α-SMA (Millipore, Temecula, CA). Following a PBS rinse, sections were then incubated with Alexa Fluor-488 or Alexa Fluor-592 conjugated secondary antibodies (Invitrogen, Carlsbad, CA). Images were obtained using an Olympus BX51 microscope.
Subcutaneous implantation
After the cells were seeded and cultured on scaffolds for 24 hours, the scaffold-cell constructs or blank scaffolds (without cells, pre-treated in the same way as for cell-containing constructs) were implanted into subcutaneous pockets of nude mice as previously reported [4]. For implantation surgery, 6–8 weeks old male nude mice (Charles River Laboratories, Wilmington, MA) were used. Surgery was performed under general inhalation anesthesia with isofluorane. Two midsagittal incisions were made on the dorsa and one subcutaneous pocket was created on each side of each incision using blunt dissection. One scaffold-cell construct or blank scaffold was implanted into each pocket. Four samples in each group were implanted randomly. After placement of implants, the incisions were closed with staples. At the end of 1 week or 2 weeks of implantation period, the mice were euthanized and the implants were harvested. The animal procedures were performed according to the protocol approved by the University of Michigan Committee of Use and Care of Laboratory Animals.
Statistical analysis
Numerical data were reported as mean ±SD. The Student’s t-test was applied to test the significance between the groups. The value of p < 0.05 was considered to be statistically significant.
Results
Generation and characterization of mouse iPSCs
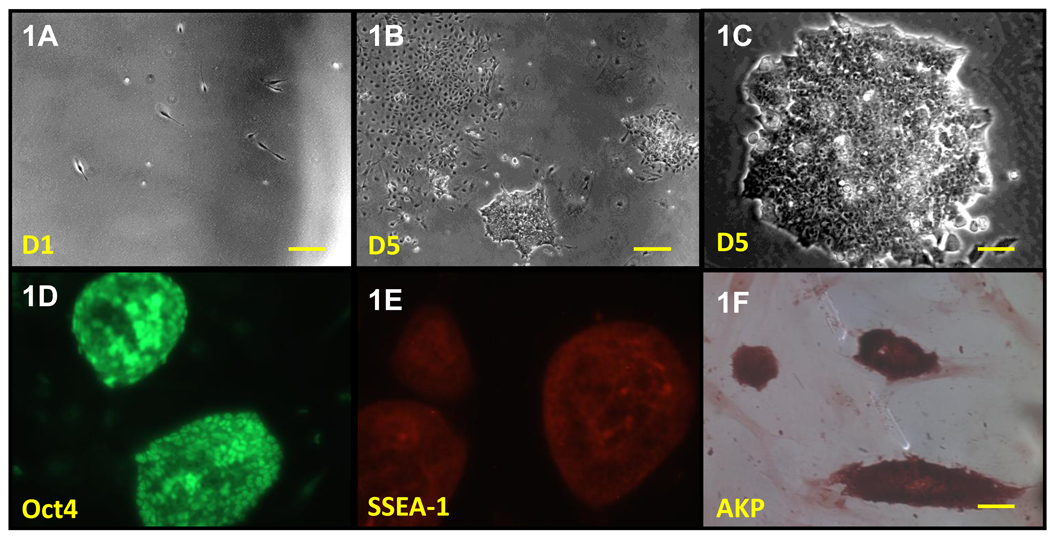
We used the original reprogramming factors defined by Dr. Yamanaka (Oct4, Sox2, Klf4 and c-Myc) [8] to construct a single polycistronic vector [18], plus p53 silence [19] to generate iPSCs from mouse embryonic fibroblasts (Fig. 1A–1C). This strategy to generate mouse iPSCs with the combination of four factors and p53 silence dramatically enhanced the efficiency of reprogramming (1.4±0.2%) and greatly reduced the time to isolate the iPSCs (from average 4–8 weeks [20, 21] to around 7 days here). Rough characterization of derived iPSCs colonies demonstrated compact mouse ESCs colony morphology (Fig. 1C) and markers of pluripotent cells, including pluripotency-associated transcription factor OCT4 (Fig. 1D), surface marker SSEA-1(Fig. 1E) and high levels of alkaline phosphatase (Fig. 1F). The tested cell lines differentiated into the three germ layer derivatives, as shown by mRNA expression in vitro formation of embryoid bodies (data no shown)
Figure 1. Induction of iPSCs from mouse embryonic fibroblasts.
(A) Morphology change of mouse embryonic fibroblasts when plating at 1×103 cells/cm2 density. Scale bar=250µm. (B) Typical image of non-ESC-like (left-corner) and ESC-like (arrow) colony after lentivirus infection for 5 days. Scale bar=250µm. (C) Typical image of established mouse iPSCs line at passage number 5. Scale bar=50µm. (D–F) Immunocytochemistry for Oct4 (D), SSEA-1 (E), alkaline phosphatase (F). Scale bar=50µm.
In vitro culture of iPSCs derived SMCs on 3D NF PLLA scaffolds
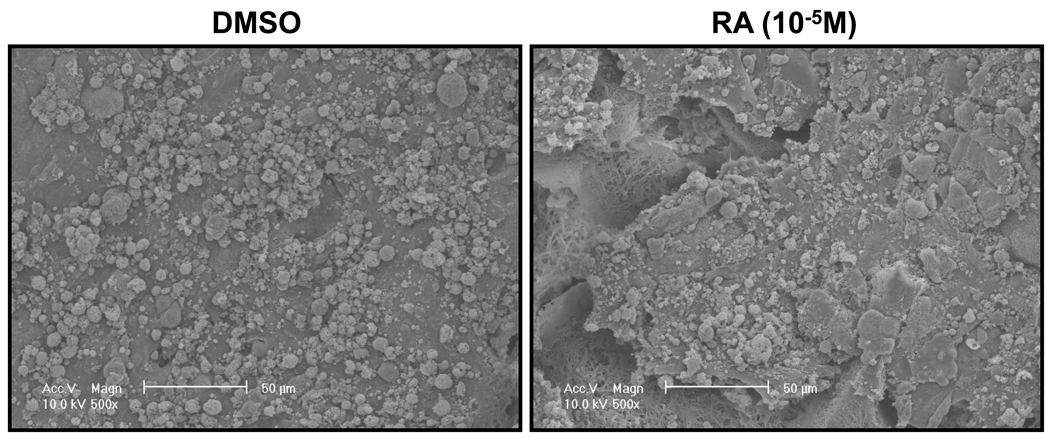
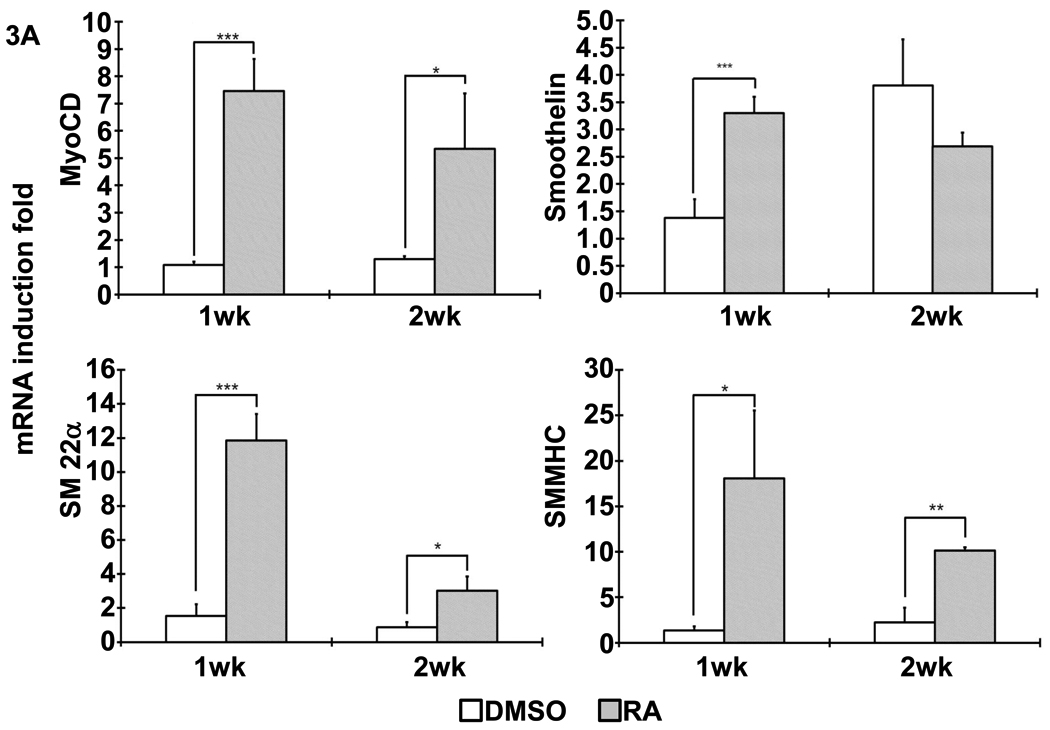
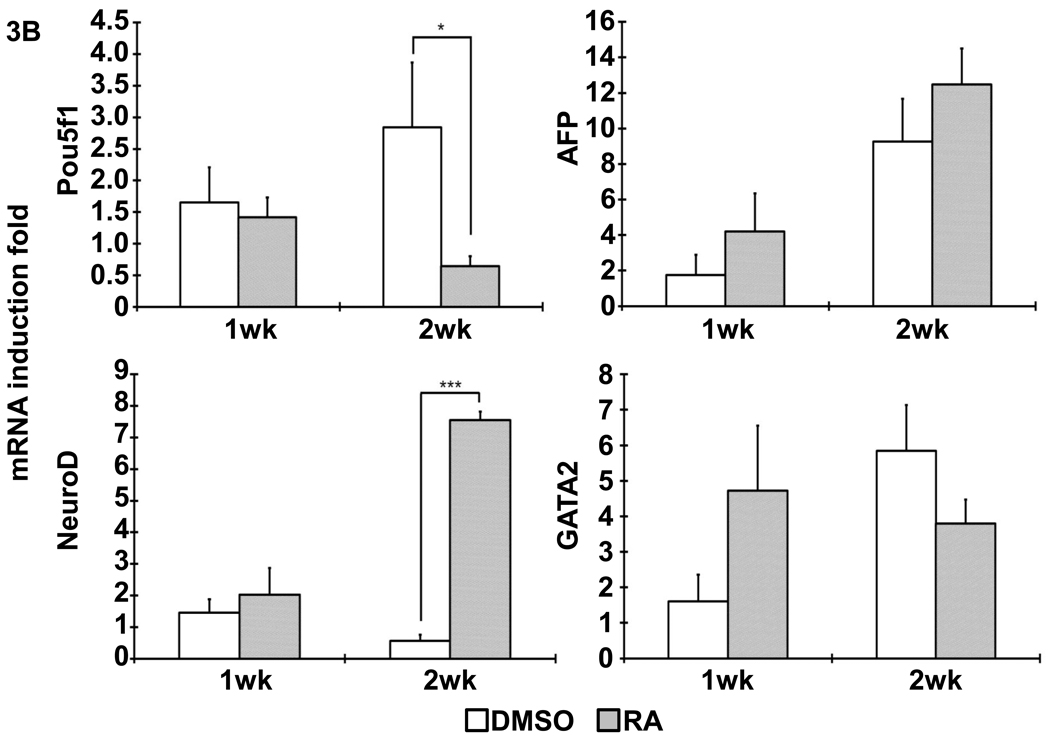
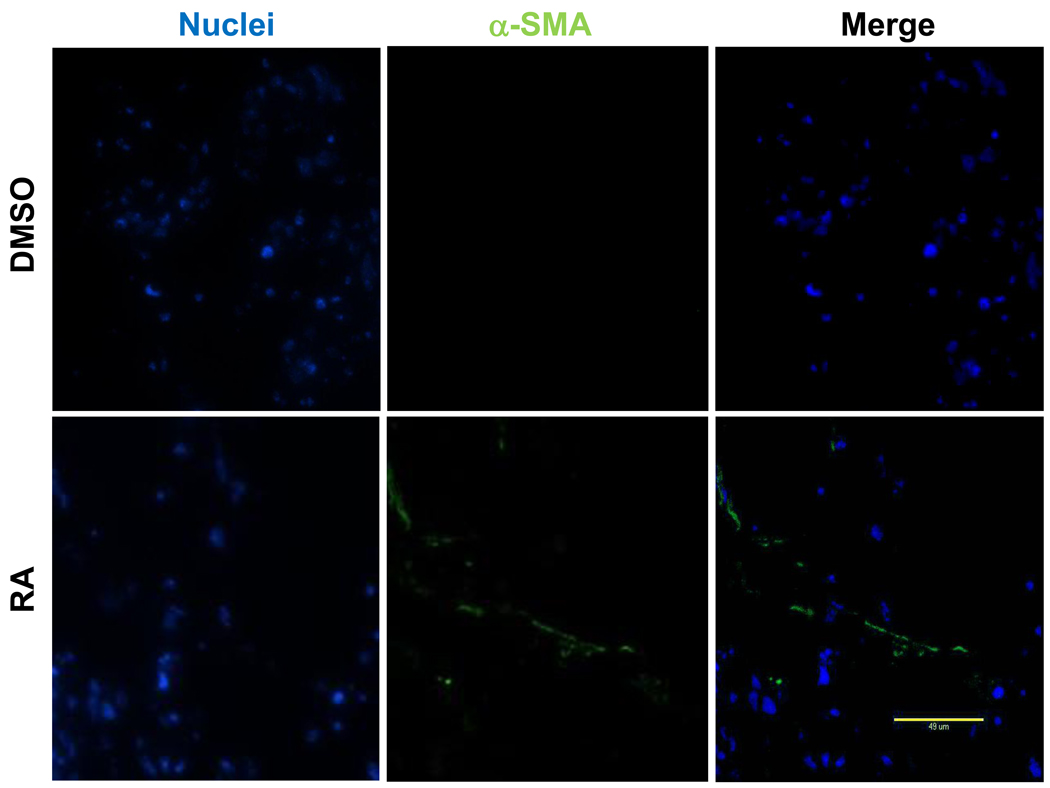
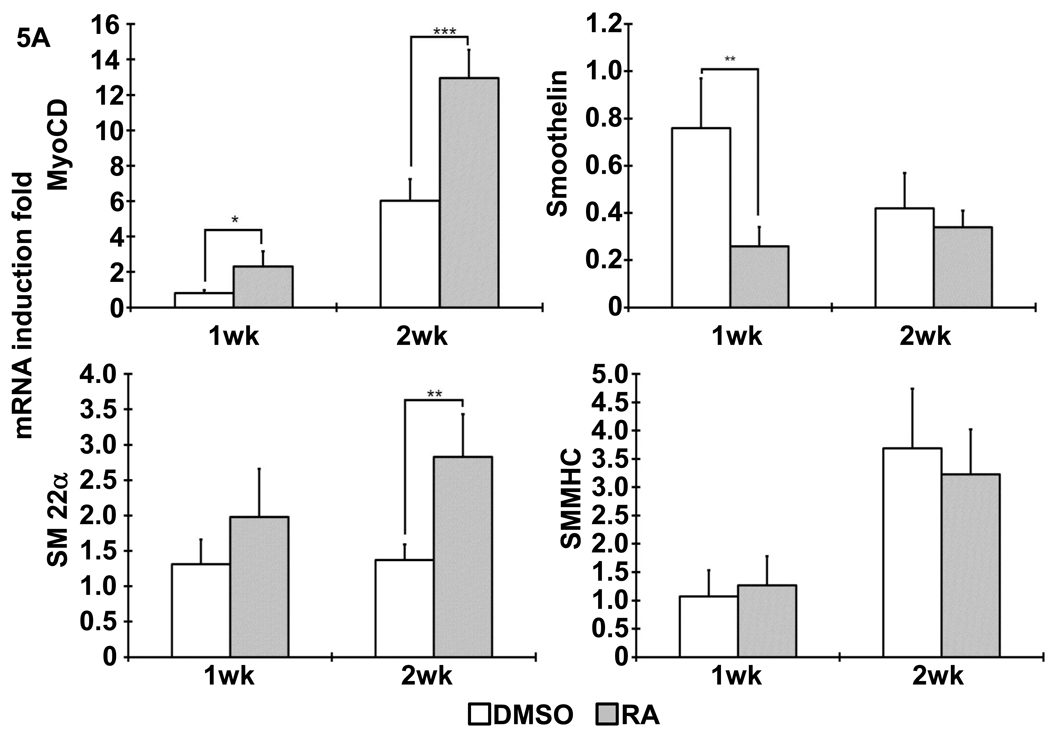
Following the previous reported protocol [9], mouse iPSCs were induced to differentiate into SMCs under the treatment of 10−5M RA for 5 days. Spontaneous differentiation control group was treated with DMSO for the same time period. After dissociation, cell suspension were seeded on 3D NF scaffolds and cultured in vitro for 2 weeks. After seeding and initial culture for 24 hours, both the DMSO- and RA-treatment derived cells aggregated inside the pores of the scaffolds (Fig. 2). Gene expression analysis showed enhanced expression of SMC marker genes, including myocardin (MyoCD), smoothelin, SM22α and SMMHC, during the in vitro 3D culture for cells originated from RA induction compared to those originated from DMSO treatment (Fig. 3A). In addition, pluoripotent gene Pou5f1 expression was significantly inhibited by RA treatment, while ectoderm marker neuroD gene expression was enhanced by RA treatment (Fig. 3B). The expression levels of the endoderm (AFP) and mesoderm (GATA2) were not statistically different between the RA and DMSO treatment groups. Immunohistochemistry analysis showed strong positive α-SMA expression in the cells originated from RA induction on NF scaffolds after 2 weeks of culture (Fig. 4).
Figure 2. Representative SEM micrographs of differentiated iPSCs seeded on 3D NF scaffolds for 24 hours.
Left panel: cells treated with DMSO; right panel: cells treated with RA. Scale bar=50µm.
Figure 3. Gene expression profile after differentiated iPSCs was cultured on NF scaffolds in vitro.
(A) The expression of SMC specific markers, including MyoCD, smoothelin, SM22α and SMMHC, was analyzed with quantitative RT-PCR, as shown by indicated times. (B) The expression of pluripotent gene Pou5f1 and other cell lineage specific markers (AFP: endoderm; NeuroD: ectoderm; GATA2: mesoderm). *, p<0.05; **, p<0.01; ***, p<0.001.
Figure 4. Immunofluorescence analysis of α-SMA expression in constructs cultured in vitro for 2 weeks.
Green: positive α-SMA expression; Blue: nuclei. Scale bar=50µm.
Subcutaneous implantation of scaffold-cell constructs
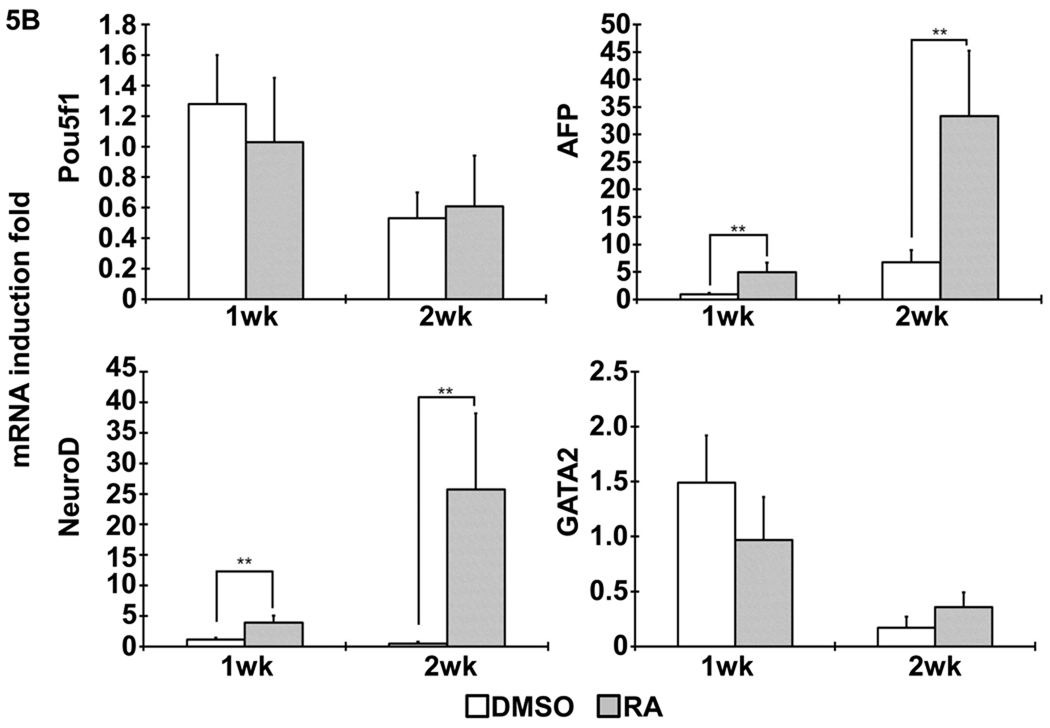
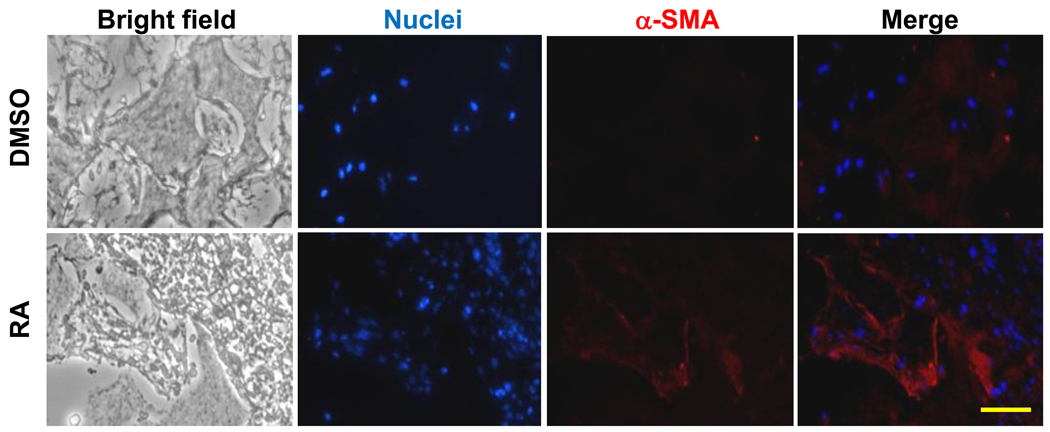
After the differentiated iPSCs, originated either from DMSO or RA treatment, were seeded on NF scaffold for 24 hours, the scaffold-cell constructs were implanted into nude mice. Gene expression analysis in the constructs after implantation showed that RA group had higher SMC markers of MyoCD and SM22α expression over DMSO group (Fig. 5A). However, smoothelin and SMMHC expression levels were not significantly different between the two groups after 2 weeks of implantation (Fig. 5A). In addition, the expression levels of endoderm marker gene AFP and ectoderm marker gene NeuroD were higher for RA group, while the mesoderm marker gene GATA2 expression was not statistically different between the RA and DMSO treatment groups (Fig. 5B). Identification of SMCs was evidenced by positive immunostaining for α-SMA (Fig.6).
Figure 5. Gene expression profile after cell-scaffold constructs implanted in vivo.
(A) The expression of SMCs specific markers, including MyoCD, smoothelin, SM22α and SMMHC, was analyzed with quantitative RT-PCR, as shown by indicated times. (B) The expression of pluripotent gene Pou5f1 and other cell lineage specific markers (AFP: endoderm; NeuroD: ectoderm; GATA2: mesoderm). **, p<0.01; ***, p< 0.001.
Figure 6. Histology analysis of constructs implanted in vivo for 2weeks.
Blue: nuclei; Red: positive α-SMA expression. Scale bar=50µm.
Discussion
The progress of current vascular disease treatment has been evidenced by the emerging surgical interventions such as implantation of stents or grafts. Among these, engineered cell-based vascular therapies offer the promising opportunities to permanently and effectively treat many vascular diseases [22]. Recently established iPSCs have been applied to regenerative medicine, due to its unlimited proliferation potential, the capacity to differentiate into all cell types of the body, and the aimed personalized therapies [23]. However, the potential of iPSCs in constructing TE vascular tissues has not been exploited.
Here, we examined the growth of SMCs derived from differentiated iPSCs on 3D NF scaffolds aiming at vascular tissue engineering. With the reprogramming strategy including 4 factors and knock-down of p53, we established a highly efficient platform to generate mouse iPSCs from fibroblasts. Then the established iPSCs were directed to differentiate into SMCs by the treatment of 10−5M RA for 5 days, following the method we reported previously [9]. Our previous study has shown that a porous NF PLLA scaffold favored contractile phenotype of primary SMCs under the in vitro culture conditions [4]. To further test the capability of the 3D NF scaffolds to support iPSCs derived SMCs growth, the cells were subsequently seeded on 3D NF scaffolds and cultured in vitro. The cells expressed high levels of SMC marker genes, including myocardin (MyoCD), smoothelin, SM22α and SMMHC, but low levels of pluoripotent marker genes. Consistently, immunofluorescence analysis showed strong positive α-SMA expression in the constructs with the cells originated from RA treatment. These data indicated that the specific SMC markers were retained in iPSCs-derived SMCs during the 3D culture. However, the detailed functional phenotype needs to be further investigated, such as secretion of vasoactive factors and contractile activity [24]. Upon implantation, the constructs with cells originated from RA induction had higher MyoCD and SM22α expression levels over those with cells treated by DMSO. However, smoothelin and SMMHC expression was not significantly different between the two groups, which may be due to the mixture of invading host cells or de-differentiation of implanted cells. Future studies are required to derive labeled SMCs for implantation studies. The results may indicate that the in vivo environment also enhanced the differentiation of RA-treated cells towards other lineages. Another concern is the retention of rare residual pluripotent cells since others have showed that embryoid bodies after 28 days of differentiation contained a small population of pluripotent cells [24]. These data strongly suggest that differentiated cells from iPSCs need to be further purified to reduce the possibility of differentiation into other cell lineages, since heterogeneous cell populations contribute to inferior tissue organization. Additional approaches, such as local growth factor delivery capacity [25] incorporated into the current system, may also be beneficial in future studies to further enhance the development of vascular tissues and eliminate un-related cell populations. Although this study is an early exploration of iPSC-based vascular tissue engineering, it has shown the promise of patient-specific or personalized vessel regeneration using iPSC technology and advanced biomimetic scaffolds.
Conclusions
Mouse iPSCs are generated by combination of over-expression of 4 iPS factors and knock-down of p53 gene. The established iPSCs are then directed to differentiate into SMCs with the treatment of RA. The vehicle DMSO treatment serves as a spontaneous differentiation control. When cultured on 3D PLLA NF scaffolds, the RA-induced iPSCs-SMCs show higher SMC specific marker gene expression level, with lower pluripotent marker gene expression level, in comparison to the spontaneously differentiated cells. Upon subcutaneous implantation, the implanted iPSCs-SMCs maintain the SMC phenotype. These findings support the potential of patient-specific vessel regeneration using biomimetic scaffolds and iPSC technology.
Supplementary Material
Acknowledgements
This work is partially funded by an Inaugural Grant (Y.E.C. and P.X.M.) from the Cardiovascular Center at University Michigan and National Institutes of Health (HL092421 and HL068878: Y.E.C.; DE015384 and DE017689: P.X.M). C.X. is supported by AHA National Scientific Development Grant (09SDG2260023). Y.E.C. is an AHA established investigator (0840025N). P.X.M. is partially supported by the Richard H. Kingery Endowed Collegiate Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflict of interest to disclose.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115(3):536–545. doi: 10.1016/S0022-5223(98)70315-0. [DOI] [PubMed] [Google Scholar]
- 3.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, et al. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33(3):628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 4.Hu J, Sun X, Ma H, Xie C, Chen YE, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 2010;31(31):7971–7977. doi: 10.1016/j.biomaterials.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou GD, et al. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008;29(10):1464–1472. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Xie CQ, Huang H, Wei S, Song LS, Zhang J, Ritchie RP, et al. A comparison of murine smooth muscle cells generated from embryonic versus induced pluripotent stem cells. Stem Cells Dev. 2009;18(5):741–748. doi: 10.1089/scd.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee TH, Song SH, Kim KL, Yi JY, Shin GH, Kim JY, et al. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ Res. 2010;106(1):120–128. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- 11.Chang LJ, Gay EE. The molecular genetics of lentiviral vectors--current and future perspectives. Curr Gene Ther. 2001;1(3):237–251. doi: 10.2174/1566523013348634. [DOI] [PubMed] [Google Scholar]
- 12.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 13.Chang LJ, Liu X, He J. Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther. 2005;12(14):1133–1144. doi: 10.1038/sj.gt.3302509. [DOI] [PubMed] [Google Scholar]
- 14.Xiong C, Tang DQ, Xie CQ, Zhang L, Xu KF, Thompson WE, et al. Genetic engineering of human embryonic stem cells with lentiviral vectors. Stem Cells Dev. 2005;14(4):367–377. doi: 10.1089/scd.2005.14.367. [DOI] [PubMed] [Google Scholar]
- 15.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77(16):8957–8961. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 17.Blelloch R, Venere M, Yen J, Ramalho-Santos M. Generation of induced pluripotent stem cells in the absence of drug selection. Cell Stem Cell. 2007;1(3):245–247. doi: 10.1016/j.stem.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey BW, Markoulaki S, Hanna J, Saha K, Gao Q, Mitalipova M, et al. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci U S A. 2009;106(1):157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotta A, Cheung AY, Farra N, Garcha K, Chang WY, Pasceri P, et al. EOS lentiviral vector selection system for human induced pluripotent stem cells. Nat Protoc. 2009;4(12):1828–1844. doi: 10.1038/nprot.2009.201. [DOI] [PubMed] [Google Scholar]
- 21.Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun G, Gerecht S. Vascular regeneration: engineering the stem cell microenvironment. Regen Med. 2009;4(3):435–447. doi: 10.2217/rme.09.1. [DOI] [PubMed] [Google Scholar]
- 23.Kusuma S, Gerecht S. Engineering blood vessels using stem cells: innovative approaches to treat vascular disorders. Expert Rev Cardiovasc Ther. 2010;8(10):1433–1445. doi: 10.1586/erc.10.121. [DOI] [PubMed] [Google Scholar]
- 24.Sinha S, Wamhoff BR, Hoofnagle MH, Thomas J, Neppl RL, Deering T, et al. Assessment of contractility of purified smooth muscle cells derived from embryonic stem cells. Stem Cells. 2006;24(7):1678–1688. doi: 10.1634/stemcells.2006-0002. [DOI] [PubMed] [Google Scholar]
- 25.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112(1):103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.