Abstract
Alcohol and chronic stress exposure, especially during adolescence, can lead to an increased risk in adulthood of developing alcohol use disorders (AUDs). To date, however, no study has assessed the potential long-term effects of chronic intermittent and unpredictable ethanol (EtOH) exposure in mice chronically stressed beginning in adolescence on brain function and anxiety-like behaviors in adulthood. In particular, alterations in function of the bed nucleus of the stria terminalis (BNST), a brain region heavily implicated in anxiety-related behaviors and altered plasticity following EtOH exposure, may play a key role in the pathological responses to chronic stress and EtOH. In the present study, adolescent and adult C57Bl/6J mice were exposed to a regimen of chronic social isolation and unpredictable stressors and EtOH (or air (sham); CSI-CUS-EtOH and CSI-CUS-Sham, respectively) for 8–10 weeks. In adulthood, mice were tested for altered anxiety-like behavior [elevated plus maze (EPM) and modified social interaction (SI) test]. Following behavioral testing, mice were re-exposed to CSI-CUS-EtOH (and CSI-CUS-Sham for controls) for an additional three days. 4–6 hours following the final EtOH (or air) exposure, field potential recordings of the dorsal-lateral (dl)BNST were performed. Mice first exposed during adolescence to CSI-CUS-EtOH displayed lower levels of anxiety-like behavior on the EPM compared to mice first exposed to CSI-CUS-EtOH during adulthood and control mice only exposed to CSI-CUS-Sham, regardless of age of first exposure. However, mice first exposed to CSI-CUS-EtOH during adulthood displayed lower levels of anxiety-like behavior on the SI test compared to mice first exposed during adolescence and control CSI-CUS-Sham mice. CSI-CUS-EtOH exposure, regardless of age, produced blunted expression of long-term potentiation (LTP) in the dlBNST compared to CSI-CUS-Sham mice. This study demonstrates age-dependent effects of chronic unpredictable ethanol exposure in chronically stressed mice on anxiety-like behaviors during adulthood. Further, CSI-CUS-EtOH exposure results in blunted LTP expression in the adult dlBNST.
Keywords: anxiety, alcohol, addiction, stress, plasticity
INTRODUCTION
In adolescents, maladaptive responses to chronic stress have been linked to an increased likelihood of consuming alcohol and later in life developing an alcohol use disorder (AUD) (Grant and Dawson, 1998; DeWit et al., 2000; Enoch, 2006; Dawson et al., 2007). Chronic stress can produce sustained increases in corticosteroid levels, depression-like symptoms, learning/memory deficits and anxiety (McEwen, 2004; Mizoguchi et al., 2000; Arborelious et al., 1999). Negative affect or stress, especially among adolescents, is associated with a two- to six-fold higher incidence of alcohol related problems (Grant et al., 2004a,b; Mulia et al., 2008). Moreover, adolescents who believe alcohol will relieve their anxieties are more likely to engage in heavy alcohol drinking (Kuntsche et al., 2005). Consistent with the link between stress and alcohol use, stressful stimuli have been effective at inducing alcohol craving in clinical studies (Breese et al., 2005; Sinha, 2001).
Behavioral testing in adolescent laboratory animals supports some of the findings from the clinical literature. In mice, adolescence spans from postnatal day (PND) 22 to PD 60 (Laviola et al., 2003). As in humans, adolescent mice can also display an enhanced susceptibility to the deleterious effects of stress (Stone & Quartermain, 1997). Adolescents show a more prolonged increase in corticosterone levels in response to an acute stressor but a more rapid recovery following chronic stressors (Choi and Kellogg, 1996; Romeo et al., 2006). Numerous studies, however, have suggested that exposure to chronic homotypic stressors given at predictable intervals may lead to habituation (Amario et al., 2004; Muir & Pfister, 1987; Grissom and Bhatnagar, 2009). One method that has been used to address this potential issue of adaptation and may better mimic the variability of type (psychological and physical), frequency, and duration of stressors that adolescent humans encounter is the chronic unpredictable stress (CUS) paradigm (Pego et al., 2008; Cerqueira et al., 2007; Willner et al., 1987; Bondi et al., 2008; Salomons et al., 2010). In adult mice, CUS results in a state of chronic hypercorticalism, enhanced anxiety- and depressive-like behavior, and reduced body weight gain (Bondi et al., 2008; Pego et al., 2008; Cerqueira et al., 2007). In addition, chronic social isolation (CSI) in adult C57 mice has also recently received additional attention as a unique model for depression and anxiety-like behavior and the behavioral effects of isolation stress may increase over time.
The central extended amydala, and in particular the bed nucleus of the stria terminalis (BNST), serves as a critical region for the processing and integration of stress and reward (Koob et al., 2008). Moreover, the BNST receives inputs from several discrete brain regions to influence the stress response via regulation of the HPA axis. Importantly, a number of studies have demonstrated that the BNST plays an integral role in the lasting response to unconditioned stressors (Fendt et al., 2003; Walker et al., 2009). Indeed, long-term activation of the BNST is associated with anxiety but not fear-conditioned behaviors (Davis et al., 1997; Walker and Davis 2008).
Reports on the anxiogenic effects of stress and EtOH in adolescent rodents have been mixed (Spear et al., 2000; Wills et al., 2008; 2009; Doremus et al., 2003; Varlinskaya and Spear, 2004). Reports of stressors administered in adolescent rats revealed an increase in anxiety-like behavior on the EPM when tested as adults (McCormick et al., 2008; Tsoory et al., 2007). Thus, although there have been some studies on the effects of acute and chronic homotypic stress and EtOH in the adolescent, mostly in rats, it is not yet known about the interactions of chronic unpredictable EtOH exposure, stress and age on unconditioned anxiety-like behavior and synaptic plasticity. Therefore, the purpose of this study was to assess in mouse the effects of chronic intermittent EtOH exposure beginning in adolescence on anxiety-like behavior and BNST plasticity in adulthood in chronically and unpredictably stressed C57Bl/6J mice.
METHODS
Subjects
All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Male C57Bl/6J mice were bred on-site at Vanderbilt University Medical Center and were used for all studies. Adult (>8 wks old) male A/J mice were used as unfamiliar ‘target’ mice in the modified SI test. The A/J strain was chosen because they are distinct from the C57Bl/6J strain on many anxiety-like behavioral assays (Moy et al., 2007).
CSI-CUS protocol
At PND28 (adolescent) or PND70–84 (adult) mice began the chronic unpredictable stress (CUS) and chronic unpredictable EtOH/air vapor chamber exposure protocol. Mice were single housed [chronic social isolation (CSI)] for the remainder of the experiment, unless otherwise noted. Four groups were used throughout all experiments: Adolescent CSI-CUS and chronic unpredictable air vapor chamber exposure (CSI-CUS-Sham-Youth), Adult CSI-CUS and chronic unpredictable air vapor chamber exposure (CSI-CUS-Sham-Adult), Adolescent CSI-CUS and chronic unpredictable EtOH vapor chamber exposure (CSI-CUS-EtOH-Youth), Adult CSI-CUS and chronic unpredictable EtOH vapor chamber exposure (CSI-CUS-EtOH-Adult). A modified version of the CSI-CUS protocol based on other studies of anxiety and depression was used in this study (Cerqueira et al., 2007; Pego et al., 2008) and included the following chronic unpredictable stressors: re-grouping, restraint stress, over-crowding, wet bedding, cage tilting; light-dark cycle inversion (see Table 1 for detailed CSI-CUS protocol; mice were singly housed at the start of the experiment so social isolation persisted, unless otherwise noted). All mice averaged 5.5 exposures to stressors and 3.75 exposures to EtOH per week. Body weight gain was monitored throughout the study. All mice were exposed to the protocol for 8–10 weeks and were then tested in adulthood for altered anxiety-like behavior. Following the behavioral testing, mice were re-exposed for another 3–5 days to the CSI-CUS-EtOH (or CSI-CUS-Sham) protocol and then examined for altered plasticity in the BNST. The same cohort of mice was used for behavioral testing and subsequent electrophysiological studies.
Table 1.
Representative 4 weeks of CSI-CUS protocol
| AGE OF MICE | 4 WKS (Youth)/10 WKS (Adult) | 5 WKS (Youth)/11 WKS (Adult) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MON | TUES | WED | THURS | FRI | SAT | SUN | MON | TUES | WED | THURS | FRI | SAT | SUN | |
| I | I | I | I | I | I | I | I | I | I | I | I | I | I | |
| 08:00–10:00 | NB | RG | ||||||||||||
| 10:00–12:00 | R | R | CT | |||||||||||
| 12:00–14:00 | CL | CT | ||||||||||||
| 14:00–16:00 | DC | RG | WB | |||||||||||
| 16:00–18:00 | WB | CT | OC | |||||||||||
| 18:00–20:00 | ||||||||||||||
| OVERNIGHT | A/E | A/E | A/E | A/E | A/E | A/E | ||||||||
| AGE OF MICE | 6 WKS (Youth)/12 WKS (Adult) | 7 WKS (Youth)/13 WKS (Adult) | ||||||||||||
| MON | TUES | WED | THURS | FRI | SAT | SUN | MON | TUES | WED | THURS | FRI | SAT | SUN | |
| I | I | I | I | I | I | I | I | I | I | I | I | I | I | |
| 08:00–10:00 | R | CI | ||||||||||||
| 10:00–12:00 | CL | RG | WB | |||||||||||
| 12:00–14:00 | NB | OC | NB | |||||||||||
| 14:00–16:00 | R | CL | CL | R | ||||||||||
| 16:00–18:00 | WB | RG | CT | |||||||||||
| 18:00–20:00 | ||||||||||||||
| OVERNIGHT | A/E | A/E | A/E | A/E | A/E | A/E | A/E | A/E | ||||||
NB = No Bedding; CL - Change Light; I = Isolated; A/E = Air//Ethanol; OC = Overcrowd; WB = Wet Bedding; RG = Regrouping; CT = Cage Tilt;R = Restraint
Chronic EtOH Procedure
Procedures were performed as outlined in the INIA-Stress standard operating procedure (www.iniastress.org). Briefly, male C57Bl/6J mice (4 or 10–12 weeks old at start of Air or EtOH exposure) were given a daily injection of either pyrazole (Air, 1 mmol/kg) or pyrazole + EtOH (EtOH group, EtOH, 1 mmol/kg + 0.8 g/kg, respectively). Thirty minutes following the injection, in their home cages, mice were placed in a chamber filled with volatilized EtOH (EtOH, 20.3 ± 0.2 mg/L) or volatilized water (Air). Airflow through the chambers was maintained at 5.5 L/min and volatilization at 1.5 L/min. Mice were allowed food and water ad libitum. After 16 hours of exposure, mice were removed from the chambers and placed in fresh care fresh cages. Mice were randomly exposed to the EtOH vapor chamber but averaged 3.75 exposures per week (see Table 1 for detailed protocol). Using these parameters we tested blood ethanol levels (BEC) following the first EtOH exposure. The CUS-EtOH-Youth group measures were 194 and 208 mg/dl (n=2) and the CUS-EtOH-Adult group 150 and 158 mg/dl (n=2; BEC levels determined as described in Healey et al., 2008). All mice were tested after 8–10 weeks of the CSI-CUS-EtOH (or CSI-CUS-Sham) paradigm for changes in anxiety-like behaviors during acute (4–6 hours) withdrawal from an EtOH/Air vapor chamber exposure. The behavioral testing occurred during the light- phase of the light/dark cycle and was conducted in a counter-balanced manner between groups. All behavior tests were performed in the Vanderbilt Murine Neurobehavioral Lab shared behavior core facilities at Vanderbilt University Medical Center (https://medschool.mc.vanderbilt.edu/mnl).
Elevated plus maze and behavioral analysis
The elevated plus maze (EPM) follows the general design described by Lister (1987). The EPM is comprised of two closed arms (30 × 10 × 5 cm) and two open arms (30 × 10 × 5 cm) that meet at the junction (5 × 5 cm) of the four arms. The floor of the EPM was made of clear plexiglass. The walls of the enclosed arms were 20 cm high and made of black plexiglass. A small (0.25 cm) edge provided grip for the animals in the open arms. The entire EPM apparatus was elevated 50 cm above floor level. To insure approximately equal light distribution, Lux levels were assessed mid-length along each of the four arms and at the junction of the EPM(~7–15 Lux). 1 hour prior to testing, the mice were brought to the EPM testing laboratory. For each mouse, EPM testing was conducted between 4–6 hours after the final vapor chamber exposure. We chose this acute withdrawal time-point based on work that has shown an increase in handling-induced convulsions (Becker et al., 1997; Jarvis and Becker, 1998) and in anxiety-like behavior in adult mice exposed to chronic intermittent EtOH vapor for 16 hours a day for 4 days and then tested on the EPM 4–6 hours after the last vapor chamber session (Kash et al., 2009). Testing began by placing the subject on the central platform (facing an open arm). The videotaped test sessions were 5 minutes in duration. Between test sessions, the maze was thoroughly cleaned with 70% EtOH. Videotapes were automatically scored with NIH Image software for open and closed arm entries, time spent in the open and closed arms and center zone, and total distance traveled. Total number of arm entries (open and closed) and distance traveled are typically used as indicators of overall locomotor activity. Although number of open arm entries may also be analyzed to look at changes in anxiety-like behavior, it is not the best indicator and time spent in the open arm is considered a more reliable and robust measure (Rodgers and Dalvi, 1997).
Social Interaction (approach-avoidance) Test
The modified Social Interaction (SI) test used in this study is based on the modified SI test reported in Berton et al., (2006). SI test, also a commonly used measure for anxiety-like behavior, assesses social behaviors that manifest in altered social communication and interactions and is influenced by familiar versus unfamiliar partners or “target” mice (File and Seth, 2003). Briefly, an experimental mouse was placed in a clear plexiglass box (40 × 40 × 40 cm) and video tracking software (ANYmaze, Stoelting) was used to score approaches to an unfamiliar adult (>8 weeks old) A/J mouse “target” that was enclosed in a cylindrical container (10 cm in diameter) located in the center of one end of the box. The designated “target area” was the 8 cm immediately outside and surrounding the container. The experiment was carried out in low-light conditions (3 lux). At the start of each phase, the experimental mouse in this study was placed in the corner farthest away from the empty container “no target’ and its movements were tracked for 150 seconds. The experimental mouse was then removed and placed back in his cage for approximately 60 seconds. For the second phase, conditions were identical except the unfamiliar ‘target’ mouse was placed in the container and the experimental mouse placed back in the box. Time the experimental mouse spent in the “target area” was calculated by 100 × (time spent with “target”)/(time spent with “no target”). The box and container were thoroughly cleaned with 70% EtOH in between tests.
Slice Preparation
CSI-CUS-EtOH and CSI-CUS-Sham mice) were decapitated under isoflurane. For field potential recordings, the brains were removed quickly and placed in ice-cold sucrose artificial cerebrospinal fluid (ACSF): (in mM) 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3 saturated with 95% O2/5% CO2 Hemisected coronal slices (300 μm)were prepared with a Vibratome (Pelco). Slices containing anterior portions of the dorsal anterolateral BNST (dlBNST; bregma, 0.26–0.02 mm) (Franklin and Paxinos, 1997)were selected using the internal capsule, anterior commissure, and stria terminalis as landmarks
Electrophysiology
Field potential recordings
After dissection, slices were transferred to an interface recording chamber where they were perfused with heated (~29°C), oxygenated (95% O2-5% CO2) artificial CSF (ACSF) (in mM: 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10 glucose, and 26 NaHCO3, pH7.2–7.4; 290–310 mOsm) at a rate of 2 ml/min. Slices were allowed to equilibrate in ACSF for at least 1 hr before experiments began. A bipolar stainless steel stimulating electrode and a borosilicate glass recording electrode filled with ACSF were placed in the dlBNST to elicit and record an extracellular field response. Baseline responses to a stimulus (50 μsec) at an intensity that produced 40% of the maximum response were recorded for no less than 20 min at a rate of 0.05 Hz. To elicit LTP, two trains of 100 Hz, 1 sec tetanus were delivered with a 20 sec intertrain interval at the same intensity as baseline test pulses. Previous studies from our lab have demonstrated that the extracellular field response in comprised of a stimulus artifact and two distinct negative deflections referred to as N1 and N2 (Weitlauf et al., 2004). The second negative deflection, N2, is abolished by 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and both the N1 (the first negative deflection) and N2 are eliminated by 1 μM tetrodotoxin (TTX) (Weitlauf et al., 2004). The elimination of the N1 by the sodium channel blocker TTX suggests that this component of the response may be similar to hippocampal fiber volleys and striatal N1 responses. Experiments in which the N1 changed by >20% were discarded. If the N1 could not be accurately estimated, input-output curves taken at the beginning and end of experiments were used to determine whether the N1 at higher stimulation intensities changed.
Statistical Analysis
All values are expressed as mean ± SEM. Data was analyzed using the Prism Software package. Statistical significance was assessed using a Two-Way ANOVA for all tests (P < 0.05) and when a significant effect was found, Bonferroni’s was used for post-hoc analysis.
RESULTS
CSI-CUS-EtOH-Youth display reduced levels of anxiety-like behavior in the EPM
After 8–10 weeks of the CSI-CUS and chronic unpredictable EtOH or air vapor exposure, mice were tested 4–6 hours after the last vapor chamber session for altered anxiety-like behavior on the EPM.
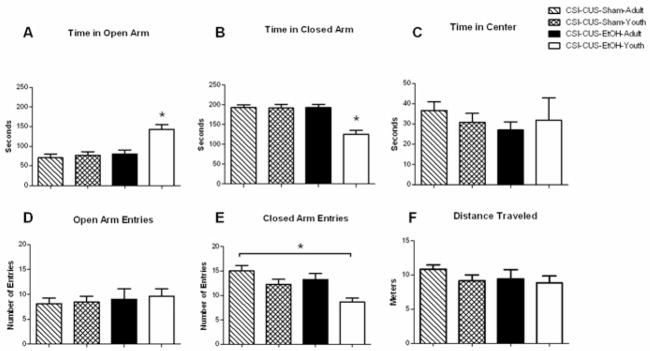
In the current study, we found that mice in the CSI-CUS-EtOH-Youth group spent a significantly greater amount of time in the open arm compared to all other groups (Fig. 1A; age F1,28=12.02; drug F1,28= 14.20; age × drug F1,28= 7.775) consistent with a decrease in anxiety-like behavior in the CSI-CUS-EtOH-Youth group. Likewise, we found that the CSI-CUS-EtOH-Youth group spent significantly less time in the closed arm of the EPM compared to all other groups (Fig. 1B; age F1,28=13.59; drug F1,28 = 13.00; age × drug F1,28 = 12.78). No differences were found in time spent at the center of the EPM apparatus (Fig. 1C). No differences were found in the open arm entries in any group (Fig. 1D). However, a significant decrease in closed arm entries was found in the CSI-CUS-EtOH-Youth compared to CSI-CUS-Sham-Adult group (Fig. 1E; age F1,28=11.51; drug F1,28= 6.251; age × drug F1,28<1). No differences were found in distance traveled in any of the groups (Fig. 1F).
Figure 1. Anxiety-like Behavior in the EPM is decreased in the CSI-CUS-EtOH-Youth group.
A. Time spent on the open arm of the EPM (seconds). All mice were tested 4–6 hours post EtOH or Air (control) vapor chamber exposure. CSI-CUS-EtOH-Youth group spent significantly more time on the open arm compared to CSI-CUS-EtOH-Adult, CSI-CUS-Sham-Adult, CSI-CUS-Sham-Youth. (n=7–9 per group), *p<0.05. Data are represented as mean ± SEM.
B. Time spent on the closed arm of the EPM. CSI-CUS-EtOH-Youth group spent significantly less time on the closed arm compared to CSI-CUS-EtOH-Adult, CSI-CUS-Sham-Adult, CSI-CUS-Sham-Youth. (n=7–9 per group), *p<0.05. Data are represented as mean ± SEM.
C. Number of open arm entries on the EPM. No significant differences were found on number of open arm entries. (n=7–9 per group). Data are represented as mean ± SEM.
D. Number of closed arm entries on the EPM. CSI-CUS-EtOH-Youth group had fewer entries on the closed arm compared to CSI-CUS-EtOH-Adult, CSI-CUS-Sham-Adult, and CSI-CUS-Sham-Youth. (n=7–9 per group), *p<0.05. Data are represented as mean ± SEM.
E. Distance Travelled on the EPM. No differences were found in the distance travelled on the EPM. (n=7–9 per group). Data are represented as mean ± SEM. Only one cohort of mice was used for all behavioral and electrophysiological studies. See Methods for additional information.
CSI-CUS-EtOH-Adult group displays increased social-interaction
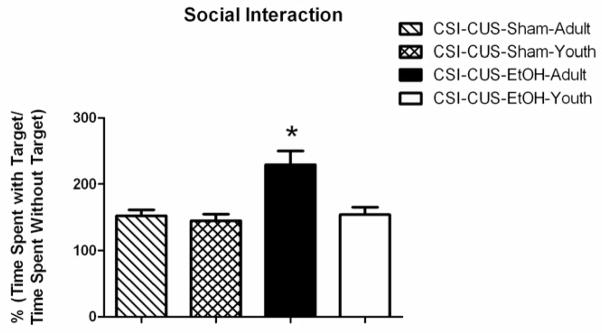
We chose the SI test based on several reports that following several cycles of intermittent but not continuous EtOH exposure, adult rats display a decrease in interaction with an unfamiliar ‘target’ animal in the social interaction test for anxiety (i.e. an increase in anxiety-like behavior; Overstreet et al., 2002; Overstreet et al., 2003). In the SI test, we found that the CSI-CUS-EtOH-Adult group spent significantly more time in the interaction zone (“target area”) when the unfamiliar “target” mouse was present (Fig. 2; age F1,28=6.858; drug F1,28 = 11.13; age × drug F1,28=10.31) compared to all other groups.
Figure 2. CSI-CUS-EtOH-Adult group spend significantly more time interacting with the target mouse compared to the CSI-CUS-EtOH-Youth, CSI-CUS-Sham-Adult, and CSI-CUS-Sham-Youth groups.
A. Time spent with the target mouse [percent of total time calculated as %(time spent with target AJ mouse/time spent without target AJ mouse)]. All mice were tested 4–6 hours post EtOH or Air (control) vapor chamber exposure but on a different day than the EPM and LD tests. CSI-CUS-EtOH-Adult mouse spent significantly more time with the target AJ mouse compared to all other groups. (n=7–9 per group), *p<0.05. Data are represented as mean ± SEM. Only one cohort of mice was used for all behavioral and electrophysiological studies. See Methods for additional information.
CSI-CUS-EtOH-Youth and Adult mice display blunted LTP in the dlBNST
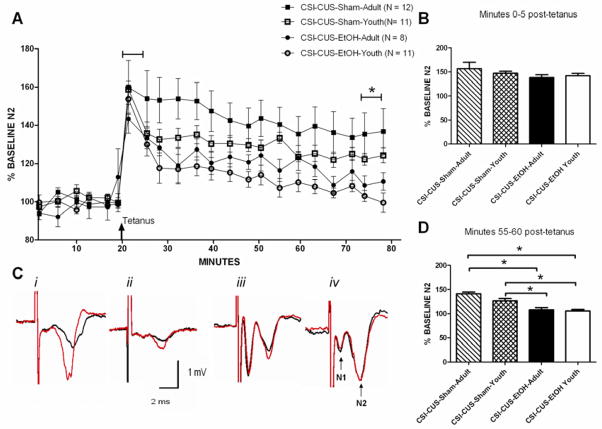
To investigate whether glutamatergic plasticity in the dlBNST would be altered following the CSI-CUS-EtOH and CSI-CUS-Sham regimens, we stimulated these afferents with a tetanus protocol (two 100 Hz, 1 sectrains with a 20 sec intertrain interval; Figure 3A) previously shown to induce NMDA receptor dependent LTP (Weitlauf et al., 2004). Only experiments in which the N1 remained stable (<20% change throughout) were included for analysis. This tetanus protocol had no effect on the early (0–5 minutes post- tetanus) component of LTP of the N2 (Fig. 3B). However, this protocol elicited significantly greater LTP of the N2 55 minutes after tetanusin the CSI-CUS-Sham but not CSI-CUS-EtOH groups (Fig. 3C; CSI-CUS-Sham-Adult: 141.5 ± 11.3%; CSI-CUS-Sham-Youth: 126.8 ± 3.67%; CSI-CUS-EtOH-Adult: 108.4 ± 5.18%; ; CSI-CUS-EtOH-Youth: 102.76 ± 3.83%; effect of treatment: F1,36 = 40.27; p< 0.05) but no effect of age or interaction of age × treatment was observed.
Figure 3. CSI-CUS-EtOH-Youth and CSI-CUS-EtOH-Adult groups displayed blunted LTP in the dlBNST compared to CSI-CUS-Sham-Youth and CSI-CUS-Sham-Adult groups.
A.  Time of tetanus [two trains (20 sec interstimulus interval); 100 Hz, 1 sec].
Time of tetanus [two trains (20 sec interstimulus interval); 100 Hz, 1 sec].
B. 0–5 minutes after administering tetanus, N2 amplitude was not significantly different among any group.
C. Representative traces i) CSI-CUS-Sham-Adult, ii) CSI-CUS-Sham-Youth, iii) CSI-CUS-EtOH-Adult, iv) CSI-CUS-EtOH-Youth and labeled N1 and N2.
D. 55–60 minutes after administering tetanus, N2 amplitude in the CSI-CUS-EtOH groups was significantly lower compared to CSI-CUS-Sham groups. Only one cohort of mice was used for all behavioral and electrophysiological studies. See Methods for additional information.
DISCUSSION
In this study, we showed that age of first exposure to chronic and intermittent unpredictable stress and EtOH can have a differential impact on anxiety-like behaviors and dramatically alter plasticity in the dlBNST in adult C57Bl/6J mice.
On the EPM test, we found that the CSI-CUS-EtOH-Youth mice displayed reduced anxiety-like behavior compared to CSI-CUS-Sham and CSI-CUS-EtOH-Adult mice. Moreover, although there were a reduced number of entries to the closed arm in the CSI-CUS-EtOH-Youth group, we think this is reflective of an overall decrease in time spent in the closed arm (indicative of a decrease in anxiety-like behavior) rather than a reduction in overall locomotor activity because the total distance traveled between groups was unchanged. Although increases, decreases and no changes in anxiety-like behaviors on the EPM has been observed in adult mice exposed to alcohol (Kliethermes, 2005), previous studies have not assessed EPM in adult chronically stressed mice exposed to long-term and intermittent EtOH beginning in adolescence. Interestingly, the CSI-CUS-Sham-Youth/Adult and CSI-CUS-EtOH-Adult mice may actually display an increase in anxiety-like behavior (measured as time spent on the open arm; ~20%) when compared to adult naïve mice (time spent on the open arm: 30–40%)run in our laboratory (Conrad KL, unpublished observations). Future studies should explore the possibility that mice subjected to the CSI-CUS paradigm display an increase in anxiety-like behavior compared to naïve controls. Further, it is important to note that it is possible that the age-related differences in our results could at least in part be derived from age-depend differences in the pharmacol kinetics of alcohol metabolism.
In contrast to the decreased anxiety-like behavior observed in CSI-CUS-EtOH-Youth group on the EPM, on the modified SI test, we found that the CSI-CUS-EtOH-Adult mice displayed reduced anxiety-like behavior. It is possible that the CSI-CUS-Sham mice display altered social interaction compared to what would be seen in naïve mice. However, we think this possibility improbable given the degree of social interaction reported for CSI-CUS-Sham mice is nearly identical to that reported in control mice in another study that used the same modified SI test (Krishnan et al., 2007).
This study also adds to our current understanding of the role altered function in the BNST plays in EtOH and stress-related adaptations. Thus, CSI-CUS-EtOH mice, regardless of age of first exposure, displayed blunting of the late (55–60 minutes post-tetanus) but not early (0–5 minutes post-tetanus) component of LTP in the dlBNST. Inclusion of chronically air but not stress exposed mice may reveal that even mice in the CSI-CUS-Sham groups display modestly blunted LTP, however, we think this result unlikely based on other work from our lab that has found normal LTP in naïve mice to be approximately what we report here for the CSI-CUS-Sham groups (~130–140% of baseline; data not shown).
There are several possible explanations for the altered anxiety-like responses in the CSI-CUS-EtOH groups. One possible interpretation for the reduced levels of anxiety-like behavior in the CSI-CUS-EtOH-Youth on the EPM test is that EtOH may offer some protective benefits against the deleterious effects of stress exposure. For example, intermittent EtOH exposure in adolescent but not adult mice can lead to a tolerance for the aversive effects of EtOH in adulthood (Diaz-Granados and Graham, 2007). Moreover, stressed rats that received EtOH displayed lower corticosterone levels compared to rats that had been stressed but given saline (Brick and Pohorecky, 1982). Several studies have assessed social interaction following differing regimens of EtOH and/or stress administration and have found, in general, that withdrawal from EtOH exposure typically results in a reduction in social interaction in adult rats (i.e. increase in anxiety-like behavior). For example, Overstreet et al., (2002) exposed adult rats to an intermittent EtOH paradigm and found reduced social interaction. Further, the authors found that neither continuous exposure to EtOH (without withdrawals) nor stress alone lead to an increase in anxiety like-behavior on the SI test, suggesting that it is the multiple withdrawals from EtOH exposure that sensitizes the anxiety-like behavior and this effect is more pronounced in adolescent rats (Overstreet et al., 2002; Breese et al., 2004; Wills et al., 2009). These studies are in line with our own findings in the CSI-CUS-Sham mice, in that stress alone was unable to modify the degree of social interaction with an unfamiliar A/J mouse and adolescents display higher levels of anxiety-like behavior on the SI test following combined stress/EtOH exposure. The modified SI test was chosen based on previous studies that successfully utilized it to assess anxiety-like behavior in C57 mice (Berton et al., 2006; Krishnan et al., 2007); however, it may not be directly comparable to many SI tests used in rats or results in different strains of mice (Moy et al., 2008). Indeed, C57Bl/6J mice may exhibit distinct trait anxiety, emotional reactivity and altered EtOH consumption compared to other inbred strains of mice (Crawley et al., 1997; Voikar et al., 2005; Milner and Crabbe, 2008). To our knowledge, only one other study has assessed social interaction in adult mice following prolonged (6 weeks) EtOH exposure that began in adolescence and found, consistent with our findings in the CSI-CUS-EtOH-Youth group, no effect on anxiety-like behavior (Zou et al., 2009). Moreover, an additional possibility for the lack of an increase in SI in the CSI-CUS-EtOH-Youth group is the fact that they were isolated from their peers at 1 week post-weaning and remained isolated for the remainder of the study (except during the social regrouping stressor and the SI test). Previous studies have demonstrated that the effects of social isolation at post-weaning/early adolescence can alter anxiety-like behavior well into adulthood, even in rodents that have been resocialized, and these effects have been especially pronounced on the SI test and EPM (Hall et al., 1998; Lukkes et al., 2009). It is important to note that although studies have reported increases in anxiety- and/or depressive-like behavior and increased EtOH consumption following chronic social isolation in C57Bl/6J mice (Kwak et al., 2005; Lopez et al., 2010), there are also reports of decreases or no effect of long-term social isolation on affective and EtOH use behaviors(Voikar et al., 2005). It is also possible that CSI-CUS-EtOH-Youth and CSI-CUS-EtOH-Adult mice may have displayed differential blood EtOH content (BEC) levels during the experiment which may have contributed to altered anxiety-like behavior. The only other study, to our knowledge, that assessed BEC levels following chronic intermittent EtOH vapor chamber exposure found no significant differences between adolescent and adult mice (Diaz-Granados and Graham, 2007). Unfortunately, a detailed and rigorous time course would be needed to address this possibility but was beyond the scope of this paper.
Previous research strongly implicated the BNST in EtOH and stress-induced adaptations. Indeed, researchers showed in the juxtacapsular BNST (jcBNST), reduced LTP of intrinsic excitability of jcBNST neurons following protracted withdrawal from EtOH self-administration (Francesconi et al., 2009). Furthermore, prior work from our lab has shown that ex vivo EtOH applied acutely to BNST slices resulted in a blunting of NMDAR-dependent LTP (Weitlauf et al., 2004). Consistent with our findings of no change in LTP in the dlBNST in CSI-CUS-Sham mice, a recent study found no effect of repeated restraint stress on excitatory postsynaptic signaling in amygdala principal neurons in adult C57Bl/6 mice, suggesting, along with our findings, that repeated stress alone may not be sufficient to alter plasticity in the amygdala and BNST regions (Holmes et al., 2010). It is unclear how blunted LTP in the dlBNST may be associated with increased anxiety-like behavior. However, the BNST is considered an important regulator and inhibitor of the paraventricular nucleus (PVN) of the hypothalamus which then activates the hypothalamic pituitary adrenal (HPA) axis (Ulrich-lay and Herman, 2009). Thus, impairment of LTP in the BNST may serve to remove its inhibitory influence on the PVN and increase output to the PVN and HPA axis activation. Moreover, dysregulation of the HPA axis is commonly observed in patients suffering from affective and substance use orders. Thus, maladaptive forms of plasticity in regions regulating the HPA axis may occur following chronic stress and EtOH use.
Taken together, this work adds to a growing body of literature on the importance of developmental differences in alcohol and stress-related studies and highlights the differential impact chronic intermittent and unpredictable stress and alcohol exposure can have on affective behavior and plasticity. In addition, in comparing our results to the available literature, our findings suggest that a CSI-CUS-Sham protocol alone is not sufficient to alter anxiety-like behavior and plasticity in the dlBNST, suggesting chronic hyper functioning of the HPA axis alone is insufficient in producing these alterations. Given the strong association between anxiety and EtOH use among adolescents, and the unique tools mice offer in the study of genetic contributions to psychiatric disorders, further investigations into the effects of chronic EtOH use in stressed adolescent laboratory mice is clearly warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews N, File SE. Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol. 1993;235:109–112. doi: 10.1016/0014-2999(93)90827-5. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Armario A, Vallès A, Dal-Zotto S, Márquez C, Belda X. A single exposure to severe stressors causes long-term desensitisation of the physiological response to the homotypic stressor. Stress. 2004;7:157–172. doi: 10.1080/10253890400010721. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–326. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharm. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharm. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Kellogg CK. Adolescent development influences functional responsiveness of noradrenergic projections to the hypothalamus in male rats. Brain Res Dev Brain Res. 1996;94:144–151. doi: 10.1016/s0165-3806(96)80005-8. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharm. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Li TK. Impact of age at first drink on stress-reactive drinking. Alcohol Clin Exp Res. 2007;31:69–77. doi: 10.1111/j.1530-0277.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Genetic and environmental influences on the development of alcoholism: resilience vs. risk Ann N Y Acad Sci. 2006;1094:193–201. doi: 10.1196/annals.1376.019. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic; 1997. [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004a;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004b;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Healey JC, Winder DG, Kash TL. Chronic ethanol exposure leads to divergent control of dopaminergic synapses in distinct target regions. Alcohol. 2008;42:179–90. doi: 10.1016/j.alcohol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharm. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Huang MM, Overtstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin Releasing Factor (CRF) Sensitization of Ethanol Withdrawal-Induced Anxiety-like Behavior is Brain Site Specific and Mediated by CRF-1 Receptors: Relation to Stress-Induced Sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Becker HC. Single and repeated episodes of ethanol withdrawal increase adenosine A1, but not A2A, receptor density in mouse brain. Brain Res. 1998;786:80–88. doi: 10.1016/s0006-8993(97)01413-3. [DOI] [PubMed] [Google Scholar]
- Karim A, Arslan MI. Isolation modifies the behavioural response in rats. Bangladesh Med Res Counc Bull. 2000;26:27–32. [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, 2nd, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharm. 2009;34:2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kwak C, Lee SH, Kaang BK. Social Isolation Selectively Increases Anxiety in Mice Without Affecting Depression-like Behavior. Korean J Physiol Pharmacol. 2009;13:357–360. doi: 10.4196/kjpp.2009.13.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Macrì S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiey in the mouse. Psychopharm. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2010 doi: 10.1016/j.alcohol.2010.08.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009;3:1–12. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnunson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Pfister HP. Time course of the corticosterone and prolactin response following predictable and unpredictable novelty stress in Rattus norvegicus, Phys. Behav. 1987;40:103–107. doi: 10.1016/0031-9384(87)90191-0. [DOI] [PubMed] [Google Scholar]
- Mulia N, Ye Y, Zemore SE, Greenfield TK. Social disadvantage, stress, and alcohol use among black, Hispanic, and white Americans: findings from the 2005 U.S. National Alcohol Survey. J Stud Alcohol Drugs. 2008;69:824–833. doi: 10.15288/jsad.2008.69.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharm (Berl) 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrin. 2006;7:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Salomons AR, Kortleve T, Reinders NR, Kirchoff S, Arndt SS, Ohl F. Susceptibility of a potential animal model for pathological anxiety to chronic mild stress. Behav Brain Res. 2010;209:241–248. doi: 10.1016/j.bbr.2010.01.050. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharm (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–145. doi: 10.1016/s0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharm. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann N Y Acad Sci. 2004;1021:459–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, et al. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharm Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Muscat R, Sophokleous S. Reduction of sucrose preference by chronic mild stress and its restoration by a tricyclic antidepressant. Psychopharm. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Differential dietary ethanol intake and blood ethanol levels in adolescent and adult rats: effects on anxiety-like behavior and seizure thresholds. Alcohol Clin Exp Res. 2008;32:1350–1360. doi: 10.1111/j.1530-0277.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2009;33:455–463. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Xie Q, Zhang M, Zhang C, Zhao G, Jin M, Yu L. Chronic alcohol consumption from adolescence-to-adulthood in mice -effect on growth and social behavior. Drug Alcohol Depend. 2009;104:119–25. doi: 10.1016/j.drugalcdep.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]