Abstract
Postmortem and genetic studies have clearly demonstrated changes in GABAB receptors in neuropsychiatric disorders such as autism, bipolar disorder, major depression, and schizophrenia. Moreover, a number of recent studies have stressed the importance of cerebellar dysfunction in these same disorders. In the current study, we examined protein levels of the two GABAB receptor subunits GABBR1 and GABBR2 in lateral cerebella from a well-characterized cohort of subjects with schizophrenia (n=15), bipolar disorder (n=14), major depression (n=13) and healthy controls (n=12). We found significant reductions in protein for both GABBR1 and GABBR2 in lateral cerebella from subjects with schizophrenia, bipolar disorder and major depression when compared with controls. These results provide further evidence of GABAergic dysfunction in these three disorders as well as identify potential targets for therapeutic intervention.
Keywords: GABAB receptor, schizophrenia, bipolar disorder, major depression, lateral cerebellum
1. Introduction
Gamma-aminobutyric acid (GABA) acts as the main inhibitory neurotransmitter in the central nervous system. There are three GABA receptor types, GABAA, GABAB, GABAC. Both GABAA and GABAC are ligand-gated Cl− channels that mediate the fast-inhibitory action of GABA (Olsen and Homanics, 2000). In contrast, the GABAB receptor is metabotropic, associated with K+/Ca2+ channels, is G-protein linked, and produces slow inhibitory signals (Bowrey, 2000). GABAB receptors are heterodimeric and consist of two subunits: GABA B receptor 1 (GABBR1) and GABA B receptor 2 (GABBR2). The GABBR1 gene produces two splice variants, GABBR1A and GABBR1B (Kaupmann et al., 1997). Functional GABAB receptors require both the GABBR1 and GABBR2 subunits. The GABAB receptor is known to modulate release of a number of neurotransmitters including dopamine, serotonin, noradrenaline, somatostatin, glutamate and GABA (Nyitrai et al., 2003; Sakamaki et al., 2003; Steiniger and Kretschmer, 2003; Takahashi et al., 2010; Waldmeier et al., 2008).
Because of its roles, GABAB receptors have been examined for their impact on psychiatric disorders. A series of studies have shown GABAB abnormalities in disorders including autism (Fatemi et al., 2009; Oblak et al., 2010), bipolar disorder (Ishikawa et al., 2005), major depression (Ghose et al., 2011; Klempan et al., 2009; Sequiera et al., 2009) and schizophrenia (Ishikawa et al., 2005; Mizukami et al., 2000, 2002; Zai et al., 2005). Our laboratory observed significant reductions in GABBR1 protein in cerebellum, superior frontal cortex, and parietal cortex of subjects with autism and a significant reduction in GABBR2 in cerebellum of subjects with autism (Fatemi et al., 2009). Oblak et al. (2010) found significantly reduced GABAB receptor density in the anterior and posterior cingulate cortex and the fusiform gyrus of subjects with autism. Reductions in GABBR1A, GABBR1B, and GABBR2 proteins were also observed in prefrontal cortex of subjects with bipolar disorder (Ishikawa et al., 2005). A series of immunohistochemical experiments similarly found reductions in GABAB immunolabeling in hippocampus, prefrontal cortex, inferior temporal cortex, and the entorhinal cortex of subjects with schizophrenia (Ishikawa et al., 2005; Mizukami et al., 2000, 2002). The loci of both the GABBR1 gene (6p21.3) and GABBR2 gene (5q34) have been established as susceptibility loci for schizophrenia (Lindholm et al., 1999; Petryshen et al., 2005). However, one study has found a weak linkage between the GABBR1 gene and schizophrenia (Zai et al., 2005), while two other studies have found no connection (Imai et al., 2002; Zhao et al., 2007). In two microarray studies of suicides, increased expression of GABBR1 and GABBR2 mRNA was observed (Klempan et al., 2009; Sequiera et al., 2009). A further study found increased mRNA for GABBR2 in dentate gyrus of subjects with depression while there was decreased mRNA expression for GABBR1A in the same region (Ghose et al., 2011).
Schizophrenia affects approximately 1% of the population (Mueser and McGurk, 2004), generally manifests itself during adolescence and early adulthood and is characterized by the presence of symptoms including hallucinations, and delusions (APA, 1994). Bipolar disorder is characterized by alternating episodes of mania and depression or mania alone (Bipolar I) or episodes of depression and hypomania (Bipolar II) (APA, 1994). Major depressive disorder is characterized by the presence of one or more episodes consisting of five or more symptoms including depressed mood, changes in sleep and appetite, loss of energy, anhedonia, indecisiveness, feelings of worthlessness, and suicidal ideation that occur during the same two week period and represent a change from previous functioning (APA, 1994).
In the current study we have examined protein levels of GABBR1 and GABBR2 in cerebellum, a brain region that has been implicated in the pathology of schizophrenia, bipolar disorder, and major depression (Andreasen et al., 1996; Baldaçara et al., 2008; Konarski et al., 2005; Krüger et al., 2003; Liotti et al., 2002; Loeber et al., 2002; Smith et al., 2002) and because of a lack of information regarding GABAB receptors in this region, particularly with regard to subjects with bipolar disorder and major depression. We used lateral cerebellar tissue from a well-characterized cohort of subjects with schizophrenia, bipolar disorder, major depression, and controls from the Stanley Medical Research Institute. We hypothesized that, similar to what has been found in other brain regions, that both GABBR1 and GABBR2 protein would be reduced in all three diagnostic groups when compared with controls.
2. Experimental/Materials and Methods
2.1. Brain Procurement
The current study has been approved by the Institutional Review Board of the University of Minnesota-School of Medicine. Postmortem lateral cerebella were obtained from the Stanley Foundation Neuropathology Consortium under approved ethical guidelines. The collection consisted of 15 subjects with schizophrenia, 14 subjects with bipolar disorder, 13 with major depression without psychotic features and 12 normal controls. All groups were matched for age, sex, race, postmortem interval and hemispheric side (Table 1). All demographic information and medical data including lifetime use of psychotropic medications and history of drug abuse were provided to us by the Stanley Foundation consortium (Table 1).
Table 1.
Demographic information for the four diagnostic groups
| Bipolar | Depression | Control | Schizophrenia | F or χ2 |
p | |
|---|---|---|---|---|---|---|
| Age | 43.6 (11.1) | 45.5 (8.35) | 47.7 (8.6) | 44.5 (13.1) | 0.35 | 0.79 |
| Sex | 5F, 9M | 5F, 8M | 4F, 8M | 6F, 9M | 0.149 | 0.99 |
| Race | 13W, 1B | 13W | 11W, 1B | 12W, 3A | 13.2 | 0.15 |
| PMI | 33.1 (16.6) | 28.3 (11.3) | 22.8 (9.08) | 33.7 (14.6) | 1.85 | 0.15 |
| pH | 6.16 (0.234) | 6.17 (0.235) | 6.25 (0.261) | 6.16 (0.256) | 0.39 | 0.76 |
| Side of Brain | 7R, 7L | 4R, 9L | 7R, 5L | 6R, 9L | 2.22 | 0.529 |
| Brain Wt | 1434 (176) | 1479 (145) | 1501 (169) | 1472 (108) | 0.45 | 0.72 |
| Family hx | 0.859 (0.77) | 0.692 (0.48) | 0 | 1.13 (0.834) | 2.91 | 0.066 |
| Suicidal death | 8 (5 violent) | 7 (2 violent) | 0 | 4 (2 violent) | 3.44 | 0.49 |
| Drug/Alc hx | 0.786 (0.802) | 0.462 (0.66) | 0.25 (0.622) | 0.533 (0.743) | 1.25 | 0.30 |
| Freezer storage time (days) | 1335 (168) | 1136 (300) | 1072 (204) | 1351 (233) | 4.93 | 0.004 |
| Age of onset | 21.6 (8.63) | 32.7 (13.9) | -- | 23.2 (7.96) | 4.51 | 0.017 |
| Duration | 21.1 (9.17) | 12.8 (11.4) | -- | 21.7 (11.2) | 2.96 | 0.064 |
| Severity of Substance abuse | 1.86 (2.03) | 1.25 (2.09) | 0.167 (0.577) | 1.2 (1.86) | 1.25 | 0.303 |
| Severity of Alcohol abuse | 2.07 (1.9) | 1.92 (2.14) | 1.17 (1.03) | 1.47 (1.6) | 0.74 | 0.532 |
| Fluphenazine (lifetime) | 21,780 (24,630) | -- | -- | 52,270 (62,060) | 2.94 | 0.098 |
2.2. SDS-PAGE and Western Blotting
Western blotting experiments for GABBR1 and GABBR2 were performed as previously described (Fatemi et al., 2009). Each gel contained samples from subjects with schizophrenia, bipolar disorder, major depression, and controls in order to minimize interblot variability. The primary antibodies used were anti-GABBR1 (NB300-160, Novus Biologicals (Littleton, CO) 1:1,000), anti-GABBR2 (56311, QED Bioscience Inc. (San Diego, CA) 1:1,000), anti-neuronal specific enolase (ab16808, (Cambridge, MA), 1:2,000), and anti-β actin (A5441, Sigma Aldrich (St. Louis, MO), 1:5,000). Secondary antibodies used were goat anti-rabbit IgG (A9169, Sigma Aldrich, (St. Louis, MO), 1:80,000) and rabbit anti-mouse IgG (A9044, Sigma Aldrich, (St. Louis, MO) 1:80,000). The molecular weights of approximately 108 kDa (GABBR1), 105 kDa (GABBR2), 46 kDa (NSE), and 42 kDa (β-actin) immunoreactive bands were quantified with background subtraction. Results obtained are based on at least two independent experiments.
2.3. Statistical Analysis
All protein measurements for each group were normalized against both NSE (Table 2) and β-actin (Table 3) and expressed as ratios of GABBR1/NSE, GABBR1/β-actin, GABBR2/NSE, and GABBR2/β-actin. Statistical analysis was performed as previously described (Fatemi et al., 2008, 2010a,b) with p<0.05 considered significant. Briefly, group comparisons were conducted using analysis of variance (ANOVA). If the group differences were significant, follow-up independent t-tests were conducted. Group differences on possible confounding factors were explored using chi-square tests for categorical variables and ANOVA for continuous variables. Where group differences were found, analysis of covariance was used to explore these effects on group differences for continuous variables and factorial ANOVA with interaction terms for categorical variables. All analyses were conducted using SPSS v.17 (SPSS Inc, Chicago, IL).
Table 2.
Western blotting results for GABBR1, GABBR2, and NSE in lateral cerebellum of subjects with schizophrenia, bipolar disorder, major depression vs. matched controls
| Group | GABBR1/ NSE | Δ | P | GABBR2/NSE | Δ | P | NSE | P |
|---|---|---|---|---|---|---|---|---|
| Control | 0.144 ± 0.087 | RG | RG | 0.182 ± 0.141 | RG | RG | 20.2 ± 3.34 | RG |
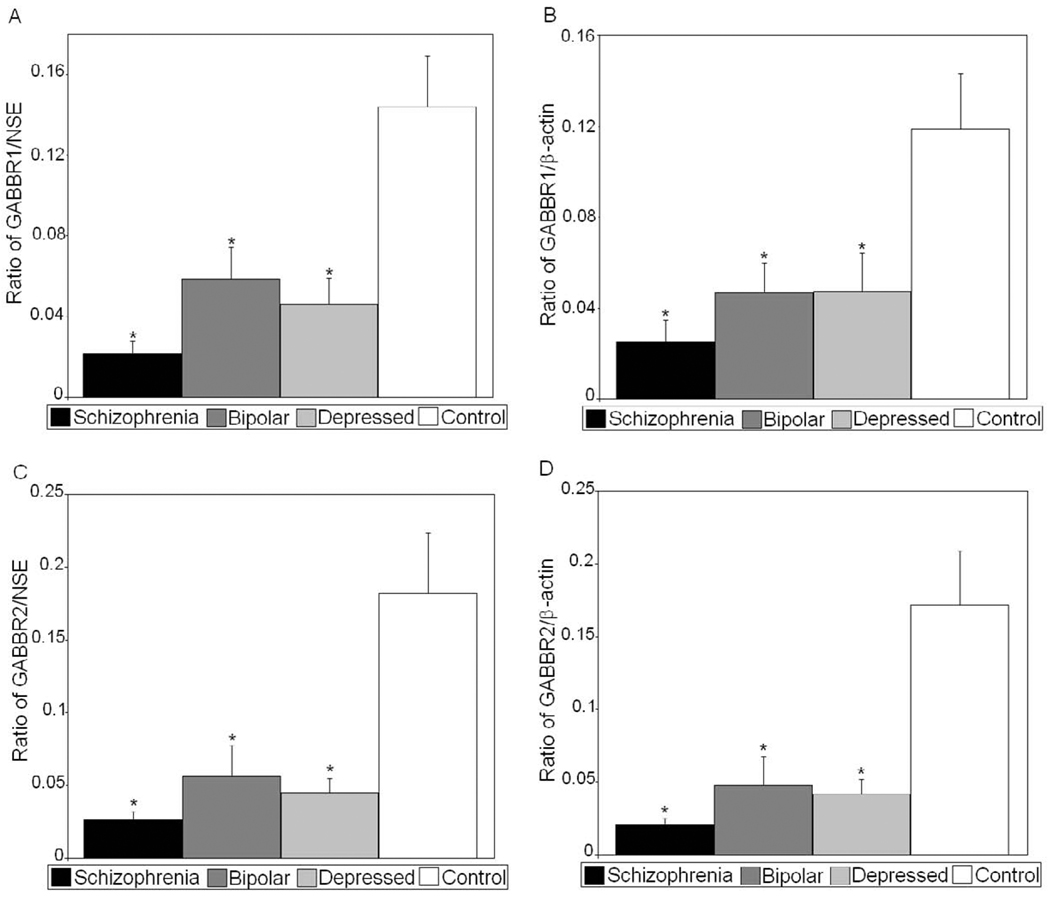
| Bipolar | 0.059 ± 0.056 | ↓59% | 0.006 | 0.056 ± 0.076 | ↓69% | 0.010 | 20.2 ± 3.24 | NS |
| Depression | 0.046 ± 0.046 | ↓68% | 0.0023 | 0.045 ± 0.036 | ↓75% | 0.0025 | 21.6 ± 2.87 | NS |
| Schizophrenia | 0.022 ± 0.022 | ↓85% | 0.0001 | 0.026 ± 0.022 | ↓86% | 0.0003 | 20.1 ± 1.67 | NS |
RG, reference group; NS, not significant
Table 3.
Western blotting results for GABBR1, GABBR2, and β-actin in lateral cerebellum of subjects with schizophrenia, bipolar disorder, major depression vs. matched controls
| Group | GABBR1/β-Actin | Δ | P | GABBR2/β-Actin | Δ | P | β-Actin | P |
|---|---|---|---|---|---|---|---|---|
| Control | 0.119 ± 0.084 | RG | RG | 0.172 ± 0.129 | RG | RG | 24.9 ± 3.57 | RG |
| Bipolar | 0.046 ± 0.048 | ↓61% | 0.012 | 0.048 ± 0.070 | ↓72% | 0.0063 | 26.5 ± 3.11 | NS |
| Depression | 0.047 ± 0.058 | ↓61% | 0.023 | 0.042 ± 0.036 | ↓76% | 0.0020 | 25.3 ± 5.25 | NS |
| Schizophrenia | 0.025 ± 0.036 | ↓79% | 0.0007 | 0.021 ± 0.016 | ↓88% | 0.0001 | 25.2 ± 4.21 | NS |
RG, reference group; NS, not significant
3. Results
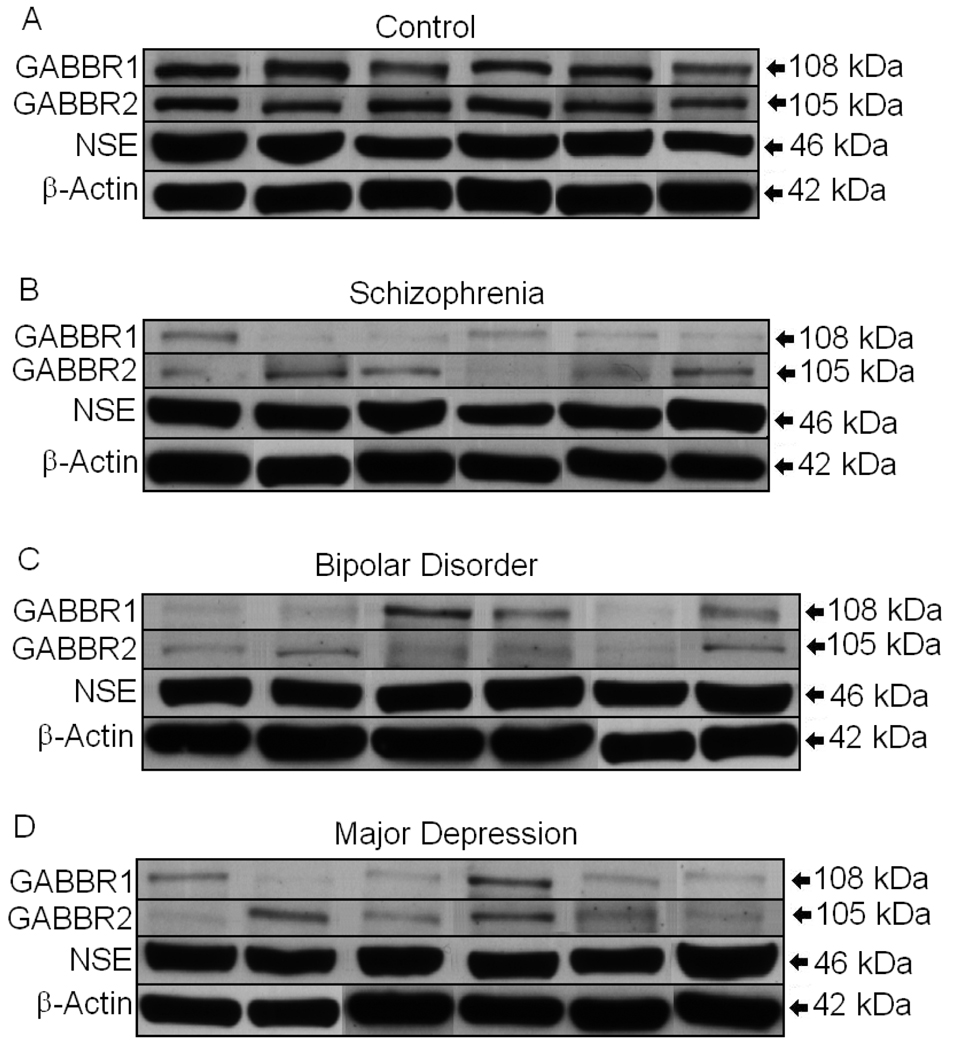
All protein measurements for GABBR1 and GABBR2 were normalized against two housekeeping proteins: neuronal specific enolase (NSE) and β-actin. Analyses of Variance identified group differences on GABBR1/β-Actin (F(3,48)=7.64, p<.001), GABBR1/NSE (F(3,48)=11.03, p<.001), GABBR2/β-Actin (F(3,48)=10.95, p<.001), and GABBR2/NSE (F(3,48)=9.90, p<.001). Individual t-tests were performed comparing control subjects vs. those with bipolar disorder, major depression, and schizophrenia. We observed a significant reduction in GABBR1 in subjects with bipolar disorder (p<0.006, GABBR1/NSE; p<0.012, GABBR1/β-actin), major depression (p<0.0023, GABBR1/NSE; p<0.023, GABBR1/β-actin), and schizophrenia (p<0.0001, GABBR1/NSE; p<0.0007, GABBR1/β-actin) when compared with controls (Figures 1 and 2; Tables 2 and 3). GABBR2 was also reduced in subjects with bipolar disorder (p<0.010, GABBR1/NSE; p<0.0063, GABBR1/β-actin), major depression (p<0.0025, GABBR1/NSE; p<0.0020, GABBR1/β-actin), and schizophrenia (p<0.0003, GABBR1/NSE; p<0.0001, GABBR1/β-actin) when compared with controls (Figures 1 and 2, Tables 2 and 3). There were no significant differences in protein levels of NSE and β-actin between controls and subjects with bipolar disorder, schizophrenia, or major depression (Figure 1, Tables 2 and 3), indicating that the decreased expression of GABBR1 and GABBR2 in cerebellum were not the result of differences in neuronal cell numbers between the four groups.
Figure 1.
GABBR1 and GABBR2 are reduced in lateral cerebella of subjects with schizophrenia (B), bipolar disorder (C), and major depression (D) when compared with healthy controls (A). NSE and β-actin are unchanged when compared across groups (A–D).
Figure 2.
Mean GABBR1/NSE (A), GABBR1/β-actin (B), GABBR2/NSE (C), and GABBR2/β-actin (D) ratios for control, bipolar, depressed, and schizophrenic subjects are shown for cerebellum. (Error bars expressed as standard error of the mean.) *, p<0.05.
No significant differences were found between groups on hemisphere side, ethnicity, gender, history of substance abuse or severity of substance abuse, post mortem interval (PMI), age, brain pH, or brain weight (Table 1). While PMI did not reach significance, the longer average PMIs for schizophrenia, bipolar disorder, and major depression when compared with controls could influence results adversely. We also compared the three diagnostic groups on family history, suicide, fluphenazine equivalents, and duration of disease and found no significant differences (Table 1). Age of onset was significantly later (32.7 years) for depressed compared to schizophrenics (23.2) and bipolar subjects (21.6), (F(2,39)=4.5, p=.017). Analyses of variance (ANOVAs), controlling for age of onset, found no effect on the protein levels of GABBR1 or GABBR2. Finally, we did find significant differences on tissue freezer time between controls (1072 days), depressed (1136 days), bipolar (1335 days) and schizophrenics (1351 days), (F(3,50)=4.93, p=.004). However, when ANOVAs were run with freezer time as a covariate, there was no impact on our results, indicating that freezer time had no impact on the observed reductions in GABBR1 and GABBR2 protein levels in the diagnostic groups.
4. Discussion
In the current study we found that there were significant reductions in GABBR1 and GABBR2 in lateral cerebellum of subjects with schizophrenia, bipolar disorder, and major depression. These results were specific for GABBR1 and GABBR2 as there were no changes in housekeeping genes β-actin or NSE. Analysis of confounds found no effect of any confound on protein data.
Consistent with our finding of reduced GABBR1 in cerebella, GABBR1 been shown to be reduced in other brain regions of subjects with schizophrenia (Ishikawa et al., 2005; Mizukami et al., 2000, 2002). Immunohistochemical studies have shown the reduction in GABBR1 immunoreactivity were specific to granular cells in the hippocampus (Mizukami et al., 2000) and pyramidal cells in the hippocampus, inferior temporal cortex, prefrontal cortex (PFC), and entorhinal cortex while there was no such reduction in interneurons (Ishikawa et al., 2005; Mizukami et al., 2000, 2002). Ishikawa et al., (2005) found no such reduction in GABBR2 immunoreactivity or protein levels in PFC of subjects with schizophrenia. The difference between GABBR1 and GABBR2 expression in this region may suggest differential expression of these subunits in PFC. Our finding of reduced GABBR2 protein in cerebella may point to regional differences in GABBR2 expression. Mizukami et al., (2002) suggested that the reduction of GABBR1 in pyramidal cells, and consequent reduction of GABAB receptors, could result in dysfunction of inhibitory mechanisms in these cells and increased signal output.
GABAergic dysfunction and consequent dysfunction of inhibitory mechanisms may underlie two of the most common forms of information processing deficits in schizophrenia, prepulse inhibition (PPI) and P50 suppression. DBA/2J mice, an animal model for schizophrenia, which display disrupted PPI also display decreased GABAB expression in both prefrontal cortex and hippocampus when compared with C57BL/6J controls (Bortolato et al., 2007). Moreover, the GABAB receptor agonist baclofen has been shown to correct PPI deficits in DBA/2J mice and in rats (Bortolato et al., 2004, 2007). Baclofen has been tested as a potential therapy for schizophrenia, but while an initial study showed that adjunctive baclofen treatment resulted in improvement of symptoms (Frederiksen, 1976), a subsequent study showed no efficacy (Bigelow et al., 1977). P50 suppression has been demonstrated to involve alpha-7 nicotinic receptor-mediated release of GABA (for a review, see Martin et al., 2004). The atypical antipsychotic drug clozapine significantly improves P50 gating in subjects with schizophrenia (Adler et al., 2004) and it has been suggested that clozapine’s efficacy may occur through the potentiation of GABAB-mediated inhibition (Daskalakis and George, 2009).
Compared with schizophrenia, there is less evidence of a link between GABAB receptors and bipolar disorder. Studies have found no differences in levels of GABA in brain of subjects with bipolar disorder, when compared with controls (Kaufman et al., 2009). Moreover, while recent research has demonstrated influence of GABAA receptors in bipolar disorder (Craddock et al., 2010), there have not been any studies implicating GABBR1 or GABBR2 in bipolar disorder. However, glutamic acid decarboxylase 65 kDa and 67 kDa proteins (GAD 65/67) are downregulated in cerebella of subjects with bipolar disorder, schizophrenia, and major depression (Fatemi et al., 2005) providing evidence of GABAergic dysfunction. Additionally there are reductions in GABBR1A, GABBR1B, and GABBR2 in prefrontal cortex of subjects with bipolar disorder as measured by western blotting (Ishikawa et al., 2005). Our finding of reduced GABBR1 and GABBR2 protein in cerebella of subjects with bipolar disorder provides further evidence of GABAergic dysfunction in bipolar disorder and supports previous findings by Ishikawa et al (2005) in the same disorder.
Altered expression of mRNA for GABBR1 and GABBR2 has been found in brains of subjects with major depression and victims of suicide (Klempan et al., 2009; Sequeira et al., 2009; Ghose et al., 2011) implicating GABAB receptor dysfunction in these disorders. A recent paper found that there was a reduction in mRNA for GABBR1A and an increase in GABBR2 mRNA in dentate gyrus while there were no changes in mRNA for GABBR1A, GABBR1B, or GABBR2 in cerebellum, cingulate cortex, or orbitofrontal cortex in subjects with major depression (Ghose et al., 2011). The discrepancy between this finding and our observation of reduced protein in cerebella of subjects with major depression may be due to several reasons including discordance between mRNA and protein levels, splicing events, and receptor downregulation in response to negative feedback loop causing increased mRNA as well as differences of measuring mRNA vs. protein. We have previously shown differences in mRNA and protein levels for GABBR1 in subjects with autism (Fatemi et al., 2009, 2010c). We found that while there was a concordant downregulation of mRNA and protein for GABBR1 in cerebellum, in parietal cortex there was a significant increase in mRNA despite significant reduction in protein levels (Fatemi et al., 2009, unpublished observations). Altered expression of GABAB receptor genes has been found in brain regions of suicide victims (Klempan et al., 2009; Sequeira et al., 2009). Importantly, Sequiera et al (2009) found that GABBR2 was upregulated in suicides who also had major depression when compared with non-depressed suicides.
Chronic administration of antidepressants has been shown to increase expression of GABAB receptors in brain of animal models of major depression (Nakagawa and Ishima, 2003; Pratt and Bowery, 1993). However, statistical analysis of our data showed lack of antidepressant effect on GABAB receptor levels in cerebellum of subjects with schizophrenia, bipolar disorder, or major depression (data not shown). GABAB receptor antagonists have been found to produce antidepressant properties in animal models, as have GABBR1 deletion studies (Nakagawa et al., 1999; Heese et al., 2000; Mombereau et al., 2005). Moreover, a study found that treatment of subjects with major depression with baclofen resulted in a worsening of symptoms (Post et al., 1991). These findings have led some to believe that an overactive GABA system contributes to major depression (Ghose et al., 2011). Further experiments are needed to determine the role of anti-depressants on GABAB receptor function.
Reports of cerebellar involvement in higher brain functions beyond motor control, such as cognition and emotion, go back several decades (reviewed by Konarski et al., 2005; Baldaçara et al., 2008). Patients with cerebellar degeneration were shown to display dementia and psychosis (Schut, 1950) and patients with cerebellar lesions have been shown to display a flattening of emotion, disinhibition of restraint, and passivity, conditions similar to what has been observed in manic and depressed states or in schizophrenia (Schmahmann and Sherman, 1998). Circuits connecting the cerebellum with regions of the prefrontal cortex associated with executive function, verbal memory and language have been identified (Schmahmann and Pandya, 1995) and disruption of prefrontal-thalamic-cerebellar circuitry has been hypothesized to result in cognitive deficits associated with schizophrenia (Andreasen et al., 1996). Reduction of cerebellar volumes have been reported in subjects with schizophrenia, bipolar disorder, and major depression (reviewed by Konarski et al., 2005; Baldaçara et al., 2008). Reduced cerebellar activation has been observed in functional imaging studies of subjects with schizophrenia (Crespo-Facorro et al., 2001, 2007), bipolar disorder (Loeber et al., 2002; Krüger et al., 2003) and major depressive disorder (Liotti et al., 2002; Smith et al., 2002). Particularly relevant to the current study, reduced activation of lateral cerebellum was observed in patients with schizophrenia on tasks that involved memory (Crespo-Facorro et al., 2001).
Our findings of reduced expression of GABBR1 and GABBR2 in lateral cerebellum provide further evidence of GABAergic dysfunction in schizophrenia, major depression, and bipolar disorder. Future studies need to examine additional brain regions such as hippocampus and prefrontal cortex. Additionally, GABAA receptors should be examined in the same brain regions to obtain a more complete picture of GABAergic dysfunction in schizophrenia and mood disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S. Hossein Fatemi, Email: fatem002@umn.edu.
Timothy D. Folsom, Email: folso013@umn.edu.
Paul D. Thuras, Email: pthuras@yahoo.com.
References
- Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, Waldo MC, Hall MH, Bowles A, Woodward L, Ross RG, Freedman R. Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am. J. Psychiatry. 2004;161(10):1822–1828. doi: 10.1176/ajp.161.10.1822. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington DC: American Psychiatric Publishing, Inc.; 1994. [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc. Natl. Acad. Sci. U.S.A. 1996;93(18):9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldaçara L, Borgio JG, Lacerda AL, Jackowski AP. Cerebellum and psychiatric disorders. Rev. Bras. Psiquiatr. 2008;30(3):281–289. doi: 10.1590/s1516-44462008000300016. [DOI] [PubMed] [Google Scholar]
- Bigelow LB, Nasrallah N, Carman J, Gillin JC, Wyatt RJ. Baclofen treatment in chronic schizophrenia: a clinical trial. Am. J. Psychiatry. 1977;134:318–320. doi: 10.1176/ajp.134.3.318. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Aru GN, Orrù M, Gessa GL. Baclofen reverses the reduction in prepulse inhibition of the acoustic startle response induced by dizoclipine, but not apomorphine. Psychopharmacology (Berl.) 2004;171(3):322–330. doi: 10.1007/s00213-003-1589-5. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Piras AP, Fà M, Tuveri A, Puligheddu M, Gessa GL, Castelli MP, Mereu G, Marrosu F. Activation of GABA(B) receptors reverses spontaneous gating deficits in juvenile DBA/2J mice. Psychopharmacology (Berl.) 2007;194(3):361–369. doi: 10.1007/s00213-007-0845-5. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptors structure and function. In: Martin DL, Olsen RW, editors. GABA in the nervous system: the view at fifty years. Philadelphia, PA: Lippincott, Williams and Wilkins; 2000. pp. 233–244. [Google Scholar]
- Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, Moskvina V, Nikolov I, Hamshere ML, Vukcevic D, Caesar S, Gordon-Smith K, Fraser C, Russell E, Norton N, Breen G, St Clair D, Collier DA, Young AH, Ferrier IN, Farmer A, McGuffin P, Holmans PA Wellcome Trust Case Control Consortium (WTCCC) Donnelly P, Owen MJ, O'Donovan MC. Strong genetic evidence for selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype. Mol. Psychiatry. 2010;15(2):146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Wiser AK, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Neural basis of novel and well-learned recognition memory in schizophrenia: a positron emission tomography study. Hum. Brain Mapp. 2001;12(4):219–231. doi: 10.1002/1097-0193(200104)12:4<219::AID-HBM1017>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Barbadillo L, Pelayo-Terán JM, Rodríguez-Sánchez JM. Neuropsychological functioning and brain structure in schizophrenia. Int. Rev. Psychiatry. 2007;19(4):325–336. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, George TP. Clozapine, GABAB, and the treatment of resistant schizophrenia. Clin. Pharmacol. Ther. 2009;86(4):442–446. doi: 10.1038/clpt.2009.115. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr. Res. 2005;72(2–3):109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, Fan YT, Paciga SA, Conti M, Menniti FS. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr. Res. 2008;101(1–3):36–49. doi: 10.1016/j.schres.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) receptors is altered in brains of Subjects with autism. Cerebellum. 2009;8:64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Vazquez G. Phosphodiesterase signaling system is disrupted in cerebella of subjects with schizophrenia, bipolar disorder, and major depression. Schizophr. Res. 2010a;119(1–3):266–267. doi: 10.1016/j.schres.2010.02.1055. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Kneeland RE, Liesch SB, Folsom TD. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr. Res. 2010b;124(1–3):246–247. doi: 10.1016/j.schres.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAalpha4, alpha5, beta1, and GABABR1 receptors are altered in brains of subjects with autism. J. Autism Dev. Disord. 2010c;40(6):743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen PK. Baclofen in schizophrenia therapy-preliminary note. Lakartidningen. 1975;72:456–458. [PubMed] [Google Scholar]
- Ghose S, Winter MK, McCarson KE, Tamminga CA, Enna SJ. The GABAB receptor as a target for antidepressant drug action. Br. J. Pharmacol. 2011;162(1):1–17. doi: 10.1111/j.1476-5381.2010.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese K, Otten U, Mathivet P, Raiteri M, Marescaux C, Bernasconi R. GABAB receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology. 2000;39(3):449–462. doi: 10.1016/s0028-3908(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Imai K, Harada S, Kawanishi Y, Tachikawa H, Okubo T, Asada T. Association analysis of an (AC)n repeat polymorphism in the GABA(B) receptor gene and schizophrenia. Am. J. Med. Genet. 2002;114(6):605–608. doi: 10.1002/ajmg.10605. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Asada T. Immunohistochemical and immunoblot analysis of gamma-aminobutyric acid B receptor in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci. Lett. 2005;383(3):272–277. doi: 10.1016/j.neulet.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, Renshaw PF, Pollack MH. Brain GABA levels in patients with bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(3):427–434. doi: 10.1016/j.pnpbp.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386(6622):239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol. Psychiatry. 2009;14(2):175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J. Psychiatry Neurosci. 2005;30(3):178–186. [PMC free article] [PubMed] [Google Scholar]
- Krüger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol. Psychiatry. 2003;54(11):1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- Lindholm E, Ekholm B, Balciuniene J, Johansson G, Castensson A, Koisti M, Nylander PO, Pettersson U, Adolfsson R, Jazin E. Linkage analysis of a large Swedish kindred provides further support for a susceptibility locus for schizophrenia on chromosome 6p23. Am. J. Med. Genet. 1999;88(4):369–377. [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am. J. Psychiatry. 2002;159(11):1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Gruber SA, Cohen BM, Renshaw PF, Sherwood AR, Yurgelun-Todd DA. Cerebellar blood volume in bipolar patients correlates with medication. Biol. Psychiatry. 2002;51(5):370–376. doi: 10.1016/s0006-3223(01)01281-1. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl.) 2005;174(1):54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Mizukami K, Sasaki M, Ishikawa M, Iwakiri M, Hidaka S, Shiraishi H, Iritani S. Immunohistochemical localization of the gamma-aminobutyric acid(B) receptor in the hippocampus of subjects with schizophrenia. Neurosci. Lett. 2000;283(2):101–104. doi: 10.1016/s0304-3940(00)00939-3. [DOI] [PubMed] [Google Scholar]
- Mizukami K, Ishikawa M, Hidaka S, Iwakiri M, Sasaki M, Iritani S. Immunohistochemical localization of the GABAB receptor in the entorhinal cortex and inferior temporal cortex of schizophrenic brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(2):393–396. doi: 10.1016/s0278-5846(01)00247-0. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Gassmann M, Bettler B, van der Putten H, Cryan JF. Altered anxiety and depression-related behavior in mice lacking GABAB(2) receptor subunits. Neuroreport. 2005;16(3):307–310. doi: 10.1097/00001756-200502280-00021. [DOI] [PubMed] [Google Scholar]
- Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Sasaki A, Takashima T. The GABAB receptor antagonist CG36742 improves learned helplessness in rats. Eur. J. Pharmacol. 1999;381:1–7. doi: 10.1016/s0014-2999(99)00567-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Ishima T. Possible involvement of GABAB receptors in action of antidepressants. Nihon Shinkei Seishin Yakurigaku Zasshi. 2003;23:83–89. [PubMed] [Google Scholar]
- Nyitrai G, Kékesi KA, Emri Z, Szárics E, Juhász G, Kardos J. GABA(B) receptor antagonist CGP-36742 enhances somatostatin release in rat hippocampus in vivo and in vitro. Eur. J. Pharmacol. 2003;478(2–3):111–119. doi: 10.1016/j.ejphar.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Decreased GABA(B) receptors in the cingulate cortex and fusiform gyrus in autism. J. Neurochem. 2010;114(5):1414–1423. doi: 10.1111/j.1471-4159.2010.06858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R, Homanics G. Function of GABAA receptors; insights from mutant and knockout mice. In: Martin D, Olsen R, editors. GABA in the nervous system: the view at fifty years. Philadelphia, PA: Lipincott Williams and Wilkins; 2000. pp. 81–96. [Google Scholar]
- Petryshen TL, Middleton FA, Tahl AR, Rockwell GN, Purcell S, Aldinger KA, Kirby A, Morley CP, McGann L, Gentile KL, Waggoner SG, Medeiros HM, Carvalho C, Macedo A, Albus M, Maier W, Trixler M, Eichhammer P, Schwab SG, Wildenauer DB, Azevedo MH, Pato MT, Pato CN, Daly MJ, Sklar P. Genetic investigation of chromosome 5q GABA receptor subunit genes in schizophrenia. Mol. Psychiatry. 2005;10(12):1074–1088. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- Post RM, Ketter TA, Joffe RT, Kramlinger KL. Lack of beneficial effects of 1-baclofen in affective disorder. Int. Clin. Psychopharmacol. 1991;6(4):197–207. doi: 10.1097/00004850-199100640-00001. [DOI] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG. Repeated administration of desipramine and a GABAB receptor antagonist, CGP 36742, discretely up-regulates GABAB receptor binding sites in rat frontal cortex. Brit. J. Pharmacol. 1993;110:724–735. doi: 10.1111/j.1476-5381.1993.tb13872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamaki K, Nomura M, Hatakenaka S, Miyakubo H, Tanaka J. GABAergic modulation of noradrenaline release in the median preoptic nucleus area in the rat. Neurosci. Lett. 2003;342(1–2):77–80. doi: 10.1016/s0304-3940(03)00242-8. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. In: Schmahmann JD, editor. The cerebellum and cognition. San Diego (CA): Academic Press; 1997. pp. 31–60. [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schut JW. Hereditary ataxia. Arch. Neurol. Psychiatry. 1950;63:535–568. [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4(8):e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Ploghaus A, Cowen PJ, McCleery JM, Goodwin GM, Smith S, Tracey I, Matthews PM. Cerebellar responses during anticipation of noxious stimuli in subjects recovered from depression. Functional magnetic resonance imaging study. Br. J. Psychiatry. 2002;181:411–415. doi: 10.1192/bjp.181.5.411. [DOI] [PubMed] [Google Scholar]
- Steiniger B, Kretschmer BD. Glutamate and GABA modulate dopamine in the pedunculopontine tegmental nucleus. Exp. Brain Res. 2003;149(4):422–430. doi: 10.1007/s00221-003-1382-z. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABA(B) receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J. Neurosci. 2010;30(35):11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmeier PC, Kaupmann K, Urwyler S. Roles of GABAB receptor subtypes in presynaptic and auto- and heteroreceptor function regulating GABA and glutamate release. J. Neural. Transm. 2008;115(10):1401–1411. doi: 10.1007/s00702-008-0095-7. [DOI] [PubMed] [Google Scholar]
- Zai G, King N, Wong GW, Barr CL, Kennedy JL. Possible association between the gamma-aminobutyric acid type B receptor 1 (GABBR1) gene and schizophrenia. Eur. Neuropsychopharmacol. 2005;15:347–352. doi: 10.1016/j.euroneuro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Zhao X, Qin S, Shi Y, Zhang A, Zhang J, Bian L, Wan C, Feng G, Gu N, Zhang G, He G, He L. Systematic study of four GABAergic genes: glutamic acid decarboxylase 1 gene, glutamic acid decarboxylase 2 gene, GABA(B) receptor 1 gene and GABA(A) receptor subunit beta 1 gene, with schizophrenia using a universal DNA microarray. Schizophr. Res. 2007;93(1–3):374–384. doi: 10.1016/j.schres.2007.02.023. [DOI] [PubMed] [Google Scholar]