Abstract
Human carcinomas can metabolically incorporate and present the dietary non-human sialic acid Neu5Gc, which differs from the human sialic acid N-acetylneuraminic acid (Neu5Ac) by one oxygen atom. Tumor-associated Neu5Gc can interact with low levels of circulating anti-Neu5Gc antibodies, thereby facilitating tumor progression via chronic inflammation in a human-like Neu5Gc-deficient mouse model. Here we show that human anti-Neu5Gc antibodies can be affinity-purified in substantial amounts from clinically-approved intravenous IgG (IVIG) and used at higher concentrations to suppress growth of the same Neu5Gc-expressing tumors. Hypothesizing that this polyclonal spectrum of human anti-Neu5Gc antibodies also includes potential cancer biomarkers, we then characterize them in cancer and non-cancer patients’ sera, using a novel sialoglycan-microarray presenting multiple Neu5Gc-glycans and control Neu5Ac-glycans. Antibodies against Neu5Gcα2–6GalNAcα1-O-Ser/Thr (GcSTn) were found to be more prominent in patients with carcinomas than with other diseases. This unusual epitope arises from dietary Neu5Gc incorporation into the carcinoma marker Sialyl-Tn, and is the first example of such a novel mechanism for biomarker generation. Finally, human serum or purified antibodies rich in anti-GcSTn-reactivity kill GcSTn-expressing human tumors via complement-dependent-cytotoxicity or antibody-dependent-cellular-cytotoxicity. Such xeno-autoantibodies and xenoautoantigens have potential for novel diagnostics, prognostics and therapeutics in human carcinomas.
Keywords: Antibodies, Biomarkers, Cancer, Neu5Gc, Sialic acids
Introduction
Altered glycosylation is common in cancer (1). One relatively tumor-specific alteration is metabolic incorporation of diet-derived N-glycolylneuraminic acid (Neu5Gc) into human cancers (2–5). N-acetylneuraminic acid (Neu5Ac) and its hydroxylated form, Neu5Gc, are the two major Sias on mammalian cell surfaces. Humans cannot synthesize Neu5Gc due to an inactive CMP-Neu5Ac hydroxylase (CMAH) (6), and lack of an alternate synthetic pathway (7). However, consumption of Neu5Gc-rich foods (particularly red meats) leads to foreign Neu5Gc incorporation into human tissue cell surfaces, especially carcinomas (2, 3), generating “xeno-autoantigens”. The humoral response against various Neu5Gc glycans shows a diverse polyclonal profile of xeno-autoantibodies in all normal human sera (5), likely induced via dietary-Neu5Gc uptake by commensal bacteria (8). Circulating anti-Neu5Gc antibodies interact with Neu5Gc-positive tumors to generate chronic inflammation and facilitate tumor progression in a mouse model of human-like Neu5Gc-deficiency (9). This is keeping with previous reports of antibody-mediated tumor stimulation via chronic inflammation in other systems (10, 11). Of course, anti-tumor antibodies are also reported as cancer therapeutics (12–14). These opposing findings can be potentially reconciled by Prehn’s hypothesis of a biphasic dose-dependent response of tumors to immune reactants (15).
Epithelial cancers (carcinomas) cause significant mortality and morbidity and survival rates improve with early diagnosis. Indeed, physical cancer screening methods have reduced mortality (16, 17), encouraging further early detection biomarker research, and guiding co-development of targeted therapies, e.g., trastuzumab (Herceptin) developed to target the biomarker HER-2/neu (12, 18). Most current biomarkers, including autoantibodies against tumor-associated antigens that appear at an early stage, lack sufficient sensitivity and specificity for early cancer diagnosis (18–22). Common approaches for biomarker discovery include global genomics, proteomics, and more recently glycomics, seeking malignancy-associated differentially-expressed targets (23).
We reasoned that Neu5Gc consumption by cancer patients could metabolically replace Neu5Ac by Neu5Gc, generating glycan xeno-autoantigens. While the corresponding xeno-autoantibodies could be involved in tumor stimulation via chronic inflammation (9), we hypothesized that they could also be novel and unique tumor biomarkers and immunotherapeutics, in line with the Prehn hypothesis of dualistic effects (15). Here we affinity-purify human anti-Neu5Gc antibodies from IVIG and use them to treat Neu5Gc-expressing tumors in vivo. Using a novel high-throughput sialoglycan-microarray containing multiple Neu5Gc-glycans with control Neu5Ac-matched glycans, we then show that human serum antibodies against Neu5Gc-sialyl-Tn (GcSTn; Neu5Gcα2–6GalNAcα1-O-Ser/Thr) are enriched in carcinoma patients over controls. Furthermore, human serum or purified antibodies with anti-GcSTn-reactivity can kill GcSTn-positive human tumor cells.
Materials and Methods
Affinity purification of anti-Neu5Gc antibodies from IVIG
Anti-Neu5Gc antibodies were purified from IVIG (GAMMAGARD LIQUID, CA) on sequential affinity columns with immobilized human or chimpanzee serum sialoglycoproteins, as described (5; chimpanzee serum obtained from Yerkes National Primate Research Center, Emory University, GA). Aliquots of IVIG diluted 1:3 in PBS were pre-cleared through a column of immobilized human serum sialoglycoproteins (Neu5Ac-containing), and the flow-through applied to a column of immobilized chimpanzee serum sialoglycoproteins (Neu5Gc-containing), columns differing primarily in the single oxygen atom that differentiates Neu5Gc from Neu5Ac (Fig. 1A). Bound antibodies were sequentially eluted with 5 mM glucuronic acid in PBS (removing non-specific charge-related binding), 0.5 mM 2-O-methyl-α-Neu5Gc (Neu5Gc2Me) in PBS, 2 mM Neu5Gc2Me in PBS, and a weak acid (0.1 M citric acid pH3), with collected fractions showing anti-Neu5Gc reactivity only when eluted by Neu5Gc2Me (Fig. 1B; Neu5Gc2Me maintains the Sia ring-form in the α-D-Sia anomer (5)). Elution with 10-fold higher concentrations of free Neu5Gc (5 and 20 mM, respectively) gave similar results (data not shown).
Figure 1. Anti-Neu5Gc IgG antibodies can be affinity-purified from IVIG.
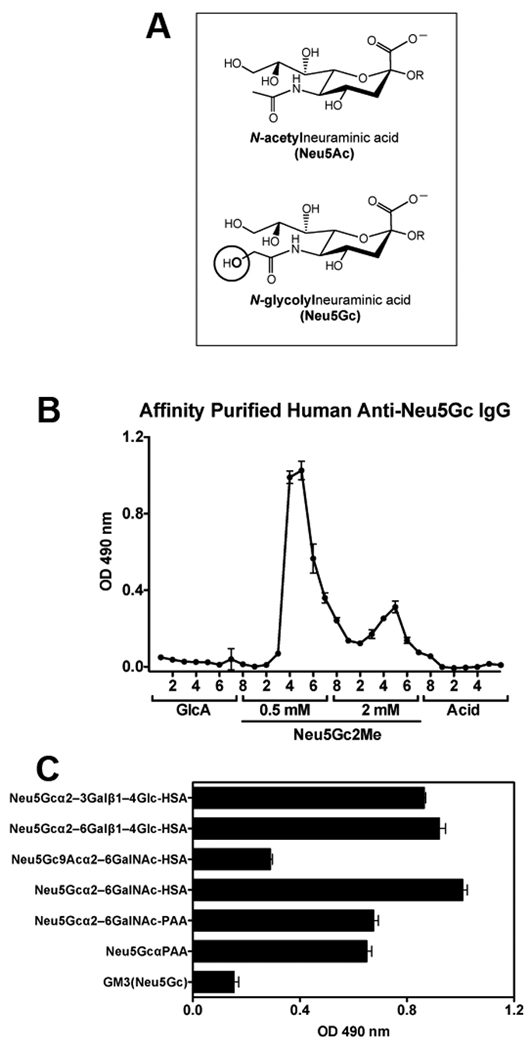
(A) Diagram of Neu5Ac and Neu5Gc. (B) Anti-Neu5Gc antibodies were affinity-purified from IVIG over sequential columns of immobilized human and chimpanzee serum sialoglycoproteins, fractions collected and analyzed by ELISA against Neu5Gcα-PAA (mean±SD of triplicates; representative of multiple independent experiments). (C) Eluted fractions were pooled, concentrated, filtered from free glycans, and analyzed by ELISA against multiple Neu5Gc-glycans (mean±SD; representative of two independent experiments).
Minimizing cross-species reactivity in tumor experiments
To avoid off-target effect of human antibodies in the experimental mice, we affinity-purified IVIG over mice sera (negative for anti-human antibodies reactivity) instead of human/chimpanzee-sera (Fig. S1). Immobilized sera sialoglycoproteins from the ‘human-like’ Cmah−/− was the pre-clearance column (removes reactivity against Neu5Ac and human-anti-mouse serum) followed by immobilized wild-type C57BL/6 sera (Neu5Gc-containing); the only major difference between these columns is Neu5Ac vs Neu5Gc (Fig. 1A). Bound antibodies were sequentially eluted with 5 mM glucuronic acid in PBS, 20 mM Neu5Gc in PBS and a weak acid, resulting in high-affinity antibodies in the Neu5Gc-eluted fractions. These were pooled, concentrated and Neu5Gc removed using 10K centrifugal-filters (Millipore).
Cell lines
Mouse colon adenocarcinoma cell line MC38 (syngeneic to C57BL/6 background) obtained from J. Schlom (National Cancer Institute, Bethesda, MD), were cultured at 37°C with 5% CO2 in DMEM with 10% FCS. All media and additives were from Life Technologies (Invitrogen), except for FCS (HyClone). Jurkat T cell leukemia clone E6.1 cells from the American Type Culture Collection were cultured in RPMI medium 1640 supplemented with 10% FCS. Both cell lines were passaged for less than 6 months, and their growth and morphology monitored by microscopy.
shRNA for CMAH
Silencing cassettes (24) consisting of an RNA polymerase III promoter (H1) expressing short hairpin RNAs for mouse CMAH (ACCESSION NM_007717) or an irrelevant control were generated for the following targets: siV1: 5'TGAGTTACCCTACCCTGA3', siV2: 5'GAAAGCTTCTGAATTACAA3' siV3: 5'CCATAACTACCATTATTCA3', siIRR: 5'CTAACACTGGGTTATACAA3'. Infectious lentiviral vector particles were produced as described (25). MC38 cells were transduced with these lenti-siRNAs viruses generating MC38si cell-lines. Long-term downregulation of CMAH was validated by qPCR using Power SYBER Green PCR Master Mix (Applied Biosystems) and CMAH–specific primer sets: Forward: 5'ATGGCAACAGGTAGACAAAAGTC3'; Reverse: 5'CACCTCCTGCGAAATCACTCA3' and cell surface expression of the end product of the CMAH enzyme, Neu5Gc, by FACS analysis (Fig. 2A–B). Cells were sorted for GFP positivity to ensure uniformity. Clone siV2 was selected for further experiments.
Figure 2. Affinity-purified anti-Neu5Gc IgG antibodies can specifically kill tumors expressing cell-surface Neu5Gc in vivo.
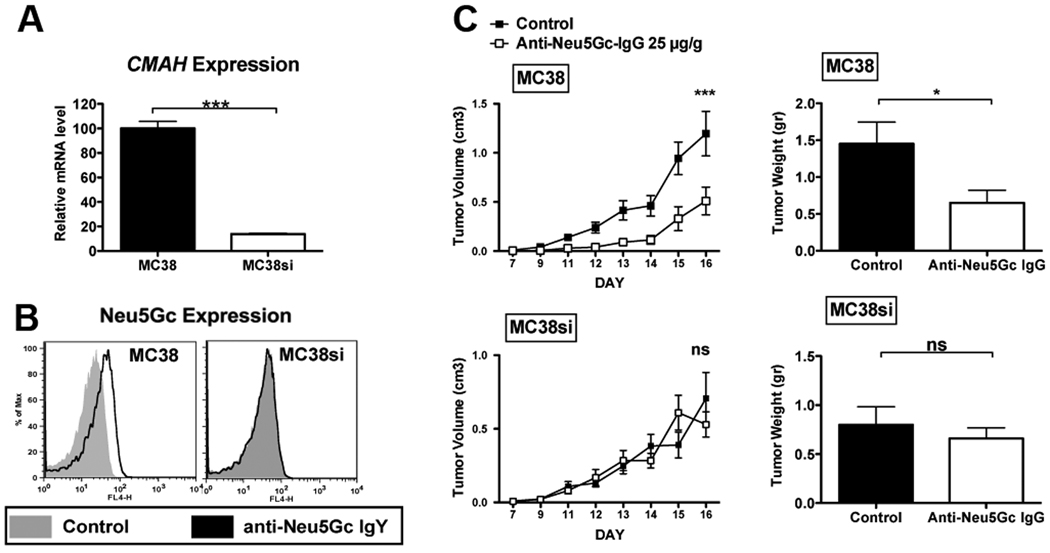
(A) qPCR reveals down regulation of CMAH gene expression in MC38 cells with siRNA to CMAH (MC38si) compared to the wild-type MC38 cells (mean±SD of triplicates; Two tailed unpaired t-test P<0.0001). (B) FACS analysis using a polyclonal chicken anti-Neu5Gc antibody confirms reduced expression of Neu5Gc on the cell surface (representative of two independent experiments). (C) Cmah−/− mice were injected subcutaneously with MC38 (right flank) and MC38si (left flank). Affinity-purified anti-Neu5Gc IgG can specifically kill tumors expressing Neu5Gc on the cell surface (MC38) (n=6) compared to the control-treated mice (n=5), but no significant effect is observed when the Neu5Gc expression on the cell surface is diminished (MC38si), as determined by daily measurements of tumor volumes (left panels; mean±SEM; Two-way ANOVA P<0.001) or terminal tumor weights on day 16 (right panels; mean±SEM; Two-tailed t-test * p=0.036), representative of 3 independent experiments. Direct comparison of the effects of anti-Neu5Gc IgG on these tumors in the same mice revealed attenuated MC38 tumors compared to the control MC38si tumors (P=0.0392, Two-way-ANOVA).
Mice and Experimental Tumor Growth Assays
Cmah−/− mice (7) were bred in a congenic C57BL/6 background and maintained according to Institutional Animal Care and Use Committee guidelines for laboratory animals. To avoid uncontrolled clearance of the human antibodies, only mice free of mouse-anti-human IgG antibodies (as determined by ELISA) were used.
Before use, MC38 or MC38si cells were released by incubation in PBS with 2 mM EDTA at 37°C for 10 min, and washed in PBS with Ca2+, Mg2+ and glucose before suspending in the same buffer for subcutaneous (s.c.) injection. Mice were injected s.c. in the flank with 1×106 MC38 cells (right flank) and MC38si (left flank). These mice were divided into two groups and on day 5, affinity-purified human anti-Neu5Gc antibodies or vehicle control (PBS) were injected i.p. at 25 µg/g weight (n=5 and n=6, respectively). Tumor growth was monitored and measured daily (tumor volume was calculated by the formula 0.5*x*y*z). Next, tumors were removed from the flank and weighed.
Feeding of human cell lines
To deplete any remaining Neu5Gc from FCS, the Jurkat T cells were split and cultured (before feeding experiments) for at least 4 days in RPMI medium 1640 supplemented with 5% heat-inactivated human serum (RPMI/5HuS) instead of FCS, resulting in chase-out of all existing Neu5Gc. Subsequently, cells were fed with 3 mM Neu5Ac/Neu5Gc in RPMI/5HuS (26).
Peripheral Blood Mononuclear Cell (PBMC) Isolation
Studies were pre-approved by the institutional review board of University of California San-Diego. Healthy human donor PBMCs were isolated using Vacutainer CPT tubes (Becton Dickinson), washed extensively with PBS to remove intrinsic human antibodies and re-suspended in culture medium RPMI 1640.
CDC and ADCC assays
CDC and ADCC were evaluated by measuring lactate dehydrogenase (LDH) release using LDH Cytotoxicity Detection kit (Roche Applied Science) according to the manufacturer's instructions. All assays (3 hours at 37°C) included maximum release controls (1% T r i t o n X-100) and % cytotoxicity was calculated as: (test release – spontaneous release) / (maximum release – spontaneous release) × 100. For CDC: Target cells (T; Jurkat cell fed with Neu5Ac/Neu5Gc) were washed extensively with PBS to remove residual human antibodies from the culture media, then plated (in triplicates) at 2×104 cells/well in 96-well round-bottom plates and supplemented with heat inactivated (H.I.) human serum S34 (10%/well or 1%/well in RPMI) or purified anti-Neu5Gc antibodies (40 µg/ml/well or 20 µg/ml/well diluted in 1%/well H.I. human serum S30 in RPMI that has low levels of anti-Neu5Gc antibodies (5)) and incubated at room temperature for 30 min. Then complement was added (10%/well fresh human serum S30 in RPMI). The plates were incubated for 3 hours at 37°C, then supernatants were transferred to a 96-well flat-bottomed plates and LDH release was determined. For ADCC: washed Target cells (T; Jurkat cells fed with Neu5Ac/Neu5Gc) were plated at 2×104 cells/well in a 96-well round-bottom plate and supplemented with 10% H.I. human sera S34, S30 or S30 containing purified 10 µg/ml/well anti-Neu5Gc IgG and incubated at room temperature for 30 min. Then, effector (E) PBMCs in RPMI were added at various E:T ratios, incubated for 3h at 37°C, then supernatants were collected and LDH release was determined.
Serum samples for glycan-microarray assays
A total of 386 cancer cases and control human sera were studied as described in Table S1, with approval from the Institutional Review Board of the University of California, San Diego. Written, informed consent was obtained in advance. We tested sera from 175 breast cancer patients and other types of carcinomas including prostate (39), ovary (29), lung (14), colon (22), pancreas (16), endometrium (11), as well as controls (80) matched for gender and, as possible, for age. Sera were tested on glycan-microarray and analyzed while blinded to the case/control status of the samples.
Sialoglycan-microarray
20 Sialoglycans pairs (Neu5Ac versus Neu5Gc; Table 1) were synthesized as described (27–29) and printed on Epoxide slides (Thermo Fisher Scientific, Corning, Pittsburgh, PA) in 250, 125, 62.5 and 12.5 µM at 4 replicates each in an optimized print buffer (300 mM phosphate buffer, pH 8.4), and sera binding to arrays tested and analyzed as detailed in supplemental methods.
Table 1.
List of glycans studied on the slide-microarray.
| Glycan Type |
O-Acetylation Status |
Glycan No. | Compound |
|---|---|---|---|
| Ac | 9OAc | 1 | Neu5,9Ac2α2–3Galβ1–4GlcNAcβProNH2 |
| Gc | 9OAc | 2 | Neu5Gc9Acα2–3Galβ1–4GlcNAcβProNH2 |
| Ac | 9OAc | 3 | Neu5,9Ac2α2–6Galβ1–4GlcNAcβProNH2 |
| Gc | 9OAc | 4 | Neu5Gc9Acα2–6Galβ1–4GlcNAcβProNH2 |
| Ac | -- | 5 | Neu5Acα2–6GalNAcαProNH2 |
| Gc | -- | 6 | Neu5Gcα2–6GalNAcαProNH2 |
| Ac | 9OAc | 7 | Neu5,9Ac2α2–3–Galβ1–3GlcNAcβProNH2 |
| Gc | 9OAc | 8 | Neu5Gc9Acα2–3Galβ1–3GlcNAcβProNH2 |
| Ac | 9OAc | 9 | Neu5,9Ac2α2–3Galβ1–3GalNAcαProNH2 |
| Gc | 9OAc | 10 | Neu5Gc9Acα2–3Galβ1–3GalNAcαProNH2 |
| Ac | -- | 11 | Neu5Acα2–3Galβ1–4GlcNAcβProNH2 |
| Gc | -- | 12 | Neu5Gcα2–3Galβ1–4GlcNAcβProNH2 |
| Ac | -- | 13 | Neu5Acα2–3Galβ1–3GlcNAcβProNH2 |
| Gc | -- | 14 | Neu5Gcα2–3Galβ1–3GlcNAcβProNH2 |
| Ac | -- | 15 | Neu5Acα2–3Galβ1–3GalNAcαProNH2 |
| Gc | -- | 16 | Neu5Gcα2–3Galβ1–3GalNAcαProNH2 |
| Ac | -- | 17 | Neu5Acα2–6Galβ1–4GlcNAcβProNH2 |
| Gc | -- | 18 | Neu5Gcα2–6Galβ1–4GlcNAcβProNH2 |
| Ac | -- | 19 | Neu5Acα2–6Galβ1–4GlcβProNH2 |
| Gc | -- | 20 | Neu5Gcα2–6Galβ1–4GlcβProNH2 |
| Ac | -- | 21 | Neu5Acα2–3Galβ1–4GlcβProNH2 |
| Gc | -- | 22 | Neu5Gcα2–3Galβ1–4GlcβProNH2 |
| Ac | 9OAc | 23 | Neu5,9Ac2α2–6GalNAcαProNH2 |
| Gc | 9OAc | 24 | Neu5Gc9Acα2–6GalNAcαProNH2 |
| Ac | -- | 25 | Neu5Acα2–3GalβProNH2 |
| Gc | -- | 26 | Neu5Gcα2–3GalβProNH2 |
| Ac | -- | 27 | Neu5Acα2–6GalβProNH2 |
| Gc | -- | 28 | Neu5Gcα2–6GalβProNH2 |
| Ac | 9OAc | 29 | Neu5,9Ac2α2–3GalβProNH2 |
| Gc | 9OAc | 30 | Neu5Gc9Acα2–3GalβProNH2 |
| Ac | 9OAc | 31 | Neu5,9Ac2α2–6GalβProNH2 |
| Gc | 9OAc | 32 | Neu5Gc9Acα2–6GalβProNH2 |
| Ac | -- | 33 | Neu5Acα2–3Galβ1–3GalNAcβProNH2 |
| Gc | -- | 34 | Neu5Gcα2–3Galβ1–3GalNAcβProNH2 |
| Ac | 9OAc | 35 | Neu5,9Ac2α2–3Galβ1–3GalNAcβProNH2 |
| Gc | 9OAc | 36 | Neu5Gc9Acα2–3Galβ1–3GalNAcβProNH2 |
| Ac | 9OAc | 37 | Neu5,9Ac2α2–6Galβ1–4GlcβProNH2 |
| Gc | 9OAc | 38 | Neu5Gc9Acα2–6Galβ1–4GlcβProNH2 |
| Ac | 9OAc | 39 | Neu5,9Ac2α2–3Galβ1–4GlcβProNH2 |
| Gc | 9OAc | 40 | Neu5Gc9Acα2–3Galβ1–4GlcβProNH2 |
Twenty Glycan pairs that differ by a single oxygen atom were synthesized and printed on epoxy-coated slides. Glycans are numbered according to terminal Sia: odd numbers indicate Neu5Ac (Ac) and even numbers are Neu5Gc (Gc). ProNH2 = O(CH2)2CH2NH2.
General statistical analyses
Statistical analyses (described in context) were performed using GraphPad Prism 5.0, with p values < 0.05 considered significant. Array statistical analysis is detailed in supplemental methods.
Results
Anti-Neu5Gc IgG can be affinity-purified from IVIG
Individual human serum samples show variable levels of anti-Neu5Gc antibodies against several Neu5Gc-epitopes (5). To better assess their prevalence in the human population, we examined IVIG, a clinically used human IgG purified from pooled-plasma of thousands of donors (30). Aliquots of IVIG were used to affinity purify anti-Neu5Gc IgG using columns differing primarily in the single oxygen atom that differentiates Neu5Gc from Neu5Ac (Fig. 1A; as detailed in Materials and Methods). Bound antibodies were sequentially eluted with increasing concentrations of 2-O-methyl-α-Neu5Gc (Neu5Gc2Me) resulting in low-affinity and high-affinity anti-Neu5Gc IgG (Fig. 1B). The overall yield was ~0.075% (0.76 ± 0.29 mg per gram IgG loaded), which is in the range described for some individual serum titers (5). These affinity-purified antibodies recognized multiple Neu5Gc-glycans (Fig. 1C). We achieved similar purifications using sequential columns of immobilized Cmah−/− or WT mouse serum sialoglycoproteins (Fig. S1).
Human Anti-Neu5Gc IgG attenuates Neu5Gc-expressing tumor outgrowth in vivo
As Neu5Gc is expressed on many human carcinomas (2, 3), it is a potential target for immunotherapy (4). To address this in vivo, we exploited the ‘human-like’ Cmah−/− mouse model, carrying the syngeneic murine carcinoma MC38 tumors that naturally express Neu5Gc at low levels, similar to human tumors (9). Tumor-engrafted mice were injected with affinity-purified human anti-Neu5Gc IgG (purified on Cmah−/− and WT mouse serum glycoproteins to minimize cross-species reactivity; Fig. S1). As a control, we also generated MC38si cells, in which a lentiviral vector coding a CMAH siRNA was stably integrated in MC38 cells, generating ~80% inhibition of CMAH mRNA (Fig. 2A) and reduced expression of Neu5Gc in those cells, especially on the cell-surface (Fig. 2B).
Cmah−/− mice were injected subcutaneously with MC38 (right flank) and MC38si (left flank), and affinity-purified human anti-Neu5Gc IgG or vehicle control were injected intraperitoneally on day 5 after tumors were established (documented histologically, data not shown). The dose of 25 µg/g body weight would result in 125.4 ± 5.2 ng/µl circulating human IgG with residual of 53 ± 1.6 ng/µl by day 16 (mean±SEM; Fig. S2A). Tumor growth was measured daily, and on day 16 tumors were removed and weighed. Compared to control-treated tumors, smaller tumors developed in anti-Neu5Gc IgG-treated MC38 tumors (expressing surface Neu5Gc), yet not in MC38si tumors (diminished surface Neu5Gc), as confirmed by reduced tumor volumes and terminal weights (Fig. 2C). This attenuated tumor growth was antibody-dependent and specific to Neu5Gc, as direct comparison of the effects of anti-Neu5Gc IgG on these tumors in the same mice revealed attenuated MC38 tumors compared to the control MC38si tumors (P=0.0392, Two-way-ANOVA). Furthermore, no growth inhibition was observed when mice were treated with 25 µg/g of lower affinity human anti-Neu5Gc IgG (eluted with 0.3 mM Neu5Gc2Me instead of 20 mM Neu5Gc; data not shown). The MC38 tumors escape by day 14 (Fig. 2C). In this regard, the injected antibody half-life is ~8 days (Fig. S2A) and is predicted to drop by day 13. Similar tumor outgrowth inhibition was observed in anti-Neu5Gc IgG treated Cmah−/− mice engrafted with MC38siIRR, containing stably integrated irrelevant siRNA that did not interfere with Neu5Gc cell surface expression (Fig. S2B–C). Taken together, these data demonstrate Neu5Gc-specific anti-tumor reactivity of human anti-Neu5Gc antibodies from IVIG.
Thus, while a low dose of human anti-Neu5Gc antibodies (1 µg/g) promotes progression of Neu5Gc-expressing tumors in Neu5Gc-deficient mice (9), a higher dose (25 µg/g) mediate tumor suppression. This further supports the theory of dualistic responses to immune reactants (15), leading us to also hypothesize that tumor-reactive antibodies against specific Neu5Gc-glycans might serve as biomarkers of human cancer.
Sialoglycan-microarray for biomarker discovery
A microarray approach permits high-throughput analysis of multiple samples and is valuable for comparative human serum profiling (31). To screen multiple anti-Neu5Gc IgGs in human sera, we used a highly efficient chemoenzymatic approach (27–29) to synthesize 40 sialylated glycans representing potentially common sialyloglycans on tumor cells. These 20 matched sialoglycan-pairs terminated with Neu5Gc or Neu5Ac, (Table 1; differing by one oxygen atom, Fig. 1A) and some of their 9-O-acetylated forms, were printed on Epoxy-coated slides in a range of concentrations. Slide print quality was monitored with polyclonal affinity-purified chicken anti-Neu5Gc IgY (32) (Fig. 3A), and with a positive control human serum (Fig. 3B using S34 (5)), both showing specific high reactivity to multiple Neu5Gc-glycans but not Neu5Ac-glycans. Next, sera from cancer or non-cancer patients were tested on the sialoglycan-microarray, and the potential of anti-Neu5Gc IgGs as cancer-biomarkers assessed.
Figure 3. Validation of sialoglycan-microarray slides for detection of anti-Neu5Gc antibodies.
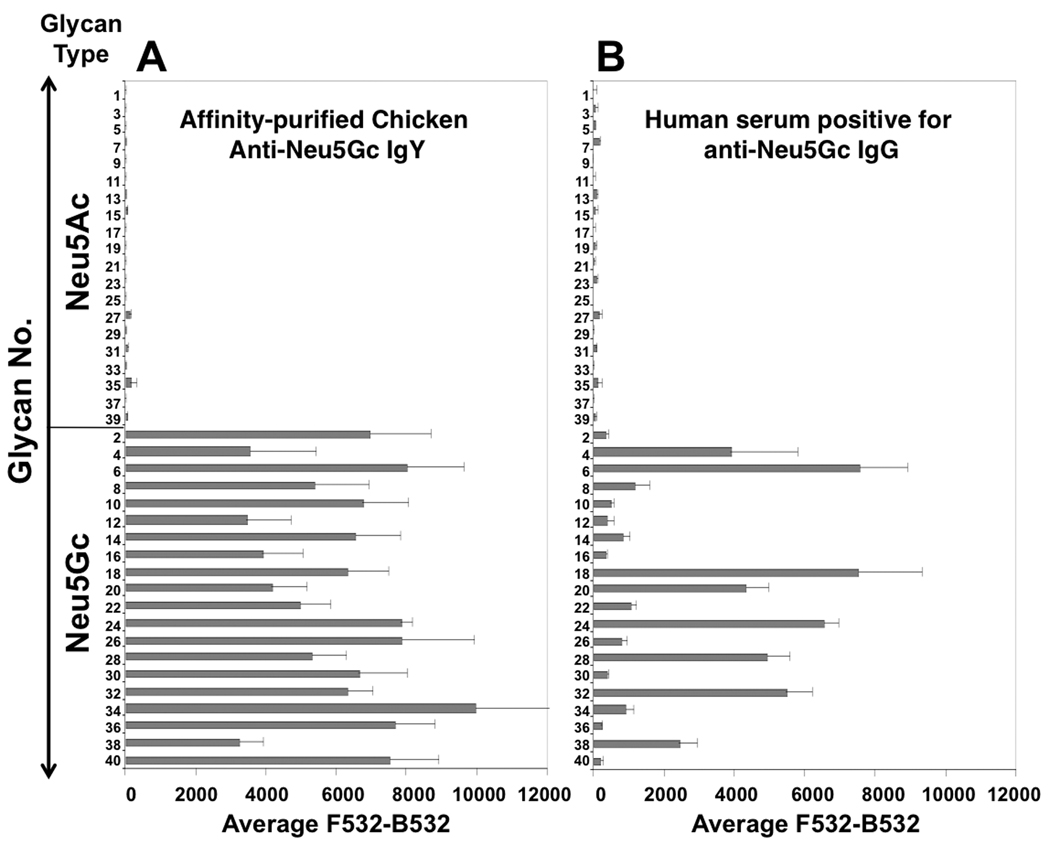
Various glycan-pairs (glycans #1–40 as detailed in Table 1) with terminal Neu5Gc or Neu5Ac were spotted on Epoxy-coated slides, then developed using (A) affinity-purified chicken anti-Neu5Gc IgY (1:10,000) (32) detected by Cy3-anti-chicken IgY (0.5 µg/ml); or, (B) human anti-Neu5Gc Ig positive human serum (1:100; S34 (5) detected by Cy3-anti-human IgG (1.5 µg/ml). Data were analyzed with an Excel pivot table, are representative of more than three independent experiments and show mean±SD of 4 replicate spots (of the glycans printed at 125 µM).
Training a classifier for cancer versus control status
We developed a classifier to distinguish cancer cases from controls using the sialoglycan-microarray. Such a classifier is a rule to call a subject as a case or control, using output from the sialoglycan-microarray assay of the subjects’ serum. We used an initial training set of 5 cases and 5 controls to develop our data standardization and filtering protocols, and to develop summary measures for each subject’s antibody response. An initial classifier trained on these preliminary data used the number of Neu5Gc-glycans (out of 20) with significantly elevated anti-Neu5Gc IgG signals for each subject, and resulted in limited sensitivity and specificity. Subsequently, each subject’s anti-Neu5Gc IgG responses on a slide were summarized with two parameters (intercept α and slope β), which describe anti-Neu5Gc IgG response as a function of Neu5Gc glycan concentration. We reasoned that using this more detailed measure of response to each glycan (α and β) could improve sensitivity and specificity. Half of the breast cancer cases and female controls with no cancer were randomly selected from 225 subjects to form the training data (Table S1; 112 breast cancer cases, including 67 non-metastatic cases and 50 controls). These data were used to screen the 20 glycans for predicitive power, and the statistical team remained blinded to the selection. The case/control status was then un-blinded and parameters α and β from each subject were used as training data for a classifier. The 20 glycans were initially screened singly for discriminatory power and ten-fold cross-validation was used to estimate the AUC (area under the receiver operator characteristic [ROC] curve) for each glycan (Table 2). A ROC curve plots the true-positive rate against the false-positive rate for the different possible cut-points of a diagnostic test. The area under the curve (AUC) measures discrimination, that is, the ability of the test to correctly classify those with and without disease. Multivariate models did not improve on univariate results; however, removing metastatic cases from the analysis slightly improved most classification results (Table 2), possibly due to absorption of anti-Neu5Gc antibodies by tumor cells upon elevated tumor burden. Glycans with mean AUC above 0.55 were selected for further validation testing (glycans 2, 6, 20, and 34).
Table 2.
Selection of significant Neu5Gc-glycans for validation testing.
| Glycan No. |
Compound |
Cross-validated mean AUC: training data |
|
|---|---|---|---|
| Including metastatic cases (87 cases, 25 controls) |
Excluding metastatic cases (67 cases, 25 controls) |
||
| 2 | Neu5Gc9Acα2–3Galβ1–4GlcNAcβProNH2 | 0.63 | 0.62 |
| 4 | Neu5Gc9Acα2–6Galβ1–4GlcNAcβProNH2 | 0.44 | 0.44 |
| 6 | Neu5Gcα2–6GalNAcαProNH2 | 0.64 | 0.63 |
| 8 | Neu5Gc9Acα2–3Galβ1–3GlcNAcβProNH2 | 0.46 | 0.47 |
| 10 | Neu5Gc9Acα2–3Galβ1–3GalNAcαProNH2 | 0.37 | 0.43 |
| 12 | Neu5Gcα2–3Galβ1–4GlcNAcβProNH2 | 0.52 | 0.53 |
| 14 | Neu5Gcα2–3Galβ1–3GlcNAcβProNH2 | 0.53 | 0.54 |
| 16 | Neu5Gcα2–3Galβ1–3GalNAcαProNH2 | 0.54 | 0.55 |
| 18 | Neu5Gcα2–6Galβ1–4GlcNAcβProNH2 | 0.48 | 0.41 |
| 20 | Neu5Gcα2–6LacβProNH2 | 0.59 | 0.58 |
| 22 | Neu5Gcα2–3Galβ1–4GlcβProNH2 | 0.31 | 0.36 |
| 24 | Neu5Gc9Acα2–6GalNAcαProNH2 | 0.51 | 0.53 |
| 26 | Neu5Gcα2–3GalβProNH2 | 0.44 | 0.46 |
| 28 | Neu5Gcα2–6GalβProNH2 | 0.34 | 0.32 |
| 30 | Neu5Gc9Acα2–3GalβProNH2 | 0.54 | 0.55 |
| 32 | Neu5Gc9Acα2–6GalβProNH2 | 0.30 | 0.29 |
| 34 | Neu5Gcα2–3Galβ1–3GalNAcβProNH2 | 0.57 | 0.58 |
| 36 | Neu5Gc9Acα2–3Galβ1–3GalNAcβProNH2 | 0.41 | 0.43 |
| 38 | Neu5Gc9Acα2–6Galβ1–4GlcβProNH2 | 0.54 | 0.53 |
| 40 | Neu5Gc9Acα2–3Galβ1–4GlcβProNH2 | 0.51 | 0.52 |
For each glycan, the two antibody response summary variables α and β, obtained from a mixed-effects model, were used to discriminate cases from controls using logistic regression. ROC curves and corresponding AUC’s were calculated for 500 ten-fold cross-validation runs. Glycans with mean AUC above 0.55 (2, 6, 20, and 34) were selected as the glycans of interest to carry into validation. ProNH2 = O(CH2)2CH2NH2.
Validating the classifier using independent breast cancer cases and controls
The validation data consisted of 74 new non-metastatic breast cancer cases and 25 new controls. The analysis plan was formalized prior to data delivery to the statistical team. Glycans 2, 6, 20, and 34 were assessed for significance as above, using cross-validation mean AUC as a measure of predictive power. We were able to independently replicate our results for two of the 4 glycans (6 and 20), as having estimated mean cross-validated AUC’s above 0.55 in these independent data (Table S2). Multivariate models (when all possible combinations of the 4 glycans were tested; 15 models in total) did not improve on univariate results, and the two final best models were those using glycans 6 and 20 alone. However, the multivariate models with the highest mean AUCs all included glycan 6 (data not shown); therefore, the classifier built using glycan 6 alone was considered to be the most promising candidate for further analysis.
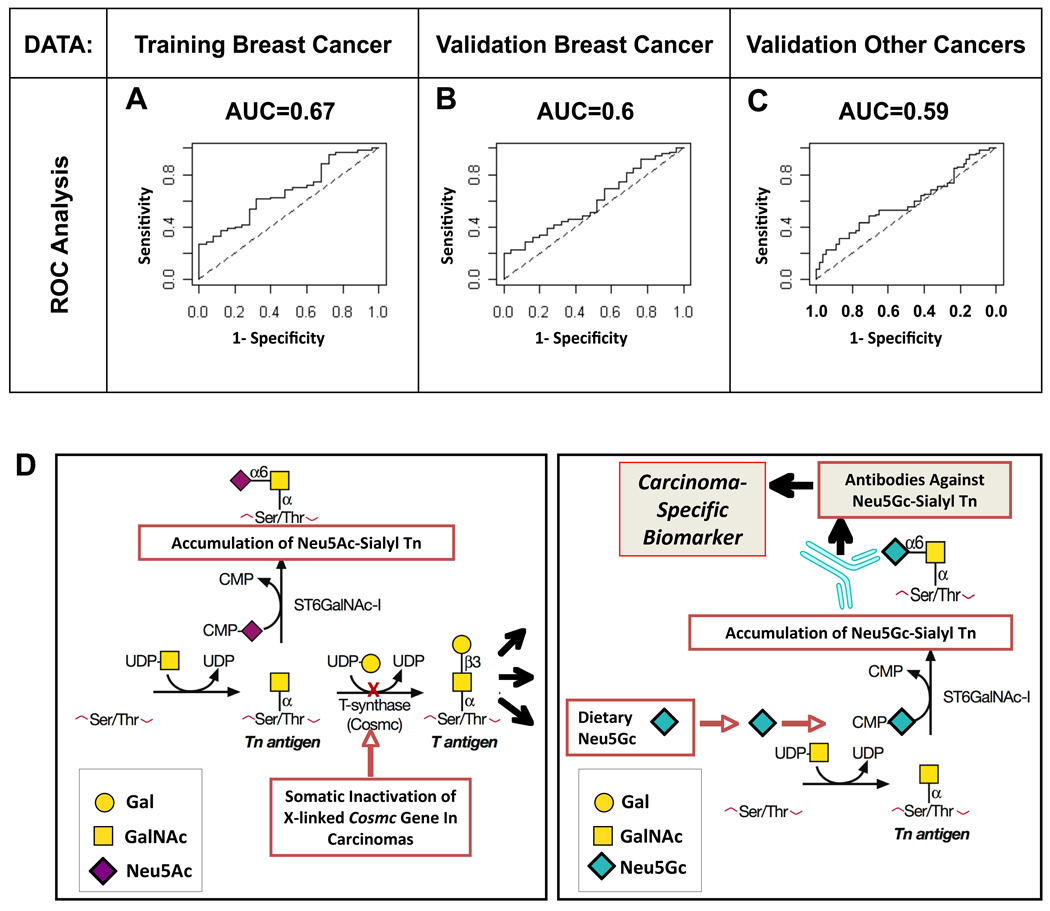
As a summary, ROC curves for glycan 6 are presented in Fig. 4, using logistic regression models estimated on training and validation data (results from the cross-validation are given in Fig. S3A–C). For the breast cancer training data (Fig. 4A), which were used to select glycan 6 from among the 20 glycans, using anti-Neu5Gc antibody response as a classifier gave an AUC of 0.67. In the breast cancer validation data, which were used to replicate results, the estimated AUC was 0.60 (Fig. 4B) with a mean AUC of 0.58 after 10-fold cross-validation (Fig. S3B; 95%CI=(0.167, 0.917), IQR=(0.458, 0.708)). These AUC values compare favorably with some common protein-based screens used today for cancer detection (33, 34). In these breast cancer validation data the estimated mean specificity was 0.86 (95%CI= (0.37, 1.00)) at a sensitivity of 0.20 and 0.76 (95%CI= (0.27, 1.00)) at a sensitivity of 0.30, respectively.
Figure 4. Anti-GcSTn is a classifier for cancer cases/controls and is suggested to be a human-specific and tumor-associated carcinoma biomarker.
Probabilities of being a cancer case were calculated using logistic regression where predictors were the two parameters, α and β, which summarized the anti-Neu5Gc antibody response to glycan 6 (Neu5Gc-sialyl-Tn; GcSTn) against the pan antibody level of 20 Neu5Ac glycans. (A) ROC curve for training data, used to select glycan 6, that had 67 non-metastatic breast cancer cases and 25 controls. (B) ROC curve for the first validation data set, which had 74 new non-metastatic breast cancer cases and 25 new controls. (C) ROC curve for a second validation data set, which had 99 cases of other cancer types and 55 controls. The biochemical and genetic rationale for the generation of the novel human carcinoma biomarker is schematically presented. (D) Somatic Cosmc mutations generate incomplete O-linked glycosylation, resulting in tumor-associated expression of the sialylated-Tn antigen in many carcinomas (left panel). Incorporation of dietary-Neu5Gc by such carcinomas generates Neu5Gc-sialyl Tn, detected by the humoral adaptive immune system as foreign, thus generating antibodies against it. Such xeno-auto-antibodies specific for Neu5Gc-sialyl Tn, are hypothesized to be novel biomarkers for early screening of carcinomas and/or potential immunotherapeutic tools (right panel).
Replication of results for glycan 6 using other types of carcinoma cases versus controls
To further validate the predictive value of glycan 6, we used a second set of independent validation data that included 55 controls (including 25 controls from the breast cancer validation and 30 new controls) and 99 cases with other types of non-metastatic cancer (Table S1). In these data, the estimated AUC was 0.59 (Fig. 4C) with a mean AUC of 0.57 after 10-fold cross-validation (Fig. S3C; 95%CI=(0.283, 0.817), IQR=(0.483, 0.683)). When the sensitivity was 0.2 and 0.3, the estimated mean specificity was 0.89 (95%CI= (0.50, 1.00)) and 0.81 (95%CI= (0.33, 1.00)), respectively (Fig. S3C). Univariate logistic regression of glycan 6 according to cancer type (Table S3) revealed predictive value in carcinomas from prostate, ovarian, lung and endometrium, while it was not correlated with colon and pancreatic cancers, however the number of cases tested in each of these cancers was very small, hence conclusions based on these small size results should be made with caution.
In summary, an unbiased glycan-microarray approach and a relatively large set of human sera allowed stringent statistical analysis to indicate that antibodies to glycan 6 show promise to classify cancer cases from controls with relatively high specificity (true negative), albeit with low sensitivity (true positive).
Novel biological rationale supports anti-GcSTn IgG as a unique human carcinoma-associated biomarker
Interestingly, glycan 6 resembles the carcinoma-associated biomarker Sialyl-Tn (STn; Neu5Acα2–6GalNAcα1-O-Ser/Thr), except that Neu5Ac is replaced with Neu5Gc (GcSTn; Neu5Gcα2–6GalNAcα1-O-Ser/Thr). STn is quite rare in normal mouse (35) or human tissues (36–38) or is cryptic due to Sia O-acetylation (39). In contrast, STn is relatively tumor-specific and abundant in many carcinomas (37) including those of the colon (39), ovary (40), breast (41) and pancreas (42). This high cancer-specificity is attributed to somatic mutations in Cosmc, the X-chromosome–encoded chaperone (Fig. 4D) (43, 44). This leads to a loss of T-synthase activity and inability to modify the Tn precursor (GalNAcα-O-Ser/Thr) with β1–3-linked Gal for further O-glycan elongation, shifting the pathway towards Tn (43, 44), and in the presence of ST6GalNAc-I (45), towards STn expression (Fig. 4D). This mutation is particularly common in human carcinomas (36, 44). Thus, dietary Neu5Gc consumption by cancer patients could replace the terminal Neu5Ac of STn by Neu5Gc, generating the novel xeno-autoantigen GcSTn, along with its corresponding specific anti-GcSTn antibodies (Fig. 4D), as novel carcinoma biomarker (based on ~400 carcinoma patients and controls). These steps likely occur at an early tumor stage suggesting anti-GcSTn antibodies may potentially be useful for early detection, or future risk, of carcinomas.
Antibody-mediated CDC and ADCC of human malignant cells expressing surface GcSTn
To further explore the immunotherapeutic potential of anti-GcSTn IgG , we used Jurkat T cells, which express STn due to a Cosmc mutation and an active ST6GalNAc-I, the enzyme capping the Tn antigen with Sia (43, 44). These cells were fed with Neu5Gc, mimicking the in vivo diet-related exchange of Neu5Ac with Neu5Gc, to generate GcSTn, confirmed by the metabolic-incorporation of Neu5Gc (Fig. 5A), and cell-surface expression with either terminal Neu5Ac or Neu5Gc (STn or GcSTn on Neu5Ac or Neu5Gc fed cells, respectively; Fig. 5A and Fig. S4). Subsequently, we tested in vitro human-tumor killing with human serum (S34) or affinity-purified human anti-Neu5Gc IgG, both rich with anti-GcSTn reactivity (recognizing Neu5Gcα2–6GalNAc; Fig. 3B and Fig. 1, respectively), revealing that both could promote complement-dependent cytotoxicity (CDC) in a Neu5Gc-specific manner (Fig. 5B). Human serum S34 was previously quantified to have ~25 µg/ml anti-GcSTn IgG in contrast to S30, which has very low levels of anti-Neu5Gc IgG (~2 µg/ml), mostly recognizing Neu5Gc2–6Galβ1–4Glc (5). Indeed, human serum S34 could promote Neu5Gc-specific antibody-dependent cellular-mediated cytotoxicity (ADCC), in contrast to human serum S30 (Fig. 5C). However, when human serum S30 was supplemented with the affinity-purified human anti-Neu5Gc IgG (10 µg/ml) it could promote ADCC in a Neu5Gc-specific manner (Fig. 5C). These results demonstrate anti-GcSTn antibodies as potential novel immunotherapeutic antibodies against tumors expressing GcSTn.
Figure 5. CDC and ADCC of cells expressing cell surface GcSTn.
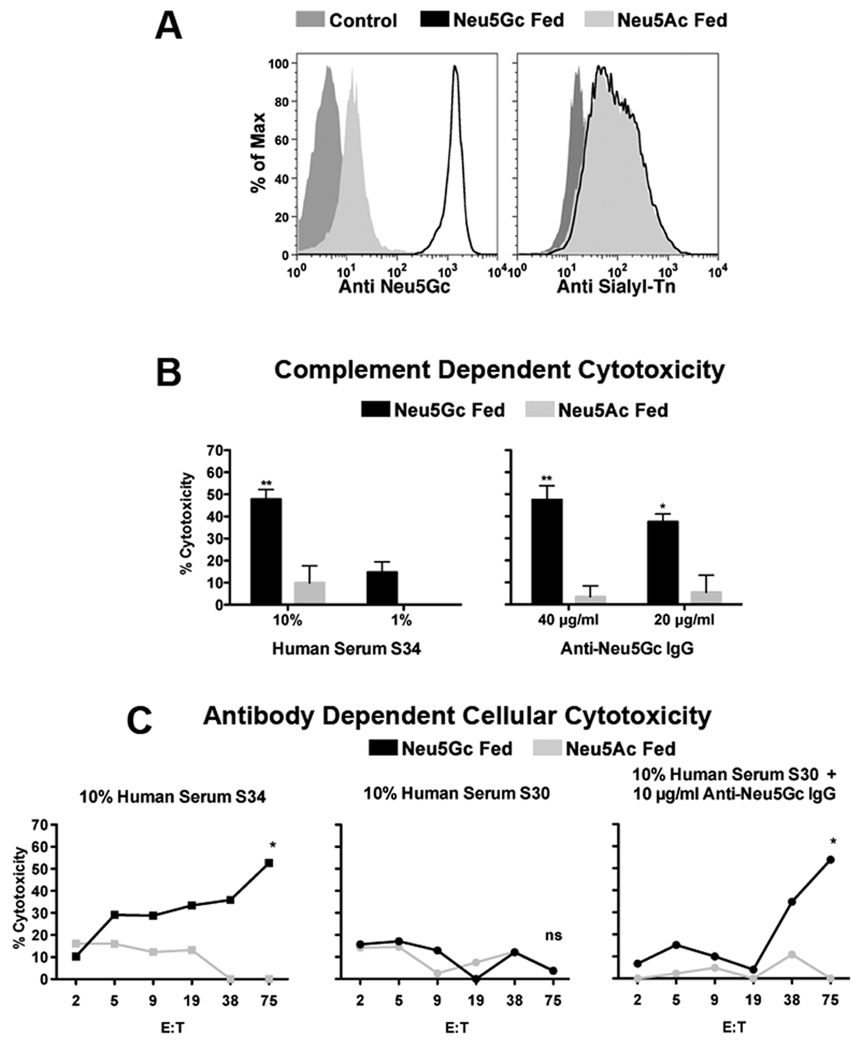
Jurkat cells were chased-out of pre-existing media-derived Neu5Gc then fed with 3 mM Neu5Ac or Neu5Gc. (A) FACS analysis using a polyclonal chicken anti-Neu5Gc antibody (highly specific to all Neu5Gc but not Neu5Ac glycans (32)) confirms feeding with Neu5Gc. A mouse monoclonal antibody specific for sialyl-Tn (STn) demonstrates cell-surface expression of this structure with either terminal Neu5Ac (STn; on Neu5Ac fed cells) or Neu5Gc (GcSTn; on Neu5Gc fed cells). (B) Human serum S34 (high in anti-GcSTn reactivity; Fig. 3B) can promote complement dependent cytotoxicity (CDC) of cells fed with Neu5Gc but not with Neu5Ac (left panel). Similarly, affinity-purified human anti-Neu5Gc IgG (high in anti-GcSTn reactivity; Fig. 1C) can promote CDC of Jurkat cells in a Neu5Gc-dependent manner (right panel; two independent experiments each; mean±SD; Two-way ANOVA, * P<0.01, ** P<0.05). (C) Human serum S34 (10%) can promote Neu5Gc-specific antibody-dependent cellular-mediated cytotoxicity (ADCC) with increasing effector:target ratios (target cells, T, Jurkat cell fed with Neu5Ac/Neu5Gc; effector cells, E, PBMCs in RPMI), in contrast to human serum S30 (10%) (5). However, when human serum S30 (10%) was supplemented with 10 µg/ml of the purified anti-Neu5Gc IgG it could promote ADCC similar to human serum S34 (representative of two independent experiments; two-way ANOVA * P<0.05).
Discussion
The immune system can either promote tumor progression or destruction depending on the balance between these opposing pathways, mediated by innate and adaptive immunity (11, 15, 15, 46). We demonstrate here that xeno-autoantibodies against an immunogenic non-human dietary xeno-autoantigen can mediate tumor growth inhibition, and serve as a potential biomarker for early carcinoma detection. The effects of anti-Neu5Gc IgG are dose-dependent: while high-affinity antibodies administered at low dose can promote tumor growth (9), we show that higher doses skew the response towards tumor regression. This dose-dependent efficacy for xeno-autoantibodies warrants further testing, especially to determine the range of concentrations by which the tumor response is shifting from stimulatory to inhibitory, thereby establishing a ‘safe’ concentration range to be used as therapeutic. These findings may also be relevant to the significant variability observed among patients’ responses to current monoclonal antibody immunotherapy (13), perhaps explaining some cases of shift in patient response towards tumor immunoescape or stimulation (13). Similar issues are potentially relevant to the use of cancer vaccines, including current clinical trials of vaccinations with STn-glycopeptides (47), Neu5Gc-GM3, or anti-idiotype antibodies against it (48).
Underglycosylation due to incomplete O-linked glycosylation can occur in some non-malignant events such as lactational mastitis and endometriosis, but it is much more common in cancer (1), specifically resulting in tumor-associated expression of the sialyl-Tn antigen in many carcinomas (37) that is rare in normal human tissues (36–39). In addition to sialyl-Tn, the most common cancer-associated sialosides are sialyl-Lex (Neu5Acα2–3Galβ1–4(Fucα1–3)GlcNAc) and its regioisomer sialyl-Lea (Neu5Acα2–3Galβ1–3(Fucα1–4)GlcNAc; and 9–O–acetyl–GD3 (Neu5,9Ac2α2–8Neu5Acα2–3Galβ1–4Glcβ1–1Ceramide) (1). Here we suggest that dietary Neu5Gc can metabolically replace Neu5Ac in STn, generating the unique neo-tumor-associated xeno-autoantigen GcSTn, specifically recognized by xeno-autoantibodies. To our knowledge, this is the first cancer biomarker related to metabolically-incorporated immunogenic dietary molecule. It is likely that Neu5Gc can also replace Neu5Ac in other tumor-associated glycan structures, thereby generating other novel biomarkers, e.g. Gc-sialyl-Lex. As high doses of the same antibody-biomarker can attenuate tumor growth, such xeno-autoantibodies might also be harnessed as novel cancer immunotherapeutics.
Novel serum biomarkers for cancer screening are needed, since current ones lack sufficient sensitivity and especially specificity for early diagnosis (18, 19), being reliably detected mainly in advanced stages, and thus used more for prognosis, staging, monitoring and therapy selection (18). While antibodies against tumor-associated antigens are commonly found in cancer patients at an early stage and could potentially be sensitive detectors for malignant transformation (21, 22), none of the previously described autoantibodies show sufficient specificity in screening. Here we demonstrate that anti-GcSTn IgG is a potentially useful biomarker for early detection of carcinomas, with an estimated AUC of 0.6 (breast cancer validation data). Prostate-specific antigen (PSA) is one of the most common protein-based screens for cancer today. In the 10 year Prostate Cancer Prevention Trial (PCPT), the AUC for PSA alone was 0.678 (95% CI= 0.666–0.689) (33), and further validated by others ranging 0.525 to 0.678 (34). Two risk calculators to predict individual risk of a positive biopsy (in the context of other factors) have recently been developed, providing some improvement of AUC (34). In any case, PSA assay sensitivity is mostly based on detection of pseudo-disease thereby leading to an AUC that overestimates the benefit of PSA screening (20).
In summary, we use a unique sialoglycan-microarray to describe antibodies against a diet-related antigen as novel type of human serum carcinoma-biomarker. Such antibodies could also mediate human tumor killing in vitro as well as in vivo. This establishes the new concept that a diet-derived antigen can metabolically-incorporate into tumors, generating a novel antigen detected by the immune system. Eventually, these antibodies can be harnessed for immunotherapy when used at the appropriate dose. Given the frequency of altered sialylation in cancer, these concepts have general potential for other important discoveries.
Supplementary Material
Acknowledgements
We thank Dr Jane Burns, UCSD Pediatrics, Rady Children's Hospital, for remnant clinical IVIG; and Rene Chow and Prof Nissi Varki, Department of Pathology, UCSD for assistance with mouse tumor experiments.
Grant support: This work was supported by ISEF postdoctoral fellowship to V.P-K., NIH grant U01 CA128442 (to A.V.), NCI SBIR Contract HHSN26120070063C (to N.H-Z.) and R01GM076360 (to X.C.) and a Tower Cancer Research Foundation, Ron Lippin Fund, grant (to R.B.S.).
Footnotes
Disclosure of potential conflicts of interest: A.V. is a co-founder of Sialix, Inc. (formerly Gc-Free, Inc.). N. H-Z. and D.G. are currently employees of Sialix, Inc.
References
- 1.Varki A, Kannagi R, Toole BP. Glycosylation Changes in Cancer. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. pp. 617–632. [PubMed] [Google Scholar]
- 2.Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–634. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- 3.Tangvoranuntakul P, Gagneux P, Diaz S, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175:228–236. doi: 10.4049/jimmunol.175.1.228. [DOI] [PubMed] [Google Scholar]
- 5.Padler-Karavani V, Yu H, Cao H, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci U S A. 2010;107 Suppl 2:8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedlund M, Tangvoranuntakul P, Takematsu H, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor RE, Gregg CJ, Padler-Karavani V, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010;207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A. 2008;105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 13.Ferris RL, Jaffee EM, Ferrone S. Tumor Antigen-Targeted, Monoclonal Antibody-Based Immunotherapy: Clinical Response, Cellular Immunity, and Immunoescape. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 15.Prehn RT, Prehn LM. The flip side of immune surveillance: immune dependency. Immunol Rev. 2008;222:341–356. doi: 10.1111/j.1600-065X.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- 16.Marcial VA. Carcinoma of the cervix: present status and future. Cancer. 1977;39:945–958. doi: 10.1002/1097-0142(197702)39:2+<945::aid-cncr2820390734>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. W237–W242. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 19.Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival - a review of the epidemiological literature. J Ovarian Res. 2009;2:13. doi: 10.1186/1757-2215-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 21.Tan HT, Low J, Lim SG, Chung MC. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009 doi: 10.1111/j.1742-4658.2009.07396.x. [DOI] [PubMed] [Google Scholar]
- 22.Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 23.Drake PM, Cho W, Li B, et al. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin Chem. 2010;56:223–236. doi: 10.1373/clinchem.2009.136333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer O, Marr RA, Rockenstein E, et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8:1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- 25.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 26.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Chokhawala HA, Huang S, Chen X. One-pot three-enzyme chemoenzymatic approach to the synthesis of sialosides containing natural and non-natural functionalities. Nat Protoc. 2006;1:2485–2492. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Highly efficient chemoenzymatic synthesis of naturally occurring and non-natural alpha-2,6-linked sialosides: a P. damsela alpha-2,6-sialyltransferase with extremely flexible donor-substrate specificity. Angew Chem Int Ed Engl. 2006;45:3938–3944. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, Chokhawala H, Karpel R, et al. A multifunctional Pasteurella multocida sialyltransferase: a powerful tool for the synthesis of sialoside libraries. J Am Chem Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 30.Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyelaran O, McShane LM, Dodd L, Gildersleeve JC. Profiling human serum antibodies with a carbohydrate antigen microarray. J Proteome Res. 2009;8:4301–4310. doi: 10.1021/pr900515y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz SL, Padler-Karavani V, Ghaderi D, et al. Sensitive and specific detection of the non-human sialic Acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE. 2009;4:e4241. doi: 10.1371/journal.pone.0004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 34.Cavadas V, Osorio L, Sabell F, Teves F, Branco F, Silva-Ramos M. Prostate Cancer Prevention Trial and European Randomized Study of Screening for Prostate Cancer Risk Calculators: A Performance Comparison in a Contemporary Screened Cohort. Eur Urol. 2010 doi: 10.1016/j.eururo.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Martin LT, Marth JD, Varki A, Varki NM. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem. 2002;277:32930–32938. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 36.Conze T, Carvalho AS, Landegren U, et al. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology. 2010;20:199–206. doi: 10.1093/glycob/cwp161. [DOI] [PubMed] [Google Scholar]
- 37.Yonezawa S, Tachikawa T, Shin S, Sato E. Sialosyl-Tn antigen. Its distribution in normal human tissues and expression in adenocarcinomas. Am J Clin Pathol. 1992;98:167–174. doi: 10.1093/ajcp/98.2.167. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol. 1996;106:197–207. doi: 10.1007/BF02484401. [DOI] [PubMed] [Google Scholar]
- 39.Ogata S, Koganty R, Reddish M, et al. Different modes of sialyl-Tn expression during malignant transformation of human colonic mucosa. Glycoconj J. 1998;15:29–35. doi: 10.1023/a:1006935331756. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi H, Terao T, Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J Clin Oncol. 1992;10:95–101. doi: 10.1200/JCO.1992.10.1.95. [DOI] [PubMed] [Google Scholar]
- 41.Imai J, Ghazizadeh M, Naito Z, Asano G. Immunohistochemical expression of T, Tn and sialyl-Tn antigens and clinical outcome in human breast carcinoma. Anticancer Res. 2001;21:1327–1334. [PubMed] [Google Scholar]
- 42.Kim GE, Bae HI, Park HU, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- 43.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju T, Lanneau GS, Gautam T, et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008;68:1636–1646. doi: 10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- 45.Sewell R, Backstrom M, Dalziel M, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and Is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 2006;281:3586–3594. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 46.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 48.de Leon J, Fernandez A, Clavell M, et al. Differential influence of the tumour-specific non-human sialic acid containing GM3 ganglioside on CD4+CD25− effector and naturally occurring CD4+CD25+ regulatory T cells function. Int Immunol. 2008;20:591–600. doi: 10.1093/intimm/dxn018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.