Abstract
Over the past decade, continent urinary diversion, especially orthotopic bladder substitutions, has become increasingly popular following radical cystectomy for bladder cancer. The ultimate goal of orthotopic bladder substitution is to offer patients the best quality of life, similar to that of patients with native bladders. To achieve that purpose, surgeons should be familiar with the characteristics of good candidates for neobladders, the possible intraoperative and postoperative problems related to the surgery, and the solutions to these problems. Postoperative surveillance and instructions given to the patients also contribute to successful, functional results. Here, we reviewed the indications, pitfalls, and solutions for orthotopic bladder substitutions and the patients' quality of life after surgery. When performed properly, orthotopic continent diversion offers good quality of life with few long-term complications. Therefore, we believe it is the best option for the majority of patients requiring cystectomy.
Keywords: Cystectomy, Quality of life, Urinary bladder, Urinary bladder neoplasms, Urinary diversion
INTRODUCTION
Radical cystectomy (RC) and urinary diversion have been the standard treatment of high-grade, invasive transitional cell carcinoma of the bladder. Ideal urinary diversion after RC should be the safest for cancer control, have the fewest complications, and provide the easiest adjustment for patients' lifestyle, thereby supporting the best quality of life (QoL). Over the past decades, since the introduction of orthotopic bladder substitution (OBS) by Camey and Le Duc in the late 1970s, orthotopic urinary diversion has been increasingly performed in both sexes.
Today, the proportion of cystectomy patients receiving a neobladder has increased to 50% to 66% at large-volume institutes [1-3]. In this article, we review the published results regarding OBS using ileum, which is the most commonly performed procedure for continent urinary diversion, specifically focusing on the indications, preoperative and intraoperative considerations, possible problems and solutions, and postoperative QoL of the patients.
PATIENT SELECTION: INDICATIONS AND CONTRAINDICATIONS
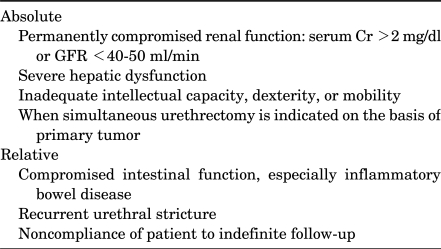
Appropriate patient selection for OBS is one of the keys to success. All cystectomy patients are possible candidates for a neobladder. Nowadays, although contraindications for OBS are fewer than in the past, it is important to identify patients in whom OBS may be less ideal (Table 1). Before determining the urinary diversion method, surgeons should consider the patient's preferences and general performance status, renal and hepatic function, primary tumor stage and location, and the need for adjuvant therapy.
TABLE 1.
Contraindications for orthotopic bladder substitution using ileum
The primary patient factor for OBS is the patient's desire for a neobladder. The patient should have a certain motivation to tolerate the initial, and sometimes lasting, inconveniences of nocturnal incontinence associated with a neobladder [4]. If patients lack the motivation to understand the new voiding techniques that will be required postoperatively, OBS is not desirable. Thus, the ileal conduit is preferred in patients with inadequate intellectual capacity, dexterity, or physical conditions that impede self-catheterization.
OBS is contraindicated in patients with permanently compromised renal function or severe hepatic dysfunction. OBS is absolutely contraindicated in patients with a glomerular filtration rate of less than 40-50 ml/min or serum creatinine greater than 2 mg/dl as a result of long-standing obstruction or chronic renal failure [5]. However, some patients with significant creatinine elevations due to bladder cancer can recover sufficient renal function to allow OBS if the obstruction is relieved. In this situation, placement of percutaneous nephrostomy before surgery may give more accurate information on true renal function. Severe hepatic dysfunction is also a contraindication to OBS because absorption of ammonia from the urine into the portal circulation markedly increases postoperatively through the intestinal mucosa of the neobladder, leading to hyperammonemia. In addition, OBS is relatively contraindicated in patients with compromised intestinal function, particularly inflammatory bowel disease.
OBS is also absolutely contraindicated in patients who are candidates for simultaneous urethrectomy on the basis of their primary tumor [6,7]. Similarly, OBS is relatively contraindicated in patients with significant benign urethral pathology, such as recurrent urethral strictures. Although prostate tumor involvement in male patients and bladder neck involvement in female patients are risk factors for urethral recurrence [8,9], OBS is possibly indicated, provided that intraoperative frozen section analysis of the urethral margin is without evidence of tumor [9-11].
With accumulated experience in the OBS procedure, advanced tumor stage is not an absolute contraindication for OBS. In a proportion of patients with locally advanced or node-positive disease, long-term survival can be achieved with a low incidence of pelvic recurrence (range, 10-13%) through RC and thorough pelvic lymph node dissection [12-14]. Thus, OBS can be performed in these patients with anticipated good results. Furthermore, it was reported that even in the presence of recurrent disease, most patients can achieve normal neobladder function until death [14]. However, if tumor involvement to adjacent organs or extensive lymph node involvement is suspected, an ileal conduit rather than OBS is preferable for prompt adjuvant therapy [15].
Notably, old age is not a contraindication for OBS. Older patients, as part of the informed consent, need to be aware that they have a greater incidence of enuresis or nocturnal incontinence than do younger men, but age by itself should not be a contraindication. In this context, physiologic rather than chronologic age must be taken into consideration [7].
INTRAOPERATIVE CONSIDERATIONS
1. Positive urethral margin and lymph node metastasis
If the tumor is located near the bladder neck in females or involves the prostatic urethra in males, intraoperative frozen section analysis of the distal urethral margin may be necessary. Because a positive urethral margin warrants total urethrectomy, all patients should be informed that diversion to the skin via an ileal conduit may be necessary owing to unexpected tumor extent, and an appropriate stoma site should be marked on the abdominal wall beforehand.
The incidence of lymph node metastasis at RC has been reported to be 14% to 32% [16-19]. Although intraoperative frozen section analysis for removed lymph nodes is not our routine practice, we do frozen section analysis in cases of palpable or grossly enlarged lymph nodes. If the results reveal positive lymph nodes, we do frozen section analysis for all lymph node specimens removed through meticulous lymph node dissection. In our practice, OBS can be performed in patients with minimal nodal disease, whereas ileal conduit is preferable in patients with extensive lymph node involvement for prompt adjuvant therapy.
2. Ureterointestinal anastomosis: refluxing or nonrefluxing?
One of the important goals of urinary diversion is preservation of renal function. The necessity of reflux prevention at a ureteroileal anastomosis is controversial [3,7,20-22]. Advocates for antireflux anastomosis reported that, on the basis of animal experiments, refluxing anastomosis was more commonly associated with reflux and pyelonephritis and subsequent renal function deterioration [23]. However, previous studies have shown that that the need for reflux prevention differs depending on the urinary diversion method. Although conduits are not always low-pressure systems [24,25], often due to obstruction at the level of the fascia immediately superficial to the external oblique muscle, OBS is associated with consistently low intra-reservoir pressure through bowel detubularization [26] and simultaneous pressure increases in the neobladder, abdomen, and renal pelvis during the Valsalva maneuver [21]. Meanwhile, many studies have reported that the risk of anastomosis stricture is significantly higher in nonrefluxing techniques than in direct refluxing anastomosis [2,21,22,27]. Hautmann et al reported that simple end-to-side, freely refluxing anastomosis to an afferent limb of a low-pressure orthotopic reconstruction, in combination with regular voiding and close follow-up, has the lowest overall complication rate [4]. Consistent with these reports, we have reported no significant difference in functional or radiographic changes between the refluxing and nonrefluxing types of OBS [28], and although reflux was more common in refluxing anastomosis in postoperative voiding cystourethrography, the development of reflux after RC does not significantly alter renal function regardless of its severity [29].
Integrating the published results and our experience, we believe that freely refluxing ureteroileal anastomosis is a safe and easy method for OBS.
3. Short mesentery
Tension-free anastomosis between a neobladder and the distal urethra may be difficult in patients with a short mesentery. In this situation, anastomosis can be performed under a more flexed position to minimize tension. If this is not enough, dissecting the mesentery to a more proximal site or making multiple, small incisions in the mesentery can lengthen the mesentery. Several investigators have suggested neourethral tube modification methods for adding extra length to reach the urethra. For example, Rawal et al reported a modification of the Studer neobladder method, the 'pitcher pot' ileal neobladder, which is the formation of a tube or neourethra by use of a part of the ileal wall, consequently providing extra length for anastomosis without tension [30]. However, note that this method is associated with urinary retention and an obstructive voiding pattern.
4. Ureteral stents and cystostomy
Placement of a ureteral stent at a ureteroileal anastomosis depends on the surgeon's preference. Most surgeons use a ureteral stent at a ureteroileal anastomosis and then remove the stent with the Foley catheter after cystography at 2-3 weeks postoperatively. In our experience, a ureteral stent is not necessarily needed in refluxing anastomosis, whereas it is helpful to prevent anastomosis obstruction in antirefluxing anastomosis.
Placement of a cystostomy into the neobladder is useful for postoperative manual irrigation and monitoring of the voiding pattern after Foley catheter removal. However, in our experience, use of a large-sized (22-24 Fr) double-lumen catheter or nephrostomy catheter without cystostomy is enough.
POSTOPERATIVE PROBLEMS AND SOLUTIONS
1. Postoperative ileus
Ileus following RC is one of the most frequent postoperative complications that cause delayed recovery and increased hospital stay [31-34]. Ileus was noted in 4-23% of patients undergoing RC [32-35]. Chang et al reported that increased blood loss during surgery and the presence of major complications were significant predictors for ileus [32]. Several perioperative managements should be considered to reduce postoperative ileus. Because the perception of pain is an acknowledged promoter of ileus [36], adequate intraoperative and postoperative pain control is important. Hypovolemia during surgery is also associated with an increased risk of postoperative ileus [31,32]. Thus, timely and adequate hydration with colloids or blood transfusions is crucial after exenteration, when blood loss exceeds 10% of the estimated total blood volume [37]. Additionally, early postoperative provision of artificial nutrients, in the form of both total parenteral nutrition and enteral nutrition, has shown beneficial effects in preventing postoperative ileus [37].
Although nasogastric tubing is helpful intraoperatively to obtain bowel decompression, routine postoperative nasogastric decompression may be unnecessary. For example, recent studies have shown that bowel resection can be performed safely without postoperative nasogastric tubing both in general surgery cases and in radical cystectomy [35,38]. Two meta-analyses including over 4,000 cases concluded that routine postoperative nasogastric decompression was unnecessary and was associated with a higher incidence of pulmonary complications than selective nasogastric decompression [39,40]. Meanwhile, two recent studies reported that gum chewing stimulates bowel motility in patients undergoing radical cystectomy with urinary diversion [41,42].
2. Urinary tract infection
Contrary to normal urothelium, which has inhibitory action against bacterial adherence, bowel epithelium lacks inhibitory action against bacterial adherence. It has been reported that about 51-67% of patients with orthotopic neobladder have a positive urine culture [43-45]. Compared with other types of urinary diversion, few studies have investigated urinary tract infection (UTI) in patients with orthotopic neobladder. In a study by Wullt et al, E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterococcus faecalis were frequently detected pathogens, and bacterial colonization was strongly correlated with residual urine [44]. However, all patients in this study were asymptomatic. In another study by Wood et al, the overall rate of UTI was 39%, and 12% of patients had urosepsis [43]. The authors suggested that prophylactic antibiotics are recommended in patients with recurrent UTI but that treating a positive urinary culture in the absence of specific voiding symptoms is not advocated. As such, the need for antibiotic treatment and the clinical significance of bacteriuria in patients with OBS are controversial. In our experience at the Asan Medical Center, the most frequent uropathogens associated with perioperative pyelonephritis and febrile UTI are Enterococcus faecalis, followed by methicillin-resistant Staphylococcus aureus. Because Enterococcous species are mostly susceptible to ampicillin, we use prophylactic antibiotics for 4 weeks postoperatively.
After RC, patients with neobladders commonly have chronic bacteriuria, which is fairly steady over many years [43,44,46,47]. Although ileal neobladders lack the native immunologic defenses of the native bladder mucosa and bacterial colonization may progress to invasive tissue-level infection, all investigators recommend against long-term prophylactic antibiotic therapy for patients with asymptomatic bacteriuria to reduce the development of drug-resistant species and to minimize cost and drug-related adverse effects. Additional studies are needed so that conclusions can be made regarding UTI in patients with orthotopic neobladder.
3. Urinary continence
Continence after OBS is affected by multiple factors, including the size and configuration of the neobladder, urethral length, patient age and mental status, intact pelvic nerve supply to the rhabdosphincter, completeness of voiding, and the presence or absence of bacteriuria [48-50]. Continence improves over time during the initial 6 to 12 months postoperatively as the compliance of the diversion increases [46,48]. In addition, after surgery, patients learn to void by performing a Valsalva maneuver in coordination with relaxation of the pelvic floor, resulting in spontaneous voiding to empty the diversion. Daytime continence is achieved earlier than nighttime continence [2]. At postoperative 1 year, the overall rate of daytime continence, defined as totally dry or the use of 1 pad per day, is approximately 85% to 90% [1,47,50-53]. Regarding the relationship between neobladder configuration and continence, Nesrallah et al found that at 3 to 6 months postoperatively, daytime incontinence and enuresis were more common in elongated ileal neobladders than in spherical neobladders but the results were the same by 1 year in both groups [53]. In that study, at 1 year, spherical neobladders tended to enlarge to a greater capacity and to have a higher prevalence of postvoid residual urine volume over 100 ml and a greater need for catheterization [53]. Thus, surgeons should be careful to not make the initial volume of a spherical neobladder too large. On the other hand, decreased functional urethral length after surgery [48] or decreased urethral sensitivity at the membranous urethra in men [54] was reported to be associated with daytime incontinence. Daytime continence rates may decrease 4 to 5 years postoperatively, partly because of decreased tone of the urethral sphincter with advanced age [55]. Persistent severe incontinence after OBS may be treated by periurethral collagen injection [56] or definitive placement of a urethral sling or artificial urinary sphincter.
In contrast with daytime continence, some degree of nocturnal leakage is a constant finding in most reports despite a technically sound operation [48]. Most series report a prevalence of nighttime leakage of 20% to 50% even after 1 year postoperatively [57-60]. Similar to daytime incontinence, nighttime incontinence resolves as the functional capacity of the neobladder increases. Rates of complete nighttime continence without any pads are reported to be 45% to 65% [60,61]. In a study based on urodynamic study [58], patients with enuresis had higher pressures, maximal volumes, postvoid residual urine volumes, and rates of positive urine culture, as well as lower maximal urethral pressures, flow rates, and compliance, than did those without enuresis by univariate analysis. However, in the multivariate analysis, only the amplitude of uninhibited contractions and increased postvoid residual volume were associated with enuresis [58]. Increased age was associated with a higher rate of enuresis in some series [51], whereas in other reports, age was not correlated [58]. For treatment of nighttime incontinence, patients are instructed initially to limit their fluid intake after the evening meal, to void before going to sleep, and to set an alarm clock to awaken and void once or twice during the night. Several studies reported that the use of imipramine hydrochloride 25 mg at bedtime decreases nighttime leakage in up to 25% of patients [57,58]. However, nighttime continence can ultimately be achieved with improvement of the patient's voiding pattern.
4. Incomplete voiding (hypercontinence)
After OBS, incomplete emptying and so-called hypercontinence requiring clean intermittent catheterization (CIC) is observed in 4% to 25% of males [44,50,62] and in 0% to 53% of females with neobladders [63,64]. Thus, voiding dysfunction after OBS is more common in women than in men. Although the cause of this finding is unclear, many studies have suggested that the primary cause of hypercontinence in female patients is formation of a "pouchocele" from lack of posterior support of the neobladder, which leads to angulation and obstruction of the neobladder-urethra junction [64-67]. Additionally, several factors such as an elongated bladder neck, the neobladder outlet not located at the most caudal portion of the reservoir, a dysfunctional bladder neck, inadequate pelvic floor relaxation, and excessive reservoir volume are suggested to be the cause of hypercontinence [62,65,67].
Although CIC is the best method for treatment of hypercontinence, compliance of male patients is low compared with female patients. In our experience, high doses of an alpha-blocker can bring subjective symptom improvement, but reduction of residual urine volume is rare. To reduce postoperative hypercontinence, patients should clearly understand the principle that lowering outlet resistance is crucial for complete emptying. Because increasing intra-abdominal pressure only is not enough for voiding, instruction on pelvic floor relaxation through biofeedback, regular voiding to prevent overdistention, and regular follow-up is essential [68]. In patients who do not respond to conservative management, cystoscopic evaluation to exclude the presence of stricture is necessary. In addition, if hypercontinence is associated with fixed sphincter tone on urodynamic study, partial sphincterotomy may be helpful. In females, techniques to prevent pouchocele formation include urethral suspension, placement of the omentum into the space posterior to the pouch, and suspension of the vaginal fornices to the Cooper ligament [69,70].
5. Mucus production
The bowel mucosa secretes mucus made up of a glycoprotein core [71], and about 35 g/day of mucus is produced in continent urinary diversions [72]. Therefore, from the first postoperative day, indwelling catheters must be carefully irrigated to prevent initial mucous buildup within the neobladder [46,73]. Because a sudden increase in mucous production may be an early sign of urinary infection [74], it is worthy of notice. Patients with good voiding and complete emptying usually pass the mucus spontaneously in the urine, whereas patients with incomplete emptying and those performing CIC may need to irrigate the neobladder to remove the retained mucus.
Ileal mucosa incorporated into urinary diversion appears to atrophy over time when exposed to urine [75]. However, it takes more than 4 years for ileal mucosa to lose its absorptive and secretive functions and acquire the function of a urinary reservoir [48,76]. Thus, during the period for structural and ultrastructural changes to the ileal mucosa, regular monitoring of the voiding pattern and complete emptying is necessary. Once a large mucous plug is formed, no drug treatment may be effective, and manual evacuation through a large resectoscope sheath is most beneficial [77]. Patients experiencing recurrent mucous retention should be instructed in periodic catheter irrigation and mucous evacuation, and oral or instillation therapy with N-acetylcysteine or urea is helpful in these patients [71,72,78].
6. Metabolic problems
Metabolic complications of OBS are common but are generally not severe. However, when renal function is insufficient or deteriorated, metabolic abnormalities can be problematic. Postoperative deterioration in renal function is most commonly associated with obstruction or infection [79]. Meanwhile, the more ileum that is used for reservoir construction, the higher the incidence of postoperative metabolic acidosis.
After urinary diversion, altered drug metabolism should be considered. Particularly, chemotherapeutic agents used in the treatment of bladder cancer require attention. Because methotrexate toxicity in patients with ileal conduits had been reported [80,81], patients with OBS who are receiving chemotherapy should be monitored closely and should stay well hydrated; the reservoir should be drained during treatment. Additionally, preservation of the terminal ileum is important to prevent vitamin B12 deficiency, which increases with age and declining renal function. Because chronic vitamin B12 deficiency is insidious and may result in irreversible neurologic and hematologic sequelae, long-term monitoring for vitamin B12 is necessary [82].
PATIENT'S QoL
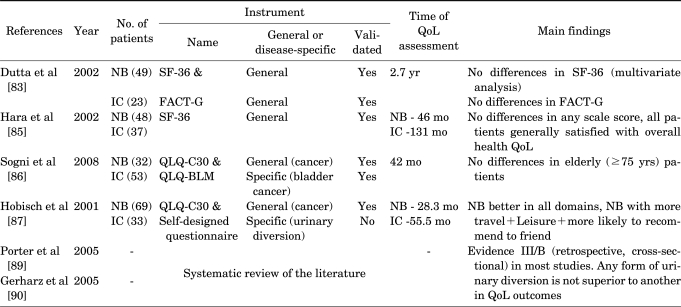
The optimal form of urinary diversion after RC in terms of patient QoL remains controversial. To date, more than 30 articles have compared the impact of different types of urinary diversion on patient QoL (Table 2), whereas no studies have examined QoL among different types of OBS. A major obstacle in assessing patient QoL after urinary diversion is the lack of a universal definition of the term "quality of life," which may differ between cultures, countries, and study groups. In addition, a patient's QoL is largely dependent on measurement modalities (open or structured face-to-face interview, telephone interview, proxy rating, self-report, etc) [4] and the QoL questionnaire itself (validated questionnaire or not, generic or cancer-specific or bladder cancer-specific QoL instruments, etc). To minimize these limitations and to evaluate QoL appropriately, it is crucial that a neutral third party be used to carry out the studies and questionnaires with validity and reliability.
TABLE 2.
Overview of studies that assessed health-related QoL after radical cystectomy and NB or IC
QoL: quality of life, NB: neobladder, IC: ileal conduit, FACT-G: Functional Assessment of Cancer Therapy-General, QLQ-C30: EORTC instruments QoL questionnaire C30, QLQ-BLM: QLQ-muscle-invasive bladder cancer module
Obviously, patients with neobladders have enhanced cosmesis and the potential for normal voiding function with no abdominal stoma and no need for a stomal appliance. However, the assumption that OBS provides better QoL than ileal conduit diversion is not supported by the results of previous studies. In a study comparing QoL between ileal conduit and OBS with 2 validated questionnaires, the RAND 36-Item Health Survey (SF-36) and the Functional Assessment of Cancer Therapy-General (FACT-G), young age and neobladder type were associated with higher QoL scores in 5 of the 9 SF-36 domains, but such significance favoring neobladder was not observed in the multivariate analysis with adjustment for age [83]. In this study, there were no significant differences in QoL between the two groups on the FACT-G. Similarly, no difference was observed in other studies comparing QoL between ileal conduit and orthotopic neobladder by use of the SF-36 questionnaire [84,85] and EORTC instruments QoL questionnaire C30 (QLQ-C30) and QLQ-muscle-invasive bladder cancer module (QLQ-BLM) [86]. To date, the superiority of OBS has been shown in only one study by Hobisch et al [87] in which patients with neobladders scored significantly better on the QLQ-C30 in all functional domains (role-physical, role-emotional, cognitive, and social) than did patients with conduits. However, a systematic review of the published literature [88-90] was insufficient to conclude that any form of urinary diversion is superior to another on the basis of QoL outcomes.
In fact, patients with an ileal conduit may discover that life with an ileal conduit is better than anticipated in terms of satisfaction, whereas patients with OBS may be dissatisfied if they anticipate the internal reservoir to function as well as the original bladder [48]. Thus, regarding patient satisfaction and QoL, it is important that the clinician provide a detailed explanation about the physical and lifestyle changes that occur after OBS. We believe that with this detailed preoperative education and discussion, most patients undergoing OBS can enjoy good QoL and have an improved self-image without an external urostomy appliance.
CONCLUSIONS
Orthotopic bladder substitution following cystectomy can provide excellent outcomes in patients who are highly motivated and carefully selected. To achieve this goal, surgeons should be familiar with possible pitfalls and their solutions as well as with adequate operative technique. When performed properly, orthotopic continent diversion offers good QoL with few long-term complications. Therefore, we believe it is the best option for the majority of patients requiring cystectomy.
Footnotes
The authors have nothing to disclose.
References
- 1.Hautmann RE, de Petriconi R, Gottfried HW, Kleinschmidt K, Mattes R, Paiss T. The ileal neobladder: complications and functional results in 363 patients after 11 years of followup. J Urol. 1999;161:422–427. doi: 10.1016/s0022-5347(01)61909-8. [DOI] [PubMed] [Google Scholar]
- 2.Studer UE, Zingg EJ. Ileal orthotopic bladder substitutes. What we have learned from 12 years' experience with 200 patients. Urol Clin North Am. 1997;24:781–793. doi: 10.1016/s0094-0143(05)70420-1. [DOI] [PubMed] [Google Scholar]
- 3.Stein JP, Skinner DG. Application of the T-mechanism to an orthotopic (T-pouch) neobladder: a new era of urinary diversion. World J Urol. 2000;18:315–323. doi: 10.1007/s003450000144. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) Consensus Conference on Bladder Cancer. Hautmann RE, Abol-Enein H, Hafez K, Haro I, Mansson W, et al. Urinary diversion. Urology. 2007;69(1 Suppl):17–49. [Google Scholar]
- 5.Kristjánsson A, Davidsson T, Månsson W. Metabolic alterations at different levels of renal function following continent urinary diversion through colonic segments. J Urol. 1997;157:2099–2103. [PubMed] [Google Scholar]
- 6.Skinner DG, Studer UE, Okada K, Aso Y, Hautmann H, Koontz W, et al. Which patients are suitable for continent diversion or bladder substitution following cystectomy or other definitive local treatment? Int J Urol. 1995;2(Suppl 2):105–112. doi: 10.1111/j.1442-2042.1995.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 7.Studer UE, Hautmann RE, Hohenfellner M, Mills RD, Okada Y, Rowland RG, et al. Indications for continent diversion after cystectomy and factors affecting long-term results. Urol Oncol. 1998;4:172–182. doi: 10.1016/s1078-1439(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 8.Stenzl A, Draxl H, Posch B, Colleselli K, Falk M, Bartsch G. The risk of urethral tumors in female bladder cancer: Can the urethra be used for orthotopic reconstruction of the lower urinary tract? J Urol. 1995;153:950–955. [PubMed] [Google Scholar]
- 9.Stein JP, Clark P, Miranda G, Cai J, Groshen S, Skinner DG. Urethral tumor recurrence following cystectomy and urinary diversion: clinical and pathological characteristics in 768 male patients. J Urol. 2005;173:1163–1168. doi: 10.1097/01.ju.0000149679.56884.0f. [DOI] [PubMed] [Google Scholar]
- 10.Stein JP, Cote RJ, Freeman JA, Esrig D, Elmajian DA, Groshen S, et al. Indications for lower urinary tract reconstruction in women after cystectomy for bladder cancer: a pathological review of female cystectomy specimens. J Urol. 1995;154:1329–1333. [PubMed] [Google Scholar]
- 11.Stein JP, Esrig D, Freeman JA, Grossfeld GD, Ginsberg DA, Cote RJ, et al. Prospective pathologic analysis of female cystectomy specimens: risk factors for orthotopic diversion in women. Urology. 1998;51:951–955. doi: 10.1016/s0090-4295(98)00099-5. [DOI] [PubMed] [Google Scholar]
- 12.Herr HW, Donat SM. Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. J Urol. 2001;165:62–64. doi: 10.1097/00005392-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 14.Hautmann RE, Simon J. Ileal neobladder and local recurrence of bladder cancer: patterns of failure and impact on function in men. J Urol. 1999;162:1963–1966. doi: 10.1016/S0022-5347(05)68079-2. [DOI] [PubMed] [Google Scholar]
- 15.Chung MK, Seo HK. Urinary diversion: ileal conduit to orthotopic neobladder substitution. Korean J Urol. 2007;48:565–573. [Google Scholar]
- 16.Leissner J, Hohenfellner R, Thüroff JW, Wolf HK. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int. 2000;85:817–823. doi: 10.1046/j.1464-410x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 17.Wiesner C, Salzer A, Thomas C, Gellermann-Schultes C, Gillitzer R, Hampel C, et al. Cancer-specific survival after radical cystectomy and standardized extended lymphadenectomy for node-positive bladder cancer: prediction by lymph node positivity and density. BJU Int. 2009;104:331–335. doi: 10.1111/j.1464-410X.2009.08403.x. [DOI] [PubMed] [Google Scholar]
- 18.Wright JL, Lin DW, Porter MP. The association between extent of lymphadenectomy and survival among patients with lymph node metastases undergoing radical cystectomy. Cancer. 2008;112:2401–2408. doi: 10.1002/cncr.23474. [DOI] [PubMed] [Google Scholar]
- 19.Park J, Park S, Song C, Doo C, Cho YM, Ahn H, et al. Effectiveness of adjuvant chemotherapy in transitional cell carcinoma of the urinary bladder with lymph node involvement and/or lymphovascular invasion treated by radical cystectomy. Urology. 2007;70:257–262. doi: 10.1016/j.urology.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 20.Kristjánsson A, Mânsson W. Refluxing or nonrefluxing ureteric anastomosis. BJU Int. 1999;84:905–910. doi: 10.1046/j.1464-410x.1999.00395.x. [DOI] [PubMed] [Google Scholar]
- 21.Studer UE, Danuser H, Thalmann GN, Springer JP, Turner WH. Antireflux nipples or afferent tubular segments in 70 patients with ileal low pressure bladder substitutes: long-term results of a prospective randomized trial. J Urol. 1996;156:1913–1917. [PubMed] [Google Scholar]
- 22.Pantuck AJ, Han KR, Perrotti M, Weiss RE, Cummings KB. Ureteroenteric anastomosis in continent urinary diversion: long-term results and complications of direct versus nonrefluxing techniques. J Urol. 2000;163:450–455. doi: 10.1016/s0022-5347(05)67898-6. [DOI] [PubMed] [Google Scholar]
- 23.Kristjansson A, Abol-Enein H, Alm P, Mokhtar AA, Ghoneim MA, Mànsson W. Long-term renal morphology and function following enterocystoplasty (refluxing or anti-reflux anastomosis): an experimental study. Br J Urol. 1996;78:840–846. doi: 10.1046/j.1464-410x.1996.02376.x. [DOI] [PubMed] [Google Scholar]
- 24.Dybner R, Jeter K, Lattimer JK. Comparison of intraluminal pressures in ileal and colonic conduits in children. J Urol. 1972;108:477–479. doi: 10.1016/s0022-5347(17)60779-1. [DOI] [PubMed] [Google Scholar]
- 25.Elder DD, Moisey CU, Rees RW. A long-term follow-up of the colonic conduit operation in children. Br J Urol. 1979;51:462–465. doi: 10.1111/j.1464-410x.1979.tb03579.x. [DOI] [PubMed] [Google Scholar]
- 26.Berglund B, Kock NG. Volume capacity and pressure characteristics of various types of intestinal reservoirs. World J Surg. 1987;11:798–803. doi: 10.1007/BF01656604. [DOI] [PubMed] [Google Scholar]
- 27.Thoeny HC, Sonnenschein MJ, Madersbacher S, Vock P, Studer UE. Is ileal orthotopic bladder substitution with an afferent tubular segment detrimental to the upper urinary tract in the long term? J Urol. 2002;168:2030–2034. doi: 10.1016/S0022-5347(05)64289-9. [DOI] [PubMed] [Google Scholar]
- 28.Song C, Kang T, Hong JH, Kim CS, Ahn H. Changes in the upper urinary tract after radical cystectomy and urinary diversion: a comparison of antirefluxing and refluxing orthotopic bladder substitutes and the ileal conduit. J Urol. 2006;175:185–189. doi: 10.1016/S0022-5347(05)00068-6. [DOI] [PubMed] [Google Scholar]
- 29.Min GE, Song C, Ahn H. Impact of vesico-ureteral reflux on renal function after a radical cystectomy: a comparison of refluxing and antirefluxing orthotopic bladder substitutes. Korean J Urol. 2007;48:933–937. [Google Scholar]
- 30.Rawal S, Kumar P, Kaul R, Raghunath SK, Julka S. The 'pitcher pot' ileal neobladder: early experiences. Jpn J Clin Oncol. 2006;36:717–722. doi: 10.1093/jjco/hyl100. [DOI] [PubMed] [Google Scholar]
- 31.Chang SS, Baumgartner RG, Wells N, Cookson MS, Smith JA., Jr Causes of increased hospital stay after radical cystectomy in a clinical pathway setting. J Urol. 2002;167:208–211. [PubMed] [Google Scholar]
- 32.Chang SS, Cookson MS, Baumgartner RG, Wells N, Smith JA., Jr Analysis of early complications after radical cystectomy: results of a collaborative care pathway. J Urol. 2002;167:2012–2016. [PubMed] [Google Scholar]
- 33.Hollenbeck BK, Miller DC, Taub D, Dunn RL, Khuri SF, Henderson WG, et al. Identifying risk factors for potentially avoidable complications following radical cystectomy. J Urol. 2005;174:1231–1237. doi: 10.1097/01.ju.0000173923.35338.99. [DOI] [PubMed] [Google Scholar]
- 34.Novotny V, Hakenberg OW, Wiessner D, Heberling U, Litz RJ, Oehlschlaeger S, et al. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51:397–401. doi: 10.1016/j.eururo.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Park HK, Kwak C, Byun SS, Lee E, Lee SE. Early removal of nasogastric tube after cystectomy with urinary diversion: Does postoperative ileus risk increase? Urology. 2005;65:905–908. doi: 10.1016/j.urology.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 36.Resnick J, Greenwald DA, Brandt LJ. Delayed gastric emptying and postoperative ileus after nongastric abdominal surgery: part I. Am J Gastroenterol. 1997;92:751–762. [PubMed] [Google Scholar]
- 37.Maffezzini M, Campodonico F, Canepa G, Gerbi G, Parodi D. Current perioperative management of radical cystectomy with intestinal urinary reconstruction for muscle-invasive bladder cancer and reduction of the incidence of postoperative ileus. Surg Oncol. 2008;17:41–48. doi: 10.1016/j.suronc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Inman BA, Harel F, Tiguert R, Lacombe L, Fradet Y. Routine nasogastric tubes are not required following cystectomy with urinary diversion: a comparative analysis of 430 patients. J Urol. 2003;170:1888–1891. doi: 10.1097/01.ju.0000092500.68655.48. [DOI] [PubMed] [Google Scholar]
- 39.Cheatham ML, Chapman WC, Key SP, Sawyers JL. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg. 1995;221:469–476. doi: 10.1097/00000658-199505000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vermeulen H, Storm-Versloot MN, Busch OR, Ubbink DT. Nasogastric intubation after abdominal surgery: a meta-analysis of recent literature. Arch Surg. 2006;141:307–314. doi: 10.1001/archsurg.141.3.307. [DOI] [PubMed] [Google Scholar]
- 41.Kouba EJ, Wallen EM, Pruthi RS. Gum chewing stimulates bowel motility in patients undergoing radical cystectomy with urinary diversion. Urology. 2007;70:1053–1056. doi: 10.1016/j.urology.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 42.Choi H, Kang SH, Yoon DK, Kang SG, Ko HY, Moon DG, et al. Chewing gum has a stimulatory effect on bowel motility in patients after open or robotic radical cystectomy for bladder cancer: a prospective randomized comparative study. Urology. 2010 doi: 10.1016/j.urology.2010.06.042. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Wood DP, Jr, Bianco FJ, Jr, Pontes JE, Heath MA, DaJusta D. Incidence and significance of positive urine cultures in patients with an orthotopic neobladder. J Urol. 2003;169:2196–2199. doi: 10.1097/01.ju.0000067909.98836.91. [DOI] [PubMed] [Google Scholar]
- 44.Wullt B, Holst E, Steven K, Carstensen J, Pedersen J, Gustafsson E, et al. Microbial flora in ileal and colonic neobladders. Eur Urol. 2004;45:233–239. doi: 10.1016/j.eururo.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Wullt B, Agace W, Mansson W. Bladder, bowel and bugs--bacteriuria in patients with intestinal urinary diversion. World J Urol. 2004;22:186–195. doi: 10.1007/s00345-004-0432-x. [DOI] [PubMed] [Google Scholar]
- 46.Varol C, Studer UE. Managing patients after an ileal orthotopic bladder substitution. BJU Int. 2004;93:266–270. doi: 10.1111/j.1464-410x.2004.04599.x. [DOI] [PubMed] [Google Scholar]
- 47.Abol-Enein H, Ghoneim MA. Functional results of orthotopic ileal neobladder with serous-lined extramural ureteral reimplantation: experience with 450 patients. J Urol. 2001;165:1427–1432. [PubMed] [Google Scholar]
- 48.Hautmann RE. Urinary diversion: ileal conduit to neobladder. J Urol. 2003;169:834–842. doi: 10.1097/01.ju.0000029010.97686.eb. [DOI] [PubMed] [Google Scholar]
- 49.Light JK. Continence mechanisms following orthotopic bladder substitution. Scand J Urol Nephrol Suppl. 1992;142:95–97. [PubMed] [Google Scholar]
- 50.Steers WD. Voiding dysfunction in the orthotopic neobladder. World J Urol. 2000;18:330–337. doi: 10.1007/s003450000146. [DOI] [PubMed] [Google Scholar]
- 51.Steven K, Poulsen AL. The orthotopic Kock ileal neobladder: functional results, urodynamic features, complications and survival in 166 men. J Urol. 2000;164:288–295. [PubMed] [Google Scholar]
- 52.Arai Y, Okubo K, Konami T, Kin S, Kanba T, Okabe T, et al. Voiding function of orthotopic ileal neobladder in women. Urology. 1999;54:44–49. doi: 10.1016/s0090-4295(99)00027-8. [DOI] [PubMed] [Google Scholar]
- 53.Nesrallah LJ, Srougi M, Dall'Oglio MF. Orthotopic ileal neobladder: the influence of reservoir volume and configuration on urinary continence and emptying properties. BJU Int. 2004;93:375–378. doi: 10.1111/j.1464-410x.2003.04620.x. [DOI] [PubMed] [Google Scholar]
- 54.Hugonnet CL, Danuser H, Springer JP, Studer UE. Urethral sensitivity and the impact on urinary continence in patients with an ileal bladder substitute after cystectomy. J Urol. 2001;165:1502–1505. [PubMed] [Google Scholar]
- 55.Madersbacher S, Möhrle K, Burkhard F, Studer UE. Long-term voiding pattern of patients with ileal orthotopic bladder substitutes. J Urol. 2002;167:2052–2057. [PubMed] [Google Scholar]
- 56.Tchetgen MB, Sanda MG, Montie JE, Faerber GJ. Collagen injection for the treatment of incontinence after cystectomy and orthotopic neobladder reconstruction in women. J Urol. 2000;163:212–214. [PubMed] [Google Scholar]
- 57.Ghoneim MA, Shaaban AA, Mahran MR, Kock NG. Further experience with the urethral Kock pouch. J Urol. 1992;147:361–365. doi: 10.1016/s0022-5347(17)37238-5. [DOI] [PubMed] [Google Scholar]
- 58.El Bahnasawy MS, Osman Y, Gomha MA, Shaaban AA, Ashamallah A, Ghoneim MA. Nocturnal enuresis in men with an orthotopic ileal reservoir: urodynamic evaluation. J Urol. 2000;164:10–13. doi: 10.1097/00005392-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Studer UE, Danuser H, Hochreiter W, Springer JP, Turner WH, Zingg EJ. Summary of 10 years' experience with an ileal low-pressure bladder substitute combined with an afferent tubular isoperistaltic segment. World J Urol. 1996;14:29–39. doi: 10.1007/BF01836342. [DOI] [PubMed] [Google Scholar]
- 60.Lee KS, Montie JE, Dunn RL, Lee CT. Hautmann and Studer orthotopic neobladders: a contemporary experience. J Urol. 2003;169:2188–2191. doi: 10.1097/01.ju.0000063941.31687.26. [DOI] [PubMed] [Google Scholar]
- 61.Stein JP, Lieskovsky G, Ginsberg DA, Bochner BH, Skinner DG. The T pouch: an orthotopic ileal neobladder incorporating a serosal lined ileal antireflux technique. J Urol. 1998;159:1836–1842. doi: 10.1016/S0022-5347(01)63170-7. [DOI] [PubMed] [Google Scholar]
- 62.Smith E, Yoon J, Theodorescu D. Evaluation of urinary continence and voiding function: early results in men with neo-urethral modification of the Hautmann orthotopic neobladder. J Urol. 2001;166:1346–1349. doi: 10.1016/s0022-5347(05)65766-7. [DOI] [PubMed] [Google Scholar]
- 63.Hautmann RE, Paiss T, de Petriconi R. The ileal neobladder in women: 9 years of experience with 18 patients. J Urol. 1996;155:76–81. doi: 10.1016/s0022-5347(01)66546-7. [DOI] [PubMed] [Google Scholar]
- 64.Ghoneim MA. Orthotopic bladder substitution in women following cystectomy for bladder cancer. Urol Clin North Am. 1997;24:225–239. doi: 10.1016/s0094-0143(05)70365-7. [DOI] [PubMed] [Google Scholar]
- 65.Mikuma N, Hirose T, Yokoo A, Tsukamoto T. Voiding dysfunction in ileal neobladder. J Urol. 1997;158:1365–1368. [PubMed] [Google Scholar]
- 66.Shimogaki H, Okada H, Fujisawa M, Arakawa S, Kawabata G, Kamidono S, et al. Long-term experience with orthotopic reconstruction of the lower urinary tract in women. J Urol. 1999;161:573–577. [PubMed] [Google Scholar]
- 67.Ali-el-Dein B, el-Sobky E, Hohenfellner M, Ghoneim MA. Orthotopic bladder substitution in women: functional evaluation. J Urol. 1999;161:1875–1880. [PubMed] [Google Scholar]
- 68.Mills RD, Studer UE. Female orthotopic bladder substitution: a good operation in the right circumstances. J Urol. 2000;163:1501–1504. doi: 10.1016/s0022-5347(05)67651-3. [DOI] [PubMed] [Google Scholar]
- 69.Stenzl A, Colleselli K, Poisel S, Feichtinger H, Bartsch G. The use of neobladders in women undergoing cystectomy for transitional-cell cancer. World J Urol. 1996;14:15–21. doi: 10.1007/BF01836339. [DOI] [PubMed] [Google Scholar]
- 70.Cancrini A, De Carli P, Fattahi H, Pompeo V, Cantiani R, Von Heland M. Orthotopic ileal neobladder in female patients after radical cystectomy: 2-year experience. J Urol. 1995;153:956–958. [PubMed] [Google Scholar]
- 71.Gillon G, Mundy AR. The dissolution of urinary mucus after cystoplasty. Br J Urol. 1989;63:372–374. doi: 10.1111/j.1464-410x.1989.tb05220.x. [DOI] [PubMed] [Google Scholar]
- 72.Bushman W, Howards SS. The use of urea for dissolution of urinary mucus in urinary tract reconstruction. J Urol. 1994;151:1036–1037. doi: 10.1016/s0022-5347(17)35170-4. [DOI] [PubMed] [Google Scholar]
- 73.Månsson W, Davidsson T, Könyves J, Liedberg F, Månsson A, Wullt B. Continent urinary tract reconstruction - the Lund experience. BJU Int. 2003;92:271–276. doi: 10.1046/j.1464-410x.2003.04330.x. [DOI] [PubMed] [Google Scholar]
- 74.Leibovitch IJ, Ramon J, Chaim JB, Goldwasser B. Increased urinary mucus production: a sequela of cystography following enterocystoplasty. J Urol. 1991;145:736–737. doi: 10.1016/s0022-5347(17)38438-0. [DOI] [PubMed] [Google Scholar]
- 75.Gatti R, Ferretti S, Bucci G, Simonazzi M, Cortellini P, Orlandini G. Histological adaptation of orthotopic ileal neobladder mucosa: 4-year follow-up of 30 patients. Eur Urol. 1999;36:588–594. doi: 10.1159/000020053. [DOI] [PubMed] [Google Scholar]
- 76.Aragona F, De Caro R, Parenti A, Artibani W, Bassi P, Munari PF, et al. Structural and ultrastructural changes in ileal neobladder mucosa: a 7-year follow-up. Br J Urol. 1998;81:55–61. doi: 10.1046/j.1464-410x.1998.00514.x. [DOI] [PubMed] [Google Scholar]
- 77.Haupt G, Pannek J, Knopf HJ, Schulze H, Senge T. Rupture of ileal neobladder due to urethral obstruction by mucous plug. J Urol. 1990;144:740–741. doi: 10.1016/s0022-5347(17)39571-x. [DOI] [PubMed] [Google Scholar]
- 78.Benderev TV. Acetylcysteine for urinary tract mucolysis. J Urol. 1988;139:353–354. doi: 10.1016/s0022-5347(17)42412-8. [DOI] [PubMed] [Google Scholar]
- 79.Mills RD, Studer UE. Metabolic consequences of continent urinary diversion. J Urol. 1999;161:1057–1066. [PubMed] [Google Scholar]
- 80.Fosså SD, Heilo A, Børmer O. Unexpectedly high serum methotrexate levels in cystectomized bladder cancer patients with an ileal conduit treated with intermediate doses of the drug. J Urol. 1990;143:498–501. doi: 10.1016/s0022-5347(17)40001-2. [DOI] [PubMed] [Google Scholar]
- 81.Bowyer GW, Davies TW. Methotrexate toxicity associated with an ileal conduit. Br J Urol. 1987;60:592. doi: 10.1111/j.1464-410x.1987.tb05050.x. [DOI] [PubMed] [Google Scholar]
- 82.Sagalowsky AI, Frenkel EP. Cobalamin profiles in patients after urinary diversion. J Urol. 2002;167:1696–1700. [PubMed] [Google Scholar]
- 83.Dutta SC, Chang SC, Coffey CS, Smith JA, Jr, Jack G, Cookson MS. Health related quality of life assessment after radical cystectomy: comparison of ileal conduit with continent orthotopic neobladder. J Urol. 2002;168:164–167. [PubMed] [Google Scholar]
- 84.Autorino R, Quarto G, Di Lorenzo G, De Sio M, Perdonà S, Giannarini G, et al. Health related quality of life after radical cystectomy: comparison of ileal conduit to continent orthotopic neobladder. Eur J Surg Oncol. 2009;35:858–864. doi: 10.1016/j.ejso.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Hara I, Miyake H, Hara S, Gotoh A, Nakamura I, Okada H, et al. Health-related quality of life after radical cystectomy for bladder cancer: a comparison of ileal conduit and orthotopic bladder replacement. BJU Int. 2002;89:10–13. doi: 10.1046/j.1464-4096.2001.01475.x. [DOI] [PubMed] [Google Scholar]
- 86.Sogni F, Brausi M, Frea B, Martinengo C, Faggiano F, Tizzani A, et al. Morbidity and quality of life in elderly patients receiving ileal conduit or orthotopic neobladder after radical cystectomy for invasive bladder cancer. Urology. 2008;71:919–923. doi: 10.1016/j.urology.2007.11.125. [DOI] [PubMed] [Google Scholar]
- 87.Hobisch A, Tosun K, Kinzl J, Kemmler G, Bartsch G, Höltl L, et al. Life after cystectomy and orthotopic neobladder versus ileal conduit urinary diversion. Semin Urol Oncol. 2001;19:18–23. [PubMed] [Google Scholar]
- 88.Somani BK, Gimlin D, Fayers P, N'Dow J. Quality of life and body image for bladder cancer patients undergoing radical cystectomy and urinary diversion--a prospective cohort study with a systematic review of literature. Urology. 2009;74:1138–1143. doi: 10.1016/j.urology.2009.05.087. [DOI] [PubMed] [Google Scholar]
- 89.Porter MP, Penson DF. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic review and critical analysis of the literature. J Urol. 2005;173:1318–1322. doi: 10.1097/01.ju.0000149080.82697.65. [DOI] [PubMed] [Google Scholar]
- 90.Gerharz EW, Månsson A, Hunt S, Skinner EC, Månsson W. Quality of life after cystectomy and urinary diversion: an evidence based analysis. J Urol. 2005;174:1729–1736. doi: 10.1097/01.ju.0000176463.40530.05. [DOI] [PubMed] [Google Scholar]