Abstract
Purpose
The prognosis of patients with malignant pheochromocytoma is poor, but the predictive factors are not well understood. We aimed to identify the clinical characteristics predictive of malignancy after initial surgical removal in patients with pheochromocytoma.
Materials and Methods
We retrospectively reviewed the records of 152 patients diagnosed with pheochromocytoma, including 5 (3.3%) with metastasis at the time of the initial surgical excision and 12 (7.9%) who developed metastasis during follow-up. To determine the factors predictive of malignancy, we compared clinical, radiographical, and urinary chemical findings between patients with benign and malignant disease. Mean follow-up was 41.5 months (range, 0.9-298 months) after surgery.
Results
Malignant tumors were significantly larger than benign tumors (11.1±4.0 cm vs. 6.2±3.4 cm, p<0.001), and postoperative persistence of arterial hypertension was more frequent after removal of malignant than benign tumors (p=0.001). Among the 147 patients without metastatic disease at diagnosis, those who developed metastasis had significantly lower concentrations of urinary catecholamine metabolites per unit of tumor, including vanillylmandelic acid (1.2 vs. 3.7 mg/day/cm, p=0.049), epinephrine (4.5 vs. 168.9 µg/day/cm, p=0.008), and norepinephrine (13.1 vs. 121.8 mg/day/cm, p<0.001). The overall 5-year metastasis-free survival rate was 84.4% and was significantly higher in patients with smaller tumors (≤5.5 vs. >5.5 cm; 90.6% vs. 81.2%, p=0.025) and higher 24-hour secretion of vanillylmandelic acid (>2.1 vs. ≤2.1 mg/day/cm; 94.9% vs. 70.9%, p=0.019).
Conclusions
Large tumor size (>5.5 cm) and minimally elevated 24-hour urinary vanillylmandelic acid (≤2.1 mg/day/cm) were significantly associated with a higher probability of a malignant pheochromocytoma portending a lower metastasis-free survival and mandating more rigorous follow-up after surgery.
Keywords: Adrenal gland neoplasms, Catecholamines, Pheochromocytoma, Tumor burden
INTRODUCTION
Pheochromocytoma is a catecholamine-secreting tumor that emerges from the chromaffin cells of the adrenal medulla, usually manifesting as a benign tumor but occasionally becoming malignant [1,2]. Because the long-term prognosis of patients with malignant pheochromocytoma is unclear or poor, identification of factors associated with malignancy could aid in modifying follow-up plans and could enhance disease-specific survival. Previous studies on malignant pheochromocytoma have mostly been anecdotal, and efforts to clinically, biochemically, and radiographically distinguish between benign and malignant pheochromocytoma have been inconsistent [3,4]. Partly, these may have been because of the low incidence of the tumor and the limited postoperative follow-up with inconsistent protocols. Therefore, from a single institutional database, we aimed to identify potential clinical characteristics predictive of differentiating malignant pheochromocytomas from benign pheochromocytomas.
MATERIALS AND METHODS
Our study consisted of 152 patients treated surgically between 1989 and 2008 at our hospital. The 152 patients consisted of 81 men and 71 women, aged 18 to 76 years (median, 46.5 years). Of these, 135 had benign tumors and 17 had malignant tumors (5 at the initial diagnosis and 12 subsequently diagnosed during follow-up). The clinical diagnosis of malignancy in the 17 patients was based on the presence of metastatic lesions either at diagnosis or during follow-up.
Preoperatively, tumors were localized by computed tomography (CT) and/or magnetic resonance imaging (MRI). Also, metaiodobenzylguanidine-131 (MIBG) scans were performed in patients with suspected extra-adrenal or malignant pheochromocytoma. Twenty-four-hour urinary concentrations of vanillylmandelic acid (VMA), epinephrine, norepinephrine, metanephrine, and dopamine were assessed in most patients. Postoperative follow-up included blood pressure measurement, CT, and MIBG scan every 6 months for 2 years and annually thereafter in the absence of recurrence. Urinary catecholamines were followed when elevated levels were observed at 6 months postoperatively. Patients were followed for a mean of 41.5 months (range, 0.9-298 months) after surgery.
Factors compared between the two groups included clinical (age, gender, symptoms, interval to diagnosis from symptom onset, associated syndromes, postoperative hypertension), radiographical (tumor location, tumor size), and biochemical (urinary catecholamine levels) variables. The 24-hour urinary catecholamine levels per unit diameter of tumor were calculated to account for the differences in tumor size.
For statistical analyses, we used the chi-squared test and the Mann-Whitney U test for comparison between the benign and the malignant characteristics. Cutoff values for tumor size and VMA per unit of tumor diameter were calculated by receiver operating characteristic (ROC) analysis for predicting the 5-year metastasis-free survival rate. Survival curves were determined by using the Kaplan-Meier method and were compared by the log-rank test. For all analyses, SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used, and a p-value<0.05 was set for significance.
RESULTS
There were 17 patients with metastatic pheochromocytomas. Initially metastatic lesions (n=5) were found in the lung (2), liver (1), bone (1), and distant lymph node (1). Metachronous metastases occurred on average 49.4 months (range, 5.9-194.8 months) postoperatively. The locations of recurrent disease were liver (6), bone (4), lung and pleura (4), and bladder (1). Patients were diagnosed with pheochromocytoma, either benign or malignant, within 0.02 months to 7.2 years (median, 1.8 years) from the onset of symptoms. Familial syndromes, including von Hippel-Lindau syndrome, were present in three patients with benign tumors but in none of those with malignancies. Of the 152 tumors, 137 (90.1%) were adrenal, 14 (9.2%) were solitary extra-adrenal, and 1 (0.7%) was multiple extra-adrenal. In particular, 11 (8%) of the 135 benign tumors were extra-adrenal, compared with 4 (24%) of the 17 malignant tumors. The single multiple extra-adrenal tumor was malignant, whereas the seven bilateral adrenal tumors were benign. Of the extra-adrenal pheochromocytomas, two malignant and five benign tumors were located in the periaortic (Zuckerkandl) bodies, and the remainder were in the retroperitoneum, bladder, and other organs (Table 1).
TABLE 1.
Comparison of clinical characteristics of patients with benign and malignant pheochromocytomas
VMA: vanillylmandelic acid, a: mean±SE
Malignant tumors were significantly larger than benign pheochromocytomas (11.1±4.0 vs. 6.2±3.4 cm, p<0.001) (Table 1). However, age and gender showed no significant correlations with malignancy. In addition, the most frequent symptoms in patients with both benign and malignant pheochromocytomas were headache, palpitations, and excessive sweating, without differences between the groups. Whereas blood pressure generally became normal after adrenalectomy, arterial hypertension persisted after removal more frequently in malignant tumors (23.5% vs. 0.7%, p=0.001).
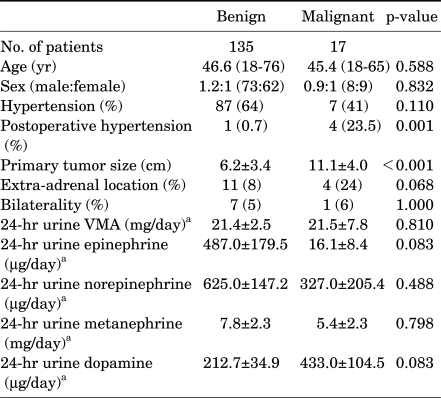
Preoperatively, total 24-hour urinary excretion of the catecholamines VMA, epinephrine, norepinephrine, metanephrine, and dopamine was significantly enhanced in patients with both malignant and benign pheochromocytomas, without differences between the groups (Table 1). However, when the urinary catecholamines excreted per unit of tumor were compared, patients with malignant tumors excreted significantly lower amounts of epinephrine (4.0 vs. 168.9 µg/day/cm, p=0.010) than did patients with benign tumors (Fig. 1A). Furthermore, among the 147 patients without metastatic disease at the initial diagnosis, those who later developed metastasis also had significantly reduced 24-hour urinary excretion of the catecholamine metabolites VMA (1.2 vs. 3.7 mg/day/cm, p=0.049), epinephrine (4.5 vs. 168.9 µg/day/cm, p=0.008), and norepinephrine (13.1 vs. 121.8 mg/day/cm, p<0.001) than did those with benign tumors (Fig. 1B).
FIG. 1.
Comparison of 24-hour urinary catecholamine secretion in patients with benign and malignant pheochromocytomas (A: benign vs. all cases malignant, B: benign vs. postoperative recurrent malignant).
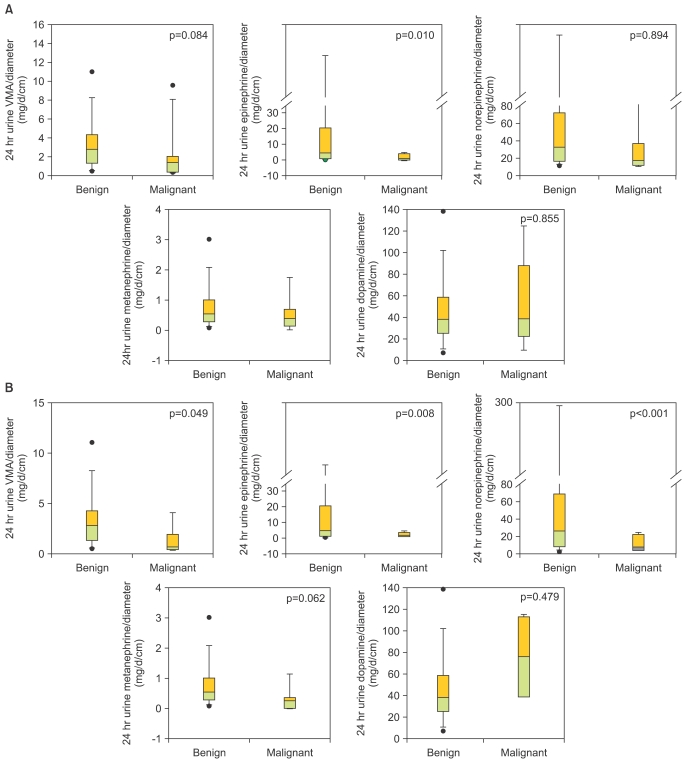
The overall 5-year metastasis-free survival rate was 84.4%. The areas under the ROC curves for predicting the 5-year metastasis-free survival rate were 0.783 (95% CI: 0.652-0.914) for tumor size and 0.767 (95% CI: 0.674-0.920) for VMA per unit of tumor diameter. The tumor size cutoff for prediction of survival rates was calculated to be 5.5 cm (sensitivity 86.7%, specificity 56.7%), and the VMA per unit of tumor diameter cutoff value was calculated to be 2.1 mg/day/cm (sensitivity 85.5%, specificity 58.1%). Survival rates were significantly higher in patients with smaller tumors (≤5.5 cm vs. >5.5 cm; 90.6 vs. 81.2%, p=0.025) and in those with higher 24-hour urinary VMA per unit of tumor diameter (>2.1 vs. ≤2.1 mg/day/cm; 94.9 vs. 70.9%, p=0.019) (Fig. 2). The 5-year survival rate of patients with malignant pheochromocytoma was 11.8%. Among patients with malignant disease, 1 patient received palliative radiotherapy and 5 received palliative MIBG therapy, resulting in modulation of the symptoms of catecholamine hyperproduction. Although six patients received palliative combined chemotherapy (cyclophosphamide/adriamycin), none demonstrated response to any chemotherapeutic agents and all progressed.
FIG. 2.
Metastasis-free survival relative to tumor size (A) and 24-hour urinary secretion of VMA/unit of tumor diameter (B).
DISCUSSION
Clinical behavior, including invasion of adjacent tissues and the presence of metastases at non-chromaffin sites, is the only currently accepted indication of malignant pheochromocytoma [5,6]. We found that the incidence of malignant pheochromocytomas among 152 patients with pheochromocytomas was 11.2%, in good agreement with the 8% to 12.5% previously reported [7-11]. We also found that the 5-year survival rate of our patients with malignant pheochromocytoma was 11.8%, which was thus somewhat lower than the 20% previously reported [12]. Although none of our patients with malignant pheochromocytomas were alive after 10 years, others have reported somewhat better results [2]. The poor prognosis of patients with malignancy is thought to be attributable to dissemination to other organs, along with the heart failure associated with hypercatecholaminemia [6,13-18]. Thus, long-term prognosis will not improve unless other features that can predict malignant behavior can be identified.
Previous studies have not been successful in identifying distinctive factors for malignant pheochromocytoma. Although some histopathological features have been suggested to be associated with an increased incidence of malignancy, including tumor necrosis, mitosis rate >3/30 HPF, capsular invasion, vascular invasion, large nests with central degeneration, a lack of hyaline globules, a high nuclear/cytoplasmic ratio, monotony of cytological pattern, and spindle cell patterns [6,17,19], none of these differences is sufficiently diagnostic. Moreover, molecular markers, including telomerase [20], inhibins, activins [21], endothelial Per-ARNT-Sim domain protein-1, vascular endothelial growth factor, endothelin receptor type B [22], and cyclooxygenase [21], have been found to be significant but unreliable and not readily applicable for distinguishing benign from malignant pheochromocytomas. Moreover, in assaying clinical parameters, we found that age, gender, symptoms, and delay in diagnosis did not correlate with the presence of either benign or malignant pheochromocytoma. Although extra-adrenal tumor location was associated with malignancy to some extent, the relationship was not significant (p=0.068).
We found, however, that malignant tumors were significantly larger than were benign tumors at presentation (11.1±4.0 vs. 6.2±3.4 cm, p<0.001). Although similar results have been reported (median tumor size, 8 cm vs. 5.1 cm, respectively), no cutoff values for size and weight were found to differentiate benign from malignant tumors [23]. In contrast, we found that the use of a size cutoff of 5.5 cm could distinguish 5-year metastasis-free survival rates in patients with benign and malignant tumors (90.6% vs. 81.2%, p=0.025). In contrast with studies reporting that malignant tumors secrete more metanephrines than do benign tumors [19,24,25], we found that the 24-hour urinary excretion per tumor diameter unit of the catecholamine metabolites VMA and epinephrine was significantly lower in patients with malignant tumors than in those with benign tumors, whereas the total excretions were similar. In particular, patients without metastatic disease at diagnosis showed more distinct differences in the secretion of the urinary catecholamine metabolites VMA, epinephrine, norepinephrine, and metanephrine. Necrosis in the tumor center, especially in large tumors, has been suggested to account for lower secretion in hormone-producing tumors [26]. However, in our study, gross tumor necrosis was observed in only 5 of the patients and our results demonstrate that the catecholamines produced per unit of tumor instead of the total mass were significantly lower. We speculate that tumors cells with malignant potential may intrinsically carry less functional capacity because of under-differentiation.
In agreement with earlier studies, which showed that persistent postoperative arterial hypertension was more common in patients with malignant than benign pheochromocytomas, we found that arterial hypertension persisted after tumor removal in 1 of 135 (0.7%) patients with benign tumors and in 4 of 17 (23.5%) with malignant pheochromocytomas, likely due to occult metastasis. Monitoring arterial blood pressure after successful surgery may provide a clue for potential development of subsequent metastasis. Postoperative metastasis or recurrence was observed within 4.2 years of surgical treatment in 9 of 12 patients (75%) with malignancy, with a median time from primary excision to metastasis of 16.8 months (range, 4.8-194.8 months). These findings suggest that patients at risk should undergo more rigorous follow-up with the use of imaging modalities such as postoperative CT and MIBG scanning for at least 2 years and with the use of biochemical modalities such as low 24-hour urinary catecholamine excretion within 4 months after surgery.
The retrospective design of our study and the relatively small number of patients from a single institution limit the interpretation of our study results. Furthermore, in the urinary catecholamine analysis, although we noted a higher significance with epinephrine and norepinephrine, we suggested a cutoff for VMA because it is the most widely and specifically used index for diagnosing pheochromocytoma, and data were not available for some of the other indexes. Thus, the predictive characteristics identified here require confirmation in a larger, prospective, multicenter study. In particular, further research on the long-term prognostic value of pre- and postoperative 24-hour urinary catecholamine excretion would seem worthwhile.
CONCLUSIONS
In patients with pheochromocytoma, large tumor size (>5.5 cm) and minimally elevated 24-hour urinary VMA (≤2.1 mg/day/cm) were significantly associated with a higher probability of the tumor being a malignancy, thus portending a lower metastasis-free survival. Thus, patients with these characteristics mandate more rigorous follow-up after surgery.
Footnotes
The authors have nothing to disclose.
References
- 1.Guerrero MA, Schreinemakers JM, Vriens MR, Suh I, Hwang J, Shen WT, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009;209:727–732. doi: 10.1016/j.jamcollsurg.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Ram CV, Fierro-Carrion GA. Pheochromocytoma. Semin Nephrol. 1995;15:126–137. [PubMed] [Google Scholar]
- 3.Ahlman H. Malignant pheochromocytoma: state of the field with future projections. Ann N Y Acad Sci. 2006;1073:449–464. doi: 10.1196/annals.1353.049. [DOI] [PubMed] [Google Scholar]
- 4.Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev. 2003;24:539–553. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- 5.Adler JT, Meyer-Rochow GY, Chen H, Benn DE, Robinson BG, Sippel RS, et al. Pheochromocytoma: current approaches and future directions. Oncologist. 2008;13:779–793. doi: 10.1634/theoncologist.2008-0043. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–436. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 7.Arnaldi G, Masini AM, Giacchetti G, Taccaliti A, Faloia E, Mantero F. Adrenal incidentaloma. Braz J Med Biol Res. 2000;33:1177–1189. doi: 10.1590/s0100-879x2000001000007. [DOI] [PubMed] [Google Scholar]
- 8.Melicow MM. One hundred cases of pheochromocytoma (107 tumors) at the Columbia-Presbyterian Medical Center, 1926-1976: a clinicopathological analysis. Cancer. 1977;40:1987–2004. doi: 10.1002/1097-0142(197711)40:5<1987::aid-cncr2820400502>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Modlin IM, Farndon JR, Shepherd A, Johnston ID, Kennedy TL, Montgomery DA, et al. Phaeochromocytomas in 72 patients: clinical and diagnostic features, treatment and long term results. Br J Surg. 1979;66:456–465. doi: 10.1002/bjs.1800660704. [DOI] [PubMed] [Google Scholar]
- 10.Scott HW, Jr, Reynolds V, Green N, Page D, Oates JA, Robertson D, et al. Clinical experience with malignant pheochromocytomas. Surg Gynecol Obstet. 1982;154:801–818. [PubMed] [Google Scholar]
- 11.Tischler AS. Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med. 2008;132:1272–1284. doi: 10.5858/2008-132-1272-PAEPU. [DOI] [PubMed] [Google Scholar]
- 12.John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999;53:679–683. doi: 10.1016/s0090-4295(98)00612-8. [DOI] [PubMed] [Google Scholar]
- 13.Guo JZ, Gong LS, Chen SX, Luo BY, Xu MY. Malignant pheochromocytoma: diagnosis and treatment in fifteen cases. J Hypertens. 1989;7:261–266. [PubMed] [Google Scholar]
- 14.Mahoney EM, Harrison JH. Malignant pheochromocytoma: clinical course and treatment. J Urol. 1977;118:225–229. doi: 10.1016/s0022-5347(17)57951-3. [DOI] [PubMed] [Google Scholar]
- 15.Mornex R, Badet C, Peyrin L. Malignant pheochromocytoma: a series of 14 cases observed between 1966 and 1990. J Endocrinol Invest. 1992;15:643–649. doi: 10.1007/BF03345808. [DOI] [PubMed] [Google Scholar]
- 16.Proye C, Vix M, Goropoulos A, Kerlo P, Lecomte-Houcke M. High incidence of malignant pheochromocytoma in a surgical unit. 26 cases out of 100 patients operated from 1971 to 1991. J Endocrinol Invest. 1992;15:651–663. doi: 10.1007/BF03345810. [DOI] [PubMed] [Google Scholar]
- 17.Schlumberger M, Gicquel C, Lumbroso J, Tenenbaum F, Comoy E, Bosq J, et al. Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest. 1992;15:631–642. doi: 10.1007/BF03345807. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida S, Hatori M, Noshiro T, Kimura N, Kokubun S. Twenty-six-years' survival with multiple bone metastasis of malignant pheochromocytoma. Arch Orthop Trauma Surg. 2001;121:598–600. doi: 10.1007/s004020100305. [DOI] [PubMed] [Google Scholar]
- 19.Linnoila RI, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol. 1990;21:1168–1180. doi: 10.1016/0046-8177(90)90155-x. [DOI] [PubMed] [Google Scholar]
- 20.Bautista CV, Felis CP, Espinet JM, García JB, Salas JV. Telomerase activity is a prognostic factor for recurrence and survival in rectal cancer. Dis Colon Rectum. 2007;50:611–620. doi: 10.1007/s10350-006-0820-y. [DOI] [PubMed] [Google Scholar]
- 21.Salmenkivi K, Haglund C, Ristimäki A, Arola J, Heikkilä P. Increased expression of cyclooxygenase-2 in malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:5615–5619. doi: 10.1210/jcem.86.11.8052. [DOI] [PubMed] [Google Scholar]
- 22.Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161:1235–1246. doi: 10.1016/S0002-9440(10)64400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551–566. doi: 10.1097/00000478-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90:2110–2116. doi: 10.1210/jc.2004-1398. [DOI] [PubMed] [Google Scholar]
- 25.Plouin PF, Chatellier G, Fofol I, Corvol P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension. 1997;29:1133–1139. doi: 10.1161/01.hyp.29.5.1133. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Obara T, Yamashita T, Kanbe M, Iihara M. Pheochromocytomas: tendency to degenerate and cause paroxysmal hypertension. World J Surg. 1996;20:923–926. doi: 10.1007/s002689900140. [DOI] [PubMed] [Google Scholar]