Abstract
Glial cell line-derived neurotrophic factor (GDNF) has been identified as a potent survival factor for both central and peripheral neurons. GDNF has been shown to be a potent survival factor for motor neurons during programmed cell death and continuous treatment with GDNF maintains hyperinnervation of skeletal muscle in adulthood. However, little is known about factors regulating normal production of endogenous GDNF in skeletal muscle. This study aimed to examine the role that motor neurons play in regulating GDNF secretion by skeletal muscle. A co-culture of skeletal muscle cells (C2C12) and cholinergic neurons, glioma × neuroblastoma hybrid cells (NG108-15) were used to create nerve muscle interactions in vitro. Acetylcholine receptors (AChRs) on nerve-myotube co-cultures were blocked with alpha bungarotoxin (α-BTX). GDNF protein content in cells and in culture medium was analyzed by enzyme-linked immunosorbant assay (ELISA) and western blotting. GDNF localization was examined by immunocytochemistry. The nerve-muscle co-culture study indicated that the addition of motor neurons to skeletal muscle cells reduced the secretion of GDNF by skeletal muscle. The results also showed that blocking AChRs with α-BTX reversed the action of neural cells on GDNF secretion by skeletal muscle. Although ELISA results showed no GDNF in differentiated NG108-15 cells grown alone, immunocytochemical analysis showed that GDNF was localized in NG108-15 cells co-cultured with C2C12 myotubes. These results suggest that motor neurons may be regulating their own supply of GDNF secreted by skeletal muscle and that activation of AChRs may be involved in this process.
Keywords: GDNF, neuromuscular junction, nerve-muscle co-culture, secretion, C2C12 cells, NG108-15 cells
1. Introduction
Glial cell line–derived neurotrophic factor (GDNF) was purified from B49 glial cells and was first identified as a potent survival factor for dopaminergic neurons in the central nervous system (CNS) (Lin et al. 1993; Schatz et al. 1999). Later, GDNF was reported to be a trophic factor for other populations of neurons in the peripheral nervous system (PNS) including spinal motor neurons (Caumont et al. 2006; Henderson et al. 1994). GDNF is widely distributed in various neuronal tissues of the central and peripheral nervous systems (Nosrat et al. 1996; Springer et al. 1995; Woodbury et al. 1998) and is produced by non-neuronal cells including skeletal muscle (Nagano and Suzuki, 2003; Suzuki et al. 1998; Trupp et al. 1995). The presence of GDNF in skeletal muscle at the neuromuscular junction (NMJ) suggests a target-derived action on motor neurons, in which GDNF is retrogradely transported along axons to the target neuron’s cell body, through a receptor-mediated process (Nguyen et al. 1998).
GDNF has been shown to be a potent survival factor for motor neurons. GDNF was found to be 75 times more potent than brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) in preventing programmed cell death in cultured motor neurons (Henderson et al. 1994). GDNF administration was associated with retention of nearly all facial motor neurons that had been deprived contact with their targets in neonatal rats (Houenou et al.1996). It was noted that only GDNF was able to prevent nearly 100% of death and atrophy of motor neurons compared to BDNF, nerve growth factor (NGF), and neurotrophin-4/5 (NT-4/5) (Henderson et al. 1994). Widespread loss of motor neurons has been observed in GDNF knockout mice (Moore et al. 1996). It was also shown that survival of motor neurons depends on GDNF secreted by skeletal muscle and it may be sufficient to provide support to the motor nerve innervating muscle during development (Angka et al. 2008). Mice lacking GDNF displayed death of motor neurons, while mice treated with GDNF in absence of skeletal muscle retained motor neurons (Angka et al. 2008). It has been shown that during synaptic formation, the number of axons innervating skeletal muscle depends on the concentration of GDNF protein available (Nguyen et al. 1998). Hyperinnervation at the NMJ is observed in transgenic mice expressing high levels of GDNF in skeletal muscle (myo-GDNF), whereas overexpression of NT-3 and NT-4 had no effect (Nguyen et al. 1998). Moreover, mice overexpressing GDNF under myosin light chain 1(MLC1) promoter were shown to maintain hyperinnervation in adulthood (Zwick et al. 2001); and multiple innervations were maintained when continuous administration of exogenous GDNF started right after birth into adulthood (Keller-Peck et al. 2001). Wang et al. (2002) found that continuous treatment with GDNF in Xenopus nerve-muscle co-culture enhanced axonal growth by increasing the length of neurites in motor neurons. From this, it can be suggested that GDNF plays a significant role in synaptic maintenance and remodeling of the NMJ; also, motor neurons depend primarily on GDNF as their trophic factor and that GDNF may be useful as a therapeutic candidate for neuromuscular diseases.
GDNF mRNA expression was shown to be altered in disease and aging in both the central and the peripheral nervous systems. GDNF protein and mRNA levels were increased in human denervated skeletal muscle and in skeletal muscle samples from individuals in the early stage of amyotrophic lateral sclerosis (Lie and Weis, 1998). GDNF is also increased in regenerating muscles with Duchenne type muscular dystrophy and polymyositis (Lie and Weis, 1998; Suzuki et al. 1998). Because GDNF maintains synaptic connections (Zwick et al. 2001), an increase in expression of GDNF in denervated tissues may indicate a response by target tissues in attempting to foster reinnervation.
Although addition of exogenous GDNF to neuromuscular synapses has been extensively studied, and the expression patterns for GDNF have been examined in skeletal muscle (Nagano and Suzuki, 2003; Suzuki et al. 1998); little is known about factors regulating normal synthesis and secretion of endogenous GDNF in skeletal muscle. In order to gain more insight concerning production of endogenous GDNF, this study is aimed at examining the role that motor neurons play in regulating GDNF secretion by skeletal muscle. A co-culture of skeletal muscle cells (C2C12) and cholinergic neurons, glioma × neuroblastoma hybrid cells (NG108-15) were used to study nerve-muscle interactions in vitro. Because of their similar characteristics to their counterparts in vivo, the cell lines used in this study have been widely used by researchers in physiological and pharmacological studies (Ling et al. 2005).
2. Results
2.1. C2C12 cells secrete GDNF into culture medium
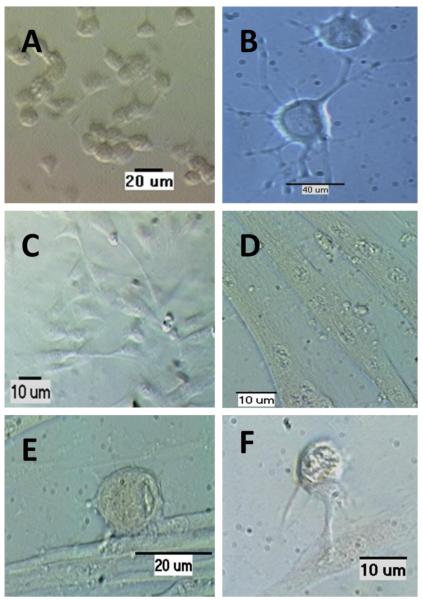
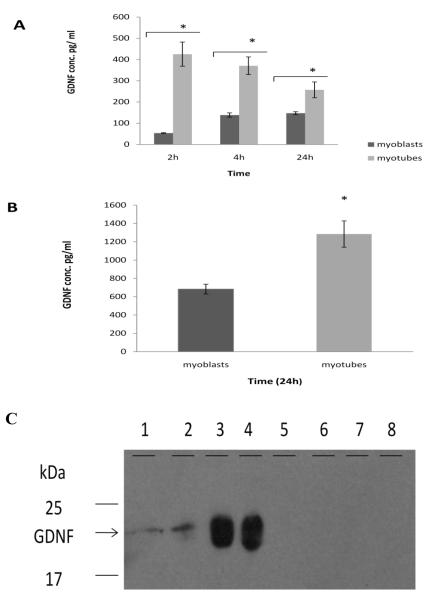
Before examining nerve-muscle co-cultures, a preliminary study was performed to investigate whether C2C12 cells or NG108-15 cells produce GDNF. To answer this question, the C2C12 cells or NG108-15 cells were cultured as described in the material and methods section and GDNF secretion and protein content of cells was studied. NG108-15 cells differentiate into nerve-like cells by cessation of cell division and growth of neurites (Fig. 1A-B). C2C12 cells resemble skeletal muscle cells therefore, they develop in two stages: a myoblast stage, which are undifferentiated skeletal muscle cells and a myotube stage, which are differentiated skeletal muscle cells (Fig. 1C-D). When grown together in co-culture, NG108-15 cells make contact with myotubes (Fig. 1E-F). The results indicated that C2C12 cells were capable of producing and secreting GDNF. However, we noted a decrease in GDNF production with increased cell passage, thus only cells at passage three were used in all experiments. Figure 2 shows GDNF content in both C2C12 myoblasts and myotubes. GDNF secreted by myoblasts into culture medium reached a maximum concentration of 140pg/ml at 4h, and this concentration did not change between 4h and 24h (Fig. 2A). Compared with myoblasts, myotubes secrete higher levels of GDNF protein (Fig. 2A).
Figure 1. C2C12 and NG108-15 cells in culture.
Cells were fixed with 4% paraformaldehyde. A. Undifferentiated NG108-15, B. differentiated NG108-15 cells, C. myoblasts, D. myotubes, myoblasts in (c) fused to form myotubes. E &F. Nerve-muscle co-cultured cells.
Figure 2. GDNF protein production in myoblasts, myotubes, and NG108-15 cells.
Samples of 3-day-old myoblasts or 10-day-old myotubes were taken at 2h, 4h, and 24h after changing medium. Myotubes were scraped from dishes at 2h, 4h, and 24h. Protein content in panel A and B was determined by ELISA. A. At all time points myotubes secreted significantly higher levels of GDNF than myoblasts. B. GDNF content within myoblasts and myotubes: Myotubes contain significantly more intracellular GDNF than myoblasts. Values are presented as mean ± S.E.M. Asterisk indicates significance (p≤ 0.05). n=4. C. Western blot: lanes 1& 2 represent GDNF secreted in culture medium by myotubes, lanes 3 & 4 represent GDNF contained within myotubes, lanes 5-6 represent GDNF in NG108-15 culture medium, and lane 7-6 represent GDNF in NG108-15 cells.
Both myoblasts and myotubes retain more GDNF inside the cells than they secreted into culture medium. However, myotubes contain more intracellular GDNF than myoblasts (Fig. 2B). Both growth stages of NG108-15 cells were examined to determine if they synthesize GDNF. Enzyme-linked immunosorbant assay (ELISA) results (Table 1) and western blot (Fig. 2C) showed no GDNF protein in differentiated NG108-15 cells. However, non-differentiated NG108-15 cells, those that are still dividing, produce low levels of GDNF (Table 1).
Table 1.
Average GDNF protein content produced by NG108-15 cells versus C2C12 cells (pg/ml)
| GDNF secreted into culture medium |
GDNF content in cells |
|
|---|---|---|
| 1. Non-differentiated NG108-15 | 74.3±7 | 217±13 |
| 2. Differentiated NG108-15 | undetectable | undetectable |
| 3. C2C12 myoblasts | 147±7 | 683±53 |
| 4. C2C12 myotubes | 487±89 | 1047±133 |
2.2. NG108-15 cells express choline acetyltransferase (ChAT) and C2C12 myotubes express myosin and GDNF
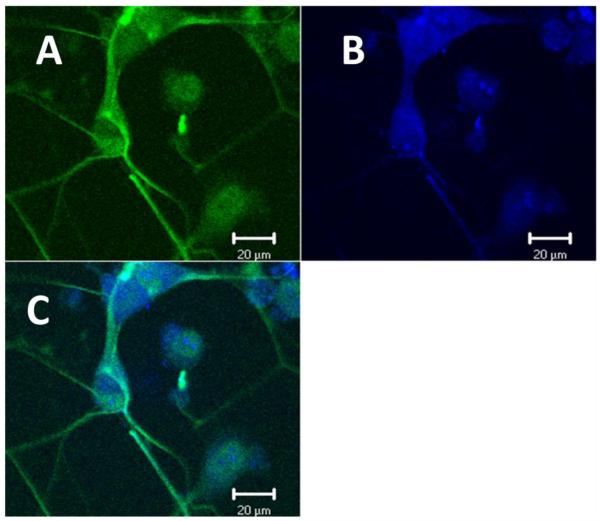
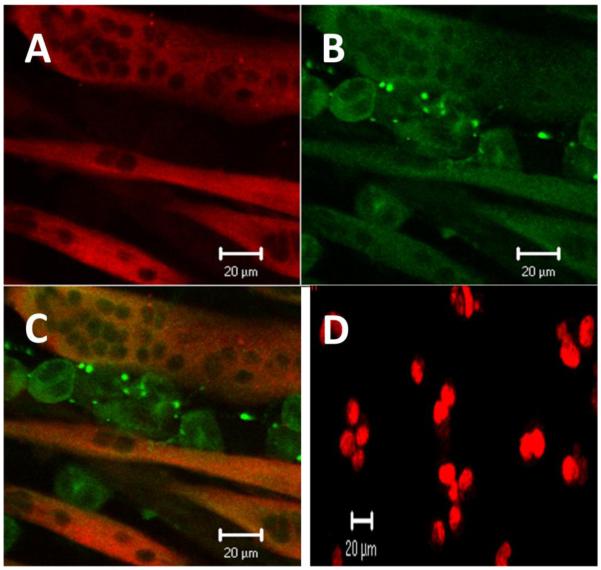
Next, we examined whether the cell lines under investigation express proteins that are normally seen in these tissues in vivo. Immunocytochemistry was performed; NG108-15 cells were confirmed to have neuronal characteristics by using Mill Marker FluoroPan Neuronal Marker; a mixture of antibodies that bind to various neuronal proteins, and were identified as cholinergic neurons by using anti-ChAT antibodies (Fig. 3A-C). Expression of myosin protein in C2C12 myotubes was examined using anti-slow myosin, indicating that C2C12 cell lines have characteristics of mammalian skeletal muscle cells (Fig. 4D). Anti-GDNF antibodies were used to detect GDNF protein within myotubes (Fig. 4B).
Figure 3. NG108-15 cells express ChAT.
Cells were cultured and fixed in 4% paraformaldehyde. A. NG108-15 cells were bound with Mill Marker FluoroPan Neuronal Marker (green), B. and anti-ChAT (blue). C. Overlay. Images were captured by Zeiss laser scanning confocal microscope.
Figure 4. GDNF in NG108-15/C2C12 myotubes co-culture.
Co-cultures of C2C12 cells and NG108-15 cells were cultured on coverslips in DMEM supplemented by 10% horse serum. The cells were fixed in 4% paraformaldehyde and mouse anti-myosin followed by donkey anti-mouse conjugated to Alexa Fluor 568 were added to localize myosin. Also, rabbit anti-GDNF followed by donkey anti-rabbit conjugated to Alexa Fluor 488 for GDNF were added to localize GDNF. A. Myosin (red), B. GDNF (green), and C. overlay, GDNF was observed in both NG108-15 cells and myotubes. D. Non-differentiated NG108-15 cells express PCNA (red), a marker for cell proliferation; identified by an anti-PCNA antibody. All images were captured by Zeiss laser scanning confocol microscope.
2.3. NG108-15 cells in co-culture contain GDNF protein
To examine GDNF in NG108-15/C2C12 co-cultures, antibodies against slow myosin protein and GDNF were used. Although ELISA and western blot results showed no GDNF in differentiated NG108-15 cells, GDNF was observed in NG108-15 cells grown in co-culture with myotubes (Fig. 4A-C). Because proliferating NG108-15 cells were shown to produce GDNF, while differentiated cells did not, we examined whether NG108-15 cells differentiate upon contact with muscle cells, by examining if these neural cells continue to divide. An antibody against proliferating cell nuclear antigen (PCNA), a characteristic of dividing cells, was used to test for NG108-15 cell division. No PCNA was observed in differentiated NG108-15 cells and in NG108-15 cells co-cultured with myotubes (data not shown). However, PCNA was observed in non-differentiated NG108-15 cells using an antibody against PCNA (Fig. 4D).
2.4. Addition of Cholinergic NG108-15 cells to C2C12 myotubes reduced GDNF protein production
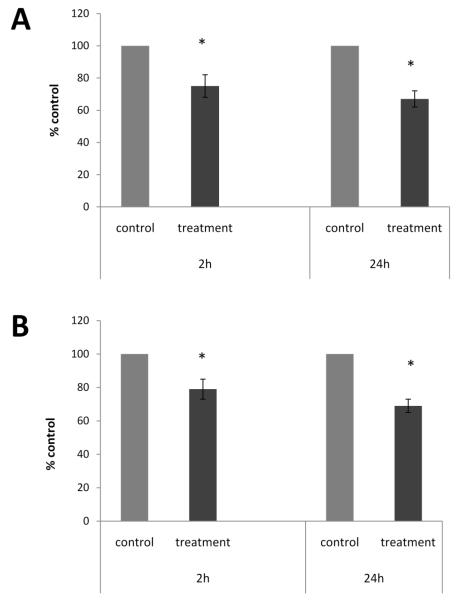
Following co-culture with myotubes, NG108-15 cells stopped dividing and extended neurites to make contact with myotubes (Fig. 1E-F). Surprisingly, the addition of cholinergic neurons on C2C12 myotubes reduced GDNF protein secretion in the culture medium by approximately 25% and 33% at 2h and 24h, respectively (Fig. 5A). GDNF protein content in cells was also reduced at 2h and 24h by 21% and 31%, respectively (Fig. 5B). Total GDNF, the sum of GDNF content in cells and GDNF secreted in culture medium, was also less in the co-culture as compared to myotubes alone, showing a decrease by 14% and 36% at 2h and 24h, respectively (data not shown).
Figure 5. NG108-15 cells inhibited GDNF production by myotubes at 2h and 24h.
The 10-day-old myotubes were co-cultured with NG108-15 neural cells and samples of harvested cells and conditioned culture medium were taken at 2h and 24h after the addition of fresh medium. Samples of conditioned culture medium were taken from cultures containing myotubes alone (control) and cultures containing myotubes and NG108-15 cells (treatment) and GDNF protein content was determined by ELISA. A. Addition of NG108-15 cells reduced GDNF concentration in culture medium by 25% and 33% at 2h and 24h, respectively. B. GDNF content in cells was reduced by 21% and 31% at 2h and 24 h, respectively. Values are presented as mean ± S.E.M. Asterisk indicates a significant difference between control and treatment groups (p ≤ 0.05). n=8.
2.5. Blocking acetylcholine receptors (AChRs) in Nerve-Muscle co-culture reversed the action of NG108-15 cells on myotubes at 2 hours
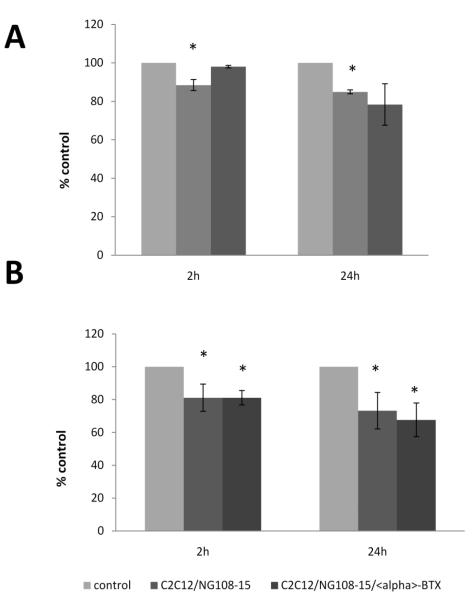
The results above suggest that addition of NG108-15 cells to myotubes inhibited both GDNF production in myotube cells and its secretion in the culture medium. Here we sought to examine whether NG108-15 cells inhibit production of GDNF through activation of AChRs. Results showed that the addition of alpha-bungarotoxin (α-BTX) reversed the effect of NG108-15 cells on GDNF secretion at 2h but not at 24h (Fig. 6A). Blocking AChRs with α-BTX failed to reverse the effect of addition of NG108-15 cells on GDNF protein content in the cells (Fig. 6B).
Figure 6. Effect of α-BTX on GDNF production in co-cultures.
The co-cultures were incubated for 24-36 hrs to allow nerve-muscle contact to form, then fresh medium containing 200nM of α-BTX was added. Culture medium samples were taken at 2h and 24h and cells were harvested and centrifuged. GDNF protein content was determined by ELISA. A. α-BTX blocked the action of neural cells on GDNF secretion in culture medium at 2h. B. α-BTX failed to reverse the effects of neural cells on GDNF content in myotubes. Data are in percentage from control and values are presented as mean ± S.E.M. Asterisk indicates significant difference (p ≤ 0.05) from control. n=3.
3. Discussion
The primary goal of the present study was to examine the normal regulation of GDNF production by skeletal muscle cells using a nerve-muscle co-culture system. Results from this study suggest that motor neurons regulate the secretion of a target-derived factor, GDNF, from skeletal muscle cells, in part, through activation of AChRs.
We examined characteristics of GDNF production in C2C12 cells. Results from these studies showed that C2C12 cells produced and secreted GDNF protein in the culture medium. This characteristic was seen in both myoblasts; undifferentiated C2C12 cells, and myotubes; differentiated C2C12 cells. These observations were consistent with previously reported observations that GDNF appears to be continuously expressed in skeletal muscle (Hase et al. 1999; Suzuki et al. 1998). Interestingly, we observed that the amount of GDNF contained within both myoblast and myotube cells was fivefold higher compared to that secreted in the culture medium. Based upon our results, which showed a significant difference between the amount of GDNF inside the cell and that secreted in culture medium, several possible explanations emerge. First, under normal conditions, skeletal muscle cells constitutively produce and secrete GDNF; and the relatively high amount of intracellular GDNF observed may be due to GDNF processing in cell compartments such as the Golgi body (Oh-hash et al. 2009; Wang et al. 2008). Second, skeletal muscle cells produce and store GDNF in cells and the stored GDNF can be secreted upon demand, such as following prolonged muscle depolarization. Up-regulation of GDNF expression by skeletal muscle has been suggested to be an indication of a need for additional GDNF by spinal motor neurons. For example, a higher amount of GDNF mRNA was expressed in mouse limb bud during neuromuscular formation (Henderson et al. 1993, 1994). In adult rats and mice, GDNF protein and its mRNA were up-regulated in denervated muscle after axotomy (Naveihan et al. 1997; Springer et al., 1995). GDNF mRNA is also elevated in the human skeletal muscle in individuals suffering from neuromuscular disease such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, and polymyosits (Lie and Weis, 1998; Suzuki et al. 1998).
Our results showed no GDNF in differentiated NG108-15 cells, suggesting that these cholinergic cells either do not produce GDNF or express GDNF at levels below the detection limit of our GDNF ELISA. Consistent with our observations, other studies have reported a very low amount of GDNF in neuronal cells (Trupp et al. 1995). Other studies show that sciatic nerves express undetectable levels of GDNF (Frostick et al. 1998), suggesting that intact motor neurons do not produce GDNF. These observations together with our results raise the possibility that some populations of neurons, including motor neurons, do not produce sufficient GDNF to sustain their own growth or regeneration. This is seen especially in conditions such as programmed cell death when motor neurons depend on GDNF secreted by skeletal muscle (Angka et al. 2008), or during nerve damage when motor neurons again depend on GDNF from other sources such as skeletal muscle and/or Schwann cells for survival (Gordon, 2009; Henderson, 1996; Naveilhan et al. 1998; Springer et al. 1995). In contrast, we observed low levels of GDNF in non-differentiated NG108-15 cells, the cells that are still dividing, suggesting that growing neural cells may be capable of expressing growth factors. GDNF expression has been reported in developing nerves (Henderson et al. 1994; Trupp et al. 1995). Therefore, from other observations and our current results, we assume that growing motor neurons may have the ability to express GDNF whereas mature motor neurons produce undetectable amounts of GDNF.
Immunocytochemical results showed that GDNF was localized in NG108-15 neural cells when in co-culture with C2C12 myotubes, suggesting that these cholinergic neurons either secrete the GDNF protein upon contact with their target or utilize GDNF secreted by skeletal muscle. Because motor neurons express GDNF receptor alpha one (GFR-α1), a GDNF receptor (Hase et al. 1999; Iwase et al. 2005; Naveilhan et al. 1997), it has been suggested that GDNF is secreted by skeletal muscle and retrogradely transported along axons to the nerve cell body, through a receptor-mediated process (Nguyen et al. 1998).
We found that C2C12 myotubes secrete GDNF in culture medium and retain approximately fivefold more GDNF within the cells. We observed GDNF production to be significantly decreased in co-cultured myotubes compared to myotubes grown alone. These results are similar to earlier reports that denervated skeletal muscle secretes a high amount of GDNF prior to reinnervation, presumably to provide trophic support for incoming neurons (Lie and Weis, 1998; Suzuki et al.1998). From this it may be assumed that upon synaptic contact, an incoming nerve provides a signal to its target, which inhibits continued production of GDNF and allows only the amount of GDNF needed for maintenance of normal structure and function to be secreted. Thus, we report that following contact with skeletal muscle, motor neurons regulate their own supply of GDNF, by inhibiting excess secretion of GDNF from the skeletal muscle they innervate.
We hypothesized that the action of NG108-15 on inhibiting GDNF production may be mediated through AChRs. α-BTX appears to abolish the action of NG108-15 cells on GDNF secretion in co-cultures at two hours. These results suggest that the decline of GDNF secretion by skeletal muscle may be related to activation of AChRs. However, the blocking agent failed to reverse the effects of neural cells at 24h. One possible explanation may be that 24h was long enough to allow the myotubes to insert new AChRs. It has been shown that insertion of newly found AChRs can be observed as early as 4h (Yang and Nelson, 2004). It was also noted that α-BTX failed to reverse the action of NG108-15 on GDNF content within skeletal muscle cells. That is, the neural cells continued to exert an inhibitory effect on GDNF content in skeletal muscle in the presence of the α-BTX. These results may indicate that processes controlling expression and secretion of GDNF by skeletal muscle may be regulated by different mechanisms. While the secretion step is directly affected by AChR activity, the expression of GDNF within the cell may involve other unknown factors.
The current study has demonstrated that cholinergic neurons regulate GDNF secretion either by utilizing the GDNF secreted by skeletal muscle and/or through activity of AChRs, suggesting that neurons may be regulating their own supply of GDNF protein.
4. Experimental Procedure
4.1 Cell Culture
Mouse skeletal muscle cells (C2C12), Glioma × Neuroblastoma hybrid cells (NG108-15), and culture medium were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Culturing procedures were performed according to the ATCC protocols.
C2C12 myoblasts, undifferentiated skeletal muscle cells, were initially seeded on a 100-mm plate (Falcon) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (Mediatech, Manassas, VA) and 1% antibiotic-antimycotic (Invitrogen-GIBCO). Cells were incubated at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. The myoblasts were subcultured after two days. About 2.5 × 105 cells were allowed to grow on 35-mm culture plates for experiments. Cultured cells were maintained in conditioned medium and in the same incubator described above. Differentiation of myoblasts to myotubes was induced by replacing the growth medium with DMEM supplemented with 10% horse serum and 1% antibiotic-antimycotic. The medium was renewed every one to two days.
NG108-15 cells were cultured on 100-mm culture dishes in DMEM supplemented with 10% fetal bovine serum, 2% HAT supplement, a mixture of hypoxanthine, aminopterin, and thymidine (Invitrogen-GIBCO), and 1% antibiotic-antimyocotic. Differentiation of NG108-15 cells was enhanced by switching from regular medium to a serum free medium. The medium was renewed every two to three days.
Nerve-muscle co-culture procedure was performed as it was first described by Chen et al. (2005) and Ling et al. (2005). Briefly, approximately 2.5 × 105 cells were grown as myoblasts on 35-mm culture dishes in culture medium: DMEM supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic for three days. Myoblasts were induced to differentiate and fuse into myotubes. Approximately 1.0 × 105 NG108-15 cells were plated onto 10-day old myotube cultures. Co-cultured cells were maintained at 37°C in a water-saturated atmosphere of 95% air and 5% CO2.
4.2 GDNF content in cultured cells
GDNF protein content in each experiment was measured by an enzyme-linked immunosorbent assay (ELISA). To determine GDNF protein content in cells or GDNF protein concentration in culture medium, culturing procedure for NG108-15 cells alone, myotubes alone, or NG108-15 co-cultured with myotubes was done as described above. NG108-15 cell and C2C12 myoblast cell samples were collected at day 3, whereas myotube only samples were collected at day 10. For nerve-muscle co-cultures, NG108-15 cells were added at day 10 and samples were collected after 24h to 36h to allow nerve-muscle contacts to form. For each experiment, a 1ml sample of culture medium was collected from each culture dish at 2, 4, and 24 hours and kept at −20°C. To remove cells on dishes, culture medium was removed followed by washing with calcium/magnesium-free saline buffer. Then 1ml of sample buffer, (a mixture of phosphate buffered saline, 0.005% Tween-20, 0.5% bovine serum albumin, 0.1mM benzethonium chloride, 2mM benzemidine, 0.4M NaCl, 2mM EDTA and 164μl/100ml aprotinin) was added to each culture dish containing cells. The cells were scraped from the dish using a cell lifter (Corning, NY). Cells were spun in a cold centrifuge and supernatant was removed and stored at −20°C.
4.3 Addition of alpha-bungarotoxin
C2C12 cells were grown and allowed to fuse into myotubes as described above. To investigate whether AChRs were involved in GDNF production we blocked the receptors with alpha-bungarotoxin (α-BTX) (Biotium, CA). The blocking procedure was adopted from Yang and Nelson (2004). Briefly, 10-day old myotubes were treated with fresh medium containing 200nM α-BTX. Cells were maintained in α-BTX for 25min. Following incubation the cells were washed twice with fresh culture medium, fresh culture medium was added and culture medium and cells were collected at intervals of 2h, 4h, and 24 h. GDNF protein content was measured by ELISA.
4.4 Immunocytochemical detection of choline acetyltransferase (ChAT)
For immunocytochemical purposes, the NG108-15 cells were cultured on coverslips (VWR Scientific) and allowed to differentiate by serum starvation as described in Pun et al. (1997). Cells were fixed with 4% parformaldehyde in phosphate buffered saline (PBS) for 30 min, washed and bound with the Mill-Marker FluoroPan Neuronal Marker conjugated to Alexa Fluor 488 (1:125; Millipore) for two hours at room temperature. The cells were washed and mounted on a glass slide with 50% glycerol/50%PBS. For detection of choline acetyltransferase (ChAT: a marker of cholinergic neurons), cells were incubated with 1:125 ratio goat anti-ChAT primary antibody (Molecular Probes) followed by labeling with a 1:100 dilution of Alexa Fluor 647 conjugated donkey anti-Goat IgG secondary antibody (Molecular Probes). Images were viewed and captured using a Zeiss laser scanning confocal microscope.
4.5 Examination of cell proliferation for NG108-15 cells
NG108-15 cells were grown on coverslips and were maintained in either DMEM supplemented with 10% FBS, 1% antimyotic-antimyocotic, and HAT supplement (neural medium), neural medium containing no FBS (serum free) or DMEM supplemented with 10% horse serum and1% antimyotic-antimyocotic (myotube medium). Detection of proliferation cell nuclear antigen (PCNA) in NG108-15 cells was performed as described in Andrade et al. (1993). Briefly, at day 3, the neural medium was switched to serum free. After 72 h, serum free medium was replaced by the neural medium for control culture dishes or myotube medium. Cells were fixed at 3h intervals with cold (−20°C) methanol for 5 min followed by acetone for 2 min at −20°C. Plates were blocked with donkey serum followed by incubation with antibody against PCNA (1:250) overnight. Cells were washed followed by 2h incubation with donkey anti mouse secondary antibody (1:125) conjugated to Alexa Fluor 568. Images were viewed and captured using a Zeiss laser scanning confocol microscope.
4.6 Detection of Myosin and GDNF in Myotubes or nerve-muscle co-cultures
Myotube culturing procedures were the same as previously described, but for immunocytochemical purposes cells were grown on coverslips placed in a 35-mm dish. The coverslips were coated with collagen to enhance myotube adhesion. 10-day old myotubes were fixed with 4% paraformaldehyde for 30 min and were incubated overnight at 4°C with mouse anti-myosin or rabbit anti-GDNF primary antibody (1:125). Cells were washed and donkey anti-mouse or donkey anti-rabbit conjugated to Alexa Fluor 488 or Alexa Fluor 568, respectively, was added (1:100) for two hours at room temperature. Cells were washed and mounted on a glass slide with 50% glycerol/50%PBS. The same immunocytochemical procedure was repeated for nerve-muscle co-cultures. Images were viewed using a Zeiss laser scanning confocal microscope.
4.7 Western Blotting
The amount of GDNF in culture medium and in cells was determined by western blotting. NG108-15 cells and C2C12 myotubes were cultured and samples of culture medium and scraped cells were collected as in previous experiments. Cells were spun in a cold centrifuge and supernatant was removed and stored at −20°C in sample buffer for western blotting analysis. Culture medium and cell samples were loaded with Laemmli 2X loading buffer to make a final volume of 20μL. Controls consisted of a protein ladder (New England BioLabs), GDNF protein (positive control) and NGF protein (negative control). The samples were boiled for 5 minutes then loaded into a 15% polyacrylamide gel. The gel was submerged and was run in separating buffer at two different voltages. The transfer of protein from the gel to the polyvinylidene difluoride (PVDF; Invitrogen) membrane was performed at 12 volts for 1 hour. The PVDF membrane was blocked with I-Block (Tropix) for 1 hour at 4°C on a shaking platform. The membrane was then incubated with a primary antibody against GDNF (Santa Cruz Biotechnologies) in I-Blocking buffer overnight at 4°C on a rotating platform. The membrane was washed 3 times, for 5, 10, and 20 minutes while shaking. The membrane was then incubated with a HRP-conjugated secondary antibody (ECL; GE Healthcare) in I-Blocking buffer for 1 hour at room temperature, while shaking. GDNF protein was detected with chemiluminescence and was visualized on BioMax XAR film (Kodak) with exposure times of 15 minutes.
Statistical Analysis
Statistical analysis was performed using a student’s t-test. P values ≤ 0.05 were considered statistically significant. All data values are reported as the mean ± standard error of the mean (SEM).
Acknowledgement
This work was funded by NIH Grant 1R15AG022908-01A2, MSU-KCMS, and Western Michigan University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Andrade LEC, Tan EM, Chan EKL. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc. Natl. Acad. Sci. 1993;90:1947–1951. doi: 10.1073/pnas.90.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angka HE, Geddes AJ, Kablar B. Differential survival response of neurons to exogenous GDNF depends on the presence of skeletal muscle. Dev. Dyn. 2008;237:3169–3178. doi: 10.1002/dvdy.21727. [DOI] [PubMed] [Google Scholar]
- Caumont A-S, Octave J-N, Hermans E. Specific regulation of rat glial cell line-derived neurotrophic factor gene expression by riluzole in C6 glioma cells. J. Neurochem. 2006;97:128–139. doi: 10.1111/j.1471-4159.2006.03711.x. [DOI] [PubMed] [Google Scholar]
- Chen S-S, Lin C-H, Chen T-J. Lead-induced attenuation in the aggregation of acetylcholine receptors during the neuromuscular junction formation. Toxicol. Lett. 2005;159:89–99. doi: 10.1016/j.toxlet.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurg. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg. Focus. 2009;26:1–9. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- Hase A, Suzuki H, Arahata K, Akazawa C. Expression of human GDNFα-1 (GDNF receptor) at the neuromuscular junction and myelinated nerves. Neurosci. Lett. 1999;269:55–57. doi: 10.1016/s0304-3940(99)00419-x. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Ruilamas J, Evans T, McMahon B, Armanini MP, Berkemeier L, Phillips HS, Rosental A. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: A potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Henderson CE. Role of neurotrophic factors in neuronal development. Curr. Opinion Neurobiol. 1996;6:64–70. doi: 10.1016/s0959-4388(96)80010-9. [DOI] [PubMed] [Google Scholar]
- Houenou LJ, Oppenheim RW, Li L, Lo AC, Prevette D. Regulation of spinal motoneuron survival by GDNF during development and following injury. Cell Tissue Res. 1996;286:219–223. doi: 10.1007/s004410050690. [DOI] [PubMed] [Google Scholar]
- Iwase T, Jung CG, Bae H, Zhang M, Soliven B. Glial Cell line-derived neurotrophic factor-induced signaling in Schwann cells. J. Neurochem. 2005;94:1488–1499. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J. Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Weis J. GDNF expression is increased in denervated human skeletal muscle. Neurosci. Lett. 1998;250:87–90. doi: 10.1016/s0304-3940(98)00434-0. [DOI] [PubMed] [Google Scholar]
- Lin L-F, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Ling KKY, Siow NL, Choi RCY, Tsim KWK. ATP potentiates the formation of AChR aggregate in the co-culture of NG108-15 cells with C2C12 myotubes. FEBS Lett. 2005;579:2469–2474. doi: 10.1016/j.febslet.2005.03.054. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:789–792. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Nagano M, Suzuki H. Quantitative analyses of expression of GDNF and neurotrophins during postnatal development in rat skeletal muscles. Neurosci. Res. 2003;45:391–399. doi: 10.1016/s0168-0102(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors ret and GDNFRα after sciatic nerve lesion in the mouse. Eur. J. Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1728. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, Ebendal T, Hoffer BJ, Olson L. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996;286:191–207. doi: 10.1007/s004410050688. [DOI] [PubMed] [Google Scholar]
- Oh-hashi K, Ito M, Tanaka YH, Kiuchi K. Biosynthesis, processing, and secretion of glial cell line-derived neurotrophic factor in astroglial cells. Mol. Cell Biochem. 2009;323:1–7. doi: 10.1007/s11010-008-9958-3. [DOI] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Saria A, Humpel C. Dopamine neurons in a simple GDNF-treated meso-striatal organotypic co-culture model. Exp. Brain Res. 1999;127:270–278. doi: 10.1007/s002210050796. [DOI] [PubMed] [Google Scholar]
- Springer JE, Seeburger JL, He J, Gabrea A, Blankenhorn EP. cDNA sequence and differential mRNA regulation of two forms of glial and rat skeletal muscle. Exp. Neurol. 1995;131:47–52. doi: 10.1016/0014-4886(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hase A, Kim BY, Miyata Y, Ikuya N, Arahata K, Akazawa C. Up-regulation of glial line-derived neurotrophic factor (GDNF) expression in regeneration muscle fibers in neuromuscular diseases. Neurosci. Lett. 1998;257:165–167. doi: 10.1016/s0304-3940(98)00817-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hase A, Miyata Y, Arahata K, Akazawa C. Prominent expression of glial cell line-derived neurotrophic factor in human skeletal muscle. J. Comp. Neurol. 1998;402:303–312. [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez C. Peripheral Expression and Biological Activities GDNF, A New Neurotrophic Factor for Avian and Mammalian Peripheral Neurons. J. cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y, Yang F, He X-P, Je H-S, Zhou J-Z, Eckermann K, Kawamura D, Feng L, Shen L, Lu B. Regulation of neuromuscular synapse development by glial cell line-derived neurotrophic factor and neurturin. J. Biol. Chem. 2002;277:10614–10625. doi: 10.1074/jbc.M106116200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Geng Z, Zhao L, Huang S-H, Sheng A-L, Chen Z-Y. GDNF isoform affects intracellular trafficking and secretion of GDNF in neuronal cells. Brain Res. 2008;1226:1–7. doi: 10.1016/j.brainres.2008.05.087. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schaar DG, Ramakrishnan L, Black IB. Novel structure of the human GDNF gene. Brain Res. 1998;803:95–104. doi: 10.1016/s0006-8993(98)00627-1. [DOI] [PubMed] [Google Scholar]
- Yang L-X, Nelson PG. Glia cell line-derived neurotrophic factor regulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells. Neurosci. 2004;128:497–509. doi: 10.1016/j.neuroscience.2004.06.067. [DOI] [PubMed] [Google Scholar]
- Zwick M, Teng L, Mu X, Springer JE, Davis BM. Overexpression of GDNF induces and maintains hyperinnervation of muscle fibers and multiple end-plate formation. Exp. Neurol. 2001;171:342–350. doi: 10.1006/exnr.2001.7753. [DOI] [PubMed] [Google Scholar]