Abstract
Eight diffusion tensor imaging (DTI) datasets of normal adult C57BL/6J mouse brains were acquired with an isotropic Nyquist limited resolution of 43 microns (voxel volume ~ 80 pl). Each specimen was scanned with a b0 image and 6 diffusion-weighted images. T1 and T2* weighted data were acquired with each specimen to aid nonlinear registration of the data to a common reference space (called “Waxholm Space”). We identified 80 different discrete landmarks in Waxholm Space to provide the gold standard for measuring the registration quality. The accuracy of the registration was established by measuring displacement of the 80 landmarks in each registered brain from the same landmarks in the reference brain. The accuracy was better than 130 microns for 95% of the landmarks (overall landmark displacement is 65±40 microns, n=640). Mean and coefficient of variation atlases of DTI indices were generated with potential application for both voxel-based and region of interest-based analysis. To examine consistency of DTI data among individual subjects in this study and difference in diffusion indices between separate brain structures within each subject, averaged values of DTI indices (axial diffusivity, radial diffusivity, fractional anisotropy, and angular deviation of the primary eigenvector) were computed in 9 white matter structures in each brain. The variation of the DTI indices across the population was very small, e.g., ~5% for axial diffusivity for each white matter structure, enabling confident differentiation of differences in these structures within each subject. ANOVA tests indicated that the current protocol is able to provide consistent DTI data of individual brains (p>0.25), and distinguish difference of diffusion indices between white matter structures (p<0.001). Power analysis was also performed to provide an estimate of the number of specimens required to detect a 10% change of the DTI indices in each white matter structure. The data provide a critical addition to Waxholm Space, the International Neuroinformatics Coordinating Facility (incf.org) online comprehensive atlas of the mouse brain.
INTRODUCTION
The existing atlases of mouse brain (Badea et al., 2007; Dhenain et al., 2001; Dorr et al., 2008; Johnson et al., 2010; Kovacevic et al., 2005; Ma et al., 2005; MacKenzie-Graham et al., 2004; MacKenzie-Graham et al., 2007; Paxinos and Franklin, 2001) offer limited information about white matter anatomy, which appears homogeneous in conventional MRI and in histology preparations. Diffusion tensor imaging (DTI) provides a unique contrast reflecting tissue diffusivity, anisotropy, and directionality that enables clearer differentiation and delineation of white matter structures, quantification of tissue integrity by scalar DTI indices such as anisotropy and diffusivity, and 3D reconstruction of fiber connectivity by the primary eigenvector of diffusion tensor (Mori et al., 2001; Song et al., 2005). The goal of this paper is to establish an atlas of the DTI indices and their variability in the normal adult C57BL/6J mouse brain.
Pooling registered DTI data across normal subjects into an atlas can identify common anatomical structures and provide a standard reference set of DTI indices. Pooling also allows one to quantitatively describe typical pattern and variation of DTI indices in the “average” brain of the normal population. This in turn establishes the baseline required for testing statistical hypotheses for voxel-based and region of interest (ROI)-based analysis, in genetic or disease models (Mori et al., 2008; Oishi et al., 2008; Oishi et al., 2009).
In this study, DTI datasets of the brains of eight male C57BL/6J mice, 9-12 weeks of age, were acquired at 43 μm isotropic resolution (Jiang and Johnson, 2010). All images were acquired with full Nyquist sampling. Each specimen was scanned with a b0 image and 6 diffusion-weighted images (b≈1.5×103 s/mm2) sensitized in 6 directions ([1, 1, 0], [1, 0, 1], [0, 1, 1], [−1, 1, 0], [1, 0, −1], [0, −1, 1]). Both T1 weighted and T2* weighted images were also included to facilitate registration. All the data were registered to a common reference space defined previously by a collection of higher resolution (21.5 μm) MR images with matching Nissl sections (Johnson et al., 2010). This standardized coordinate system, which we have called Waxholm Space (WHS), has been developed by Task Force members of the Digital Atlasing Program of the International Neuroinformatics Coordinating Facility (INCF) <http://www.incf.org/core/programs/atlasing> to allow scientists to share different types of images of the mouse brain in a common reference space. http://www.incf.org/core/programs/atlasing/projects/waxholm-space (Johnson et al., 2010).
We determined the registration quality by measuring landmark displacement between individual brains and the gold standard WHS reference brain. After verifying the registration accuracy, a DTI atlas containing diffusion indices and brain anatomy was constructed from the mean and the coefficient of variation (CV) of several of the most useful DTI indices, e.g. anisotropy and diffusivity. To examine variability of DTI data among individual normal subjects in this study and differences in diffusion indices between separate brain structures within the same subject, averaged values of DTI indices were computed in 9 white matter structures (derived from the WHS reference) (Johnson et al., 2010) in each brain and analyzed by ANOVA test. A probabilistic white matter map was created to determine the reproducible regions on a voxel by voxel basis (i.e., cores) of each white matter structure. These regions were applied with the same ANOVA test to further decide if the quality of manual ROI delineation has any influence on the statistical findings. Finally, given the mean and standard deviation of diffusion indices in the normal group, power analysis was performed to estimate the number of specimens required to detect a 10% change of each diffusion index in each one of the 9 white matter structures.
The DTI data is now part of the Biomedical Informatics Research Network (BIRN) (Johnson et al., 2007), which enables investigators from any place on the globe to access quantitative mouse brain anatomy in a routine and standardized fashion. DTI data from this paper is available at <http://www.civm.duhs.duke.edu/neuroYJ201009>.
MATERIAL AND METHODS
All animal procedures were approved by the Duke University Institutional Animal Care and Use Committee. Eight normal C57BL/6J male mice (9–12 weeks of age) were actively stained with a mixture of formalin and ProHance (Gadoteridol, Bracco Diagnostics, Inc., Princeton, NJ) to enhance MR signal, while preserving the tissue (Johnson et al., 2002). The sample tissues were trimmed (brain still in the cranium) to fit into an acrylic sample holder and immersed in fomblin, a perflorocarbon that minimizes susceptibility artifacts at the interface and limits specimen dehydration (Solvay Solexis, West Deptford, NJ).
All scans were performed on a 9.4 T vertical bore magnet interfaced to a GE console running Epic 12.4X (GE Medical Systems, Milwaukee, WI). The system is equipped with Resonance Research gradients (Resonance Research, Inc., Billerica, MA), which achieve peak gradients of 2000 mT/m. Specimens were scanned in a 12-mm diameter × 25-mm-long solenoid radio frequency coil. A diffusion-weighted spin-echo pulse sequence was used to acquire 3D volume images (field of view [FOV]=22×11×11 mm, matrix size=512×256×256 resulting in 43 μm isotropic resolution, TR=100 ms, TE=11.8 ms, NEX=2). Diffusion encoding was performed using a pair of half-sine gradient waveforms (width=1.3 ms, separation=6.4 ms, gradient amplitude=1600 mT/m). One b0 (i.e., b≈0) and 6 diffusion-weighted images (b≈1.5×103 s/mm2) sensitized in 6 directions ([1, 1, 0], [1, 0, 1], [0, 1, 1], [−1, 1, 0], [1, 0, −1], [0, −1, 1]) were acquired over approximately 28 hours. T1 and T2* anatomical images with the same FOV and resolution were also acquired with 1-hour scan time (TR=50 ms, NEX=1) (Jiang and Johnson, 2010). A novel acquisition strategy that amplified the high-frequency information by selectively altering the receiver gain during the phase-encoding steps was applied to extend the dynamic range of the system, capture the higher-frequency components, and limit saturation in the center of k-space (Johnson et al., 2007).
All b0, DWI, and T2*-weighted images for each subject were co-registered to the corresponding T1-weighted image and skull-stripped using 64-bit MATLAB (MathWorks, Natick, MA) (Jiang and Johnson, 2010). After skull-stripping, the T1 and T2* images were used to register each subject to the reference brain in WHS, which was performed in 64-bit DiffeoMap (X. Li, H. Jiang, and S. Mori, Johns Hopkins University), using a series of steps in the following order: rigid transformation, 12-mode linear affine transformation with Automated Image Registration (AIR) (Woods et al., 1998), and two-channel (T1 and T2* images) Large Deformation Diffeomorphic Metric Mapping (LDDMM) (Miller et al., 2005). One overall transformation matrix was generated for each subject and applied to the calculated diffusion tensor field to properly map and reorient the diffusion tensors (Alexander et al., 2001; Xu et al., 2003). The registration parameters of all the steps are provided in the supplementary material online at <http://www.civm.duhs.duke.edu/neuroYJ201009>.
The transformed diffusion tensors were diagonalized into three eigenvalues and three eigenvectors in 64-bit DTIStudio (Jiang et al., 2006) (Johns Hopkins University, Kennedy Krieger Institute). Selected diffusion indices, i.e., fractional anisotropy (FA) (Pierpaoli and Basser, 1996), axial diffusivity (AD, which is the primary eigenvalue), and radial diffusivity (RD, which is the average of the secondary and tertiary eigenvalues) (Song et al., 2005), were calculated. A 24-bit, color-coded orientation map was also generated by assigning red, green, and blue channels to the rostral-caudal, left-right, and dorsal-ventral components of the primary eigenvector (i.e., ev0, which represents local fiber orientation), where intensity was proportional to fractional anisotropy.
To measure registration quality during the normalization process, 80 anatomical landmarks were manually selected on prominent brain structures in the T1 image using DiffeoMap—36 in seven axial planes; 32 in five coronal planes; and 12 in two sagittal planes. A text file specifying all landmark coordinates is provided in the supplementary material online at <http://www.civm.duhs.duke.edu/neuroYJ201009>. The same set of 80 landmarks was placed in the reference brain (WHS), which served as the gold standard, and in each individual subject brain. The landmarks on each subject brain were mapped on to the reference coordinate by the corresponding overall transformation matrix. Displacements between landmarks mapped from each subject and landmarks of the reference brain (i.e., landmark displacement) were measured to quantify registration quality, which represented the residual anatomical difference between each transformed subject and the reference brain.
After measuring accuracy of registration, a high-resolution atlas of selected diffusion indices with full brain coverage was created as maps of mean or coefficient of variation for each diffusion index (AD, RD, and FA) as well as the mean map of angular deviation of ev0 (in degrees), which is the angle between ev0 of each brain and the averaged ev0 of all 8 brains quantifying ev0 consistency across all 8 subjects. Since angular deviation of ev0 already represents the variability of ev0, the CV map was not included.
We performed two separate analyses of the variation of DTI indices of white matter structures that differ in how the boundaries for the white matter structures are defined. In the first method 9 3D white matter structures for each registered brain were defined using the delineations performed in WHS. We have previously evaluated the robustness of automatically defined regions based on registration vs. manual vetting in a population of 6 animals of the identical strain, gender, and age as used in this study, using traditional DICE coefficients (Badea et al., 2007). We have more recently added 14 animals of the same strain, gender and age in WHS using diffeomorphic registration. A full description is available in (Johnson et al., 2010). In this study, the boundaries for these white matter structures were determined by normalizing each DTI set into WHS using the transformation matrix derived from registration of the T1 and T2* images for each brain to WHS. The 9 3D white matter structures are listed in Table 2 of this manuscript and Figure 3 of (Johnson et al., 2010). The averaged values of the diffusion indices including FA, AD, RD, and angular deviation of ev0 were measured in each white matter structure in each subject to provide a compact description of these indices. One-way ANOVA and post-hoc Tukey-Kramer tests were performed to examine variations of each diffusion index across 8 subjects or across 9 white matters (WM) for the regional average.
Table 2.
Averaged values (mean±standard deviation) of AD, RD, FA, and angular deviation of ev0 across the 8 subjects for each white matter region, including lateral lemniscus (ll), anterior commissure (ac), cerebral peduncle (cp), internal capsule (ic), optic tract (ot), fimbria (fi), corpus callosum (cc), fornix (f), and spinal trigeminal tract (sp5).
| Diffusion indices | ll | ac | cp | ic | ot | fi | cc | f | sp5 |
|---|---|---|---|---|---|---|---|---|---|
|
AD (×10−3 mm2/s) |
0.59± 0.02 |
0.65± 0.04 |
0.80± 0.04 |
0.64± 0.03 |
0.81± 0.08 |
0.66± 0.05 |
0.64± 0.01 |
0.58± 0.03 |
0.88± 0.03 |
|
RD (×10−3 mm2/s) |
0.33± 0.02 |
0.25± 0.02 |
0.22± 0.02 |
0.25± 0.02 |
0.25± 0.02 |
0.25± 0.02 |
0.28± 0.01 |
0.27± 0.03 |
0.28± 0.02 |
| FA | 0.39± 0.02 |
0.56± 0.03 |
0.69± 0.02 |
0.55± 0.03 |
0.64± 0.03 |
0.58± 0.01 |
0.50± 0.02 |
0.47± 0.04 |
0.60± 0.02 |
| ev0 (°) | 14±2 | 15±3 | 10±1 | 18±1 | 14±2 | 11±0 | 14±1 | 16±2 | 15±2 |
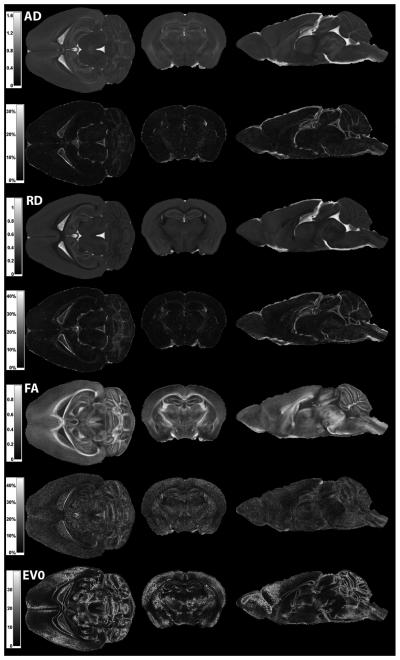
Fig. 3.
Mean (upper row) and coefficient of variation (lower row, except for ev0) maps of AD, RD, FA, and angular deviation of ev0 across 8 brains, displayed in three orthogonal views. Colorbars for AD and RD are in the unit of mm2/s, colorbar for FA is unitless, and colorbar for angular deviation of ev0 is in the unit of degrees.
A second analysis, i.e. an alternative way to define the ROIs for white matter structures with less subjectivity, was performed to determine if the original manual delineations of white matter structures in WHS had any influence on the statistical variation of the DTI indices. The method is based on a probabilistic white matter map (PWM) reported by (Oishi et al, 2009). A binarized FA map was generated for each subject in its native space, i.e. before registering to WHS, by thresholding at 1.2-times the mean FA over entire brain. The mean FA over the entire brain was found to be very consistent for all the subjects (0.33±0.01, where n=8). FA values higher than the threshold were labeled as WM (1) and FA values lower than the threshold were labeled as non-WM (0). The transformation matrices for each specimen derived previously based on the T1 and T2* images were then applied to the binarized FA maps to register them into WHS. Finally, the 8 registered, binarized FA maps were averaged at each voxel to obtain the PWM in the WHS coordinate. The PWM quantifies the relative frequency with which WM was present in each voxel. For example, a 50% value of WM probability in a certain voxel in the reference coordinate indicates that WM was present in that voxel in 4 out of the 8 subjects. The same ANOVA and Tukey-Kramer tests were performed on the reproducible regions (i.e., cores) of the 9 manually defined WM ROIs with WM probability of 0.5, 0.625, 0.75, 0.875, or 1.
Using the mean and standard deviation of each diffusion index measured in each white matter region within the normal group, we performed power analysis to estimate the number of specimens which would be required for detecting a 10% change, assuming that the control group and the disease group have the same number of specimens and the same standard deviation. The statistical significance was determined by an alpha error probability less than 0.05 and a power greater than 0.95 of a t-test. The power analysis was performed by G*Power (Faul et al., 2007).
Each DTI dataset resulted in an aggregate of more than 4 GB of data, including the initial b0 and DWI images, and derived data such as the diffusion tensors, eigenvalues, eigenvectors, and anisotropy indices. All the related images of each brain were added into the primary archive for the Center for In Vivo Microscopy and linked to the original animal metadata via an Oracle database, which can be accessed via the Internet (Johnson et al., 2007).
RESULTS
Fig. 1 shows a representative slice from the group average of T1 and T2* images of 8 mouse brains after registering into the Waxholm Space, as well as an averaged DTI color map. The anatomical details and sharp boundaries of brain structures provide visual feedback on the normalization quality.
Fig. 1.
Averaged T1, T2* images, and DTI color map (marked as CM) of 8 normal mouse brains, displayed in three orthogonal views.
The quality of spatial normalization of the 8 brains was further measured quantitatively by manually placed landmarks. Mean displacement of all 640 landmarks (80 landmarks on each subject and 8 subjects in total) was found to be 1.5±1.0 voxels (i.e., 65±40 μm, n=640, 1 voxel=43 μm isotropic). More specifically, mean displacement of 80 landmarks on each subject is 1.5±0.8, 1.3±0.9, 1.4±1.3, 1.6±1.3, 1.6±0.9, 1.6 ±0.9, 1.5±0.9, and 1.6±1.1 voxels (n=80), demonstrating that the registration quality for each subject is similar. Fig. 2 shows the cumulative distribution of the 640 landmark displacements, which reveals that approximately 95% of the landmarks mapped from each subject are within about 3 voxels (i.e. 130 μm) of corresponding landmarks in the reference brain.
Fig. 2.
Cumulative fraction curve of all 640 landmark displacements (in voxels, where 1 voxel=43 μm isotropic). The arrow indicates the mean of all the landmark displacements.
After measuring registration quality, mean and CV maps of AD, RD, and FA, as well as mean map of angular deviation of ev0, were computed across the 8 subjects. The results are illustrated in Fig. 3 in three orthogonal views. The mean maps of AD, RD, and FA reveal the reproducible pattern of the mouse brain anatomy and diffusion indices, while the CV maps of AD, RD, and FA and the mean map of angular deviation of ev0 provide an estimate of the variability of diffusion indices or fiber orientation at each voxel across the population of 8 brains. This is particularly useful when trying to use the atlas to analyze voxel-based differences between two population groups. There is high variation in both AD and RD at the brain edges, CSF, and at other interfaces, where geometric distortions are high. FA variation tends to be lowest at the center of a major pathway, increasing towards the edge. Similarly, the mean of angular deviation of ev0 is smallest within major white matter pathway and becomes more variable approaching the edges, which indicates fiber direction is more stable within the larger tracts.
The averaged values over the entire brain of each mean map are 0.61×10−3 mm2/s for AD, 0.37×10−3 mm2/s for RD, 0.33 for FA, and 19.3° for angular deviation of ev0 (n=6,782,619). The averaged values of each CV map are 9.8% for AD, 13% for RD, and 22% for FA (n=6,782,619). The higher CV of AD, RD, FA or higher mean of angular deviation of ev0 leads to lower statistical power, when the method is used to detect abnormalities. In general, variation of diffusion indices at any voxel will be determined by several factors, including real anatomical and physiological variations, noise in the diffusion index estimation, and registration inaccuracy resulting in residual geometric mismatch. The high variability observed at the edge of structures could be true biological variability as well as manifestation of registration errors due to sharp anatomical transitions. The previous measurement of landmark displacement indicates that the contribution of registration inaccuracy should be limited. Moreover, contribution of random errors should also have been diminished through the group-averaging process.
Thirty-seven brain structures have been manually labeled in Waxholm Space for public use (Badea et al., 2009; Johnson et al., 2010). In the study reported here, 9 white matter structures were chosen to compare DTI indices of different WM structures. Table 1 and Table 2 list the averaged values of AD, RD, FA, and angular deviation of ev0 across the 9 WM regions for each subject, and the averaged values across the 8 subjects for each WM region, respectively.
Table 1.
Averaged values (mean±standard deviation) of axial diffusivity (AD), radial diffusivity (RD), fractional anisotropy (FA), and angular deviation of ev0 across the 9 white matter regions for each subject.
| Diffusion indices | brain1 | brain2 | brain3 | brain4 | brain5 | brain6 | brain7 | brain8 |
|---|---|---|---|---|---|---|---|---|
|
AD (×10−3 mm2/s) |
0.67± 0.09 |
0.73± 0.10 |
0.71± 0.10 |
0.68± 0.11 |
0.70± 0.10 |
0.70± 0.12 |
0.72± 0.11 |
0.65± 0.11 |
|
RD (×10−3 mm2/s) |
0.27± 0.04 |
0.29± 0.03 |
0.27± 0.04 |
0.26± 0.03 |
0.26± 0.03 |
0.25± 0.03 |
0.28± 0.03 |
0.25± 0.04 |
| FA | 0.54± 0.08 |
0.54± 0.10 |
0.55± 0.10 |
0.55± 0.08 |
0.57± 0.09 |
0.59± 0.09 |
0.55± 0.09 |
0.55± 0.08 |
| ev0 (°) | 13±2 | 14±3 | 13±2 | 15±3 | 13±2 | 13±2 | 14±3 | 16±3 |
Table 3 lists p-values of the one-way ANOVA test of AD, RD, FA, and angular deviation of ev0 across the 8 subjects or across the 9 WM regions. No significant difference was found across the 8 subjects for any diffusion index, which demonstrates the consistency of the 8 DTI datasets. In other words, the 8 subjects of normal population have very similar diffusion properties. In contrast, there exists significant difference across the 9 WM regions for all indices, i.e., these WMs have different diffusion characteristics (e.g., cellular composition, myelination, axon integrity, or fiber bundle organization) within the same subject. For these statistically significant ANOVA findings, subsequent Tukey-Kramer tests (Table 4) provide additional information of the pair-wise comparisons. For example, the lateral lemniscus was found to have significantly lower anisotropy than all the other WMs, while the cerebral peduncle exhibits higher anisotropy than all the other WMs. Anatomical indication and use of the findings need further investigation, but it provides a valuable reference for the anatomy and DTI indices in the normal mouse brain population.
Table 3.
p-values of ANOVA test of diffusion indices across the 8 subjects or across the 9 white matters.
| Diffusion indices | Across 8 subjects | Across 9 WMs |
|---|---|---|
| AD | 0.84 | 0* |
| RD | 0.34 | 4.6e-13* |
| FA | 0.94 | 0* |
| Angular deviation of ev0 | 0.28 | 3.3e-13* |
indicates statistical significance.
Table 4.
Subsequent Tukey-Kramer tests of AD, RD, FA, and angular deviation of ev0 for pair-wise comparison of the 9 white matter regions, including lateral lemniscus (ll), anterior commissure (ac), cerebral peduncle (cp), internal capsule (ic), optic tract (ot), fimbria (fi), corpus callosum (cc), fornix (f), and spinal trigeminal tract (sp5).
| AD | ll | ac | cp | ic | ot | fi | cc | f | sp5 |
|---|---|---|---|---|---|---|---|---|---|
| ll | * | * | * | * | |||||
| ac | * | * | * | * | |||||
| cp | * | * | * | * | * | * | * | ||
| ic | * | * | * | ||||||
| ot | * | * | * | * | * | * | |||
| fi | * | * | * | * | * | ||||
| cc | * | * | * | ||||||
| f | * | * | * | * | * | ||||
| sp5 | * | * | * | * | * | * | * |
| RD | ll | ac | cp | ic | ot | fi | cc | f | sp5 |
|---|---|---|---|---|---|---|---|---|---|
| ll | * | * | * | * | * | * | * | * | |
| ac | * | * | * | ||||||
| cp | * | * | * | * | |||||
| ic | * | * | * | ||||||
| ot | * | ||||||||
| fi | * | ||||||||
| cc | * | * | * | * | |||||
| f | * | * | |||||||
| sp5 | * | * | * | * |
| FA | ll | ac | cp | ic | ot | fi | cc | f | sp5 |
|---|---|---|---|---|---|---|---|---|---|
| ll | * | * | * | * | * | * | * | * | |
| ac | * | * | * | * | * | ||||
| cp | * | * | * | * | * | * | * | * | |
| ic | * | * | * | * | * | * | |||
| ot | * | * | * | * | * | * | * | ||
| fi | * | * | * | * | * | ||||
| cc | * | * | * | * | * | * | * | ||
| f | * | * | * | * | * | * | * | ||
| sp5 | * | * | * | * | * |
| ev0 | ll | ac | cp | ic | ot | fi | cc | f | sp5 |
|---|---|---|---|---|---|---|---|---|---|
| ll | * | * | * | ||||||
| ac | * | * | |||||||
| cp | * | * | * | * | * | * | * | ||
| ic | * | * | * | * | * | ||||
| ot | * | * | * | ||||||
| fi | * | * | * | * | * | * | * | ||
| cc | * | * | * | ||||||
| f | * | * | |||||||
| sp5 | * | * |
indicates statistical significance.
Note that these tables are symmetric because statistical difference is mutual.
To decide if the quality of manual ROI delineation has any influence on the statistical findings, a probabilistic white matter map was constructed by averaging the 8 thresholded FA maps of individual subjects, which is shown in Fig. 4. White matter structures that are appreciable in the PWM represent reproducible structures (i.e., core regions) among normal adult subjects and their locations in the standardized WHS coordinates. As can be seen in the upper row in Fig. 4, all prominent white matter tracts can be clearly identified in this averaged map. The PWM can be thresholded at different probability levels to get white matter core regions of the mouse brain at different certainty, and thus different size, which was also illustrated in Fig. 4 at probability levels of 0.5, 0.75, and 1. The higher is the probability, i.e. certainty of being WM, the smaller the resulted WM core region is. The same ANOVA analysis of diffusion indices in core regions of the manually defined WMs at probability levels of 0.5, 0.625, 0.75, 0.875 or 1 was performed across 9 WMs and across 8 subjects, which reveals almost identical statistical findings (data not shown) as the ANOVA test on the manually defined WMs. This demonstrates that statistical analysis of diffusion indices in this study has good tolerance to certain ROI inaccuracy, and the statistical findings are quite robust and reliable.
Fig. 4.
Probabilistic white matter map of the 8 mouse brains (top row) and the corresponding thresholded versions at probability=0.5, 0.75, or 1.
Fig. 5 illustrates color map images that complement the quantitative data in Table 1 and Table 2, and demonstrate the reproducibility and precision of the data. Fig. 5a and 5b are representative color maps from two individual subjects at a level comparable to plate 45 of (Paxinos and Franklin, 2001). Fig. 5c shows the same level of the averaged color map from the population of 8 subjects and Fig. 5d is a section of 5c magnified by 2.5-times. Note in Fig. 5d, the very fine structures that are visible, such as those shown by the numbered arrows: (1) extension of the external capsule; (2) fiber tracks in the caudate putamen; (3) the alveus; (4) two separate bundles in the corpus callosum; (5) the nigrostriatal bundle. Fig. 2 demonstrates that the alignment of the data across the population is ~1.5 voxels (~65 μm). T he structures listed above are all a couple of voxels across in the averaged color map. It is remarkable that these structures appear in the same position across the individual subjects and the averaged brain with such consistency, underscoring the reproducible anatomy achieved at microscopic resolution in this population-based atlas. Moreover, the individual data quality is sufficiently high and the registration is sufficiently accurate that the resulting averaging of diffusion indices provides a high level of confidence in their absolute values for quantitative analysis. For example, the variability of the axial diffusivity across the 8 different specimens is ~ 5% for the 9 different WMs listed in Table 2. Given this precision one has confidence that the observed difference of mean AD between the lateral lemniscus (0.59×10−3 mm2/s) and the cerebral peduncle (0.80×10−3 mm2/s) is representative of significant differences in these two structures. Table 3 and Table 4 present a more in depth and complete description of the statistically significant differences that can be revealed between the white matters as a consequence of the high degree of precision in this data.
Fig. 5.
Panel a and b are color maps from two individual subjects in axial view, panel c is the same level of the averaged color map from the population of eight subjects, and d is a section of panel c magnified by 2.5 times, showing some very fine structures indicated by the numbered arrows: (1) extension of the external capsule; (2) fiber tracks in the caudate putamen; (3) the alveus; (4) two separate bundles in the corpus callosum; (5) the nigrostriatal bundle. With careful scrutiny, one can actually notice that these structures consistently appear on all panels a, b, and c.
Table 5 listed the power analysis results of the number of specimens required to detect a 10% change in AD, RD, FA, or angular deviation of ev0 for each white matter region. Overall, FA needs fewer specimens (mostly less than 10) to detect the same percentage change than the other 3 indices. The angular deviation of ev0 requires more specimens (usually more than 25). This shows that different diffusion indices demonstrate different amount of variability under the same experimental settings. Moreover, certain white matter structure, such as the corpus callosum (cc), requires fewer specimens to detect diffusion changes than most other structures, showing that cc may have less anatomical and cellular variability across the normal population.
Table 5.
Number of specimens required to detect a 10% change in AD, RD, FA, or angular deviation of ev0 for each white matter region, via power analysis with α=0.05 and power of 0.95. Note that the result listed was number of specimens of one group (e.g., the control group). The other group (e.g., the disease group) was assumed to have the same number of specimens.
| Diffusion indices | ll | ac | cp | ic | ot | fi | cc | f | sp5 |
|---|---|---|---|---|---|---|---|---|---|
| AD | 5 | 11 | 8 | 7 | 27 | 16 | 3 | 9 | 5 |
| RD | 11 | 18 | 23 | 18 | 18 | 18 | 5 | 34 | 15 |
| FA | 8 | 9 | 4 | 9 | 7 | 3 | 6 | 20 | 5 |
| ev0 | 55 | 105 | 27 | 10 | 55 | 7 | 15 | 42 | 48 |
DISCUSSION
A number of investigators have produced atlases of the mouse brain using individual specimens, populations, in vivo, ex vivo, MR, and CT, with widely varying contrasts and widely varying spatial resolutions. These all complement the long-standing reference atlases based on conventional histology. Our long-term goal is to develop a comprehensive atlas available online in a common reference space (Waxholm Space), so that investigators can share their observations regardless of modality (Johnson et al., 2010). A population-based DTI atlas is clearly crucial to achieving this goal. Several groups have published DTI images of the adult mouse brain, usually at voxel volume (Δv) > 1.5 nl (Verma et al., 2005; Zhang et al., 2005). Aggarwal et al. have developed a population-based atlas combining in vivo T2 weighted images, micro-CT, and ex vivo DTI at spatial resolution as high as 113 μm (Δv ~ 1.4 nl) (Aggarwal et al., 2009). More recently, the same group has acquired DTI data of adult mouse brain at higher spatial resolution of 55 μm (Δv ~ 166 pl) within 45.5 hours scan time, and also imaged excised mouse brain cerebellum across limited FOV at 50 μm resolution (Δv ~ 125 pl) (Aggarwal et al., 2010). The work presented here created high-quality mean and variation atlases of normal mouse brain by pooling DTI data at 43 μm resolution (Δv ~ 80 pl) using elastic spatial normalization into the standard coordinate Waxholm Space (WHS), providing a reference set of DTI indices and their variation in the normal population. The registration quality measurement by landmark displacement (Fig. 2) yields excellent results indicating registration error is quite limited in this study. Thus the atlases provide the cross-subject anatomical similarity and variability. The current work differs from these previous superb studies in four ways. The resolution is to the best of our knowledge the highest yet achieved (Δv ~ 80 pl), which can help reduce partial volume effects, resolve finer structures, and improve accuracy of fiber tracking. The data is registered to WHS, so that one has reference to even higher-resolution (Δv ~ 10 pl) T1-weighted, T2*-weighted MRI, Nissl volumes, and other upcoming contrasts and information available in WHS. The data has been acquired with the active staining protocol that we believe will allow reasonable acquisition time (28 hours) at the achieved high resolution. Finally, even with the higher spatial resolution, the reproducibility of the data is better than any previous data of which we are aware. There may still be room for improvement with optimized gradient directions and the use of other diffusion models, such as higher order tensors (Liu et al., 2005). Such work is under investigation.
The atlas can be used in several ways. The most obvious use is for conventional voxel-based analysis (Abe et al., 2004; Kumra et al., 2005; Park et al., 2004) to compare across subjects at the same anatomical location to identify abnormalities. The results of the normal variations should provide important information to perform power analysis and judge sensitivity to detect abnormalities (Oishi et al., 2009; Wakana et al., 2007), as we have illustrated in this study. The coefficient of variation atlas shows that the variance of each diffusion index is inhomogeneous across the brain (Fig. 3). Consequently, smaller differences can be detected in some regions compared to others. This uneven sensitivity pattern should be part of the interpretation of any DTI result in group data, including the scalar diffusion indices and fiber tracking (Dougherty et al., 2005).
The voxel-based method often suffers from low statistical power because information from each voxel can be noisy and the inherent variations across subjects may complicate distinguishing subtle abnormalities from normal variants. Manual ROI definition is another widely adopted approach with some limitations, because it is time-consuming, and shows inter- and intra-operator variability of the manual delineation. Combining pre-defined white matter labels (such as those in Waxholm Space) to individual subjects provides an efficient means to segment and quantify the large number of white matter structures automatically and reproducibly within the accuracy level of normalization, which can be used for initial assessment of the whole brain and bring our attention to sensitive brain regions for more refined investigation of anatomy and diffusion indices (Mori et al., 2008; Oishi et al., 2009). Previous studies have found the variability of diffusion measurements was very similar (Hua et al., 2008; Mori et al., 2008) or even slightly decreased (Hagler et al., 2009) for atlas-derived automated ROIs compared to manually defined ROIs. In this study, the ANOVA tests of diffusion indices across 9 WMs or across 8 subjects illustrated one exemplary use of the DTI atlas based on automated white matter ROIs (derived from WHS). The robustness of the statistical tests performed on the core regions of the automated ROIs further validated that small errors in the initial ROI delineation and subsequent image transformation could be acceptable.
Using the protocol and infrastructure described previously (Jiang and Johnson, 2010), we were able to acquire high-resolution DTI data of mouse brain in a robust and repeatable fashion, allowing us to normalize individual brains onto a common reference space with high accuracy, provide statistically consistent data of individual brains, and discover what may be meaningful anatomical difference between white matters, lending validity to atlas-based representation of DTI indices. This data has now been added to Waxholm Space to expand the utility of this common reference data. It will hopefully serve as a foundation to quantitatively study mouse brain integrity and white matter architecture for both voxel-based and ROI-based analysis, at what we believe to be the highest spatial resolution yet attained. Using a single mouse strain provides only limited insight into the variability of diffusion properties in the mouse brain. This atlas of the C57BL/6J mouse brain provides the requisite foundation for more extensive studies of variation in DTI indices induced by genetic and environmental variation.
ACKNOWLEDGMENTS
We are grateful to Boma Fubara for assistance in specimen preparation and definition of anatomical regions, Gary Cofer for assistance in MR acquisition, Alexandra Badea for assistance in definition of anatomical regions, and Sally Zimney for assistance in manuscript preparation. All work was performed at the Duke Center for In Vivo Microscopy, an NCRR national Biomedical Technology Research Center (P41 RR005959) and NCI Small Animal Imaging Resource Program (U24 CA092656), with specific support from the Mouse Bioinformatics Research Network (U24 RR021760).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe O, Yamada H, Masutani Y, Aoki S, Kunimatsu A, Yamasue H, Fukuda R, Kasai K, Hayashi N, Masumoto T, Mori H, Soma T, Ohtomo K. Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel-based analysis. NMR in Biomedicine. 2004;17:411–416. doi: 10.1002/nbm.907. [DOI] [PubMed] [Google Scholar]
- Aggarwal M, Zhang J, Miller MI, Sidman RL, Mori S. Magnetic resonance imaging and micro-computed tomography combined atlas of developing and adult mouse brains for stereotaxic surgery. Neuroscience. 2009;162:1339–1350. doi: 10.1016/j.neuroscience.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal M, Mori S, Shimogori T, Blackshaw S, Zhang J. Three-dimensional diffusion tensor microimaging for anatomical characterization of the mouse brain. Magnetic Resonance in Medicine. 2010;64:249–261. doi: 10.1002/mrm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Transactions on Medical Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Badea A, Ali-Sharief AA, Johnson GA. Morphometric analysis of the C57BL/6J mouse brain. Neuroimage. 2007;37:683–693. doi: 10.1016/j.neuroimage.2007.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea A, Johnson GA, Williams RW. Genetic dissection of the mouse brain using high-field magnetic resonance microscopy. Neuroimage. 2009;45:1067–1079. doi: 10.1016/j.neuroimage.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhenain M, Ruffins SW, Jacobs RE. Three-dimensional digital mouse atlas using high-resolution MRI. Developmental Biology. 2001;232:458–470. doi: 10.1006/dbio.2001.0189. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage. 2008;42:60–69. doi: 10.1016/j.neuroimage.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch G, Potanina P, Bammer R, Wandell BA. Ulmer JL, Parsons L, Moseley M, Gabrieli J, editors. Occipital-callosal pathways in children - Validation and atlas development. White Matter in Cognitive Neuroscience: Advances in Diffusion Tensor Imaging and its Applications, Annals of the NY Academy of Sciences. 2005;1064:98–112. doi: 10.1196/annals.1340.017. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM. Automated white-matter tractography using a probabilistic diffusion tensor atlas: application to temporal lobe epilepsy. Human Brain Mapping. 2009;30:1535–1547. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang JY, Wakana S, Jiang HY, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, van Zijl PCM, Kim J, Pearlson GD, Mori S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Johnson GA. Microscopic diffusion tensor imaging of the mouse brain. Neuroimage. 2010;50:465–471. doi: 10.1016/j.neuroimage.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GA, Ali-Sharief A, Badea A, Brandenburg J, Cofer G, Fubara B, Gewalt S, Hedlund LW, Upchurch L. High-throughput morphologic phenotyping of the mouse brain with magnetic resonance histology. Neuroimage. 2007;37:82–89. doi: 10.1016/j.neuroimage.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GA, Badea A, Brandenburg J, Cofer G, Fubara B, Liu S, Nissanov J. Waxholm Space: An image-based reference for coordinating mouse brain research. Neuroimage. 2010;53:365–372. doi: 10.1016/j.neuroimage.2010.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GA, Cofer GP, Gewalt SL, Hedlund LW. Morphologic phenotyping with MR microscopy: The visible mouse. Radiology. 2002;222:789–793. doi: 10.1148/radiol.2223010531. [DOI] [PubMed] [Google Scholar]
- Kovacevic N, Henderson JT, Chan E, Lifshitz N, Bishop J, Evans AC, Henkelman RM, Chen XJ. A three-dimensional MRI atlas of the mouse brain with estimates of the average and variability. Cerebral Cortex. 2005;15:639–645. doi: 10.1093/cercor/bhh165. [DOI] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, Wu JH, Clarke T, Thaden E, Kane JM, Rhinewine J, Lencz T, Diamond A, Ardekani BA, Szeszko PR. White matter abnormalities in early-onset schizophrenia: A voxel-based diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:934–941. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- Liu C, Bammer R, Moseley ME. Characterizing non-Gaussian diffusion by using generalized diffusion tensors. Magn Reson Med. 2004;51:924–937. doi: 10.1002/mrm.20071. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hof PR, Grant SC, Blackband SJ, Bennett R, Slatest L, McGuigan MD, Benveniste H. A three-dimensional digital atlas database of the adult C57BL/6J mouse brain by magnetic resonance microscopy. Neuroscience. 2005;135:1203–1215. doi: 10.1016/j.neuroscience.2005.07.014. [DOI] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Lee EF, Dinov ID, Bota M, Shattuck DW, Ruffins S, Yuan H, Konstantinidis F, Pitiot A, Ding Y, Hu GG, Jacobs RE, Toga AW. A multimodal, multidimensional atlas of the C57BL/6J mouse brain. Journal of Anatomy. 2004;204:93–102. doi: 10.1111/j.1469-7580.2004.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie-Graham AJ, Lee EF, Dinov ID, Yuan H, Jacobs RE, Toga AW. Multimodal, multidimensional models of mouse brain. Epilepsia. 2007;48:75–81. doi: 10.1111/j.1528-1167.2007.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Itoh R, Zhang JY, Kaufmann WE, van Zijl PCM, Solaiyappan M, Yarowsky P. Diffusion tensor imaging of the developing mouse brain. Magnetic Resonance in Medicine. 2001;46:18–23. doi: 10.1002/mrm.1155. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang HY, Jiang L, Li X, Akhter K, Hua KG, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang JY, Huang H, Miller MI, Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang HY, Li X, Akhter K, Zhang JY, Hsu JT, Miller MI, van Zijl PCM, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: Application to normal elderly and Alzheimer's disease participants. Neuroimage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang HY, Li X, Akhter K, Hua KG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang JY, Huang H, Miller MI, van Zijl PCM, Mazziotta J, Mori S. Human brain white matter atlas: Identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; New York: 2001. [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Verma R, Mori S, Shen DG, Yarowsky P, Zhang JY, Davatzikos C. Spatiotemporal maturation patterns of murine brain quantified by diffusion tensor MRI and deformation-based morphometry. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6978–6983. doi: 10.1073/pnas.0407828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua KG, Zhang JY, Jiang HY, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Xu DR, Mori S, Shen DG, van Zijl PCM, Davatzikos C. Spatial normalization of diffusion tensor fields. Magnetic Resonance in Medicine. 2003;50:175–182. doi: 10.1002/mrm.10489. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Miller MI, Plachez C, Richards LJ, Yarowsky P, van Zijl P, Mori S. Mapping postnatal mouse brain development with diffusion tensor microimaging. Neuroimage. 2005;26:1042–1051. doi: 10.1016/j.neuroimage.2005.03.009. [DOI] [PubMed] [Google Scholar]