Abstract
Stress and anxiety are commonly thought to be detrimental to sexual function. Several studies in both the human and animal literature, however, have found that inducing anxiety can enhance sexual function in women. The mechanisms that explain a negative relationship between physical and psychological stress and sexual functioning are well documented, but little is known about how stress or anxiety might have a facilitatory effect on sexual arousal. As an initial step in exploring the relationship between anxiety and sexual arousal, the present study examined the role of the autonomic nervous system, and the adrenal hormones cortisol and dehydroepiandrosterone-sulfate (DHEA-S) in response to a sexual film, an anxiety-inducing film, and a humorous film. Nineteen premenopausal women (mean age 24.4 years) who were free from sexual difficulties came into the lab on three separate days. At each session they were shown an anxiety-inducing, sexually arousing, or humorous (control) film while their physiological arousal was measured. They also provided saliva samples before and after each film. Cortisol significantly decreased, while DHEA-S increased in the sexual and humorous conditions. Neither hormone changed significantly in the anxiety-inducing condition. Autonomic nervous system activity measured by heart rate and heart rate variability did not change in response to the sexual or anxiety-inducing films, but heart rate variability increased significantly in response to the humorous film. The cortisol/DHEA-S ratio at the post-sexual film time point was significantly negatively correlated with genital arousal (measured by vaginal pulse amplitude). Anxiety-inducing films did not result in a physiological stress response, which can explain why they do not impair sexual function.
Keywords: Cortisol, Dehydroepiandrosterone-sulfate, Human sexuality, Stress, Anxiety, Arousal, Humor
Introduction
Stress and anxiety are commonly thought to be detrimental to sexual function, and this assertion is supported by many empirical studies. Survey studies have generally found a negative relationship between psychological stress or anxiety and sexual functioning in the general population (e.g., Bodenmann et al., 2006; Dunn et al., 1999) and in clinical populations (e.g., Figueira et al., 2001; van Minnen and Kampman, 2000). Studies that have induced anxiety in the laboratory have shown mixed results with some demonstrating that stress can inhibit women's sexual arousal (e.g., Brauer et al., 2007; ter Kuile et al., 2007) and others finding moderate levels of stress or anxiety can enhance sexual arousal (e.g., Bradford and Meston, 2006; Hoon et al., 1977; Palace and Gorzalka, 1990). There are numerous studies spanning both the animal and human literature outlining the reasons for a negative relationship between both physical and psychological stress and sexual functioning. Specifically, corticosteroids (cortisol in humans) can interfere with hypothalamic-pituitary-gonadal (HPG) axis functioning (for reviews see Rivier and Rivest, 1991; Welsh et al., 1999). However, little is known about how stress or anxiety might have a facilitatory effect on sexual arousal.
Two previous studies used anxiety-inducing films to increase subsequent genital arousal (Hoon et al., 1977; Palace and Gorzalka, 1990). As this type of stimuli increases both anxiety and subsequent genital sexual arousal, we were interested in exploring potential facilitatory mechanisms that may explain this relationship. One possible mechanism could be the activity of the cardiovascular components of the autonomic nervous system (ANS). Stressors generally increase sympathetic nervous system(SNS) activity, and SNS activation has been repeatedly shown to increase subsequent genital arousal in studies using exercise (Meston and Gorzalka, 1995, 1996), administration of ephedrine (Meston and Heiman, 1998), and hyperventilation (Brotto and Gorzalka, 2002). Further support for the role of the SNS in genital arousal was found in a study showing an increase in alpha-amylase, a marker of norepinephrine (NE), after exercise that continued to increase during a sexually arousing film (Hamilton et al., 2008). Although the mechanisms through which the SNS and the parasympathetic nervous system(PNS) influence sexual arousal are still debated (for a reviewsee Meston and Bradford, 2007), it is clear that a moderate increase in SNS results in increased genital arousal in most women.
Stress and sexual arousal are considered to have opposing hormonal responses based on research demonstrating the suppressive effects of the hypothalamic-pituitary-adrenal (HPA) axis on the hypothalamic-pituitary-gonadal (HPG) axis. During sexual arousal, cortisol generally decreases (e.g., Exton et al., 2000), and during stress it increases. During stress, cortisol released from the adrenal gland inhibits release of gonadotropin releasing hormone (GnRH), luteinizing hormone (LH) and follicle stimulating hormone (FSH) at the hypothalamic and pituitary levels resulting in decreased levels of sex steroids (for review, see Welsh et al., 1999). This model or similar disruptions of the HPG axis by hormones released from the HPA axis has been demonstrated in several species (e.g., Breen and Karsch, 2004; Gore et al., 2006; Olster and Ferin, 1987). Other adrenal hormones, including norepinephrine (NE), and dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S) can potentially counteract the negative effect of cortisol on sexual arousal.
DHEA and particularly DHEA-S have been increasingly implicated in women's sexual function (Spark, 2002). Studies of women with sexual dysfunctions have shown that women with both sexual arousal and desire problems have lower levels of DHEA-S compared to women without sexual difficulties (Basson et al., 2010; Guay et al., 2004). Randomized placebo-controlled studies that have administered DHEA to women (most of whom were postmenopausal) have shown mixed results with some reporting increases in sexual function and some reporting no change (reviewed by Panjari and Davis, 2010). DHEA is found in low quantities in women and has a fairly short half-life, while DHEA-S is present in much higher levels in plasma and has a long half-life, making it a more stable measure of DHEA activity (Labrie et al., 2005; Panjari and Davis, 2010). Although exogenous administration of DHEA has been linked with increased DHEA-S and genital arousal in some studies (e.g., Hackbert and Heiman, 2002), to our knowledge, no one has looked at the relationship between endogenous DHEA-S and sexual arousal in the laboratory.
DHEA-S has also been shown in non-sexual studies to be a protective factor against the negative effects of cortisol and/or stress (reviewed by Bonne et al., 2004). Of particular interest has been the cortisol/DHEA-S ratio, for which lower values are linked to more positive outcomes. The beneficial effects of low cortisol/DHEA-S ratios have been shown in various conditions including clinical depression (Michael et al., 2000), startle response (Grillon et al., 2006), and stressful military training (Morgan et al., 2004). It is possible that the beneficial functions of DHEA-S and a low cortisol/DHEA-S ratio would also extend to sexual functioning.
As a first step in exploring the role of DHEA-S and cortisol in the relationship between anxiety and sexual arousal, the present study was designed to explore the underlying ANS and adrenal hormone changes that occur in response to these states of arousal. Although much of the literature indicates that anxiety and sexual arousal have differing physiological responses, we hypothesized that there would be several similarities that could explain a complimentary effect of anxiety on sexual arousal. In regards to ANS arousal, we measured heart rate with the prediction that there would be similar ANS responses to the anxiety-inducing stimuli and the sexual stimuli, specifically that both would increase SNS activity. We predicted that while cortisol would decrease in response to sexual stimuli and increase in response to anxiety-inducing stimuli, DHEA-S would increase in both conditions. Since lower cortisol/DHEA-S ratios have been linked with more positive outcomes in other domains, we hypothesized that this ratio would also correlate with higher genital and subjective sexual arousal.
Method
Participants
Twenty-five women were enrolled in the study. Of these women, three did not complete all sessions and three more had problems with data collection. All six women were excluded from data analysis. The remaining participants were 19 women between the ages of 18 and 47 (M = 24.4, SD = 6.8) who were recruited from the community via flyers and online advertisements. All women had been sexually active with a male partner within the month before the study began and reported they were exclusively or predominantly heterosexual. Five women were single and 14 were in committed relationships ranging in length from six months to five years. Reported ethnicity was Caucasian (11), Latina (5), African American or Black (2), and Asian (1).
Participants were all screened to ensure they met the inclusion and exclusion criteria. Inclusion criteria were as follows: between the ages of 18 and 50, currently sexually active with men, sexual intercourse within the past month, fluent in English, regular menstrual cycles over the past six months (i.e. no evidence of menopause, pregnancy, or menstrual irregularities). Exclusion criteria were as follows: use of any hormonal medication, use of medications known to interfere with cardiovascular or genital functioning, currently pregnant or breastfeeding, current distress about sexual abuse or assault, and any report of sexual problems, endocrine abnormalities, major genital/pelvic surgery, or current sexually transmitted infection.
Materials and apparatus
Stimuli
Film sequences
To explore the women's physiological response to anxiety and sexual arousal, we chose to use an anxiety-inducing film as our stimulus because two previous studies have shown that similar film clips enhanced subsequent genital sexual arousal (Hoon et al., 1977; Palace and Gorzalka, 1990). Unlike previous studies that have looked at anxiety and sexual arousal within the same session, we wanted to isolate these states of arousal (anxiety and sexual) in separate sessions to get a clear picture of each condition. As a comparison, we used a humorous video as a positive-affect, non-sexual control that was also emotionally arousing to contrast the anxiety-inducing and sexual conditions.
All three of the film sequences were approximately 12 min long, and all began with an introductory sequence of a 1 min display of the word “Relax” followed by 3 min of a neutral film (a travel documentary). In the sexual arousal condition, the introductory sequence was followed by 8 min of a woman-centered erotic film. The erotic film was drawn from the Sexual Psychophysiology Laboratory film library. All films in this library have been standardized in terms of length of different types of sexual scenes (i.e. foreplay, oral sex and vaginal intercourse). None of the films show sexual violence or fellatio. These films were selected from erotic films produced and directed by women and are intended to be sexually appealing to women. The film had been shown to elicit significant subjective and genital arousal in women in previous studies (e.g., Rellini et al., 2005). The anxiety-inducing film consisted of an 8 min clip from the movie Bully (Clark, 2001). The clip depicted the lead up to and murder of a teen bully by a group of other teens and was ranked as highly unpleasant and moderately stressful during a pilot test. The humorous film consisted of 8 min of stand-up comedy by Dane Cook. The clip was the unedited version of Cook's appearance at the Bar Mitzvah Bash from his Retaliation DVD (2005). This film has been effectively used by other labs to induce humor in studies where several comedy films were piloted (David Gilden, personal communication, November 15, 2005).
Questionnaires
Demographics
The demographics questionnaire asked participants their age, level of education, relationship status, sexual orientation, ethnicity, and length of relationship with their current partner.
Screening questionnaire
The screening questionnaire documented that participants met inclusion/exclusion criteria. It included items on current drug use, medical conditions, distress from sexual abuse, menstrual cycle dates and irregularities, and whether the participant had eaten, drank, smoked or exercised in the past hour. To verify that participants were free of sexual arousal problems, we also included the Arousal subscale of the Female Sexual Function Index (FSFI; Rosen et al., 2000). The FSFI is a validated 19-item questionnaire designed to assess sexual functioning in women. A shortened version of the Screening questionnaire was administered during sessions 2 and 3 to verify that participants had not engaged in any behaviors in the hour prior that would affect their hormonal or cardiovascular responses.
Subjective Response Scale
Subjective responses to the films were measured using the Subjective Response Scale, which was derived from the Film Scale (Heiman and Maravilla, 2007) and the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). The scale consisted of 58 items, which were divided into four subscales: subjective experience of physiological sexual arousal (e.g., “genital sensations”), mental sexual arousal (e.g., “turned on”), positive affect (e.g., “happy”), and negative affect (e.g., “guilty”). Items were rated on a 7-point Likert scale ranging from “not at all” to “intensely.” This scale was used as a manipulation check to verify that the films were inducing appropriate affect and sexual arousal response for the conditions.
Apparatus
Vaginal photoplethysmograph
Genital arousal in response to all films was measured using a vaginal photoplethysmograph (Sintchak and Geer, 1975). The vaginal photoplethysmograph is a clear, acrylic, tampon-shaped device that contains an infrared light-emitting diode as a light source, and a photosensitive light detector. When inserted into the vagina, the light source illuminates the capillary bed of the vaginal wall and the blood circulating within it. Upon contact with the vaginal wall, some of the light is absorbed, while the remainder is backscattered. The amount of backscattered light is related to the transparency of the tissue, which is affected by blood flow. The measure of interest derived from the photoplethysmograph was the vaginal pulse amplitude (VPA). This A/C signal was band pass filtered at 0.5 to 30 Hz. VPA is a sensitive and specific indicator of sexual arousal; it is not influenced by other non-sexual affective states (e.g., Laan et al., 1995). VPA was sampled at 80 Hz and results were measured in millivolts (mV). VPA data were acquired in real time using the software program AcqKnowledge III, Version 3.7.3 (BIOPAC Systems, Inc., Santa Barbara, CA) and a Model MP100WS data acquisition unit (BIOPAC Systems, Inc., Santa Barbara, CA) for analog/digital conversion.
Electrocardiograph (ECG)
Heart rate and heart rate variability (HRV) were measured via an ECG, which consisted of three disposable electrodes that were attached to the participant's body and connected by leads to a BIOPAC Systems ECG100 module. We used heart rate as an indicator of SNS activity with increased heart rate indicating increased SNS activity. As the SNS speeds up the heartbeat, beat-to-beat intervals become more regularly spaced, while the PNS returns the body to a regular sinus rhythm, allowing the beat-to-beat intervals to become more variable. Measuring the standard deviation of these intervals, called the R–R intervals (SDRR) has been demonstrated to be a fairly accurate measure of ANS activity. The SDRR measure encompasses all components of HRV, so an increase in variability (SDRR) indicates higher PNS activity, while a decrease indicates higher SNS activity (Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology., 1996). The signal from the ECG100 module was recorded in real time using the AcqKnowledge software program. ECG was also sampled at 80 Hz.
Saliva samples
Salivary assays provide a relatively noninvasive way to examine biomarkers of interest. Participants were asked to passively drool without stimulation directly into untreated, polystyrene centrifuge tubes while alone in a research room. After the study procedures were completed, saliva samples were frozen until assay, when they were thawed and centrifuged at 3000 rpm for 15 min. DHEA-S and cortisol were assayed in-house using commercially available enzyme immunoassay kits purchased from Salimetrics (State College, PA). All assays were run in duplicate. For DHEA-S, inter-assay C.V. was 8.42% at 9.43 pg/ml and 5.62% at 538 pg/ml, and intra-assay C.V. was 2.65%. For cortisol, inter-assay C.V. was 4.2% at 0.02 µg/dl and 5.3% at 0.95 µg/dl, and intra-assay C.V. was 3.4%.
Procedures
The University of Texas Institutional Review Board approved all procedures. Trained female research assistants screened participants over the phone to determine eligibility for the study. To control for menstrual cycle variations in hormones, all experimental appointments took place between days 5 and 10 of the women's menstrual cycles. To control for diurnal variations in hormone levels, all women were tested between the hours of 2:00 pm and 6:00 pm when cortisol is known to be relatively stable. After the initial phone screening, participants who qualified were scheduled to come into the lab on three separate days. Participants were asked to refrain from eating or drinking anything but water, smoking, or exercising for at least one hour prior to their scheduled appointments.
Upon arrival, participants were given a cup of water to drink while the study procedures were explained. After reading and signing the consent form, participants had three electrodes placed on their skin for the ECG. After the placement of the electrodes, participants were left alone in the research room until the completion of the study. All subsequent communication was via intercom. Participants then filled out the demographics and screening questionnaires. Twenty minutes after they began the questionnaires, they provided the first saliva sample. Once the first saliva sample was completed, participants inserted the vaginal photoplethysmograph and attached the electrode wires for the ECG, as previously instructed. Once the vaginal photoplethysmograph signal stabilized, they were asked to fill out the pre-film Subjective Response Scale indicating their feelings of affect and arousal at that moment. Participants then watched one of the three film sequences. Immediately following the film sequence, they filled out the post-film Subjective Response Scale, indicating their affect and arousal during the last film. Upon completion of the Subjective Response Scale, the participants were told to remove the photoplethysmograph and the electrodes and get dressed. Ten minutes after the end of the film, they provided a second saliva sample. This time frame allowed for 20 min from the onset of the stimulus; salivary cortisol has been shown to peak 10–30 min after the onset of a stressor in previous studies (Kirschbaum and Hellhamer, 1989; Kirschbaum et al., 1992).
Sessions 2 and 3 proceeded in the same sequence as the first session, with the exception of the demographics and screening questionnaires, which were only administered at the start of the first session. During each of the three sessions, participants saw one of three different films after the neutral film: anxiety-inducing, sexual, or humorous. The three films were presented in a counterbalanced order.
Data analysis
Preprocessing
VPA data
VPA data were reduced by calculating the total change in amplitude for each heart beat. This was done by finding the peak and nadir for each pulse wave and computing the differences between the two, using AcqKnowledge software. Artifacts in the data were identified visually by the researcher and removed manually, as per past studies of this nature (Laan et al., 1995; Rellini et al., 2005). VPA was averaged across the neutral film and final three minutes of the experimental portions of each film. In order to control for individual variability in VPA signals, a VPA difference score for each person and each condition was calculated as the percent change in VPA during the experimental film over the neutral film.
EGC data
Heart rate was determined by calculating a difference score between the average heart rate for the neutral and the final three min of each experimental film for each participant. Heart rate variability was calculated from the ECG signal by determining the time interval between each heart beat (R–R interval). The R–R intervals from the neutral segment and from the experimental segment (final three minutes) were entered into a MATLAB based program, Biosignal (Niskanen et al., 2004) to determine the SDRR.
Hormonal data
In order to control for individual variability in basal hormone levels, hormonal data was calculated as percent change over baseline.
Subjective Response Scale
A difference score was calculated for each item on the Subjective Response Scale by subtracting the pre-film score from the post-film score. These difference scores were then averaged for the items within each of the four subscales: subjective perception of physical arousal, mental arousal, positive affect and negative affect.
Statistical analyses
To look at differences between conditions, all percent change and difference scores were entered as dependent variables into separate repeated-measures ANOVAs with Condition as the within-subjects variable. Significant ANOVAs were further tested using post hoc paired samples t-tests. All post hoc tests were done comparing all three conditions. To look at changes over baseline, one sample t-tests were conducted for each condition. A Bonferroni correction was used for each set of tests to control family wise error at 0.05. The significance level for all post hoc t-tests was rounded to 0.02. For correlations, the alpha level was set at 0.01 to control for multiple tests.
Results
Manipulation check
Subjective arousal
The means for the four subscales of the SRS are show in Table 1. The overall ANOVAs were significant for all four subscales: subjective perceptions of physical arousal, F(2, 36) = 39.58, p<0.001, mental arousal, F(2, 36) = 28.01, p<0.001, positive affect, F(2, 36) = 6.32, p = 0.004, negative affect, F(2, 36) = 10.16, p<0.001. As expected, only the erotic film showed a significant increase in subjective perceptions of physical arousal, and in mental arousal. The erotic and humorous films showed increases in positive affect and no significant change in negative affect. The anxiety-inducing film showed an increase in negative affect and no significant change in positive affect. To test specifically for anxiety, repeated measures ANOVAs were run on the anxious item, F(2, 36) = 23.98, p<0.001, and the relaxed item, F(2, 36) = 14.45, p<0.001, individually, and the results showed that participants were significantly more anxious and less relaxed after the anxiety-inducing film compared to the sexual and humorous films.
Table 1.
Mean difference scores for vaginal pulse amplitude, subjective response scale, and heart rate.
| Mean difference scores (S.E.M.) | |||
|---|---|---|---|
| Sexual | Anxiety | Humorous | |
| VPA percent change | 37.92% (8.35)b,c | −0.02% (1.63)a | 1.11% (1.71)a |
| SRS — physical arousal | 3.308 (0.58)b,c | 0.26 (0.12)a | 0.15 (0.07)a |
| SRS — mental arousal | 2.54 (0.77)b,c | −0.31 (0.13)a | 0.25 (0.14)a |
| SRS — positive affect | 2.40 (0.93)b | −0.44 (0.13)a,c | 2.33 (0.76)b |
| SRS — negative affect | −0.07 (0.09)b | 2.77 (0.58)c | −0.09 (0.12)b |
| Heart rate (BPM) | 0.32 (1.84) | 0.78 (1.43) | −1.34 (1.08) |
Significantly different from the sexual condition at p = 0.02.
Significantly different from the anxiety-inducing condition at p = 0.02.
Significantly different from the humorous condition at p = 0.02.
Vaginal photoplethysmography
As noted earlier, the vaginal photoplethysmograph is specific to sexual arousal and does not change in response to non-sexual arousal. Thus, as expected the VPA percent change was significantly different between conditions, F(2,36) = 19.85, p<0.001. The change in VPA was significantly higher in the sexual condition compared to the anxiety-inducing condition, t(18) = −4.44, p<0.001, and the humorous condition, t(18) = 4.57, p<0.001. There were no significant differences between the anxiety-inducing and humorous conditions, t (18) = −0.55, p = 0.59. VPA in both the anxiety-inducing and humorous conditions was not significantly different from zero (Table 1).
Autonomic responses
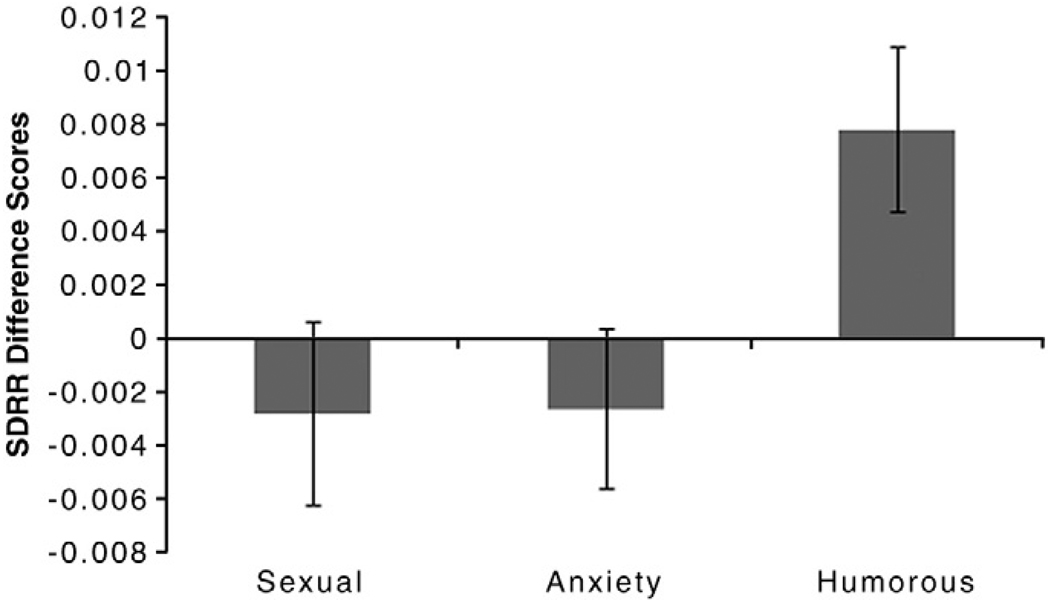
The change in heart rate was not significantly different between conditions, F(2, 36) = 2.67, p = 0.09, and none of the conditions showed any significant increase or decrease above neutral (Table 1). The measure of heart rate variability, SDRR, was significantly different across conditions, F(2, 36) = 4.16, p = 0.02. The sexual and anxiety-inducing conditions were not significantly different from one another, t(18) = 0.042, p = 0.97, indicating that the cardiovascular components of the PNS were similar during these two affective states. The increase in the humorous condition was significantly higher than both the sexual, t(18) 2.43, p = 0.02 and the anxiety-inducing conditions, t(18) 2.56, p = 0.02, indicating increased PNS activation (Fig. 1).
Fig. 1.
Heart rate variability across film conditions: Mean difference scores (Experimental–Neutral) for the Standard Deviation of the R–R intervals (SDRR) +/− S.E.M. Humor film induced increased SDRR, which is indicative of increased parasympathetic nervous system activity. There was no change for the sexual or anxiety-inducing films.
Hormonal responses
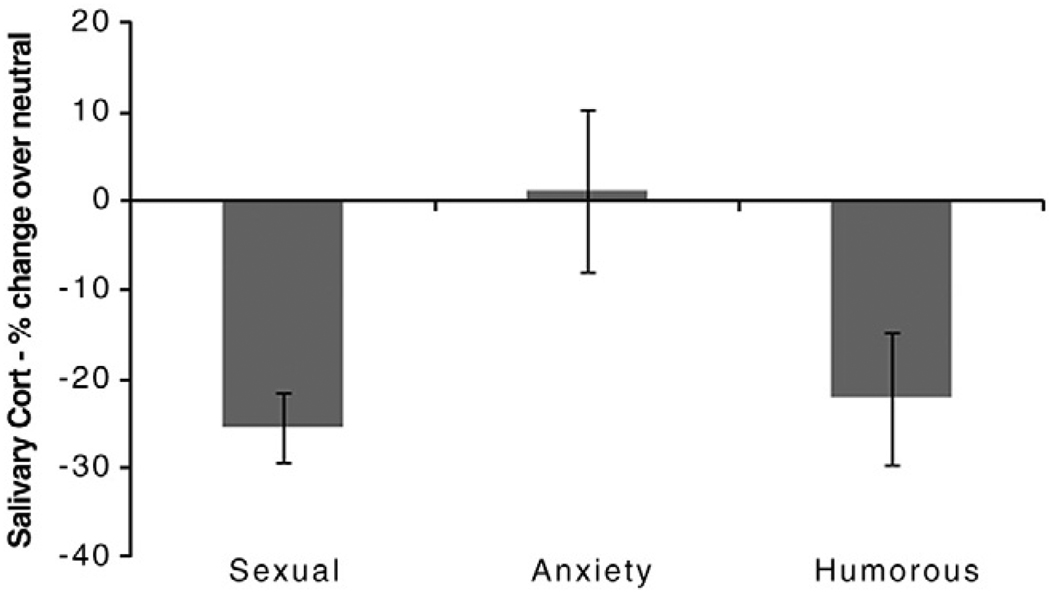
Means for the hormonal data are reported in Table 2. The change in cortisol in response to the experimental films was significantly different across conditions, F(2, 36) = 4.06, p = 0.03. Post hoc tests showed that the anxiety-inducing film was significantly different from the sexual film, t(18) = −2.93, p = 0.01, but not the humorous film, t(18) = 1.64, p = 0.12. Cortisol decreased significantly in the sexual, t(18) = 6.37, p<0.001 and the humorous conditions, t(18) −2.97, p = 0.01. There was no significant change in the anxiety-inducing condition, t(18) = 0.18, p = 0.86 (Fig. 2).
Table 2.
Mean salivary cortisol and DHEA-S in before and after the sexual, anxiety-inducing, and humorous films.
| Salivary cortisol and DHEA-S values in pg/ml (S.E.M.) | ||||
|---|---|---|---|---|
| Sexual | Anxiety | Humorous | ||
| Cortisol | Pre-film | 1763 (459) | 1521 (270) | 1343 (230) |
| Post-film | 1258 (311) | 2184 (639) | 1659 (410) | |
| DHEA-S | Pre-film | 5625 (1133) | 5621 (1144) | 3470 (614) |
| Post-film | 6504 (1121) | 4979 (722) | 4234 (552) | |
Fig. 2.
Salivary cortisol change across film conditions: Mean percent change over neutral +/− S.E.M. Cortisol declined significantly in the sexual and humorous conditions, but did not change in the anxiety condition.
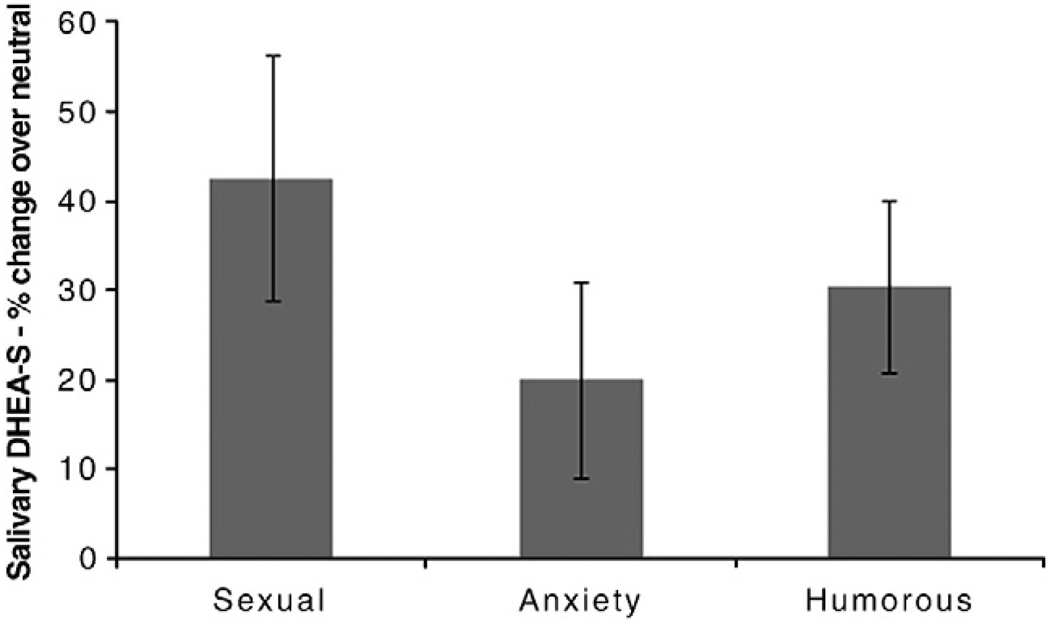
One participant had DHEA-S levels that were too high to read in one sample; her data were excluded from these analyses. The change in DHEA-S in response to the experimental films was not significantly different across conditions, F(2, 34) = 1.29, p = 0.29. Although DHEA-S increased in all three conditions, the increase was only significant for the sexual, t(17) = 3.09, p = 0.001 and humorous, t(17) = 3.39, p = 0.004 conditions. The anxiety-inducing video did not significantly change DHEA-S, t(17) = 1.38, p = 0.19 (Fig. 3).
Fig. 3.
Salivary DHEA-S change across film conditions: Mean percent change over neutral +/− S.E.M. DHEA-S increased significantly in the sexual and humorous conditions, but did not change in the anxiety condition.
The cortisol/DHEA-S ratio for the baseline sample for the erotic condition was not significantly correlated with subsequent change in VPA r(18) = −0.38, p = 0.16, or with subjective reports of physical r(18) = −0.44, p = 0.10 or mental arousal r(18) = −0.35, p = 0.16. The post sexual video cortisol/DHEA-S ratio was significantly, negatively correlated with the amount of increase seen in VPA r(18) = −0.60, p = 0.01, but not for subjective reports of physical r(18) = 0.17, p = 0.51, or mental arousal r(18) = 0.11, p = 0.68. For exploratory purposes, we calculated the cortisol/DHEA-S ratio for each condition at baseline for each person. The correlations within people, across conditions were highly significant, indicating that the cortisol/DHEA-S ratios are likely a stable trait factor (Table 3).
Table 3.
Mean cortisol/DHEA-S by condition and correlations between conditions at the pre-film sample.
| Sexual | Anxiety | Humorous | |
|---|---|---|---|
| Mean (SD) | 0.50 (0.62) | 0.89 (1.04) | |
| Correlations | 0.43 (0.36) | ||
| Anxiety | 0.76* | ||
| Humorous | 0.73* | 0.92* |
p<0.01.
Discussion
The present study examined the underlying autonomic and hormonal components of three distinct types of arousal: sexual, anxious, and humorous. The primary purpose of the study was to understand the role of underlying mechanisms that might explain a positive relationship between anxiety and sexual arousal. Genital and subjective reports of arousal and affect showed responses in the expected directions. Participants were more sexually aroused in the sexual condition compared to the anxiety-inducing and humorous conditions. Positive affect was highest for the sexual and humorous conditions, while negative affect was highest for the anxiety-inducing condition. Participants reported being more relaxed after the sexual and humorous films, and more anxious after the anxiety-inducing film.
Neither the anxiety-inducing or sexual films altered heart rate or heart rate variability; however, previous studies have also shown no change in heart rate in response to their sexual or anxiety inducing stimuli (e.g., Hoon et al., 1977). Additionally, Exton et al. (2000) found that while heart rate did not increase in response to sexual arousal in women, NE significantly increased, providing evidence for SNS activation in response to sexual arousal. It is possible that heart rate via ECG is not an appropriate measure for the changes in SNS activity that occur in response to sexual arousal and to a mild stressor like an anxiety-inducing film. Although there was no increase in SNS activity as hypothesized, the heart rate and HRV responses for the sexual and anxiety-inducing films were similar. This similarity demonstrates a common physiological response between two states of arousal that are often considered to have opposite responses.
Cortisol response was significantly different between the sexual and the anxiety-inducing conditions. Both the sexual condition and the humorous condition showed sharp decreases in cortisol over the course of the film. There was no significant change in the level of cortisol in response to the anxiety-inducing film, suggesting that a film may not be a strong enough stressor to elicit a cortisol response. As cortisol has been demonstrated to interfere with sexual function in animals, the lack of cortisol response to this film stimulus could explain why the anxiety-inducing films used by Hoon et al. (1977) and Palace and Gorzalka (1990) did not interfere with sexual arousal.
The DHEA-S response was not significantly different across conditions, but the pattern of response indicated there was a similar response (increase) for the sexual and humorous conditions. We hypothesized that DHEA-S would increase in response to both sexual arousal and anxiety, but similar to the cortisol results, there was no significant change in DHEA-S in response to the anxiety-inducing film. While the effect sizes for the other analyses allowed for enough power to see differences between the film conditions, the sample size of this study may not have been large enough to detect changes in DHEA-S, which was highly variable across participants. That DHEA-S did not increase in response to the anxiety-inducing film in this study indicates that it may not play a role in the relationship between anxiety and sexual arousal. The lack of increase could also be due to the strength of the stimuli, which did not increase cortisol or SNS activity either. The low power for this particular measure indicates that the role of DHEA-S in anxiety responses should not be ruled out.
This is the first study to examine the effects of sexual arousal on DHEA-S, and the first to show a significant increase in an androgenic hormone in response to sexual arousal in women. Previous studies of testosterone response to film-induced sexual arousal have repeatedly shown no change in testosterone (e.g., Exton et al., 1999; Hamilton et al., 2008). One recent study that examined several androgenic compounds in relation towomen's sexual function suggests that ifDHEA-S does play a role in female sexual function it may be via non-androgenic pathways, as increases in DHEA-S did not correspond with increases in other androgens or their metabolites (Basson et al, 2010). The increase in DHEA-S in response to sexual arousal further supports the theory that this hormone may play an important role in female sexual function (e.g., Basson et al., 2010; Spark, 2002).
The cortisol/DHEA-S ratio for the sample taken after the sexual film was negatively correlated with the women's VPA response, while the baseline ratio was not. This suggests that it is the hormonal response to the stimuli that is related to sexual response, not the baseline levels. The relationship between the ratio and VPA suggests that the protective benefits of a low cortisol/DHEA-S ratio that have been seen in other areas (e.g., Morgan et al., 2004) may also extend to sexual situations.
A key limitation of the present study was the use of discrete sampling methods for the hormonal measures. Using salivary sampling is a less invasive, less stressful method of assaying hormones, which important for stress-related research, but saliva samples have limitations as well. Hormones take longer to diffuse into saliva than into blood, so the timing of the measurement is an issue for all studies using salivary samples. In order to get a more complete picture of the cortisol and DHEA-S responses of women to different stimuli, blood would need to be sampled continuously.
This study was a first step in understanding the role of adrenal steroid hormones in the relationship between anxiety and stressful arousal. Previous studies have shown that anxiety-inducing films increased subsequent sexual arousal, and one reason for this may be that these films do not induce a physiological stress response, which has been shown to interfere with sexual arousal. Although the results of the DHEA-S response across conditions were somewhat unclear, the cortisol/DHEA-S ratio was related to sexual response in sexually functional women.
Acknowledgments
This research was supported, in part, by Grant Number R01 HD51676 from the National Institute for Child Health and Human Development to Cindy M. Meston and, in part, by a doctoral fellowship from the Natural Sciences and Engineering Research Council of Canada to Lisa Dawn Hamilton. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute for Child Health and Human Development or the Natural Sciences and Engineering Research Council. The authors would like to thank our research assistants Ashley Garner, Sheila Molina, Eve Andrews, and Taylor Anne Morgan for their assistance with data collection for this study.
References
- Basson R, Brotto LA, Petkau AJ, Labrie F. Role of androgens in women's sexual dysfunction. Menopause. 2010;17:962–971. doi: 10.1097/gme.0b013e3181d59765. [DOI] [PubMed] [Google Scholar]
- Bodenmann G, Ledermann T, Blather D, Galluzzo C. Associations among everyday stress, critical life events, and sexual problems. J. Nerv. Ment. Dis. 2006;194:494–501. doi: 10.1097/01.nmd.0000228504.15569.b6. [DOI] [PubMed] [Google Scholar]
- Bonne O, Grillion C, Vythilingam M, Neumeister A, Charney DS. Adaptive and maladaptive psychobiological responses to severe psychological stress: implications for the discovery of novel pharmacotherapy. Neurosci. Biobehav. Rev. 2004;28:65–94. doi: 10.1016/j.neubiorev.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bradford A, Meston CM. The impact of anxiety on sexual arousal in women. Behav. Res. Ther. 2006;44:1067–1077. doi: 10.1016/j.brat.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, ter Kuile MM, Janssen SA, Laan E. The effect of pain-related fear on sexual arousal in women with superficial dyspareunia. Eur. J. Pain. 2007;11:788–798. doi: 10.1016/j.ejpain.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145:692–698. doi: 10.1210/en.2003-1114. [DOI] [PubMed] [Google Scholar]
- Brotto LA, Gorzalka BB. Genital and subjective sexual arousal in postmenopausal women: influence of laboratory-induced hyperventilation. J. Sex Marital Ther. 2002;28 Suppl. 1:39–53. doi: 10.1080/00926230252851186. [DOI] [PubMed] [Google Scholar]
- Clark L. Bully. USA: Blacklist Pictures; 2001. [Motion Picture] [Google Scholar]
- Cook DJ. Retaliation. USA: Comedy Central; 2005. Unedited Bar Mitzvah Bash. [DVD] [Google Scholar]
- Dunn KM, Croft PR, Hackett GI. Sexual problems: a study of the prevalence and need for health care in the general population. Fam. Pract. 1999;15:519–524. doi: 10.1093/fampra/15.6.519. [DOI] [PubMed] [Google Scholar]
- Exton MS, Bindert A, Krüger T, Scheller F, Hartmann U, Schedlowski M. Cardiovascular and endocrine alterations after masturbation-induced orgasm in women. Psychosom. Med. 1999;61:280–289. doi: 10.1097/00006842-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Exton NG, Truong TC, Exton MS, Wingenfield SA, Leygraf N, Saller B, Hartmann U, Schedlowski M. Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology. 2000;25:187–199. doi: 10.1016/s0306-4530(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Figueira I, Possidente E, Marques C, Hayes K. Sexual dysfunction: a neglected complication of panic disorder and social phobia. Arch. Sex. Behav. 2001;30:369–377. doi: 10.1023/a:1010257214859. [DOI] [PubMed] [Google Scholar]
- Guay A, Jacobsen J, Munarriz R, Traish A, Talakoub L, Quirk F, Goldstein I, Spark R. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: part B: reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int. J. Impot. Res. 2004;16:121–129. doi: 10.1038/sj.ijir.3901176. [DOI] [PubMed] [Google Scholar]
- Gore AC, Attardi B, DeFranco DB. Glucocorticoid repression of the reproductive axis: effects on GnRH and gonadotropin subunit mRNA levels. Mol. Cell. Endocrinol. 2006;256:40–48. doi: 10.1016/j.mce.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Baas JMP, Lawley M, Ellis V, Charney DS. Cortisol and DHEA-S are associated with startle potentiation during aversive conditioning in humans. Psychopharmacology. 2006;186:434–441. doi: 10.1007/s00213-005-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackbert L, Heiman JR. Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J. Womens Health Gend. Based Med. 2002;11:155–161. doi: 10.1089/152460902753645290. [DOI] [PubMed] [Google Scholar]
- Hamilton LD, Fogle EA, Meston CM. The roles of testosterone and alpha-amylase in exercise-induced sexual arousal. J. Sex. Med. 2008;5:845–853. doi: 10.1111/j.1743-6109.2007.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman JR, Maravilla KR. Female sexual arousal response using serial magnetic resonance imaging with initial comparisons to vaginal photoplethysmography. In: Janssen E, editor. The Psychophysiology of Sex. Bloomington, Indiana: Indiana University Press; 2007. pp. 103–128. [Google Scholar]
- Hoon PW, Wincze JP, Hoon EF. A test of reciprocal inhibition: are anxiety and sexual arousal in women mutually inhibitory? J. Abnorm. Psychol. 1977;86:65–74. doi: 10.1037//0021-843x.86.1.65. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhamer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer DH. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W, Evers A. Assessment of female sexual arousal: response specificity and construct validity. Psychophysiology. 1995;32:476–485. doi: 10.1111/j.1469-8986.1995.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Belanger A, Lin S-X, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J. Endocrinol. 2005;187:169–196. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- Meston CM, Bradford A. Autonomic nervous system influences: the role of the sympathetic nervous system in female sexual arousal. In: Janssen E, editor. The Psychophysiology of Sex. Bloomington, Indiana: Indiana University Press; 2007. pp. 66–82. [Google Scholar]
- Meston CM, Gorzalka BB. The effects of sympathetic activation following acute exercise on physiological and subjective sexual arousal in women. Behav. Res. Ther. 1995;33:651–664. doi: 10.1016/0005-7967(95)00006-j. [DOI] [PubMed] [Google Scholar]
- Meston CM, Gorzalka BB. The effects of immediate, delayed and residual sympathetic activation on physiological and subjective sexual arousal in women. Behav. Res. Ther. 1996;34:143–148. doi: 10.1016/0005-7967(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Meston CM, Heiman JR. Ephedrine-activated sexual arousal in women. Arch. Gen. Psychiatry. 1998;55:652–656. doi: 10.1001/archpsyc.55.7.652. [DOI] [PubMed] [Google Scholar]
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol. Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Southwick S, Hazlett G, Rasmusson A, Hoyt G, Zimolo Z, Charney D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch. Gen. Psychiatry. 2004;61:819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- Niskanen J-P, Tarvainen MP, Ranta-aho PO, Karjalainen PA. Software for advanced HRV analysis. Comput. Meth. Programs Biomed. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Olster DH, Ferin M. Corticotrophin releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. J. Clin. Endocrinol. Metab. 1987;65:262–267. doi: 10.1210/jcem-65-2-262. [DOI] [PubMed] [Google Scholar]
- Palace EM, Gorzalka BB. The enhancing effects of anxiety on arousal in sexually dysfunctional and functional women. J. Abnorm. Psychol. 1990;99:403–411. doi: 10.1037//0021-843x.99.4.403. [DOI] [PubMed] [Google Scholar]
- Panjari M, Davis SR. DHEA for postmenopausal women: a review of the evidence. Maturitas. 2010;66:172–179. doi: 10.1016/j.maturitas.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Rellini AH, McCall KM, Randall PK, Meston CM. The relationship between self-reported and physiological measures of female sexual arousal. Psychophysiology. 2005;42:116–124. doi: 10.1111/j.1469-8986.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivest S. Effects of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol. Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Rosen R, Brown C, Heiman JR, Leiblum S, Meston CM, Shabsigh R, Ferguson D, D'Agostino R., Jr The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Sintchak G, Geer JH. A vaginal plethysmograph system. Psychophysiology. 1975;12:113–115. doi: 10.1111/j.1469-8986.1975.tb03074.x. [DOI] [PubMed] [Google Scholar]
- Spark RF. Dehydroepiandrosterone: a springboard hormone for female sexuality. Fertil. Steril. 2002;77 Suppl. 4:S19–S25. doi: 10.1016/s0015-0282(02)02987-4. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- ter Kuile MM, Vigeveno D, Laan E. Preliminary evidence that acute and chronic daily psychological stress affect sexual arousal in sexually functional women. Behav. Res. Ther. 2007;45:2078–2089. doi: 10.1016/j.brat.2007.03.006. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Kampman M. The interaction between anxiety and sexual functioning: a controlled study of sexual functioning in women with anxiety disorders. Sex. Relation. Ther. 15. 2000:47–57. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsh TH, Kemper-Green CN, Livingston KN. Stress and reproduction. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. San Diego: Academic Press; 1999. pp. 662–674. [Google Scholar]