Abstract
Orientational anisotropy of collagen molecules is integral for the mechanical strength of collagen-rich tissues. We have previously reported a novel methodology to synthesize highly oriented electrochemically aligned collagen (ELAC) threads with mechanical properties converging upon those of native tendon. Decorin, a small leucine rich proteoglycan (SLRP), binds to fibrillar collagen and has been suggested to enhance the mechanical properties of tendon. Based on the structure of natural decorin, we have previously designed and synthesized a peptidoglycan (DS-SILY) that mimics decorin both structurally and functionally. In this study, we investigated the effect of the incorporation of DS-SILY on the mechanical properties and structural organization of ELAC threads. The results indicated that the addition of DS-SILY at a molar ratio of 30:1 (Collagen:DS-SILY) significantly enhanced the ultimate stress and ultimate strain of the ELAC threads. Furthermore, differential scanning calorimetry revealed that the addition of DS-SILY at a molar ratio of 30:1 resulted in a more thermally stable collagen structure. However, addition of DS-SILY at a higher concentration (10:1 Collagen:DS-SILY) yielded weaker threads with mechanical properties comparable to collagen control threads. Transmission emission microscopy revealed that the addition of DS-SILY at a higher concentration (10:1) resulted in pronounced aggregation of collagen fibrils. More importantly, these aggregates were not aligned along the long axis of the ELAC thereby compromising on the overall tensile properties of the material. We conclude that incorporation of an optimal amount of DS-SILY is a promising approach to synthesize mechanically competent collagen based biomaterials for tendon tissue engineering applications.
Keywords: collagen, decorin, biomimetic materials, tissue engineering, tendon
1. Introduction
Tendons, ligaments, bladder and many other tissues depend on collagen as their load carrying framework. Most existing collagen constructs lack the desired mechanical strength needed for their applications to reconstruct load-bearing collagen-rich tissues. Precise alignment and orientation of the collagen fibrils along the direction of habitual physiological loads are critical parameters that govern the mechanical properties of collagen constructs. We have previously developed and reported a novel methodology using the principles of isoelectric focusing to align collagen molecules in the presence of an electrochemically induced pH gradient to form highly oriented dense electrochemically aligned collagen (ELAC) threads [1]. These ELAC threads are mechanically stronger and more amenable to cell migration and population compared to randomly oriented collagen threads, making it suitable for tendon/ligament repair [2]. While ELAC mimics the orientational anisotropy of tendon, it can further be improved by the incorporation of small leucine-rich proteoglycans (SLRPs) which form 3% of tendon’s dry weight.
SLRPs are believed to direct the functionality of tendon by binding to collagen fibrils and regulating collagen fibrillogenesis [3]. Decorin, a major constituent of the SLRP family, contains a leucine-rich protein core and a chondroitin sulfate/dermatan sulfate glycosaminoglycan (GAG) chain [4]. The protein component of the decorin molecule binds non-covalently to the surface of the collagen fibrils at the D-band [5], while the GAG component on neighboring collagen fibrils associate with one another to form inter-fibrillar crosslinks [6–8]. These functional inter-fibrillar crosslinks prevent the lateral fusion of collagen fibrils [9] and have been suggested to enhance the strength of the tendon by facilitating the transmission of tensile stresses [10]. Tendon in decorin deficient mice has been shown to exhibit fiber asymmetry and size variability caused by the lateral fusion of the collagen fibrils [11].
Since the applicability of natural decorin is associated with difficulties such as extractability, purity and cost, we have previously designed a peptidoglycan (DS-SILY) containing a collagen binding peptide (SILY) and a covalently attached dermatan sulfate (DS) GAG [12, 13]. DS-SILY is a decorin biomimetic that closely mimics natural decorin both structurally and functionally. Similar to natural decorin, DS-SILY has been shown to modulate collagen fibrillogenesis and enhance the stiffness of collagen gels [13]. Furthermore, DS-SILY presents several advantages that include ease of synthesis and design control with respect to the peptide sequence, number of peptides and GAG identity. In this study, we investigated the effect of the incorporation of this novel decorin biomimetic on the mechanical properties and the structural organization of ELAC threads, a collagen platform that is comparable to natural tissues in terms of packing density and orientational anisotropy. Dialyzed collagen solution was mixed with DS-SILY at different molar ratios and subjected to the electric field to form DS-SILY incorporated ELAC threads. The incorporation of DS-SILY within ELAC threads was confirmed by 1,9-dimethylmethylene blue (DMMB) staining of sections obtained via cryo-histology. The mechanical properties and structural organization of these threads were characterized by monotonic tensile tests, differential scanning calorimetry, atomic force microscopy and electron microscopy.
2. Materials and Methods
2.1 Materials
Pepsin digested monomeric collagen solution (Nutragen, 6.4 mg/ml) from bovine hide was purchased from Advanced Biomatrix (Tucson, AZ). Dermatan sulfate (DS) was obtained from Celsus Laboratories (Cincinnati, OH). The SILY peptide was purchased from Genscript (Piscataway, NJ). The 3-(2-Pyridyldithio) propionyl hydrazyde (PDPH) crosslinker was obtained from Pierce (Rockford, IL). All other chemicals and reagents were bought from Sigma-Aldrich unless noted otherwise.
2.2 DS-SILY Synthesis
DS-SILY was synthesized as previously described with slight modifications [13]. The synthesis process involved three steps: 1) DS Oxidation: Ten mg/ml DS (41 kDa) was oxidized by reacting with sodium metaperiodate in 0.1 M sodium acetate buffer (pH 5.5). The reaction was carried out for two hours protected from light. After two hours, the polysacharride was separated from smaller species by size exclusion chromotography (SEC) using a column packed with Sephadex G-25 medium with an exclusion limit of 5,000 Da (GE Healthcare, Piscataway, NY). 2) Crosslinking with 3-(2-Pyridyldithio) propionyl hydrazyde (PDPH): PDPH is a heterofunctional crosslinker containing a hydrazide that reacts with the aldehyde group on the polysaccharide and a pyridyltithio group that reacts with the thiol on the peptide. Oxidized DS was reacted with 10 fold molar excess of PDPH protected from light in 1X PBS for 2 hours. After two hours, the DS-PDPH was separated from the excess PDPH by SEC, frozen and lyophilized. 3) Coupling DS-PDPH with SILY: DS-PDPH was reacted with 5 fold molar excess of SILY peptide protected from light in 1X PBS for four hours. The DS-SILY synthesized was separated by SEC in ultrapure water, and was then frozen and lyophilzed until further use. The number of peptides bound per DS molecule was back calculated by quantifying the number of moles of PDPH consumed using a standard curve.
2.3 Rheological Testing of DS-SILY Incorporated Collagen Gels
Rheological testing of collagen gels incorporated with DS-SILY was performed for quick screening of the effects of different collagen/DS-SILY molar ratios. Briefly, acid soluble collagen (Nutragen) was brought to physiologic pH and ionic strength on ice to a final concentration of 4 mg/mL. DS-SILY dissolved in 1xPBS pH 7.4 was added to the gel solutions in varying concentrations including 1:1, 10:1, 30:1, and 50:1 molar ratio of collagen to DS-SILY. Control gels received a 1×PBS pH 7.4 addition. The cold collagen solution was then pipetted (200 μL) onto hydrophobic printed slides (Tekdon, Myakka City, FL) with a 20 mm circular wettable center, and were then warmed to 37 °C and allowed to gel overnight in a cell culture incubator with 5% CO2. Rheological measurements were made on an AR-G2 stress controlled rheometer (TA Instruments, New Castle, DE) with stress of 0.5 Pa, and comparisons of storage modulus G′ were made at a frequency of 0.1 Hz.
2.4 Rheological Testing of DS-SILY Incorporated Dialyzed Collagen
The stock acid soluble collagen solution (Nutragen) used for the electrochemical alignment experiments was dialyzed with distilled water for three days to remove salts. Therefore, the rheology measurements were repeated for dialyzed collagen. The dialyzed collagen solution (6 mg/ml) was mixed with DS-SILY at different molar ratios: 1) Collagen control (no DS-SILY), 2) 30:1 Collagen:DS-SILY and 3) 10:1 Collagen:DS-SILY. Molar ratios were calculated based on estimated molecular weights: Collagen - 285 kDa and DS-SILY −50.2 kDa. The rheological mechanical properties of dialyzed collagen samples containing peptidoglycan DS-SILY were measured on an AR-G2 rheometer (TA Instruments, New Castle, DE). Samples (50 μL) were loaded from a syringe onto the peltier plate and a 20 mm 1 cone geometry was lowered onto the sample. Stress and frequency sweeps were first performed to determine the linear range for the samples. Conditions for the oscillatory procedures performed at 25 °C included a frequency sweep from 1 Hz to 10 Hz with constant stress of 5.0 Pa.
2.5 Synthesis of DS-SILY Incorporated ELAC Threads
The protocol followed for the synthesis of the DS-SILY incorporated ELAC threads was modified from previously published literature [1]. Briefly, DS-SILY was dissolved in ultrapure water at a concentration of 10 mg/ml to make up the stock solution. Composite mixtures of collagen and DS-SILY were prepared prior to loading into the electrochemical cell. Based on the desired concentration (30:1 or 10:1 Collagen:DS-SILY), an appropriate volume of stock DS-SILY was diluted in 100 μl of ultrapure water and added to 1 ml of dialyzed collagen solution. For the collagen control, 100 μl of ultrapure water (without DS-SILY) was added to 1 ml of dialyzed collagen solution. Homogenization was achieved via simple mixing by syringing the solution in and out several times using a syringe equipped with a 16 gauge needle. Dialyzed collagen or composite mixtures of dialyzed collagen and peptiodoglycan DS-SILY (30:1 and 10:1) were loaded between two stainless steel wires (electrodes) and an electric field of 20 V was applied for 12 hours. In the presence of an electric field, a pH gradient develops between the anode and the cathode and the collagen molecules align within the gradient at their isoelectric point to form highly oriented electrochemically aligned collagen (ELAC) threads. The collagen only and DS-SILY incorporated ELACs were incubated with 10X PBS overnight to facilitate their recovery from the electrochemical cells. All threads were examined under a polarized microscope (Olympus BX 51) to confirm alignment by verifying that the collagen molecules appear blue when oriented along the slow axis of the gypsum plate. The average dimensions of each thread were approximately 8 cm in length, 200–400 μm in width and 200–300 μm in thickness. The samples for the following experiments are named based on the amount of DS-SILY added prior to electrochemical alignment (30:1 and 10:1 Collagen:DS-SILY).
2.6 Cryo-Histology
Small fragments (~1 cm length) of DS-SILY incorporated and collagen control threads were embedded in OCT embedding medium by snap freezing in liquid nitrogen. Thin sections (5 – 7 μm) were cut using a cryostat, mounted onto a glass slide and washed with DI water. The 1,9-dimethylmethylene blue (DMMB) dye was prepared by mixing DMMB in water with 40 mM NaCl and 40 mM glycine. DMMB is a cationic dye that binds to sulfated GAGs with high affinity. The sections were stained with DMMB for 45 minutes, washed with DI water, air dried, dipped quickly in xylene and coverslipped with permanent mounting media. The stained sections were imaged to qualitatively assess the presence and distribution of DS-SILY within the ELAC threads.
2.7 Quantitative Analysis of DS-SILY Incorporation into ELAC Threads
To determine the amount of DS-SILY incorporated into ELAC threads, a biochemical assay using 1,9-dimethylmethylene blue (DMMB) dye was performed. The DMMB dye was prepared by adding 1.6 mg DMMB, 0.304 g glycine and 0.237 g NaCl to 9.5 ml of 0.1 M HCl and then adding DI water to make up a 100 ml solution (pH 3.0). Two cm long samples of collagen control and DS-SILY incorporated ELAC threads were immersed individually in 500 μl of 10 mM HCl and heated at 60 °C for 4 hours to completely dissolve the thread. Standards with known concentrations of DS-SILY in 10 mM HCl were also heated at 60 °C for 4 hours. Fifty μl of sample (standard or unknown) was added to each well of a 96 well plate. To this, 200 μl of DMMB dye was added and the absorbance was recorded at 525 nm. A standard curve was generated by plotting the known DS-SILY concentration and absorbance data and the corresponding linear equations were generated. These linear equations were used to calculate the amount of DS-SILY incorporated into the ELAC threads.
2.8 Monotonic Tensile Testing
Collagen control and DS-SILY incorporated ELAC threads were subjected to monotonic mechanical testing to determine their tensile properties (n=9–12 samples/group). At first, the threads were hydrated with DI water and 2 cm long samples were cut from each thread using a scalpel blade. The average area of each sample in wet state was calculated by measuring the area (width x thickness) at six different locations along the length of the sample using a confocal microscope (Olympus FV1000). Following this, the excess surface water on the samples was removed using Kimwipes and the samples were glued onto tabs made out of transparency sheets in a hydrated state using a medical grade adhesive (Loctite 4851). The samples and the glue were air dried and the samples were hydrated in 1X PBS for 1 hour prior to testing. The samples were mounted onto the fixtures of an ARES rheometer (TA Instruments, New Castle, DA) equipped with a 250 g load cell and capable of handling miniature samples. The samples were monotonically loaded to failure under displacement control (10 mm/min) and the load and displacement data were recorded. Stress was calculated by dividing the load measured with the average area of the sample. Strain was computed by normalizing the change in gauge length with the original length of the sample. Young’s Modulus was determined by calculating the slope of the linear elastic region of the stress-strain curve using MATLAB.
2.9 Differential Scanning Calorimetry
The heat of denaturation and denaturation temperature of the ELAC threads and DS-SILY incorporated ELAC threads were measured by differential scanning calorimetry using TA Instruments (Q 2000-0811 DSC) with refrigerated cooling system. The temperature and peak area were calibrated with a known standard, Indium. Wet specimens weighing 7 – 10 mg were placed in the specimen pans and hermetically sealed with the lids. The specimens were heated at a rate of 10 °C/min from 20 °C to 70 °C.
2.10 Swelling Ratio
Collagen control and DS-SILY incorporated ELAC threads were cut into 2 cm long samples using a scalpel blade. Samples were vacuum dried for 24 hours and weighed to obtain the dry weight (Wd). Following this, 1 ml of 1X PBS was added to each sample in a microcentrifuge tube and the samples were incubated at room temperature for 24 hours. The excess surface water was removed using Kimwipes and the samples were weighed to obtain the wet weight (Ww). The swelling ratio of each sample is the ratio of wet weight to dry weight and the swelling degree (SD) percentage was determined using the following equation:
2.11 Transmission Electron Microscopy of DS-SILY Incorporated ELAC threads
The structural organization of the collagen fibrils in the collagen control and DS-SILY incorporated collagen threads was observed using TEM. ELAC threads were cut into small pieces (1 mm length) using a scalpel blade and fixed in 2% glutaraldehyde (in PBS) for 1 hour. After washing with PBS, the samples were post-fixed in 1% OsO4 (in PBS) for 1 hour. The samples were then washed and dehydrated in a graded series of ethanol, infiltrated with Spurr’s epoxy resin and flat embedded in a mold by polymerization at 60 °C for 48 hours. Ultrathin sections were cut in the transverse direction and collected on grids. The sections were stained with 2% uranyl acetate in 70% methanol for 5 minutes and lead citrate for 3 minutes, dried and examined with TEM at 80 kV (FEI/Philips CM-10, FEI Company, Hillsboro, OR).
2.12 Turbidity Assay
Since the opacity of a solution may suggest the presence of aggregates, turbidity assay was performed to quantify the opacity of dialyzed collagen and composite mixtures of Collagen:DS-SILY (30:1 and 10:1) samples and to determine whether the addition of DS-SILY led to the formation of aggregates prior to electrochemical alignment. Samples were diluted 5-fold in ultrapure water and kept on ice. Collagen-DS-SILY composite samples were prepared as described, and the optical density was measured at 313nm by loading 50 μL/well into a 96-well plate. Composite samples immediately become turbid upon mixing and thus measurements over time were not possible.
2.13 Transmission Electron Microscopy of DS-SILY Incorporated Dialyzed Collagen
TEM was performed on dialyzed collagen and composite mixtures of dialyzed collagen and DS-SILY to visually confirm the presence of aggregates prior to electrochemical alignment. Samples were diluted 20 times with distilled water and a droplet was deposited onto a 400 mesh Cu grid. Following this, the sample was allowed to attach to the grid for ~30 seconds and the excess sample was rinsed off. The sample attached to the grid was stained with 1% phosphotungstic acid, dried and examined with TEM (Philips CM-100, FEI Company, Hillsboro, OR).
2.14 Atomic Force Microscopy
A Veeco Instruments, Inc., MultiMode atomic force microscope (AFM) with Nanoscope IV controller, PicoForce scanner, and fluid cell was used to perform pull-off force measurements of DS-SILY incorporated and collagen control ELAC threads in high purity DI water (Milli-Q model XYZ, 17.3 m-ohms). One nanoscale pyramidal probe on a triangular AFM cantilever, model OTR8 purchased from Veeco, was used for all pull-off force experiments. The pyramidal probe was made of silicon nitride (Si3N4) with a radius curvature (ROC) of 20 nm at the apex. Threads were immobilized on separate silicon coupons using a two-part epoxy purchased from Veeco (model 1580-00). Pull-off force measurements were conducted by bringing the cantilever probe into and out of contact with each sample in the radial direction of the thread. In other words, the pull-direction of the AFM probe was perpendicular to the longer axis of collagen molecules. Force data were collected by making a minimum of 10 measurements in each of 10 locations along each sample. The cantilever spring constant was 0.1138 nN/nm and the deflection sensitivity was 56.28 nm/V as calibrated in high purity DI water against a silicon coupon using the thermal tune method [14–16].
2.15 Statistical Analysis
The results of rheological testing (n=3/group), turbidity assay (n=3/group) and swelling ratio (n=3–4/group) were analyzed with one way ANOVA for repeated measures with Fisher post-hoc comparisons. Data obtained from monotonic tensile testing (n=9–12/group), differential scanning calorimetry (n=5–6/group) and atomic force microscopy (n=5/group) were analyzed using Mann-Whitney U-test. Statistical significance threshold was set at p < 0.05. All data are expressed as mean ± standard deviation.
3. Results
3.1 Rheological Testing
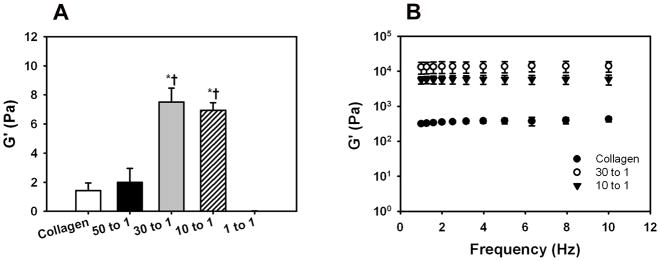
The G′ of DS-SILY incorporated collagen gels (physiological salt concentration and pH) at 30:1 and 10:1 (Collagen:DS-SILY) molar ratios was significantly greater (p < 0.05) compared to the 50:1 and collagen control groups (Fig. 1A). However, increasing the concentration of DS-SILY to 1:1 molar ratio prevented gel formation.
Fig 1.
Rheological mechanical testing of (A) collagen gels and (B) dialyzed collagen at different collagen to DS-SILY molar ratios. (A) Addition of DS-SILY at a molar ratio of 30:1 and 10:1 Collagen:DS-SILY significantly increased the G′ of collagen gels compared to collagen control and 50:1 Collagen:DS-SILY. However, further increasing the concentration of DS-SILY to 1:1 resulted in inhibition of collagen fibrillogenesis. *Comparison with collagen control, p<0.05. †Comparison with 50:1 Collagen:DS-SILY. (B) The G′ values of DS-SILY incorporated dialyzed collagen at 30:1 and 10:1 (Collagen:DS-SILY) molar ratios were significantly greater (p < 0.05) compared to the dialyzed collagen control group. Furthermore, the G′ values of DS-SILY incorporated dialyzed collagen at 30:1 were significantly higher (p < 0.05) compared to 10:1 (Collagen:DS-SILY).
The G′ values of DS-SILY incorporated dialyzed collagen at 30:1 and 10:1 (Collagen:DS-SILY) molar ratios were significantly greater (p < 0.05) compared to the dialyzed collagen control group (Fig. 1B). Furthermore, the G′ values of DS-SILY incorporated dialyzed collagen at 30:1 were significantly higher (p < 0.05) compared to 10:1 (Collagen:DS-SILY).
3.2 Cryo-histology
Staining the cryosections of the DS-SILY incorporated ELAC threads with the DMMB dye indicated that DS-SILY was distributed throughout the volume of the thread (Fig. 2). The uptake of the DMMB dye increased with the amount of DS-SILY such that 10:1 Collagen:DS-SILY threads were observed to stain darker (Fig. 2C) compared to the 30:1 Collagen:DS-SILY threads (Fig. 2B). The collagen control did not take up any dye and was hardly visible under the microscope due to the transparency of the sample (Fig. 2A).
Fig 2.
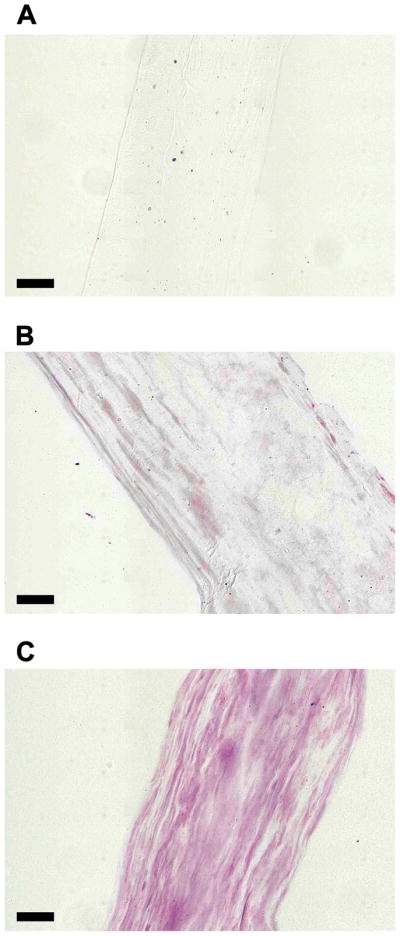
DMMB stained cryo-sections of ELAC. (A) Collagen Only (B) 30:1 Collagen:DS-SILY and (C) 10:1 Collagen:DS-SILY. DMMB staining revealed that DS-SILY was distributed throughout the length of the ELAC thread for both the 30:1 (B) and 10:1 (C) groups. The collagen only control (A) did not stain and was hardly visible under the microscope due to the transparency of the sample. (Scale bar: 50 μm).
3.3 Quantitative Analysis of DS-SILY Incorporation into ELAC Threads
Each electrochemical cell is 8 cm long and requires approximately 300 μl of sample to synthesize a thread measuring its entire length. Based on the estimated molecular weights (Collagen: 285 kDa and DS-SILY: 50.2 kDa), the initial amount of DS-SILY added to dialyzed collagen to synthesize a 2 cm long ELAC thread was determined. The results from the DMMB assay showed that around 20% of the DS-SILY initially added is incorporated within the ELAC thread (Fig. 3) for both 30:1 and 10:1 groups and the remaining is lost during the alignment process.
Fig 3.
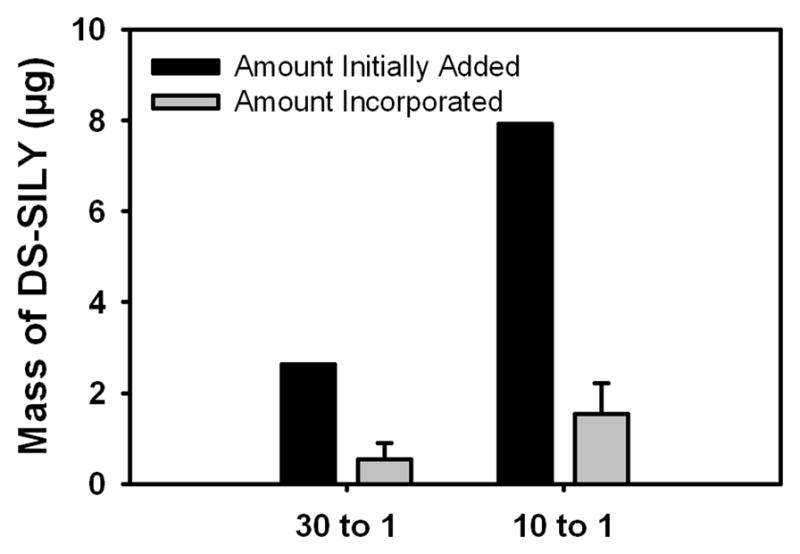
Quantitative analysis of DS-SILY incorporation into ELAC threads. The results of the DMMB assay showed that around 20% of the DS-SILY initially added is incorporated within the ELAC thread.
3.4 Tensile Testing
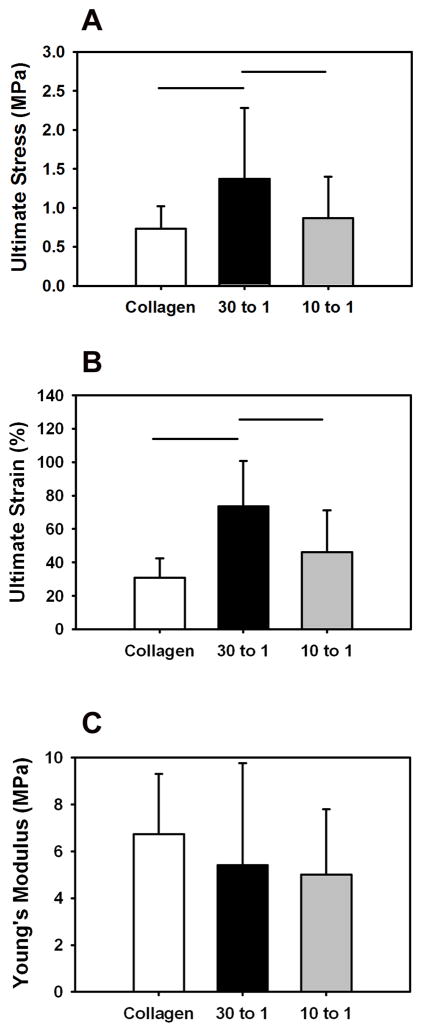
The cross-sectional area of collagen control (0.047±0.006 mm2), 30:1 Collagen:DS-SILY (0.049±0.010 mm2) and 10:1 Collagen:DS-SILY (0.040±0.010 mm2) threads in the wet state was comparable. The ultimate stress and ultimate strain of the 30:1 Collagen:DS-SILY threads were two-fold greater (p < 0.05) compared to both the 10:1 and collagen control threads (Fig. 4A and 4B). The Young’s modulus was comparable between all groups (Fig. 4C).
Fig 4.
Monotonic mechanical properties of ELAC at different collagen to DS-SILY molar ratios. (A) Ultimate Stress, (B) Ultimate Strain and (C) Young’s Modulus. The ultimate stress and ultimate strain of 30:1 Collagen:DS-SILY threads was significantly greater compared to the 10:1 and collagen control threads. The Young’s modulus was comparable. The horizontal lines connecting groups indicate statistical significance (p < 0.05).
3.5 Differential Scanning Calorimetry
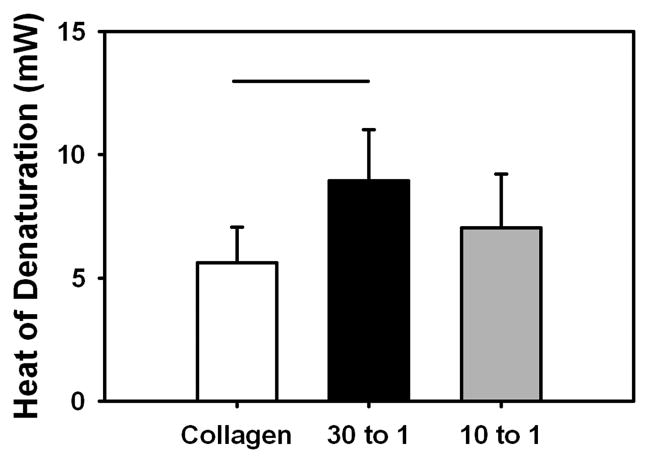
The denaturation temperatures of the DS-SILY incorporated ELAC threads and collagen control threads were comparable (data not shown). However, the heat of denaturation (ΔH, enthalpy) was significantly higher (p < 0.05) for the 30:1 Collagen:DS-SILY threads compared to the collagen control threads (Fig. 5).
Fig 5.
Differential scanning calorimetry of ELAC at different collagen to DS-SILY molar ratios. The enthalpy of denaturation was significantly higher for the 30:1 Collagen:DS-SILY threads compared to collagen control threads. The horizontal lines connecting groups indicate statistical significance (p < 0.05).
3.6 Swelling Ratio
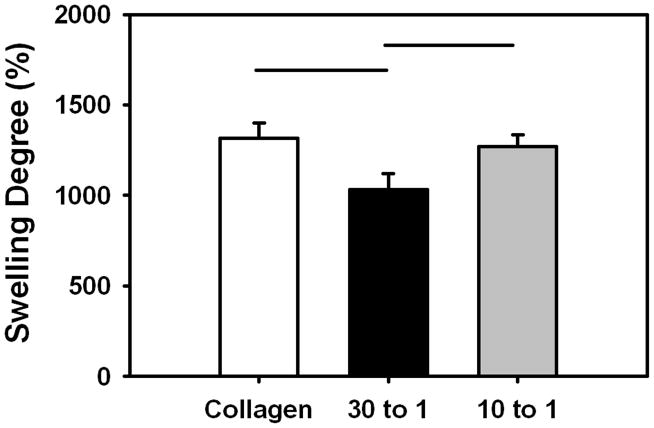
The swelling ratio of the collagen control and 10:1 Collagen:DS-SILY threads was comparable. However, the swelling ratio of the 30:1 Collagen:DS-SILY threads was significantly lower (p < 0.05) compared to both the collagen control and 10:1 Collagen:DS-SILY threads (Fig. 6).
Fig 6.
Swelling degree percentage of ELAC at different collagen to DS-SILY molar ratios. The swelling ratio of the 30:1 Collagen:DS-SILY threads was significantly lower compared to both the collagen control and 10:1 Collagen:DS-SILY threads. The horizontal lines connecting groups indicate statistical significance (p < 0.05).
3.7 Transmission Electron Microscopy of DS-SILY incorporated ELAC threads
Previous work using TEM on the longitudinal sections of the ELAC threads demonstrate fibril alignment in the direction of the long axis [1]. In agreement with these findings, transverse sections of the ELAC threads analyzed by TEM in the current study show well-defined and circular fibrils in the collagen control group (Fig. 7A), suggesting that the fibrils are aligned orthogonal to the cut and therefore oriented preferentially in the direction of the long axis. In contrast, DS-SILY incorporated threads contained pockets of collagen fibrils that appear as lines instead of circles on the transverse section (indicated by * in Fig. 7C) suggesting that these pockets are not oriented along the long axis of the ELAC thread. A greater number of these pockets were found in the 10:1 group compared to the 30:1 group (Fig. 7B and 7C).
Fig 7.
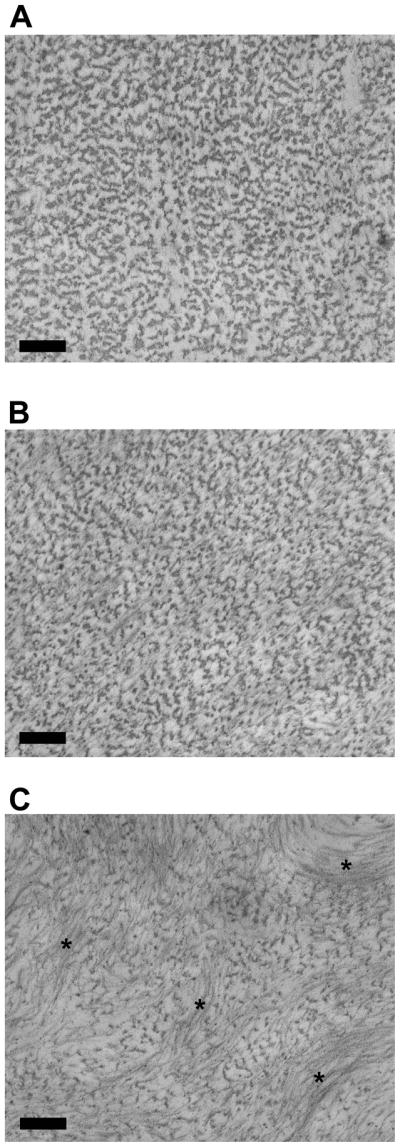
Transmission emission microscopy images of ELAC at different collagen to DS-SILY molar ratios. (A) Collagen Only, (B) 30 to 1 and (C) 10 to 1. Addition of DS-SILY in the molar ratio of 10:1 resulted in pronounced aggregation and precipitation of collagen fibrils (indicated by “*’). These aggregates were locally aligned but not oriented along the long axis of the ELAC thread. Scale bar: 200 nm.
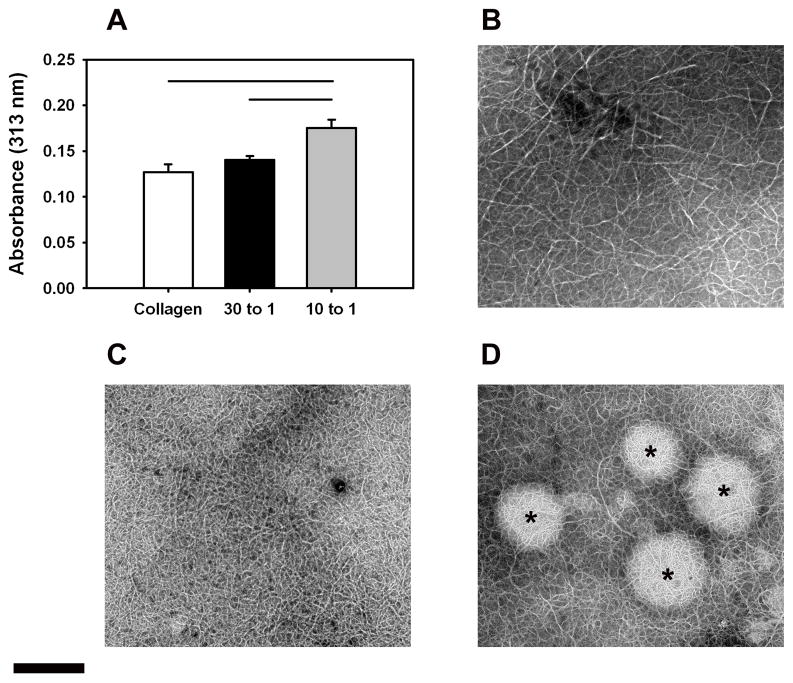
3.8 Turbidity
The addition of peptidoglycan DS-SILY at a molar ratio of 10:1 Collagen:DS-SILY significantly increased the opacity of collagen (p < 0.05) compared to both the collagen control and at 30:1 Collagen:DS-SILY groups (Fig. 8A). The lower molar ratio of 30:1 also increased the turbidity of the sample, but the result was not significant compared to the collagen control.
Fig 8.
(A) Turbidity measurements at 313 nm of collagen gels at different collagen to DS-SILY molar ratios. The addition of DS-SILY at a molar ratio of 10:1 Collagen:DS-SILY significantly increased the opacity of collagen compared to both the collagen control and at 30:1 collagen:DS-SILY groups. The horizontal lines connecting groups indicate statistical significance (p < 0.05). (B, C, D) Transmission electron microscopy of dialyzed collagen (B), 30:1 Collagen:DS-SILY (C) and 10:1 Collagen:DS-SILY (D). Addition of DS-SILY in the molar ratio of 10:1 resulted in the formation of aggregates prior to electrochemical alignment (indicated by ‘*’). Scale bar: 500 nm.
3.9 Transmission Electron Microscopy of DS-SILY incorporated Dialyzed Collagen
Collagen fibril precipitation after dialysis was observed in all groups including the collagen control. However, the addition of DS-SILY resulted in the formation of a tighter network of fibers (Fig. 8C and 8D). Additionally, large aggregates were observed in the composite mixture of 10:1 Collagen:DS-SILY (Fig. 8D). No such aggregates were present in the collagen control (Fig. 8B). These results indicate that the addition of DS-SILY triggers the formation of aggregates prior to electrochemical alignment.
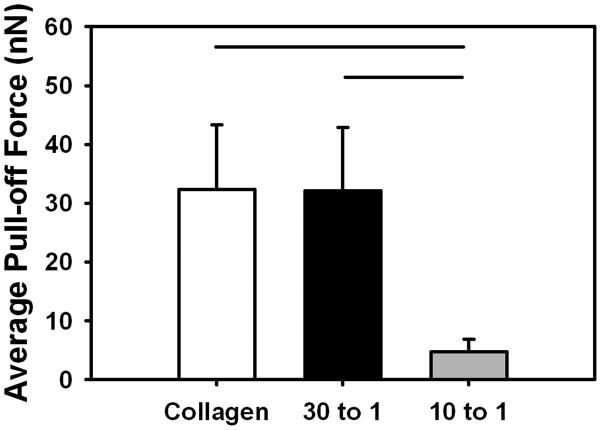
3.10 Atomic Force Microscopy
The average pull off forces of the silicon nitride probe against the collagen control threads and 30:1 Collagen:DS-SILY threads were comparable (Fig. 9). However, addition of DS-SILY at a higher concentration (10:1) decreased the average pull of force by a factor of 5 compared to both the 30:1 and collagen control threads (p < 0.05).
Fig 9.
The average pull-off force for a pyramidal silicon nitride probe with ELAC at different collagen to DS-SILY molar ratios. The pull-off force was effectively equal for collagen control and 30 to 1 Collagen:DS-SILY threads. The pull-off force for 10 to 1 was lower by nearly a factor of 5. The horizontal lines connecting groups indicate statistical significance (p < 0.05).
4. Discussion
The present study focused on elucidating the effects of the incorporation of a collagen binding peptidoglycan, DS-SILY, on the mechanical properties and structural organization of ELAC threads. The key findings of this study are as follows: 1) Addition of DS-SILY at optimal concentrations significantly enhances the stiffness of both collagen gel and dialyzed collagen, 2) DS-SILY can be successfully incorporated within the ELAC thread by mixing it with dialyzed collagen solution before subjecting it to the electric field, 3) Monotonic tensile testing revealed that addition of DS-SILY at a molar ratio of 30:1 Collagen:DS-SILY significantly enhances the tensile strength and extensibility of the ELAC threads with no effect on the stiffness of the threads, 4) Significantly higher heat of denaturation (ΔH, enthalpy) for the 30:1 threads compared to the collagen control threads is indicative of a more thermally stable collagen structure, 5) Addition of DS-SILY at a molar ratio of 30:1 Collagen:DS-SILY significantly reduced the swelling ratio of ELAC threads compared to both the collagen control and 10:1 Collagen:DS-SILY threads, 6) Transmission electron microscopy revealed that the addition of DS-SILY at higher concentration (10:1) triggered pronounced aggregation of collagen fibrils thereby affecting the structural organization of ELAC threads, 7) Turbidity assay and TEM of DS-SILY incorporated dialyzed collagen indicate that the aggregation of collagen fibrils takes place prior to electrochemical manipulation, and 8) Atomic force microscopy divulged that the addition of DS-SILY at higher concentration (10:1) is detrimental to the mechanical properties of the ELAC threads when loaded in the transverse direction.
For the synthesis of ELAC threads, it is imperative that the collagen solution is free of salts to facilitate the formation of a pH gradient between the anode and the cathode on application of the electric field. Therefore, acid soluble monomeric collagen solution was dialyzed with distilled water before exposure to the electric field. The initial goal of the current study was to successfully incorporate DS-SILY into ELAC threads by aligning composite mixtures of dialyzed collagen and DS-SILY at different concentrations. To structurally mimic natural decorin, DS-SILY was synthesized with a single peptide (SILY) per GAG (DS) chain. However, the addition of DS-SILY with one peptide per GAG chain to dialyzed collagen solution resulted in the lateral fusion of collagen molecules and precipitation of fibrils. It has been previously reported that at low ionic conditions, interactions between dermatan sulfate and collagen molecules results in fibril formation [17]. To overcome this limitation, the reaction conditions for the synthesis of DS-SILY were modified to yield four peptides per DS molecule. Incorporation of the modified DS-SILY into dialyzed collagen minimized the lateral fusion of collagen molecules and the precipitation of fibrils. Therefore, in the current study, DS-SILY with four peptides per GAG chain was used as a decorin biomimetic.
Rheological testing of DS-SILY incorporated collagen gels at physiological conditions illustrated a bell shaped response in the storage modulus (G′) of the collagen gel with increasing concentrations of DS-SILY (Fig. 1A). Addition of DS-SILY in the 30:1 and 10:1 (Collagen:DS-SILY) ratios significantly increased the G′ of the collagen gels. However, further increasing the concentration of DS-SILY to 1:1 ratio significantly decreased the G′ due to inhibition of collagen fibrillogenesis at high concentrations of DS-SILY [13]. Based on these results, 30:1 and 10:1 molar ratios were chosen as optimal concentrations of DS-SILY to be incorporated into the ELAC threads.
The addition of DS-SILY resulted in up to 45-fold increase in the G′ of dialyzed collagen (Fig. 1B). The higher G′ of DS-SILY incorporated dialyzed collagen at a molar ratio of 30:1 (Collagen:DS-SILY) compared to 10:1 suggests that there is an optimal concentration of collagen to DS-SILY beyond which further addition of DS-SILY is detrimental. An excessive dose of DS-SILY may dilute the concentration of free collagen in solution and dampen the efficiency of fibril formation.
Previous work in our laboratory (unpublished data) has shown that almost 100% of collagen initially added is recovered in the form of an ELAC thread. DMMB assay was performed to quantify the actual amount of DS-SILY incorporated within the ELAC thread. It is important to note that the molar ratios of Collagen:DS-SILY (30:1 and 10:1) reported in this study refer to the amount of DS-SILY added to dialyzed collagen prior to electrochemical alignment. The results from the DMMB assay showed that post electrochemical alignment only 20% of DS-SILY initially added gets incorporated within the ELAC thread for both the 30:1 and 10:1 groups (Fig. 3). It is likely that on application of the electric field, some amount of DS-SILY gets entrapped within the ELAC thread while the collagen molecules align along the isoelectric point. The remaining DS-SILY not entrapped within the ELAC thread is possibly lost along with the water molecules that are driven out during the alignment process. Therefore, based on the DMMB assay results, addition of DS-SILY at a molar ratio of 30:1 and 10:1 Collagen:DS-SILY prior to electrochemical alignment resulted in ELAC threads with approximately 150:1 and 50:1 Collagen:DS-SILY respectively.
The macroscale mechanical tests demonstrated that the ultimate stress and ultimate strain of 30:1 Collagen:DS-SILY threads were two-fold greater compared to the collagen control threads (Fig. 4). The synchronized increase in both stress and strain without a change in Young’s modulus suggests that DS-SILY allows the collagen fibrils to slip past one another possibly via sacrificial bonds between GAG chains on adjacent fibrils. These results are in agreement with the sliding filament model put forward by Scott, which postulated that the interaction between the GAG chains of the decorin molecule on adjacent collagen fibrils facilitates sliding and thereby maintains the mechanical stresses elastically [18]. Furthermore, Pins et al. also hypothesized that the increase in the tensile stress of uncrosslinked decorin incorporated collagen fibers is due to the fibrillar slippage facilitated by decorin [19]. Therefore, it is likely that the biomimetic employed in our study facilitates shear flow between molecules and their supramolecular aggregates when the threads are challenged in shear.
The structural stability of the ELAC threads incorporated with DS-SILY was investigated using differential scanning calorimetry (DSC). DSC is a quantitative technique and is sensitive to local and global conformational changes in collagen with respect to temperature [20, 21]. The results showed that the heat of denaturation (ΔH, enthalpy) for 30:1 Collagen:DS-SILY threads was significantly higher compared to the collagen control threads (Fig. 5). The higher ΔH values illustrate that more heat is needed to denature the secondary collagen structure suggesting that the addition of DS-SILY at a molar ratio of 30:1 resulted in a more thermally stable collagen organization within the ELAC thread.
Previous studies have reported that the GAG components of the decorin molecule on adjacent collagen fibrils form interfibrillar crosslinks [6–8]. The lower swelling ratio of 30:1 Collagen:DS-SILY threads compared to the collagen control (Fig. 6) suggests that the addition of an optimal amount of DS-SILY may result in the formation of inter-fibrillar crosslinks within the ELAC thread. These crosslinks restrict the uptake of water and facilitate fibril sliding thereby enhancing mechanical properties of the DS-SILY incorporated ELAC threads. Furthermore, the presence of these functional crosslinks might also explain the higher amount of heat needed to denature the secondary collagen structure of these threads as evidenced by DSC.
Addition of DS-SILY at a concentration of 10:1 (Collagen:DS-SILY) yielded weaker threads with mechanical properties comparable to collagen control threads. The diminished mechanical properties of the ELAC threads with higher DS-SILY concentration might be due to differences in the structural organization of the collagen fibrils within the ELAC threads. Transmission electron microscopy revealed that the addition of DS-SILY at higher concentration (10:1) resulted in pronounced aggregation of collagen fibrils (Fig. 7). More importantly, these aggregates were observed to be off axis and not aligned longitudinally along the length of the ELAC thread. The results from the turbidity assay divulged that the opacity of dialyzed collagen with DS-SILY added at the molar ratio of 10:1 was significantly higher compared to collagen control indicating that some degree of aggregation of collagen fibrils takes place prior to electrochemical alignment (Fig. 8A). This finding was further validated by visually confirming the presence of large aggregates in the 10:1 Collagen:DS-SILY solution prior to electrochemical alignment (Fig. 8D). On exposure to the electric field, the collagen monomers along with the aggregates are driven to the isoelectric point; however, the aggregates may fail to orient along the long axis thereby compromising the overall alignment of the ELAC thread. At the lower DS-SILY concentration (30:1), the decrease in the sample opacity indicates that fewer fibrils aggregated, thereby allowing better alignment in the longitudinal direction while still incorporating the peptidoglycan. Therefore, the disruption in the overall alignment of collagen can explain the weak mechanical properties of the ELAC thread incorporated with the higher concentration of DS-SILY.
The average pull-off force of the silicon nitride probe against the collagen control and 30:1 Collagen:DS-SILY threads was comparable as determined by AFM (Fig. 9). The divergence of these results from the findings of the rheology and macroscale mechanical tests can be explained by the anisotropy of the material. As an aligned material, ELAC is anisotropic such that its material properties and deformation patterns differ between the longer axis of the thread (along which molecules are oriented) and the axes transverse to the longer axis. The macroscale mechanical tests and rheology incite shear in the bulk of the material (Mode II loading), whereas AFM loading splits/pulls the collagen fibrils in the transverse direction (Mode I loading). The AFM results confirm that the addition of DS-SILY in the molar ratio of 30:1 does not affect the mode I performance of the ELAC whereas it benefits the shear mode. On the other hand, addition of DS-SILY at a higher concentration (10:1) is detrimental to the mechanical properties of ELAC in Mode-I loading.
Recent studies have challenged the traditional belief that GAGs contribute to the mechanical properties of tendons and ligaments [22, 23]. Tensile tests performed on human medial collateral ligaments before and after the enzymatic removal of GAGs by chondroitinase B have been reported to show no significant differences in its mechanical properties. It is difficult to compare these results with the outcomes of the current study because the collagen fibril diameters of ELAC threads are significantly less small compared to mature tendons. Collagen in the ELAC threads mostly resides in the form of microfibrils (5 – 10 nm in diameter) and may not instill the tissue level dynamics prevalent in tendons and ligaments. Furthermore, decorin may contribute to the mechanical properties of native tendon by modulating the collagen fibril diameter and organization [24]. It might be possible that the presence of decorin during fibril formation is more critical to the mechanical properties of tendons and ligaments compared to its presence after the formation of fibrils.
In conclusion, this study demonstrates that the collagen binding peptidoglycan DS-SILY, which mimics the native proteoglycan decorin, can be successfully incorporated into the ELAC threads. Addition of an optimal amount of DS-SILY imparted structural and thermal stability to the ELAC threads and significantly enhanced the deformation range of collagen potentially by facilitating shear sliding between fibrils. Overall, incorporation of DS-SILY is a viable strategy to yield mechanically competent collagen based biomaterials for tendon/ligament tissue engineering applications.
Acknowledgments
This study was funded by a grant from the National Institute of Health (NIH 1R21AR056060). The authors would like to thank Dr. Gudrun Schmidt for access to the ARES rheometer and Akhilesh K. Gaharwar for technical help with the macroscale mechanical tests of the DS-SILY incorporated ELAC threads.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng X, Gurkan UA, Dehen CJ, Tate MP, Hillhouse HW, Simpson GJ, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29:3278–88. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Gurkan UA, Cheng X, Kishore V, Uquillas JA, Akkus O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J Biomed Mater Res A. 2010;94:1070–9. doi: 10.1002/jbm.a.32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 4.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity of function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- 5.Scott JE, Orford CR. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981;197:213–6. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raspanti M, Alessandrini A, Ottani V, Ruggeri A. Direct visualization of collagen-bound proteoglycans by tapping-mode atomic force microscopy. J Struct Biol. 1997;119:118–22. doi: 10.1006/jsbi.1997.3865. [DOI] [PubMed] [Google Scholar]
- 7.Scott JE. Proteoglycan: collagen interactions and corneal ultrastructure. Biochem Soc Trans. 1991;19:877–81. doi: 10.1042/bst0190877. [DOI] [PubMed] [Google Scholar]
- 8.Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–23. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules. 2006;7:2388–93. doi: 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- 10.Cribb AM, Scott JE. Tendon response to tensile stress: an ultrastructural investigation of collagen:proteoglycan interactions in stressed tendon. J Anat. 1995;187 ( Pt 2):423–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paderi JE, Sistiabudi R, Ivanisevic A, Panitch A. Collagen-binding peptidoglycans: a biomimetic approach to modulate collagen fibrillogenesis for tissue engineering applications. Tissue Eng Part A. 2009;15:2991–9. doi: 10.1089/ten.tea.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paderi JE, Panitch A. Design of a synthetic collagen-binding peptidoglycan that modulates collagen fibrillogenesis. Biomacromolecules. 2008;9:2562–6. doi: 10.1021/bm8006852. [DOI] [PubMed] [Google Scholar]
- 14.Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Rev Sci Instrum. 1993;64:1868–73. [Google Scholar]
- 15.Kumar G, Smith S, Jaiswal R, Beaudoin S. Scaling of Adhesion Forces from the Micro- to the Nano-scale. J Adhesion Sci Tech. 2008;22:407–28. [Google Scholar]
- 16.Ohler B. Cantilever spring constant calibration using laser Doppler vibrometry. Rev Sci Instrum. 2007;78:063701. doi: 10.1063/1.2743272. [DOI] [PubMed] [Google Scholar]
- 17.Obrink B. The influence of glycosaminoglycans on the formation of fibers from monomeric tropocollagen in vitro. Eur J Biochem. 1973;34:129–37. doi: 10.1111/j.1432-1033.1973.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 18.Scott JE. Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol. 2003;553:335–43. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997;73:2164–72. doi: 10.1016/S0006-3495(97)78247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JM, Pereira CA, Abdulla D, Naimark WA, Crawford I. A multi-sample denaturation temperature tester for collagenous biomaterials. Med Eng Phys. 1995;17:115–21. doi: 10.1016/1350-4533(95)91882-h. [DOI] [PubMed] [Google Scholar]
- 21.McClain PE, Wiley ER. Differential scanning calorimeter studies of the thermal transitions of collagen. Implications on structure and stability. J Biol Chem. 1972;247:692–7. [PubMed] [Google Scholar]
- 22.Lujan TJ, Underwood CJ, Henninger HB, Thompson BM, Weiss JA. Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament. J Orthop Res. 2007;25:894–903. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- 23.Lujan TJ, Underwood CJ, Jacobs NT, Weiss JA. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol. 2009;106:423–31. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–55. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]