Abstract
Mefloquine is an effective and widely used anti-malarial drug; however, some clinical reports suggest that it can cause dizziness, balance, and vestibular disturbances. To determine if mefloquine might be toxic to the vestibular system, we applied mefloquine to organotypic cultures of the macula of the utricle from postnatal day 3 rats. The macula of the utricle was micro-dissected out as a flat surface preparation and cultured with 10, 50, 100, or 200 μM mefloquine for 24 h. Specimens were stained with TRITC-conjugated phalloidin to label the actin in hair cell stereocilia and TO-PRO-3 to visualize cell nuclei. Some utricles were also labeled with fluorogenic caspase-3, -8, or -9 indicators to evaluate the mechanism of programmed cell death. Mefloquine treatment caused a dose-dependent loss of utricular hair cells. Treatment with 10 μM caused a slight reduction, 50 μM caused a significant reduction, and 200 μM destroyed nearly all the hair cells. Hair cell nuclei in mefloquine-treated utricles were condensed and fragmented, morphological features of apoptosis. Mefloquine-treated utricles were positive for the extrinsic initiator caspase-8 and intrinsic initiator caspase-9 and downstream executioner caspase-3. These results indicate that mefloquine can induce significant hair cell degeneration in the postnatal rat utricle and that mefloquine-induced hair cell death is initiated by both caspase-8 and caspase-9.
Keywords: Mefloquine, Utricles, Hair cells, Caspase-8, Caspase-9, Caspase-3
Introduction
Malaria is a serious infectious disease caused by plasmodium parasites and spread by mosquitoes. Malaria is most common in developing countries in tropical and subtropical regions in parts of the Americas, Asia, and Africa (Russell 1956; Angus 2001). Malaria is a serious health concern for military personnel and travelers entering infectious regions of the globe (Chen et al. 2006; Tepper et al. 2007; Sauvet et al. 2009; Schmid et al. 2009); however, small outbreaks of locally acquired, mosquito-transmitted malaria also occur in developed countries (Filler et al. 2006). A number of antimalarial drugs are used prophylactically against or as a treatment for malaria (Nyunt and Plowe 2007; Dow et al. 2008; Carmargo et al. 2009). Mefloquine (Lariam®), a structural derivative of quinine, but less toxic, is highly effective against many types of malaria and is the drug of choice because of its long half-life that requires less frequent dosing (Dow et al. 2004). However, mefloquine has been reported to cause neurological side effects such as depression, nausea, nightmares, fatigue, anxiety, hearing loss, dizziness, and vertigo (Phillips-Howard and ter Kuile 1995; Fusetti et al. 1999; Jha and Kumar 2006; Wise and Toovey 2007). Mefloquine prophylaxis causes severe CNS disturbances in roughly 1 in 10,000 patients whereas mefloquine therapy produces serious CNS problems ranging from 1:200 to 1:1200 (Phillips-Howard and ter Kuile 1995). In addition, a single oral dose of mefloquine-induced behavioral and motor disturbances and degeneration in brainstem nuclei of rats; many of these changes were associated with plasma concentrations of mefloquine similar to those observed in human (Dow et al. 2006). The difficulties traversing a bean in this study were attributed to proprioceptive impairments, but could also arise from vestibular disturbances. Aggression and convulsion have been observed in mice treated with 300 mg/kg (Fletcher and Shepard 2003).
Although reports are infrequent, mefloquine has been suggested as being ototoxic based largely on case reports where it has been associated with hearing loss, tinnitus, vertigo, and dizziness in patients (Roland and Rutka 2004). Three cases of high-frequency hearing loss and tinnitus were reported after mefloquine prophylaxis; tinnitus persisted in all three patients and only one reported hearing recovery after terminating treatment (Fusetti et al. 1999). Another case of severe temporary hearing loss and tinnitus was reported after a single 250 mg dose of mefloquine prophylaxis (Wise and Toovey 2007). Approximately 26% of travelers undergoing mefloquine prophylaxis reported vertigo, nausea, and headaches raising the specter of vestibulotoxicity (Mizuno et al. 2006). Vertigo, nausea, and headaches were also the most common complaints in a well controlled clinical study using prophylaxis doses of mefloquine (Kollaritsch et al. 2000). In another study with 22 health volunteers taking a therapeutic dose (1250 mg/day), 96% developed vertigo; those suffering from severe, grade 3 vertigo (73%) required bed rest and medication for 1–4 days. Patient symptoms resolved 3 weeks after cessation of treatment (Rendi-Wagner et al. 2002). While some clinical reports suggest that mefloquine may be toxic to the inner ear, few laboratory studies have evaluated its toxic effects on the sensory cells or neurons of the inner ear. Using zebrafish larvae to screen for ototoxicity with more than a 1000 FDA approved drugs, mefloquine was identified as toxic to hair cells in lateral line sensory organ (Chiu et al. 2008). More recently, using postnatal cochlear organotypic cultures, we reported that mefloquine concentrations exceeding 10 μM resulted in a dose-dependent loss of cochlear hair cells and spiral ganglion neurons (Ding et al. 2009). Given that mefloquine has been reported to cause vertigo and nausea in patients, we carried out additional studies to evaluate the vestibulotoxic effects of mefloquine on hair cells in the maculae of the utricle.
Methods
Vestibular Organotypic Cultures
Our procedures for preparing postnatal cultures of the vestibular sensory epithelia have been described previously (Ding et al. 2002a; Zhang et al. 2003a). F344 rats were decapitated on postnatal days 3 or 4; the macula of the utricle was carefully dissected out and the otoliths overlying the macula were carefully removed. A 15 μl drop of rat-tail collagen was placed on a 35 mm culture dish (Falcon 3001, Becton Dickinson) and allowed to gel. Type I rat-tail collagen (Collagen, BD Biosciences) was mixed with 10× basal medium Eagle (BME, Sigma) and 2% sodium carbonate in a 9:1:1 ratio prior to use. Then 1.2 ml of serum-free BME, containing serum-free supplement (Sigma), 1% bovine serum albumin, 2 mM glutamine, 5 mg/ml glucose, and 10 U/ml of penicillin was added to the culture dish. The utricular maculae were positioned on the collagen as a flat surface preparation and held in place by surface tension from the thin layer of culture medium. Explants were placed in an incubator (Forma Scientific 3029, 37°C, 5% CO2) for 24 h. On the second day the medium was replaced with fresh control medium or medium containing 10, 50, 100 or 200 μM mefloquine (Sigma M-2319) and cultured for another 24 h. Controls and mefloquine-treated samples were run concurrently (n = 10/group). After 24 h treatment, the cultures were fixed with 4% formaldehyde in 0.1 M phosphate buffer (pH 7.4) and stained with different methods as described below. Utricular maculae were mounted in glycerin on glass slides, cover slipped and evaluated by confocal microscopy.
Staining
After fixation, some specimens were stained with TRITC-conjugated phalloidin (Sigma P1951, 1:200) in PBS for 30 min to label the stereocilia and cuticular plate of the hair cells, as described previously (Ding et al. 2002a). To visualize the nuclei, some specimens were incubated for 20 min in a working solution of TO-PRO-3 as described in our earlier publications (Jamesdaniel et al. 2009; Zhou et al. 2009). To investigate the role of apoptotic signaling, cultures were labeled with cell permeable carboxyfluorescein caspase-3, -8, and -9 (Cell Technology Inc.) which preferentially label each of their respective activated caspase; labeling was carried out according to the manufacturer's recommendations as described in our previous descriptions (Qi et al. 2008; Zhou et al. 2009; Ding et al. 2010). Specimens were incubated with one of the carboxyfluorescein caspases for 1 h at 37°C; afterwards the tissue was fixed with 4% formaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 h. After fixation, specimens were double or triple stained with phalloidin and/or To-Pro-3.
Confocal Microscopy
Specimens were examined with a confocal microscope and data acquisition software (LSM510, Zeiss) using filters appropriate for caspase (absorption: 488 nm, emission: 529 nm), TRITC (absorption: 543 nm, emission: 571 nm) or To-Pro-3 (absorption: 642 nm, emission: 661 nm) fluorescent probes. Images were processed offline with Advanced Imaging Microscopy software (version 4.0, Carl Zeiss) and Adobe Photoshop (version 5.0).
Quantification of Vestibular Hair Cell Loss
Specimens were examined with a microscope (Zeiss Axioskop) equipped with epifluorescence using filters appropriate for TRITC fluorescence (absorption: 543 nm, emission: 571 nm). The stereocilia bundles and the circumferential ring of the cuticular plate of the vestibular hair cells were heavily labeled with TRITC-conjugated phalloidin. To quantify hair cell loss in vestibular cultures, hair cell counts were obtained from a 0.01 mm2 region in three standardized areas in each specimen. Hair cells were counted as normal if the stereocilia bundle was largely intact and considered as damaged if the stereocilia bundle was largely absent. Hair cell counts were obtained from six or more cultures for each experimental condition and the means and standard deviations calculated for each condition.
This research was approved by the University of Buffalo Institutional Animal Care and Use Committee in accordance with NIH guidelines.
Results
Mefloquine-Induced Vestibular Hair Cell Loss
The photomicrograph in Fig. 1a shows the normal vestibular hair cells in the macula of the utricle. TRITC-conjugated phalloidin intensely labeled the stereocilia bundle and the circumferential ring surrounding the hair cell cuticular plate allowing easy identification of hair cells. Adding 10 μM of mefloquine to the culture medium resulted in a slight reduction of hair cell density, some disorganization of the ciliary tufts and moderate fading of the hair cell circumferential ring (Fig. 1b). Increasing the concentration of mefloquine to 100 μM resulted in considerable disarray of the stereocilia bundles and significant loss of staining of the hair cell circumferential ring (Fig. 1c); hair cell density was also greatly reduced. When the mefloquine dose was increased to 200 μM, nearly all the stereocilia bundles were missing (Fig. 1d). The circumferential rings decorating the remaining cells were much smaller than normal.
Fig. 1.
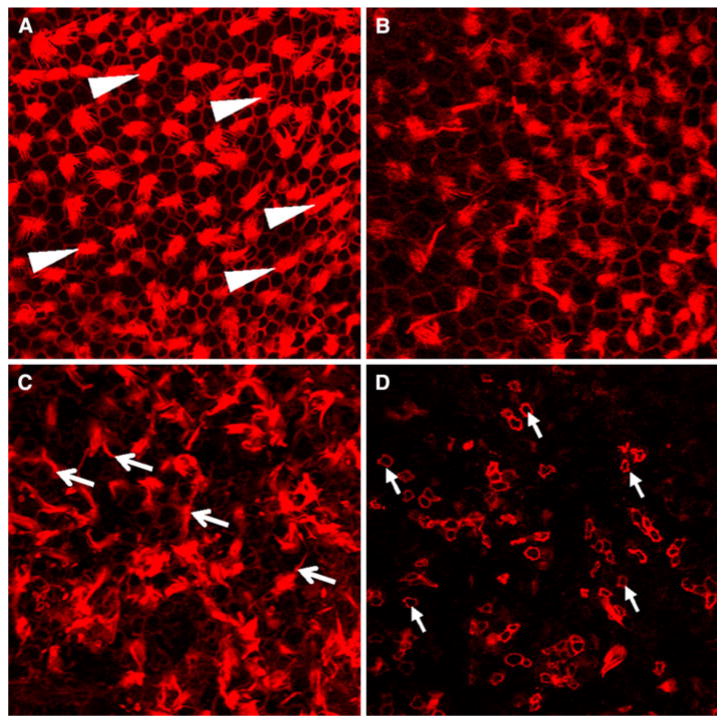
Representative photomicrographs of the macula of the utricle labeled with TRITC-conjugated phalloidin. a Normal macula of utricle. Note phalloidin labeling of stereocilia tufts (arrowheads) and hexagonal ring of phalloidin labeling around hair cells and supporting cells. b Hair cell density reduced in cultures treated with 10 μM mefloquine; circumferential ring surrounding some cell less intense than normal. c Many stereocilia tufts in disarray (arrows) or disorganized on cultures treated with 100 μM mefloquine. d Nearly all hair cells missing with 200 μM mefloquine; circumferential ring on remaining cell shrunken (arrows)
To quantify the hair cell loss induced by different doses of mefloquine, hair cells were counted in three separate regions (0.01 mm2) of the macula of each utricle and the values from each specimen averaged. Figure 2 shows the mean (n = 6/group) hair cell density in normal and mefloquine-treated macula of the utricle. In normal control maculae, the hair cell density was 65.2 cells per 0.01 mm2. Treatment with 10 μM mefloquine reduced hair cell density to 57.7 cells per 0.01 mm2. Increasing the dose to 50 μM reduced the hair cell density to 28.3 cells per 0.01 mm2. When the mefloquine dose increased to 100 μM, the hair cell density declined to 26.3 cells per 0.01 mm2 and when the dose increased to 200 μM, hair cell density declined to 5.2 cells per 0.01 mm2. A one-way analysis of variance showed that the effect of mefloquine treatment was statistically significant (F = 33.27, P < 0.001). A Tukey's post hoc multiple comparison indicated that hair cell densities with the 50, 100, and 200 μM doses of mefloquine were significantly different from control (0 μM) (P < 0.05). Hair cell densities for 50 and 100 μM were significantly different from 200 μM (P < 0.05) and hair cell density for 10 μM was significantly different from 50, 100, and 200 μM (P < 0.05).
Fig. 2.
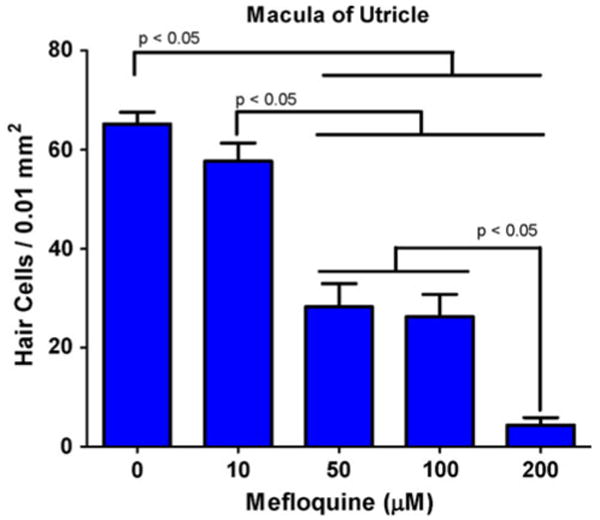
Mean (+1 SD) number of hair cells per 0.01 mm2 in the maculae of the utricle as a function of mefloquine dose. A one-way analysis of variance showed a significant effect of treatment (P < 0.001). Tukey's post hoc analysis indicated that 50, 100, and 200 μM were significantly different (P < 0.05) from control (0 mM); 10 mM was significantly different (P < 0.05) from 50, 100, and 200 mM, and 200 μM was significantly different (P < 0.05) from 50 to 100 μM
Mefloquine-Induced Apoptotic Changes in Vestibular Hair Cells
Two prominent anatomical features of cells undergoing programmed cell death are nuclear shrinkage and fragmentation (Lang and Liu 1997; Sidhu et al. 1997). To determine if mefloquine-induced hair cell death was occurring by apoptosis, specimens were labeled with TO-PRO-3 to visualize the nuclei (n = 3/group). Figure 3a shows the characteristic shape of cell nuclei from a normal utricle; the nuclei are large and round characteristic of healthy cells. Figure 3b shows the typical shape of nuclei from a utricle treated with 50 μM mefloquine for 24 h. After mefloquine treatment, the nuclei of many cells were darkly stained and severely shrunken or fragmented, morphological features of cells undergoing apoptosis.
Fig. 3.
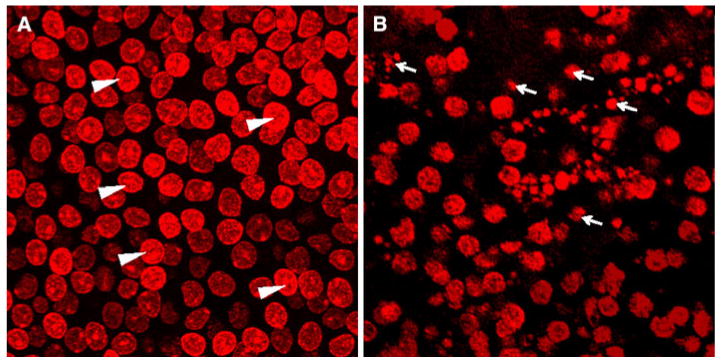
Photomicrographs of surface preparations from the maculae of utricle stained with TO-PRO-3. a TO-PRO-3 labeled nuclei of hair cells and supporting cells in control maculae are large and round (arrowheads). b Many nuclei the hair cells and supporting cells in macula treated with 50 μM mefloquine are shrunken and fragmented (arrows); many areas devoid of TO-PRO-3 labeling
To determine if programmed cell death was caspase mediated, mefloquine-treated vestibular cultures were labeled with cell permeable, fluorogenic probes that specifically label caspase-3, -8 or -9 (n = 3/group). In normal cultures of the utricle, large, round nuclei were present throughout the macula and there was no activation of caspase-3 (Fig. 4a) or caspase-8 or -9 (data not shown). However, after 24 h treatment with 50 μM mefloquine, the cell nuclei in the maculae of the utricle were shrunken and fragmented (Fig. 4b–d) and there was extensive labeling of extrinsic, initiator caspase-8 (Fig. 4b), the intrinsic, initiator caspase-9 (Fig. 4c), and the downstream executioner caspase-3 (Fig. 4d), caspase-9 (Fig. 4c).
Fig. 4.
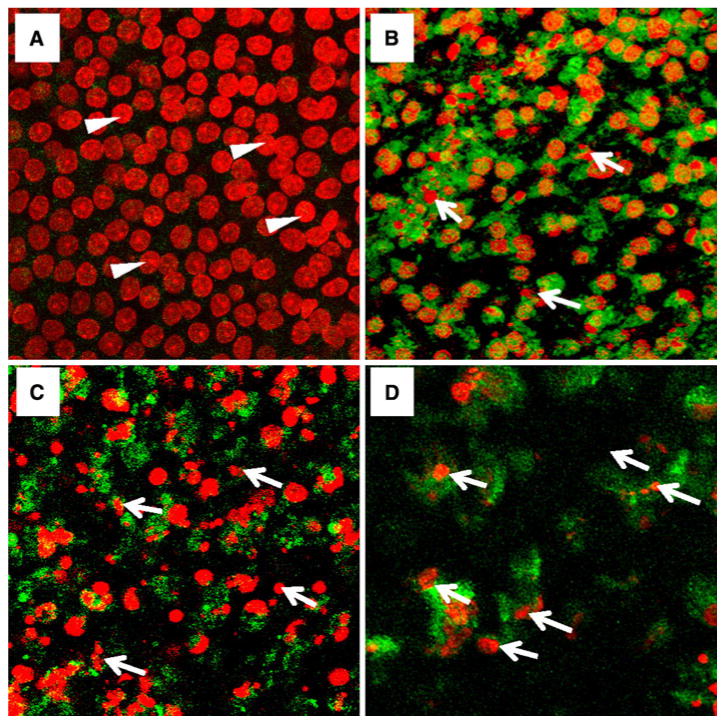
Maculae of utricle double-labeled with TO-PRO-3 (red, large round, arrowhead) and fluorogenic probes against caspase-3, -8 or -9 (green, around nuclei). a Normal macula shows large round nuclei labeled with TO-PRO-3 (red, arrowhead); note absence of executioner caspase 3. b Macula treated for 24 h with 50 μM mefloquine. Cell nuclei (red, arrows) are shrunken and fragmented; note intense cytoplasmic, green labeling of extrinsic, initiator caspase-8 present throughout much of the macula. c Macula treated for 24 h with 50 μM mefloquine. Note shrunken and fragmented nuclei (red, arrows); widespread labeling of intrinsic, initiator caspase-9. d Macula treated for 24 h with 50 μM mefloquine. Note severely, shrunken and fragmented nuclei (red, arrows); extensive labeling of executioner caspase 3 surrounding shrunken nuclei (Color figure online)
Discussion
Clinical reports and the manufacturer's drug insert suggest that mefloquine (Lariam®) may cause vertigo and vestibular disturbances (Phillips-Howard and ter Kuile 1995; Rendi-Wagner et al. 2002; Roche 2003). In addition, mefloquine is known to destroy hair cells in the Zebrafish lateral line organ (Chiu et al. 2008). However, we are unaware of any experimental data directly linking mefloquine treatment to damage of the mammalian vestibular sensory epithelium. The results presented here are the first to demonstrate the vestibulotoxic effects of mefloquine in postnatal organotypic cultures of the maculae of the utricle. Mefloquine-induced vestibular hair cell damage in a dose-dependent manner (Fig. 2). Treatment for 24 h with 50 μM mefloquine caused a significant (57%) loss of hair cells; increasing the dose to 200 μM resulted in nearly 100% hair cell loss.
The doses of mefloquine that induced significant hair cell damage in our study are comparable to the levels of mefloquine observed in various regions of the brain (27–111 μM) when rats were treated with 50 mg/kg/day of mefloquine (Baudry et al. 1997). When mefloquine is used as a treatment for malaria, a single dose of 1250 mg is given; this corresponds to 25 and 12.5 mg/kg for humans weighing 50 and 100 kg, respectively (Dow et al. 2006). After applying a species scaling factor of 6 to go from human to rats, the corresponding treatment dose for rat would scale up to 75 and 150 mg/kg. These treatment doses in rats would produce concentrations in the brain comparable to those used in our organ cultures. Thus, the doses used in our organ cultures seem clinically relevant.
Apoptosis
The 50 μM dose of mefloquine caused significant nuclear shrinkage and nuclear fragmentation, classic morphologic features of programmed cell death (Lang and Liu 1997; Sidhu et al. 1997). Nuclear swelling, a morphologic feature of necrotic cell death, was not observed in mefloquine-treated vestibular cultures (Morrison et al. 2002). Thus, the primary pathway for mefloquine-induced cell death in our organotypic cultures appears to be apoptotic. Caspases, a family of cysteine-aspartic proteases, appear to play a major role in mefloquine-induced cell death in vestibular hair cells. Mefloquine activated both initiator caspase-8 and initiator caspase-9 both of which lead to downstream activation of executioner caspase-3 (Beer et al. 2001; Cowan et al. 2001; Ding et al. 2002b; Zhang et al. 2003b; Meller et al. 2006). These results are consistent with mefloquine's ototoxic effects on cochlear hair cells (Ding et al. 2009). Early activation of caspase-8 suggests the engagement of death receptors on the cell's membrane with specific ligands such as FADD, TRADD, Fas or tumor necrosis factor (TNF) (Lin et al. 1999; Ding et al. 2007). The early activation of intrinsic caspase-9 is associated with the release of cytochrome c from damaged mitochondria which subsequently interacts with apoptosis protease activating factor, Apaf-1, causing self-cleavage and activation of caspase-9 (Bratton et al. 2001; Henshall et al. 2001). Our results show that downstream executioner caspase-3 is activated leading to cell death.
Although the 10 μM dose of mefloquine caused a slight reduction in vestibular hair cell density, only doses of 50 μM or more caused a significant decrease in hair cell density (Fig. 2). These results suggest that the “threshold dose” of mefloquine that is toxic to vestibular hair cells is somewhere between 10 and 50 μM under the culture conditions employed here. In our study, 50 μM of mefloquine destroyed 57% of vestibular hair cells (Fig. 2) whereas in our earlier study of the organ of Corti, the same dose destroyed approximately 90% of cochlear hair cells (Ding et al. 2009). The implications of these findings are that that mefloquine is slightly more toxic to cochlear hair cells than vestibular hair cells; however, the reasons for this are unclear.
Mechanisms
Mefloquine is a potent blocker of connexins which form gap junction proteins. Mefloquine blocked connexin 50 with an IC50 near 1 μM and connexin 36 at an IC50 of approximately 300 μM; both of these gap junctions are highly expressed in the brain (Cruikshank et al. 2004). Thirty millimolar of mefloquine almost completely blocked connexin 26 which is highly expressed in the vestibular system (Masuda et al. 2001; Todt et al. 2005). Thus, mefloquine blockade of gap junction proteins represents one potential trigger for initiating cell death in the vestibular system. Mefloquine is also a potent blocker of L-type calcium channels (Coker et al. 2000) which are expressed in the vestibular system (Bao et al. 2003; Almanza et al. 2007). Blockade of L-type calcium channels represents a second pathway for initiating cell death. Finally, mefloquine and related anti-malarial drugs such as quinine and chloroquine, generate toxic reactive oxygen and nitrogen species which could induce cell death (Clerici et al. 1996; Park et al. 2004; Park et al. 2008; Hood et al. 2010).
High doses of mefloquine have been shown to cross the blood–brain barrier and to cause neurotoxicity in rats and to damage to the nucleus gracilis, nucleus cuneatus and solitary tract; these effects were seen with plasma concentrations in the rat that are in the clinical range for humans (Dow et al. 2006). While our results clearly indicate that mefloquine is toxic to vestibular hair cells in postnatal day 3 vestibular organ cultures, it remains to be seen whether it is toxic in vivo either in postnatal or adult animals. However, there have been several reports of hearing loss, tinnitus and dizziness in patients taking clinically relevant doses of mefloquine (Karbwang et al. 1994; Phillips-Howard and ter Kuile 1995; Fusetti et al. 1999; Wise and Toovey 2007); however, other studies have failed to identify hearing and vestibular problems in healthy volunteers (Carrara et al. 2008). On the other hand, certain patients being treated with mefloquine may be more susceptible to adverse effects because of other medical problems, genetic predisposition or concomitant drug treatments (Karbwang et al. 1994; Phillips-Howard and ter Kuile 1995; Pussard et al. 2007; Thompson et al. 2007).
Our results clearly show that mefloquine begins to damage vestibular hair cells at doses at low a 10 μM and causes significant hair cell damage at 50 μM. Our results are consistent with previous studies showing that 24 h treatment with 10 μM mefloquine causes significant loss of cultured cortical neurons and 50 μM leads to nearly 100% neuronal loss (Hood et al. 2010); these changes were associated with increases in oxidative stress. In addition, the doses at which mefloquine begins to damage vestibular hair cells are in the same range known to block connexin 26, a gap junction protein expressed in the vestibular system. Mefloquine vestibulotoxicity in vivo will depend on many factors such dose and duration of treatment and uptake of the compound across the blood–brain barrier. These results raise the possibility that high dose, long term treatment with mefloquine might be vestibulotoxic in adulthood or prenatally.
Acknowledgments
Supported in part by NIH Grant R01DC006630.
Contributor Information
Dongzhen Yu, Center for Hearing and Deafness, State University of New York at Buffalo, 137 Cary Hall, Buffalo, NY 14214, USA; Six People's Hospital of Shanghai Jiao Tong University, Shanghai, China.
Dalian Ding, Center for Hearing and Deafness, State University of New York at Buffalo, 137 Cary Hall, Buffalo, NY 14214, USA; Six People's Hospital of Shanghai Jiao Tong University, Shanghai, China; Third Xiangya Hospital of Central South University, Changsha, China.
Haiyan Jiang, Center for Hearing and Deafness, State University of New York at Buffalo, 137 Cary Hall, Buffalo, NY 14214, USA.
Daniel Stolzberg, Center for Hearing and Deafness, State University of New York at Buffalo, 137 Cary Hall, Buffalo, NY 14214, USA.
Richard Salvi, Email: salvi@buffalo.edu, Center for Hearing and Deafness, State University of New York at Buffalo, 137 Cary Hall, Buffalo, NY 14214, USA.
References
- Almanza A, Navarrete F, Vega R, Soto E. Modulation of voltage-gated Ca2+ current in vestibular hair cells by nitric oxide. J Neurophysiol. 2007;97:1188–1195. doi: 10.1152/jn.00849.2006. [DOI] [PubMed] [Google Scholar]
- Angus BJ. Malaria on the World Wide Web. Clin Infect Dis. 2001;33:651–661. doi: 10.1086/322683. [DOI] [PubMed] [Google Scholar]
- Bao H, Wong WH, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J Neurophysiol. 2003;90:155–164. doi: 10.1152/jn.00244.2003. [DOI] [PubMed] [Google Scholar]
- Baudry S, Pham YT, Baune B, Vidrequin S, Crevoisier C, Gimenez F, Farinotti R. Stereoselective passage of mefloquine through the blood–brain barrier in the rat. J Pharm Pharmacol. 1997;49:1086–1090. doi: 10.1111/j.2042-7158.1997.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Beer R, Franz G, Krajewski S, Pike BR, Hayes RL, Reed JC, Wang KK, Klimmer C, Schmutzhard E, Poewe W, Kampfl A. Temporal and spatial profile of caspase 8 expression and proteolysis after experimental traumatic brain injury. J Neurochem. 2001;78:862–873. doi: 10.1046/j.1471-4159.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Walker G, Roberts DL, Cain K, Cohen GM. Caspase-3 cleaves Apaf-1 into an approximately 30 kDa fragment that associates with an inappropriately oligomerized and biologically inactive approximately 1.4 MDa apoptosome complex. Cell Death Differ. 2001;8:425–433. doi: 10.1038/sj.cdd.4400834. [DOI] [PubMed] [Google Scholar]
- Carmargo LM, de Oliveira S, Basano S, Garcia CR. Antimalarials and the fight against malaria in Brazil. Ther Clin Risk Manag. 2009;5:311–317. doi: 10.2147/tcrm.s4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara VI, Phyo AP, Nwee P, Soe M, Htoo H, Arunkamomkiri J, Singhasivanon P, Nosten F. Auditory assessment of patients with acute uncomplicated Plasmodium falciParum malaria treated with three-day mefloquine–artesunate on the north-western border of Thailand. Malar J. 2008;7:233. doi: 10.1186/1475-2875-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Wilson ME, Schlagenhauf P. Prevention of malaria in long-term travelers. JAMA. 2006;296:2234–2244. doi: 10.1001/jama.296.18.2234. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Cunningham LL, Raible DW, Rubel EW, Ou HC. Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol. 2008;9:178–190. doi: 10.1007/s10162-008-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Coker SJ, Batey AJ, Lightbown ID, Diaz ME, Eisner DA. Effects of mefloquine on cardiac contractility and electrical activity in vivo, in isolated cardiac preparations, and in single ventricular myocytes. Br J Pharmacol. 2000;129:323–330. doi: 10.1038/sj.bjp.0703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CM, Thai J, Krajewski S, Reed JC, Nicholson DW, Kaufmann SH, Roskams AJ. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J Neurosci. 2001;21:7099–7109. doi: 10.1523/JNEUROSCI.21-18-07099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci USA. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002a;164:115–126. doi: 10.1016/s0378-5955(01)00417-8. [DOI] [PubMed] [Google Scholar]
- Ding WX, Shen HM, Ong CN. Calpain activation after mitochondrial permeability transition in microcystin-induced cell death in rat hepatocytes. Biochem Biophys Res Commun. 2002b;291:321–331. doi: 10.1006/bbrc.2002.6453. [DOI] [PubMed] [Google Scholar]
- Ding D, Jiang H, Wang P, Salvi R. Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res. 2007;226:129–139. doi: 10.1016/j.heares.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ding D, Qi W, Yu D, Jiang H, Salvi R. Ototoxic effects of mefloquine in cochlear organotypic cultures. J Otology. 2009;4:29–38. [Google Scholar]
- Ding D, Jiang H, Salvi RJ. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res. 2010;259:16–23. doi: 10.1016/j.heares.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow GS, Koenig ML, Wolf L, Gerena L, Lopez-Sanchez M, Hudson TH, Bhattacharjee AK. The antimalarial potential of 4-quinolinecarbinolamines may be limited due to neurotoxicity and cross-resistance in mefloquine-resistant Plasmodium falciparum strains. Antimicrob Agents Chemother. 2004;48:2624–2632. doi: 10.1128/AAC.48.7.2624-2632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow G, Bauman R, Caridha D, Cabezas M, Du F, Gomez-Lobo R, Park M, Smith K, Cannard K. Mefloquine induces dose-related neurological effects in a rat model. Antimicrob Agents Chemother. 2006;50:1045–1053. doi: 10.1128/AAC.50.3.1045-1053.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow GS, Magill AJ, Ohrt C. Clinical development of new prophylactic antimalarial drugs after the 5th Amendment to the Declaration of Helsinki. Ther Clin Risk Manag. 2008;4:803–819. doi: 10.2147/tcrm.s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler SJ, MacArthur JR, Parise M, Wirtz R, Eliades MJ, Dasilva A, Steketee R. Locally acquired mosquito-transmitted malaria: a guide for investigations in the United States. MMWR Recomm Rep. 2006;55:1–9. [PubMed] [Google Scholar]
- Fletcher A, Shepard R. Use of (+)-mefloquine for the treatment of malaria. 6664397. USPO; USA: 2003. pp. 1–11. [Google Scholar]
- Fusetti M, Eibenstein A, Corridore V, Hueck S, Chiti-Batelli S. Mefloquine and ototoxicity: a report of 3 cases. Clin Ter. 1999;150:379–382. [PubMed] [Google Scholar]
- Henshall DC, Bonislawski DP, Skradski SL, Araki T, Lan JQ, Schindler CK, Meller R, Simon RP. Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ. 2001;8:1169–1181. doi: 10.1038/sj.cdd.4400921. [DOI] [PubMed] [Google Scholar]
- Hood JE, Jenkins JW, Milatovic D, Rongzhu L, Aschner M. Mefloquine induces oxidative stress and neurodegeneration in primary rat cortical neurons. Neurotoxicology. 2010;31:518–523. doi: 10.1016/j.neuro.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Jamesdaniel S, Ding D, Kermany MH, Jiang H, Salvi R, Coling D. Analysis of cochlear protein profiles of Wistar, Sprague-Dawley, and Fischer 344 rats with normal hearing function. J Proteome Res. 2009;8:3520–3528. doi: 10.1021/pr900222c. [DOI] [PubMed] [Google Scholar]
- Jha S, Kumar R. Mefloquine toxicity presenting with polyneuropathy—a report of two cases in India. Trans R Soc Trop Med Hyg. 2006;100:594–596. doi: 10.1016/j.trstmh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Karbwang J, Bangchang KN, Thanavibul A, Wattanakoon Y, Harinasuta T. Quinine toxicity when given with doxycycline and mefloquine. Southeast Asian J Trop Med Public Health. 1994;25:397–400. [PubMed] [Google Scholar]
- Kollaritsch H, Karbwang J, Wiedermann G, Mikolasek A, Na-Bangchang K, Wernsdorfer WH. Mefloquine concentration profiles during prophylactic dose regimens. Wien Klin Wochenschr. 2000;112:441–447. [PubMed] [Google Scholar]
- Lang H, Liu C. Apoptosis and hair cell degeneration in the vestibular sensory epithelia of the guinea pig following a gentamicin insult. Hear Res. 1997;000:1–7. doi: 10.1016/s0378-5955(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Usami S, Yamazaki K, Takumi Y, Shinkawa H, Kurashima K, Kunihiro T, Kanzaki J. Connexin 26 distribution in gap junctions between melanocytes in the human vestibular dark cell area. Anat Rec. 2001;262:137–146. doi: 10.1002/1097-0185(20010201)262:2<137::AID-AR1018>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Meller R, Clayton C, Torrey DJ, Schindler CK, Lan JQ, Cameron JA, Chu XP, Xiong ZG, Simon RP, Henshall DC. Activation of the caspase 8 pathway mediates seizure-induced cell death in cultured hippocampal neurons. Epilepsy Res. 2006;70:3–14. doi: 10.1016/j.eplepsyres.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Kudo K, Kano S. Chemoprophylaxis according to the guidelines on malaria prevention for Japanese overseas travelers. Southeast Asian J Trop Med Public Health. 2006;37(Suppl 3):11–14. [PubMed] [Google Scholar]
- Morrison RS, Kinoshita Y, Johnson MD, Ghatan S, Ho JT, Garden G. Neuronal survival and cell death signaling pathways. Adv Exp Med Biol. 2002;513:41–86. doi: 10.1007/978-1-4615-0123-7_2. [DOI] [PubMed] [Google Scholar]
- Nyunt MM, Plowe CV. Pharmacologic advances in the global control and treatment of malaria: combination therapy and resistance. Clin Pharmacol Ther. 2007;82:601–605. doi: 10.1038/sj.clpt.6100361. [DOI] [PubMed] [Google Scholar]
- Park J, Choi K, Jeong E, Kwon D, Benveniste EN, Choi C. Reactive oxygen species mediate chloroquine-induced expression of chemokines by human astroglial cells. Glia. 2004;47:9–20. doi: 10.1002/glia.20017. [DOI] [PubMed] [Google Scholar]
- Park BC, Park SH, Paek SH, Park SY, Kwak MK, Choi HG, Yong CS, Yoo BK, Kim JA. Chloroquine-induced nitric oxide increase and cell death is dependent on cellular GSH depletion in A172 human glioblastoma cells. Toxicol Lett. 2008;178:52–60. doi: 10.1016/j.toxlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Phillips-Howard PA, ter Kuile FO. CNS adverse events associated with antimalarial agents. Fact or fiction? Drug Saf. 1995;12:370–383. doi: 10.2165/00002018-199512060-00003. [DOI] [PubMed] [Google Scholar]
- Pussard E, Merzouk M, Barennes H. Increased uptake of quinine into the brain by inhibition of P-glycoprotein. Eur J Pharm Sci. 2007;32:123–127. doi: 10.1016/j.ejps.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236:52–60. doi: 10.1016/j.heares.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendi-Wagner P, Noedl H, Wernsdorfer WH, Wiedermann G, Mikolasek A, Kollaritsch H. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 2002;81:167–173. doi: 10.1016/s0001-706x(01)00210-8. [DOI] [PubMed] [Google Scholar]
- Roche . LARIAM brand of mefloquine hydrochloride. Roche Laboratories Inc; Nutely, NJ: 2003. [Google Scholar]
- Roland P, Rutka . Ototoxicity. BC Decker; Hamilton, ON: 2004. [Google Scholar]
- Russell PF. World-wide malaria distribution, prevalence, and control. Am J Trop Med Hyg. 1956;5:937–965. doi: 10.4269/ajtmh.1956.5.937. [DOI] [PubMed] [Google Scholar]
- Sauvet F, Lebeau C, Foucher S, Flusain O, Jouanin JC, Debonne JM. Operational impact of health problems observed during a four-month military deployment in Ivory Coast. Mil Med. 2009;174:921–928. doi: 10.7205/milmed-d-05-1008. [DOI] [PubMed] [Google Scholar]
- Schmid S, Chiodini P, Legros F, D'Amato S, Schoneberg I, Liu C, Janzon R, Schlagenhauf P. The risk of malaria in travelers to India. J Travel Med. 2009;16:194–199. doi: 10.1111/j.1708-8305.2009.00332.x. [DOI] [PubMed] [Google Scholar]
- Sidhu RS, Tuor UI, Del Bigio MR. Nuclear condensation and fragmentation following cerebral hypoxia-ischemia occurs more frequently in immature than older rats. Neurosci Lett. 1997;223:129–132. doi: 10.1016/s0304-3940(97)13426-7. [DOI] [PubMed] [Google Scholar]
- Tepper M, Schofield S, Anderson J. Malaria chemoprophylaxis for coalition troops in Afghanistan. JAMA. 2007;298:1275. doi: 10.1001/jama.298.11.1275-a. (author reply 1275–1276) [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lochner M, Lummis SC. The antimalarial drugs quinine, chloroquine and mefloquine are antagonists at 5-HT3 receptors. Br J Pharmacol. 2007;151:666–677. doi: 10.1038/sj.bjp.0707238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt I, Hennies HC, Basta D, Ernst A. Vestibular dysfunction of patients with mutations of connexin 26. Neuroreport. 2005;16:1179–1181. doi: 10.1097/00001756-200508010-00009. [DOI] [PubMed] [Google Scholar]
- Wise M, Toovey S. Reversible hearing loss in temporal association with chemoprophylactic mefloquine use. Travel Med Infect Dis. 2007;5:385–388. doi: 10.1016/j.tmaid.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neurosci. 2003a;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Graham SH, Kochanek PM, Marion DW, Nathaniel PD, Watkins SC, Clark RS. Caspase-8 expression and proteolysis in human brain after severe head injury. FASEB J. 2003b;17:1367–1369. doi: 10.1096/fj.02-1067fje. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ding D, Kraus KS, Yu D, Salvi R. Functional and structural changes in the chinchilla cochlea and vestibular system following round window application of carboplatin. Audiological Med. 2009;7:189–199. doi: 10.3109/16513860903335795. [DOI] [PMC free article] [PubMed] [Google Scholar]


