Abstract
The auditory system exhibits differences by sex and by sexual orientation, and the implication is that relevant auditory structures are altered during prenatal development, possibly by exposure to androgens. The otoacoustic emissions (OAEs) of newborn male infants are weaker than those of newborn females, and these sex differences persist through the lifespan. The OAEs of nonheterosexual females also are weaker than those of heterosexual females, suggesting an atypically strong exposure to androgens some time early in development. Auditory evoked potentials (AEPs) also exhibit sex differences beginning early in life. Some AEPs are different for heterosexual and nonheterosexual females, and other AEPs are different for heterosexual and nonheterosexual males. Research on non-humans treated with androgenic or anti-androgenic agents also suggests that OAEs are masculinized by prenatal exposure to androgens late in gestation. Collectively, the evidence suggests that prenatal androgens, acting globally or locally, affect both nonheterosexuality and the auditory system.
Keywords: Sexual orientation, otoacoustic emissions (OAEs), auditory evoked potentials (AEPs), prenatal androgen exposure, prenatal development, localized effects of hormones, nonmonotonic effects of hormones, homosexuality, heterosexuality
Introduction
A central tenet of modern neuroscience is that all behaviors, and thus, all differences in behavior, must have a corresponding basis somewhere in the structures of the brain. So, when you learn a new fact about Texas wines, for example, or how to do a new rope trick, something in your brain must be different from before (how could it be any other way?). From this tenet, it follows that there must be something about the brains of nonheterosexuals that is different from the brains of heterosexuals, and it is interesting to ponder the origins of that difference. At conception, was there a configuration of the genes that predisposed the person to a nonheterosexual orientation because of some atypical brain structure? During prenatal development, were there physiological events that rendered some neural circuits atypical and predisposed the person to a nonheterosexual orientation? During early childhood, were there environmental factors that somehow altered the brain and predisposed the person to a nonheterosexual orientation? Or sometime prior to, or soon after, puberty did the person make a conscious decision to live as a nonheterosexual and the atypical brain structure(s) followed from that? These examples differ not just in when, during development, the critical event occurred in the brain; they differ as to whether that atypicality in the brain was a cause or an effect.
In modern society worldwide, there exists a full spectrum of sexual orientations extending from exclusively heterosexual to exclusively homosexual, with individual people falling all throughout the intervening range, and it is likely that this was true historically as well. While many of the distinctions between sub-groups are interesting to know about and interesting to consider in regard to their origins and implications, for current purposes, these distinctions will be ignored and the issue of sexual orientation treated as if there were only two categories. Here it will be sufficient to acknowledge that the vast majority of modern humans is primarily heterosexual in regard to sexual thoughts and behaviors, the remainder is not to varying degrees, and this clearly has been true for a long time in human society. Thus, for this discussion, I will treat what clearly is not a simple dichotomy as if it were by using the overly simplistic terms heterosexual and nonheterosexual to characterize what clearly is a complex spectrum of sexual thoughts and behaviors.
Some investigators imply that any physiological measure that differs with sexual orientation is inherently more interesting, and potentially more informative about the origins of orientation, than are various behavioral or cognitive measures that are commonly studied. One reason is that physiological measures carry the appearance of being evidence that nonheterosexuality has a biological basis and is not simply a conscious choice. Care must be taken with this approach, however. Some physiological characteristics, including differences in brain structures, may not be informative about the causes of nonheterosexuality but may themselves be results. This is because the activities in which a person engages as a young adult (say, after the realization of being nonheterosexual and perhaps even because of that realization) can lead to increased development in certain brain regions and the decline of certain others. Similarly, differences in the voice, in carriage, or in certain sensory-motor skills clearly might be a consequence of a person's experiences after beginning to live a nonheterosexual lifestyle. So, while finding differences with orientation for physiological measures of this sort certainly is interesting, it clearly would be an error to assume that those physiological differences are in some way related to the reason for the person being nonheterosexual in the first place.
However, for some physiological measures it is difficult to imagine how lifestyle-related experiences or conscious decisions could change the magnitude or quality of the measure. For example, how might someone intentionally make her index fingers slightly shorter than her ring fingers? The ratio of those two finger lengths (called the 2D:4D ratio) does differ between the sexes (summarized in [8]; [66]), and that sex difference exists beginning in the early weeks of prenatal development [25,44]. (The direction of effect is that, for females, the lengths of the index and ring fingers are similar, but in males, the ring finger is a bit longer than the index finger.) Although the literature is mixed, there is some evidence that the finger-length ratios (FLRs) of nonheterosexuals are different from those of heterosexuals (see, e.g., [56]). Because of the apparent constancy of FLRs through life, the implication is that heterosexuals and nonheterosexuals differed in FLRs at birth, well before any conscious decision about sexual orientation could be made. So, it appears that some physiological measures may be informative about the biological factors contributing to nonheterosexuality while others only can be informative about how differences in lifestyle for heterosexuals and nonheterosexuals can alter the physiology of the body or brain.
This review will concentrate on two physiological measures, otoacoustic emissions (OAEs) and auditory evoked potentials (AEPs), that also are difficult to imagine changing through conscious effort or lifestyle preferences, and that also exhibit sex differences at birth. My belief is that these measures have the potential to be informative about mechanisms operating during early development that are responsible for nonheterosexuality as well as mechanisms underlying other special populations in humans.
Background for Auditory Measures
OAEs are sounds produced in the cochlea that propagate out through the middle-ear system into the external ear canal where they can be recorded and measured using small microphone systems [33,42]. Multiple forms of OAEs exist; here we will be concerned primarily with spontaneous OAEs and click-evoked OAEs. (To conserve space here, some of the references have been omitted for factual assertions made below; those references can be found in [50,51,52].)
Spontaneous OAEs (SOAEs) are essentially continuous pure tones that are present in the ear canal in quiet environments. An individual ear can have as many as several dozen SOAEs, but smaller numbers are more common. Generally, the right ear exhibits more, and stronger, SOAEs than the left ear. Although the strengths of individual SOAEs (measured in decibels of sound-pressure level, or dB SPL) can vary across measurement sessions, the frequencies of those SOAEs are highly stable across time [11]. In humans, approximately 75% of females and 50% of males have at least one SOAE (e.g., [4]; reviewed in [50,51,52]). This sex difference in the number of SOAEs also exists in newborn humans [12,13,72,73,84]; that fact, plus the marked stability of SOAEs through life suggests that the SOAEs measured in young adults are a good representation of what was present at birth. SOAEs are not the basis for the “ringing in the ears” experienced after exposure to intense sounds or after ingestion of certain drugs; that tinnitus is a sign of a damaged cochlea, and SOAEs are a characteristic of normal cochleas. SOAEs are unusual among OAEs in that they are common in humans but are found only rarely in non-human species, small and large. The standard measure of SOAEs is their number, but some investigators measure their level as well. (The adjective “spontaneous” indicates that SOAEs are present without any special action by the investigator; the other forms of OAE require presentation of sounds.)
Click-evoked OAEs (CEOAEs) are brief sounds that can be recorded in the ear canal immediately after the presentation of an acoustic stimulus. They can be thought of as echo-like sounds whose characteristics depend, in part, upon the stimulus used to elicit them. As the term “click-evoked” indicates, the most common stimulus for CEOAEs is brief sounds (1 ms or shorter) having wide bandwidths. A brief acoustic stimulus can give rise to a CEOAE waveform that lasts several tens of milliseconds. Unlike typical echoes, where all frequency components are reflected back essentially simultaneously, CEOAEs are frequency-dispersed; the highest frequencies are reflected back first and successively lower frequencies are reflected back with successively longer latencies. Sometimes some frequencies are emitted multiple times. This behavior suggests that the reflections are originating, at least in part, from different locations along the length of the tonotopically organized cochlear partition. The standard measure of CEOAEs is their root-mean-square (rms) amplitude expressed in decibels. CEOAEs (and SOAEs) are quite weak and require averaging techniques to be detected. CEOAEs are generally stronger in females than in males, and generally stronger in right ears than in left ears, but essentially all normal-hearing ears have CEOAEs. Common practice nowadays is to use CEOAEs as a rapid screening for hearing loss before a newborn infant leaves the hospital. CEOAEs are rarely observed in small, non-human species, such as rodents, presumably because their short cochleas lead to echoes having such short latencies that they are cloaked by the persistence of the stimulus in the ear canal. However, CEOAEs easily are recorded from larger mammals. Examples of CEOAE waveforms and frequency spectra containing SOAEs can be found in [51].
Another commonly measured form of OAE, especially in non-humans, is the distortion-product OAE (DPOAE; see [42]), but the absence of a substantial sex difference in DPOAEs (see [58]; [86]) and a shortage of space here discourage a discussion of this measure.
We are far from a full understanding of the various mechanisms underlying the production of OAEs [81], but it is clear that one of the two populations of receptor cells in the cochlea – the outer hair cells (OHCs) – plays a crucial role. The outer hair cells are substantially more numerous than the inner hair cells (approximately 14,000 and 4,000, respectively, in human cochleas), but the preponderance of the afferent innervation goes to the inner hair cells (approximately 95% and 5% for IHCs and OHCs, respectively). OHCs are unique among cells in the cochlea in that they are electromotile [10]. As their stereocilia are bent back and forth by the up and down movements of the basilar and tectorial membranes, the OHCs exhibit rapidly alternating phases of depolarization and hyperpolarization. Accompanying these changes in state of polarization are small changes in the lengths of the OHCs. Because the columnar-shaped OHCs are held tightly at both ends by supporting structures, these changes in length alter the local micromechanics of the cochlea, and as a consequence, the magnitude of displacement of the basilar membrane is increased from what it would be without the actions of the OHCs. That is, the OHCs contribute to a mechanical amplification of weak sounds in the cochlea, and as a consequence, they have come to be called cochlear amplifiers. The OHCs are quite delicate and are the first to be damaged by exposure to intense sounds and ototoxic drugs. When the OHCs are damaged temporarily or permanently, hearing sensitivity is reduced and OAEs are diminished or lost (e.g., [59]); hence the association between healthy OHCs and strong OAEs, even though many details have yet to be worked out.
Also summarized in this review are data on AEPs (auditory evoked potentials), which are brain waves evoked by an acoustic stimulus and measured using scalp electrodes and averaging techniques (see [30]). A brief click can give rise to a succession of peaks over the course of a couple hundred milliseconds. Standard procedure is to measure the elapsed time from the click to the peak (the latency) and, for some peaks, the peak-to-trough amplitude also is measured. The earliest waves are categorized as the auditory brainstem response (ABR), next are the middle-latency response (MLR), and last are the long-latency response (LLR). The peaks of the ABR have latencies from the click stimulus of about 10 ms and shorter; the peaks of the MLR have latencies of about 10 to 50 ms; the peaks of the LLR have latencies of about 50 to 300 ms. Various peaks exhibit sex differences (e.g., [53]), and that is true in newborns as well as in adults [30].
All of the research described below was approved in advance by the relevant university committees on human or animal research.
Some Relevant Results
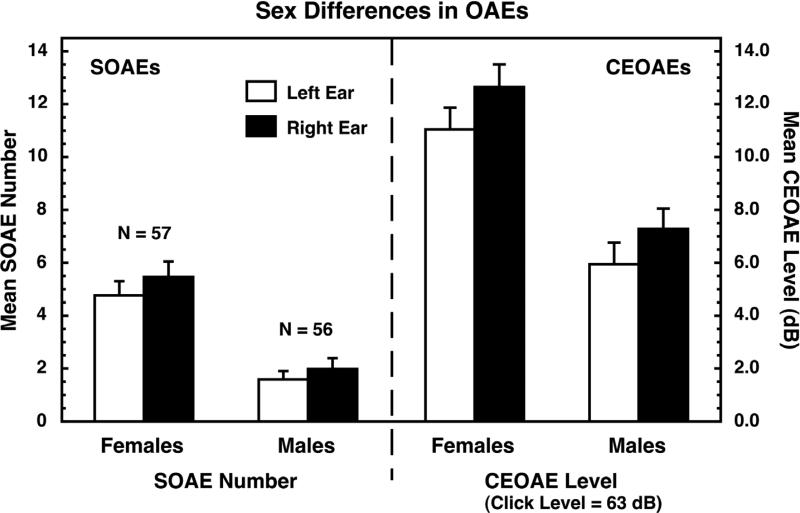
As noted, both SOAEs and CEOAEs exhibit moderately large sex and ear differences (e.g., e.g., [4]; reviewed in [50,51,52]), and the same patterns of differences exist in newborns as in adults (e.g., [12,13,72,73,84]). Namely, females have more (and stronger) SOAEs and stronger CEOAEs than males, and right ears generally have more SOAEs and stronger CEOAEs than left ears. Examples of human sex and ear differences are shown in Figure 1 (from [60,61]). The SOAEs and CEOAEs were measured in the same subjects, and the correlation between the number of SOAEs and the strength of the CEOAEs was 0.76 [61].
Figure 1.
Sex differences in SOAEs (left) and CEOAEs (right) measured in the same ears. Human females generally have more and stronger OAEs than males, and those differences are substantially larger than the differences between the ears. Similar differences have been reported for newborn infants. Data are from [60,61], and are reproduced with permission.
Sex differences often are expressed as effect sizes [16]. We calculate effect size as the difference between the means for females and males divided by the square root of the weighted mean of the variances for the two groups. By convention, effect sizes of 0.2, 0.5, and 0.8 are taken as small, medium, and large, respectively [16]. For the data shown in Figure 1, the effect sizes for sex differences in SOAEs were about 0.98, and those for CEOAEs were about 0.76, both relatively large effects.
So, why do OAEs differ between the sexes? The fact that the same patterns of sex and ear differences existing in the OAEs of young adults also exist in newborns [12,13,72,73,84] suggests that this sex difference is attributable to some process(es) occurring during prenatal development. Among the various possibilities is that the sex difference in OAEs is the result of the same basic events that are responsible for so many other sex differences in body, brain, and behavior: the degree of exposure to androgens prenatally. In all male mammals, including humans, the SRY gene becomes active early in prenatal development. Among the consequences of this activation is the development of embryoni c testes which begin producing androgens, including testosterone. These androgens are responsible for masculinizing the bodies, brains, and eventually the behaviors of males. In the absence of these androgens (as in females), prenatal development proceeds along an alternative path, and different bodies, brains, and behaviors are the result. Because no androgens are necessary for this alternative, female path of development, it long has been common to see the developmental path of females characterized as being the “default” condition in mammals. [Various recent discoveries reveal that the process of producing a female mammal is complex and not merely a matter of implementing a simpler archetype [1]; lacking a suitable synonym, I will acknowledge this important realization by using “default” in quotation marks here.]
In passing, note that if female is the “default” condition, then the “default” choice of sex partner is male; the typical female prefers male sex partners. The fact that the typical male has the opposite choice for sex partners (females) suggests that one of the changes accomplished in the developing male brain (presumably by the prenatal exposure to androgens) is to flip the switch for choice of sex partner from “default” to non-“default.” Under this simplistic view, nonheterosexual females are exhibiting the male-typical choice in sex partners (it is as if the switch was thrown in error) and nonheterosexual males are exhibiting the female-typical/“default” choice in sex partners (it is as if the switch failed to be thrown). Whether or not this view eventually proves to be an appropriate way of conceptualizing (some forms of?) nonheterosexuality, it does provide a simple framework for discussing the data of interest here. (As noted, the idea of a two-position switch for choice of sex partner clearly is too simplistic because, when it comes to sexual thoughts and behaviors, individual humans can fall almost anywhere along a continuum of categories between strictly heterosexual females to strictly heterosexual males – e.g., [39].)
Although sex differences in exposure to androgens is a reasonable explanation for both the sex differences in the positioning of the switch determining choice of sex partner and the sex differences in OAEs, there is an alternative explanation: One or both of these sex differences may be the result of direct effects of sex-chromosome genes themselves (not “just” sex-chromosome genes operating to control the degree of androgen exposure). The number of sex differences in mammals that can be linked solely to genes has been increasing recently (see [1]), so direct gene effects is a logical possibility. As will be seen below, however, experimental manipulations of hormones can affect OAEs and AEPs in various species in ways that lead me to conclude that, for the sex differences and other group differences in the auditory system, the correct explanation is more likely to be degree of hormone exposure than direct gene effects. Thus, the working hypothesis here is that the high concentrations of androgens experienced by male humans during prenatal development leads somehow to a weakening of their cochlear amplifiers, and thus to a weakening of their OAEs and a diminution of their hearing sensitivity [50,51,52]. Furthermore, that androgen exposure also is assumed to alter parts of the auditory brain, and as a consequence, sex differences in OAEs and AEPs exist at birth and persist through life. This collection of assumptions will be called the prenatal-androgen-exposure explanation for the sex differences in the auditory system. This explanation is noncommital on whether the differences in strength of the cochlear amplifiers are caused by genes, by other factors and mechanisms, or a combination of the two; however, the presumption is that, if genes are involved, they play their role indirectly via androgen exposure.
Note that, at birth, the androgen levels of male and female humans are essentially identical [82], so it is the long-term (organizational) effects of prenatal androgens that presumably are responsible for the sex differences in OAEs and AEPs in newborn infants [12,13,72,73,84], not differences in existing hormone levels (activational effects). Also note that the auditory sex differences in newborn humans exist before, or very early in, the so-called second surge of androgens that begins soon after birth in males and persists until about postnatal week 24 [82]. That is, the sex differences in human OAEs and AEPs are established prior to the second surge. In other species, some of the masculinization of the auditory system likely does occur after birth.
OAEs and sexual orientation
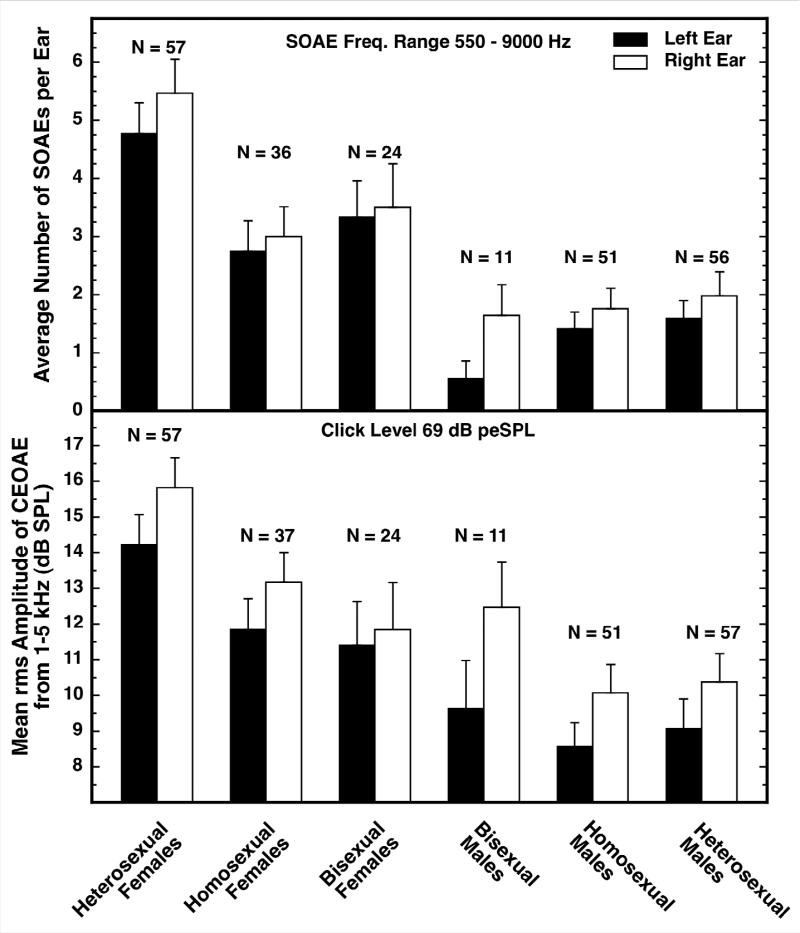
There are group differences in the OAEs of nonheterosexuals and heterosexuals [60,61]. The evidence is shown in Figure 2. Data for both SOAEs (top panel) and CEOAEs (bottom panel) are shown. At the far left are the data for heterosexual females, at the far right are the data for heterosexual males, and in the middle are the data for the nonheterosexuals. Homosexuals and bisexuals were not independently recruited; those categorizations resulted from detailed examination of the answers given to a collection of questionnaire items that included the two traditional Kinsey items on sexual fantasies and experience, plus additional items on past relationships and activities. Because most of these subjects were still college age, I suspect that some may have moved to a different category with age and experience, but I am confident that, as a group, the nonheterosexual subjects were different from the heterosexual subjects at the time our measurements were made.
Figure 2.
Number of SOAEs (top) and strength of CEOAEs (bottom) for people of differing sexual orientations. The data for the heterosexual females and males are shown at the far left and right, respectively, and the data for the various nonheterosexual groups are in between. The OAEs of homosexual and bisexual females are shifted toward those of heterosexual males (are masculinized), but the OAEs for nonheterosexual and heterosexual males are not different. The Ns for bisexual males are too small to permit credible conclusions. Based on Figure 3 in [50] and reproduced with kind permission of Springer Science and Business Media.
The differences between the black and white bars in Figure 2 reveal that the ear differences were much the same for all the subject groups: namely, right ears had more SOAEs and stronger CEOAEs than did left ears. Comparison of the bars at the far left and the far right illustrates again the basic sex difference in SOAEs and CEOAEs (these are the same data as shown in Figure 1). The new information contained in Figure 2 is that SOAE number and CEOAE strength were diminished in the homosexual and bisexual females; they were shifted in the direction of the males. Simply as a descriptive term, the OAEs of these females were masculinized. The effect sizes for the differences between heterosexual and nonheterosexual females were approximately 0.57 and 0.41 for SOAEs and CEOAEs, respectively. By contrast, the OAEs of the nonheterosexual males were not different from those of the heterosexual males.
How might this masculinization of the OAEs of nonheterosexual females be explained? One plausible possibility is that their cochlear amplifiers were weakened during prenatal development in much the same way they apparently are in normal males – by exposure to androgens. In this case, the origins of these anomalous androgens are unknown, but some suggestions are made below. Note that the directionality of this effect is the same as for the simplistic explanation of nonheterosexuality described above. Both th e weakening of the cochlear amplifiers and the throwing of the switch determining choice of sex partner from its “default” position appear to require exposure to androgens. In a recent review of the various theories of the origins of homosexuality, LeVay [37] also concluded that atypical exposure to prenatal androgens likely plays a crucial role. Note that the difference in androgen exposure (if that is the right explanation) had to be small; our nonheterosexual females are fully female, structurally and functionally. Indeed, one of the most fascinating characteristics of nonheterosexuality is how extremely subtle the differences are. As noted, there is some evidence that the FLRs of nonheterosexual females also are masculinized (e.g., [28,56,66]).
If there is any truth to this prenatal-androgen-exposure idea about the origins of nonheterosexuality in females, then we have the curious situation that the auditory system is, for some peculiar reason, sensitive to the same mechanisms that are involved in changing the “default” choice for sex partner. Why the auditory system should be sensitive to these mechanisms is not clear, but the existence of this sensitivity does provide us with what appears to be a valuable window on hormonal events occurring during prenatal development.
If the prenatal-androgen-exposure idea is correct, then why are the OAEs of nonheterosexual females affected but the OAEs of nonheterosexual males not different from those of heterosexual males? There are at least three possible reasons, and they are not mutually exclusive. One possibility is procedural; before making any OAE measurements, we used an audiometer to screen prospective subjects for hearing loss. If the cochlear amplifiers of nonheterosexual males are weaker than those of heterosexual males (hyper-masculinized), then their hearing sensitivity would be reduced, meaning that more nonheterosexual males than hetereosexual males may have failed the hearing screening test and been excluded from the study. As a consequence, the OAEs of those two groups did not differ even though they would have if more of the nonheterosexual applicants had been included. In our defense, if we had not used common audiometric standards to assure that only “normal-hearing” subjects were included in our study, our reports surely would not have been accepted for publication out of a concern that our heterosexual and nonheterosexual groups may have been noncomparable for hearing sensitivity. Hindsight is perfect; in retrospect, we could have obtained the audiometric measurements on all prospective subjects but not used those results for excluding subjects in advance, and then analyzed the OAE data both for all subjects and only for those subjects having “normal” hearing. Note that this possibility is contradicted by the fact that some AEPs of nonheterosexual males were different from those of heterosexual males (see below; also [53]), even though hearing screening was used in that study as well.
Another possible reason for the OAEs of nonheterosexual males not being different from those of heterosexual males is that the cochlea may be less sensitive to the processes responsible for nonheterosexuality in males than it is to the corresponding processes in females. For example, some of the masculinization of the structures and mechanisms underlying female nonheterosexuality may coincide temporally with the development of cochlear structures relevant to the production of OAEs, whereas in males the masculinization of sexuality may coincide less well with relevant cochlear development. In support of this possibility, aspects of the auditory brain (revealed by the AEPs; see below) are different in nonheterosexual and heterosexual males even though OAEs are not. That difference may be simply a matter of timing during development.
A third possible reason for the OAEs of nonheterosexual males not being different from those of heterosexual males is that the structures or mechanisms themselves underlying nonheterosexuality in females are fundamentally different from those in males. The ways heterosexual and nonheterosexual females differ behaviorally are not the same as the ways heterosexual and nonheterosexual males differ behaviorally (reviewed in [3]). For example, nonheterosexual females exceed nonheterosexual males in all categories of heterosexual experience; also, there are far more female than male bisexuals. Perhaps these behavioral differences reflect fundamental differences in the brain structures and mechanisms involved in nonheterosexuality in the two sexes that somehow protect the cochlea in males but not in females. As noted, these three possibilities are not mutually exclusive.
AEPs and sexual orientation
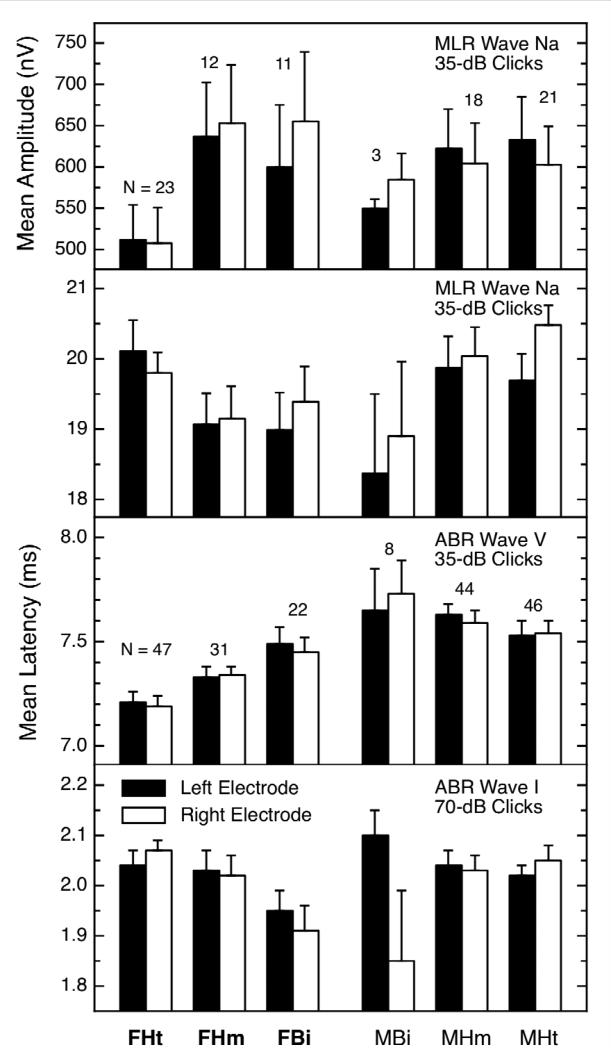
If the cochleas of nonheterosexual females are different from those of heterosexuals, what about the rest of the auditory brain? The answer is that some peaks of the AEP also are masculinized in nonheterosexual females [53]. The evidence is shown in the left half of Figure 3. The format of this figure is like that of Figure 2, with the data from the heterosexual females and heterosexual males being at the extreme left and right, respectively, and the data for the nonheterosexual subjects in between. Each panel contains the data for a different AEP measure; some are latencies and some are amplitudes. The ear differences (actually, side-of-head differences) are not as consistent as they were for OAEs, but that can be ignored for the moment. The important feature of Figure 3 is that, for each measure shown, the data for the nonheterosexual females are different from those for the heterosexual females. Only some of these measures showed a basic sex difference, but when one existed, the nonheterosexual females were shifted toward the heterosexual males; that is, they were masculinized, just as were their OAEs (Figure 2). The effect sizes for sexual orientation in females ranged between about 0.4 and 0.6.
Figure 3.
AEP measures showing differences by sexual orientation for females (F = female, M = male, Ht = heterosexual, Hm = homosexual, Bi = bisexual). The data for the heterosexual females and males are shown at the far left and right, respectively, and the data for the various nonheterosexual groups are in between. All females were non-users of systemic contraceptives. Note in the left half of the figure that, for every panel, the values for the nonheterosexual females were significantly different from those for the heterosexual females even though there were significant sex differences only in panels 1 and 3. In general, the AEPs of nonheterosexual females were masculinized. Figure from [53] and reproduced with permission.
The implication of the results shown in Figure 3 is that, not just the cochleas, but also parts of the auditory brain, are masculinized in nonheterosexual females. When in development this occurs is not yet known, but one possibility is that both the cochlea and the auditory brain were masculinized during prenatal development.
One obvious question about the results shown in Figure 3 is whether they represent “only” an obligatory perpetuation of the OAE results. That is, does the diminution of the strength of the cochlear amplifiers necessarily lead to the additional masculinizations in the AEP measures – a “pass-through” effect? One counterargument is that the very first measure in the AEP chain, Wave I of the ABR, did not show a difference for the nonheterosexual females. Another counterargument is that not all AEP measures showing a sex difference also showed a difference for the nonheterosexual females. Finally, the side-of-head differences for AEPs did not always favor the right ear, where OAEs were stronger.
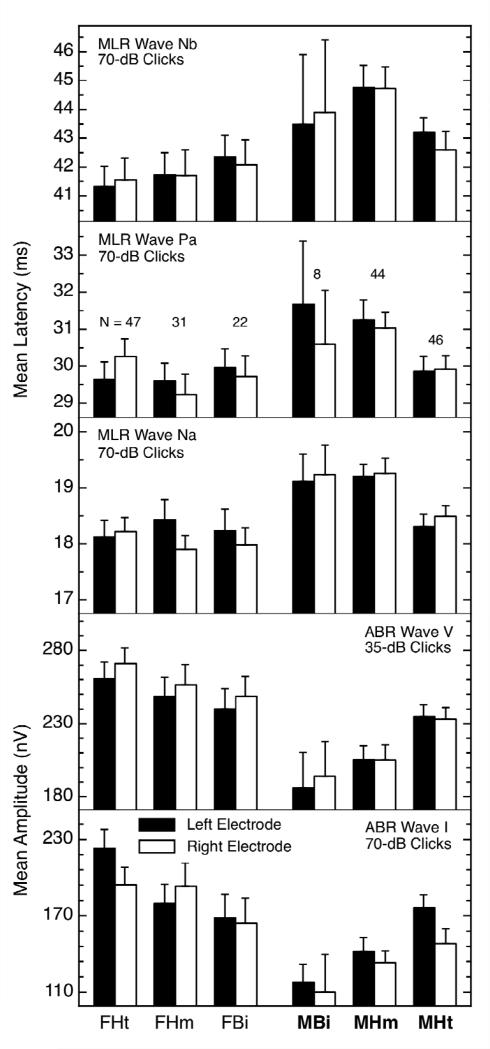
The AEPs for heterosexual and nonheterosexual males also were different for some measures, as the summary in Figure 4 reveals. As was true for the females, not all of the measures showing differences with orientation also showed a basic sex difference, but when the latter did exist, the values for the nonheterosexual males were shifted away from those for the heterosexual females. That is, the values for the nonheterosexual males were hyper-masculinized (other examples of hyper-masculinization in nonheterosexual males are discussed below). The effect sizes for sexual orientation in males ranged between about 0.4 to 0.6. The presence of AEP differences for males (Figure 4) in the absence of OAE differences for males (Figure 2) also suggests that the AEP differences are not simply “pass-through” effects.
Figure 4.
AEP measures showing differences by sexual orientation for males. Again, the data for the heterosexual females and males are shown at the far left and right, respectively, and the data for the various nonheterosexual groups are in between. The abbreviations are the same as for Figure 3. Note in the right half of the figure that, for every panel, the values for the nonheterosexual males were significantly different from those for the heterosexual males even though there were significant sex differences only in panels 1, 4, and 5. In general, the AEPs of nonheterosexual males were hyper-masculinized. Figure from [53] and reproduced with permission.
AEP interpeak intervals and sexual orientation
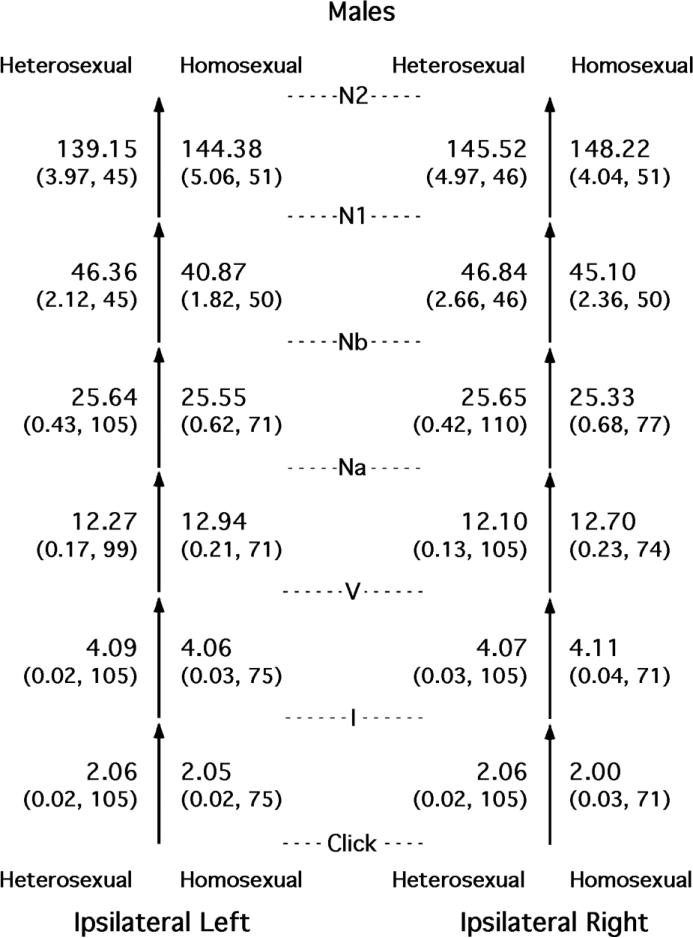
AEP waveforms are sometimes summarized by calculating the times between successive peaks, called interpeak intervals. Such intervals are used clinically because they can be longer than normal (prolonged) in some special populations [27]. Because we had collected ABRs, MLRs, and LLRs on the same subjects, we had the capability of calculating a succession of interpeak intervals and comparing them across sexual orientation (see [54]). The results for males are shown in Figure 5, where two sets of stacked arrows represent the succession of interpeak intervals for the two ears. The entries shown are for the peaks obtained from the electrodes on the same side of the head as the ear being stimulated acoustically, called ipsilateral left and ipsilateral right.
Figure 5.
Time intervals between successive peaks in the AEPs of heterosexual and nonheterosexual (homosexual plus bisexual) males. Shown for each interval for both groups and for both sides of the head are means and standard errors (both in milliseconds) and the Ns. Figure from [54] and reproduced with permission.
All of the differences between the heterosexual and nonheterosexual males in Figure 5 clearly are quite small, and the same was true for the corresponding comparisons for the females (see [54]). Effect sizes for sexual orientation are shown in Table 1 for all the relevant comparisons for both sexes. As a way to gain perspective on these differences, the data were resampled (see [54] for details), and those effect sizes that were rare occurrences are shown in bold font. Only one of the 12 comparisons made between heterosexual and nonheterosexual females was unlikely to be attributable to chance: the interval Click → I for the right side of the head. The mean duration of that interval for the nonheterosexual females was shifted away from the mean for the heterosexual males, so descriptively, it was a hyper-feminization. For the males, four of the 12 comparisons between heterosexuals and nonheterosexuals were unlikely to be attributable to chance. For the interval V → Na on both sides of the head, the mean intervals for the nonheterosexual males were shifted away from those for the heterosexual females, so descriptively, these were hyper-masculinizations. The other two rare intervals were hypo-masculinizations in the nonheterosexual males.
Table 1.
Effect sizes for sexual orientation (heterosexual minus nonheterosexual females or heterosexual minus nonheterosexual males).
| Females | Males | |||
|---|---|---|---|---|
| Side of Head | Side of Head | |||
| Interval | Left | Right | Left | Right |
| Click -> I | + 0.19 | + 0.36† | + 0.06 | + 0.32‡ |
| I -> V | - 0.09 | - 0.20 | + 0.10 | - 0.12 |
| V -> Na | - 0.20 | - 0.13 | - 0.38§ | - 0.37§ |
| Na -> Nb | + 0.32 | + 0.12 | + 0.02 | + 0.06 |
| Nb -> N1* | - 0.03 | + 0.15 | + 0.41‡ | + 0.10 |
| N1 -> N2* | + 0.07 | - 0.20 | - 0.16 | - 0.09 |
Bold font indicates that fewer than 5% of 20,000 resamples had an effect size whose absolute value was equal to or greater than the obtained effect size shown.
Measurements obtained in study 1 only.
Hyper-feminized
Hypo-masculinized
Hyper-masculinized
Note that the raw latencies themselves necessarily are less variable, and thus psychometrically preferable to, these inter-peak intervals. So these results provide only weak confirmation of the AEP differences already reported above [53]. However, the fluctuating directionality of the results provides additional examples of a phenomenon previously noted in research on sexual orientation: A mixture of hyper- and hypo-masculinizations in the same subjects. This mixture admittedly seems quite odd, but it is not unprecedented. Typically, when nonheterosexual males are found to be different from heterosexual males on some measure, the directionality of effect is a hypo-masculinization. That is, the measures for the nonheterosexual males are intermediate to those of heterosexual females and heterosexual males (e.g., [5,31,36,41,46,80,91]; and [76] review numerous examples). Less commonly, nonheterosexual males also have been reported to be hyper-masculinized: for example, on measures such as handedness and penis size (e.g., [6,35,95]) as well as the AEP measures shown in Figure 4 (from [53]). Outcomes of this sort are perplexing if nonheterosexuality is caused by an anomaly in the global exposure to androgens at some point early in development. If the circulating levels of androgens are anomalously and globally high or low, then all relevant parts of the brain and body seemingly should be affected similarly. So, perhaps global exposure is not the correct way to think about nonheterosexuality, and perhaps nonheterosexuality, at least in males, originates from mechanisms acting locally in the brain (see [50]), a topic that is discussed below.
Other Characteristics of OAEs
There are a number of characteristics of OAEs and AEPs that deserve consideration when evaluating the working hypothesis that early, perhaps prenatal, exposure to androgens can affect the cochlea and the auditory segments of the brain.
In humans, SOAE number and CEOAE strength are largely attributable to genes. Heritability (the proportion of phenotypic variance that is attributable to genetic differences among individuals) is about 0.75 for those two OAE measures [55,57]. That makes them less heritable than height and fingerprint characteristics, more heritable than psychological traits like honesty and religiousity, and about equally as heritable as adult IQ [68]. The implication is that something about the strength of the cochlear amplifiers is affected by the genes, and, as noted above, I presume that the degree of androgen exposure is the ultimate mechanism of implementation. Heritabilities for AEPs appear to be somewhat smaller than for OAEs (e.g., [85]).
The OAEs of females having male co-twins are masculinized; the number of their SOAEs and the strength of their CEOAEs are shifted towards those of males [47,57]. One interpretation is that the cochlear amplifiers of these opposite-sex dizygotic (OSDZ) females were weakened because they were exposed to higher-than-normal levels of androgens (for females) because of the simultaneous presence of a male in the womb. That is, perhaps some of the androgens produced by the male co-twin diffused into the intrauterine fluid and thus reached the OSDZ female, where they somehow weakened the cochlear amplifiers. While this may seem rather far-fetched upon first hearing, intrauterine effects of this sort are well-known in other mammals, where it is called the intrauterine-position phenomenon (see [14,78,88]. Dozens of physiological and behavioral characteristics can be masculinized in these species. In humans, OSDZ females also have been reported to have masculinized dentition and to be less prolific, among other differences (see [7,19,26,43,71], but not to be atypical on other likely characteristics [32,40,71]. Early reports suggested that finger-length ratios might differ between females from same-sex and opposite-sex twin pairs [87,89], but other investigators have found no difference [17,70]. Even so, masculinization of the female co-twin by the male co-twin is the simplest explanation of the weak OAEs in OSDZ females that I can think of, and it is a well-documented phenomenon in other mammals.
There is some evidence for activational as well as organizational effects of hormones on human OAEs and AEPs. In humans, AEPs are stronger during the midluteal phase of the menstrual cycle than during menses [22,23,24], although there is little or no change in OAEs across the cycle [29,94]. Second, both AEPs and OAEs are masculinized in women using oral contraceptives [49]; these effects were greater for AEPs than for OAEs, but were not large in either case. Although not tested directly, the presumption is that both measures would return to normal upon cessation of the drug. Third, OAEs gradually strengthened in an adult male taking high levels of estrogens to suppress his androgens prior to sex-change surgery [62].
There is some evidence that OAEs vary with ethnic background. Specifically, people with dark skin seem to have more SOAEs than people with light skin, and Asians seem to be intermediate to those two groups (reviewed in [48]. Previous reports had indicated that dark-skinned people also have better hearing sensitivity and are less susceptible to noise-induced hearing loss than fair-skinned people (reviewed in [67]), so it appears that melanin concentration is correlated with mechanisms that strengthen and/or protect the cochlear amplifiers. The absolute values of finger-length ratios also vary with skin color [45], so greatly in fact that tight control of ethnicity is required in FLR research. Typically, a sex difference does exist in the FLRs of other ethnic groups and is in the same direction as for Caucasians.
The CEOAEs of rhesus monkeys (Macaca mulatta) are like those in humans in that they are stronger in females than in males [63]. Interestingly, the magnitude of this sex difference fluctuates seasonally because the CEOAEs of the males are weaker during the breeding season (when male androgen levels are high) than during the birthing season (when male androgen levels are low). That is, both organizational and activational effects of hormones were seen in this species. The effect size for sex difference was about 1.2 during breeding season. In accord with the prenatal-androgen-exposure explanation, rhesus monkeys administered additional androgens late in prenatal development had weaker OAEs than those of untreated monkeys, and that was true of both sexes. The cochlear amplifiers seemingly were weakened by additional androgens, as the working hypothesis suggests. In addition, male monkeys administered an anti-androgenic agent (flutamide) during prenatal development had slightly stronger CEOAEs than untreated males, an outcome also in accord with the idea that androgens weaken the cochlear-amplifier system. (The anti-androgenic agent did not produce OAE differences in females, however.)
Sheep (Ovis aries) also exhibit a small sex difference in CEOAE strength that favors the females [64]. The effect size was about 0.4. Also, the CEOAEs of females administered androgens during prenatal development were weaker than those of untreated females, as is predicted by the prenatal-androgen-exposure explanation. However, the CEOAEs of androgen-treated males were not weakened (possibly due to a systemic reduction in androgen production that left the overall concentration near the normal range?).
The spotted hyena (Crocuta crocuta) is an interesting species because females normally are exposed to high levels of androgens prenatally, and as a consequence, the females are larger than the males, will dominate some males, and have an elaborated clitoris that is erectile and appears similar to a penis. In accord with the prenatal-androgen-exposure explanation, the CEOAEs of female spotted hyenas were not stronger than those of males [65]. Furthermore, hyenas administered flutamide (which blocks androgen receptors) and finasteride (which blocks the conversion of testosterone into its active metabolite dihydrotestosterone) exhibited stronger CEOAEs than untreated hyenas, suggesting that these agents did protect their recipients’ cochlear amplifiers from being fully weakened by their normally high prenatal androgen exposure. In both the rhesus and hyena colonies, there were animals that had experienced normal gestations but then were gonadectomized at varying times after birth, and in both species, their CEOAEs were similar in strength to those of untreated animals. This outcome confirms that it is the organizational effects of prenatal hormonal exposure that are most important to OAE expression in adults, not the currently circulating hormone levels.
This summary reveals that there are a number of lines of evidence supporting the prenatal-androgen-exposure explanation of the sex difference in OAEs and the masculinization of the OAEs in OSDZ females. Accordingly, it seems reasonable to assume that the differences in OAEs and AEPs observed in nonheterosexuals also exist because of atypical hormonal processes during prenatal development. No one yet knows if, at birth, the OAEs or AEPs of people who become nonheterosexual as adults are different from those of people who become heterosexual, but that implication exists in the data collected to date. The magnitudes of the group differences are small, the individual differences are large, and OAEs and AEPs can be similarly affected by other factors (such as minor hearing loss), so it is highly unlikely that OAEs ever could be used to predict sexual orientation later in life, and there is no obvious need for predictive measures of this sort. Nonetheless, there is a strong implication that the auditory systems of nonheterosexual humans are subtly different at birth, and thus, that the brain structure(s) responsible for sexual orientation likely are different at birth as well.
The Working Hypothesis
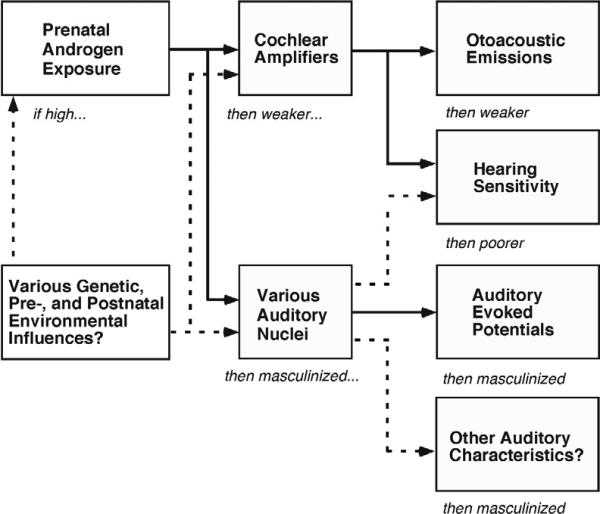
Figure 6 summarizes the prenatal-androgen-exposure explanation of sex differences, orientation differences, and OSDZ-twin differences in OAEs and AEPs. Beginning at the top left, high levels of prenatal androgens are presumed to weaken the cochlear amplifiers and also to alter auditory structures in the brain, leading to changes in OAEs, hearing sensitivity, AEPs, and perhaps other auditory characteristics. The dotted lines indicate additional possible factors in the overall process. Direct-gene effects [1,34] ultimately may be discovered for some of the group differences discussed here, but for the moment the most parsimonious explanation appears to be prenatal androgen exposure.
Figure 6.
A summary of the prenatal-androgen-exposure explanation for the basic sex difference in OAEs and AEPs and the reduction of OAEs in females from OSDZ twin pairs. Beginning at the top left, prenatal androgen exposure is presumed to affect the strength of the cochlear amplifiers, with the result that both hearing sensitivity and OAEs are affected. In addition, androgen exposure also apparently affects nuclei in the brain that are responsible for certain components of the AEP. The dotted lines indicate that there may be additional genetic and environmental influences affecting the various processes, mechanisms, and locations illustrated in the diagram. The box at the bottom right acknowledges that additional, as yet undiscovered, aspects of auditory function also might be affected by the androgen and other influences.
Prenatal timelines
In order to evaluate the plausibility of the prenatal-androgen-exposure explanation for various group differences in the auditory system, it is necessary to know something about the timing of both cochlear development and androgen exposure. In humans, a developing male fe tus begins to produce testosterone about week 8 of gestation, the testosterone levels peak about week 16, this first surge of testosterone ends about prenatal week 24, and the testosterone levels in males then become low and similar to the levels in females until birth (see [82]). At its peak, the plasma testosterone concentration in the male fetus is about that seen in adult males and is about 3 – 10 times higher than the level in the female fetus. The fetus is recognizable as male at about prenatal week 10, and the masculinization of the external genitalia appears complete between about weeks 16 and 20. As noted, there is a second surge of testosterone that begins soon after birth, that peaks about postnatal week 8, and is complete by about postnatal week 24. At its peak, the second surge in testosterone is about half the concentration reached in prenatal week 16.
Pujol and Lavigne-Rebillard [75] have summarized the prenatal development of the cochlea. The coiling of the human cochlea is complete by prenatal week 9, which is about the time testosterone production begins in male fetuses. The sensory surface on the basilar membrane remains undifferentiated until the sensory hair cells begin to differentiate between about prenatal weeks 11 and 12. There are two gradients of development for the hair cells: from base to apex of the cochlea, and from the single row of inner hair cells (IHCs) toward the three rows of outer hair cells (OHCs). The IHCs appear to mature somewhat faster than the OHCs, but both are beginning to receive afferent fibers between about prenatal weeks 12 and 14. Efferent fibers are beginning to appear near the bases of the OHCs by week 20, but mature efferent synapses are not present until about prenatal weeks 26 – 30 (by which time the testosterone levels in male fetuses have fallen to the levels in female fetuses). Prenatal week 30 is believed to mark the completion of cochlear maturation, although myelination of auditory nerve fibers continues for weeks after birth. The human cochlea is believed to begin functioning (i.e., sending afferent impulses to the brain) by about prenatal week 20, and cortical evoked potentials have been found in premature infants at prenatal week 25. Whitlon [92] provides details about the developmental processes in hair cells.
As noted, a number of facts suggest that the critical structures in the cochlea for OAEs are the OHCs. They are electromotile [10], which allows them to alter cochlear micromechanics, and they receive efferent synapses directly, meaning that their effects can be modulated by higher brain centers. A plausible hypothesis is that something about the OHCs is different in the two sexes because of the actions, direct or indirect, of androgens prenatally (other factors possibly contributing to the sex differences in OAEs and AEPs at birth were discussed in [52]. The two timelines above reveal no obvious conflict with such an idea. The androgen levels in human males are high during the time the OHCs are differentiating and receiving efferent contacts. Androgen receptors do exist in both IHCs and OHCs of adult mammals (B. Canlon, personal communication, 1 July 2009), and assuming that those receptors also are expressed during prenatal development, then the opportunity exists for an androgen-induced effect when and where it is needed to explain the relevant facts. [Estrogen receptors also exist in the cochlea [83], so, logically, the cochlear masculinization might be accomplished by estradiol aromatized from testosterone, but Wallen and Baum [90] argued that there is little evidence for estradiol being a masculinizing agent in humans.]
Localized and nonmonotonic effects
Now let us return to the topic of both hyper-masculinization and hypo-masculinization in the same nonheterosexual individuals. For the sake of argument, imagine that the differences exhibited in sex-related characteristics and traits by nonheterosexual males and females do not originate from global, relatively long-lasting differences in prenatal androgen levels, but rather from differences in androgen uptake or post-uptake androgen action in certain localized structure(s) during certain localized time(s) during prenatal development (see [50]). For example, this could occur if, for some reason, the number of active androgen receptors in these localized circuits was atypically large or small for a (perhaps short) period of time, and as a consequence, the androgen uptake by the relevant cells was atypical. If receptor anomalies of this sort were to occur at multiple localized regions of the brain, and at slightly different times in prenatal development, then it becomes easier to understand how some characteristics or traits could be hypo-masculinized and others hyper-masculinized in the same people; the androgen uptake (and masculinization) was anomalously low or anomalously high in different localized structures at critical times in development. Alternatively, one or more of the molecular aftereffects of androgen uptake – the stages leading to and involving transcription and translation -- might be atypical. This way, global androgen levels become subordinate to local events; two fetuses having exactly the same global androgen levels (as measured in the intrauterine fluid or in the mother's or fetus's blood supply or cerebrospinal fluid) could end up with differences in the brain that lead to differences in sexual orientation because of differences in localized androgen uptake or post-uptake androgen action. Woodson and Gorski [93] also argued for localized mechanisms for sexual differentiation, and [76] discussed localized effects in the context of sexual orientation.
Related to the idea of localized effects of hormones is the topic of nonmonotonic effects of hormones. (In this context, a nonmonotonic response is one in which some dependent variable changes regularly with increases in some independent variable up to some point but then, with further increases in the independent variable, the dependent variable reverses its direction of change.) In numerous animal studies, androgens have been administered to males either prenatally or perinatally and various dependent variables monitored. Often the additional androgens produce an additional masculinization on some measures, as would be expected, and sometimes there is no effect on some measures. More importantly, investigators occasionally report that some measures are less-than-fully masculinized, and this hypo-masculinization is seen in the same animals for which there is hyper-masculization (or no effect) on some other measures.
To summarize the examples given elsewhere [50]: female Mongolian gerbils injected with androgens shortly after birth exhibited the male-typical tripodal stance, as might be expected, but when male gerbils were similarly injected, they exhibited the female-typical stance [15], which is contrary to expectation. When male ferrets were given additional androgens early in development, testicular descent was incomplete and the number of intromissions was lower than in untreated males [2]. Male rats administered additional androgens perinatally were hyper-masculinized on some sexually dimorphic traits and hypo-masculinized on others [79]. Similar examples can be found in [20,69,74] (and [21] reported a nonmonotonic response to estrogen administration in females). In all of these cases, the hormone level presumably was higher than normal everywhere in the bodies and brains of the treated animals, yet some structures seemingly were affected differently from others. Apparently, localized effects can follow global exposures, at least in non-humans. These various reports suggest that some male brain structures can exhibit nonmonotonic responses to androgen dosage. The details of this mechanism are unclear, but the phenomenon appears real. Note that mixed outcomes of the sort described cannot be attributed solely to the well-known down-regulation of androgen production that can occur in the face of high androgen levels (e.g., [9]), because that is a global change, meaning that the directionality of effect ought to be the same for all measures, which is not the case.
For me, the concepts of localized effects and nonmonotonic effects are closely related. Both have the ability to explain the existence of hypo- and hyper-masculinization in nonheterosexual males, and in other special populations. Note that nonmonotonic effects is not a necessary mechanism for understanding these mixed outcomes because the mechanism of localized effects appears adequate for that. However, nonmonotonic responses to androgen concentration have been reported by more than one investigator, so the phenomenon belongs in our thinking and theorizing. Clearly, there is nothing to prevent both mechanisms from working together in certain circumstances. Investigators should be encouraged to watch for additional examples of nonmonotonic responses to hormones, and for evidence of localized effects of hormones. If the idea of localized effects proves to be incorrect, then we will need another explanation for how the same group of subjects can be hyper-masculinized on some measures, hypo-masculinized on others, and no different on yet others.
Additional special populations
There are additional special populations of humans that could be studied in order to advance knowledge about the prenatal effects of androgens on the auditory system. In congenital adrenal hyperplasia (CAH), the adrenal gland behaves atypically during prenatal development, with the result that the developing fetus exposes itself to abnormally high levels of androgens (e.g., [18]). For male fetuses, there are no obvious morphological consequences of this over-exposure; CAH males are not hypermasculinized, perhaps because of some negative feedback mechanism that reduces the production of testicular androgens (e.g., [9]). However, the bodies and behaviors of CAH females can be affected more or less greatly by this over-exposure to androgens. At birth, the genitals of CAH females can be partially masculinized, and later in life various masculine behaviors can be evident (e.g., [18]). The prediction from the prenatal-androgen-exposure hypothesis suggested above is that CAH females ought to have weakened cochlear amplifiers, and, thus, masculinized OAEs. To the extent the brain also is affected, CAH females also ought to have masculinized AEPs.
Some fetuses having a Y chromosome do begin to produce androgens early in prenatal development, but they have defective androgen receptors, and that prevents the androgen from doing its typical job of masculinizing the body and brain. This androgen-insensitivity syndrome (AIS) can be complete or partial; when complete, the person appears fully female at birth, is raised female, and typically is not recognized as atypical until puberty, when menstruation fails to begin. The clear prediction from the prenatal-androgen-exposure hypothesis is that AIS females ought to have OAEs and AEPs that are like those of normal females even though they are chromosomally male.
Another special population of interest in the current context is people diagnosed with autism spectrum disorders (ASD; [38]). ASD is far more common in males than in females and some of its signature features are evident beginning early in life. This pair of characteristics suggests that prenatal androgens may play a central role in the etiology of ASD, and if true, then the prediction again is straight-forward: The OAEs, and perhaps the AEPs, of males with ASD ought to be hyper-masculinized and those of females with ASD ought to be masculinized. Over the years, I have made numerous attempts to collaborate with experts on CAH, AIS, and ASD to obtain auditory measures from these special populations, but so far funding has been elusive.
Conclusions
A number of lines of circumstantial evidence suggest that the cochlear-amplifier system in mammals can be masculinized during prenatal development. A parsimonious conclusion to draw is that the relevant mechanism is androgen exposure. If that is the correct conclusion, then the basic sex differences in OAEs and AEPs (at birth and in young adults) are likely attributable to a difference in genes that in turn leads to the global difference in prenatal androgen exposure. Also, if that conclusion is correct, then it is likely that the OAE differences seen in nonheterosexual (and OSDZ) females are correlated effects of atypical androgen exposures; these atypical exposures could be either direct effects of some still-unknown genes or (more likely to me) some congenital mechanism operating to produce either a global or local over-exposure to androgens during some, perhaps critical, period in prenatal development. Note that if genes are involved, they need not be ones explicitly involved in normal sexual differentiation as long as they have the ability to affect androgen production, androgen uptake, or post-uptake androgen action in some way in some critical location(s) in the brain. Again, if that original conclusion is correct, the AEP differences seen in nonheterosexual females and males also are likely to be attributable to anomalous androgen exposure prenatally. Research on human special populations having CAH, AIS, and ASD would provide valuable additional information about the prenatal effects of androgens on the auditory system. The literatures on early androgen exposure in both humans and non-humans contain enough examples of apparently local and apparently nonmonotonic effects that these topics deserve greater consideration by the research community.
Acknowledgments
This invited article covers some of the same material covered in previous reviews [48,50,51,52]; the differences are organization, emphasis, and audience. This work was supported by a research grant awarded to DM by the National Institute on Deafness and other Communication Disorders (NIDCD; RO1 DC000153). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIDCD or the National Institutes of Health. E. Grenwelge helped with the references, and commented on a prior version; M.M. Maloney helped with the figures; J.C. Loehlin provided helpful comments and advice.
Abbreviations appearing in this article
- ABR
auditory brainstem response
- AEP
auditory evoked potential
- AIS
androgen insensitivity syndrome
- ASD
autism spectrum disorder
- CAH
congenital adrenal hyperplasia
- CEOAE
click-evoked otoacoustic emission
- dB SPL
decibels sound-pressure level
- DPOAE
distortion-product otoacoustic emissions
- FLR
finger-length ratio
- IHCs
inner hair cells
- IQ
intelligence quotient
- LLR
long-latency response
- MLR
middle-latency response
- OAE
otoacoustic emission
- OHCs
outer hair cells
- OSDZ
opposite-sex dizygotic twin
- rms
root-mean-square
- SOAE
spontaneous otoacoustic emission
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
**FOOTNOTE 1 -- Note that the existence of receptors for sex hormones in the cochleas of adult animals is not uninteresting—because of the activational effects on OAEs noted above—but for the prenatal-androgen-exposure explanation to be correct, those receptors would need to be expressed during those prenatal weeks when both OHCs are developing and androgen levels are high.
**FOOTNOTE 2 -- Previously, I suggested that localized anomalies in the rate of aromatization of testosterone into estradiol also might be a plausible mechanism for localized effects in the brain [50], but current thinking is that, in humans, estradiol is not the strong masculinizing agent it is in other mammalian brains [90].
**FOOTNOTE 3 – When several measures are shifted in the expected direction by an experimental manipulation and one or two are inexplicably shifted in the opposite direction, it is easy to interpret the latter as error variance, and to minimize or ignore them in published reports. This reaction has been called the file-drawer problem [77]. It is possible that additional examples of mixed outcomes will be reported for both animal and human research once experimenters realize that exceptions of this sort are precedented and have the potential to provide important insights.
Research Highlights For Sexual Orientation and the Auditory System By Dennis McFadden University of Texas, Austin
Invited Contribution to Frontiers in Neuroendocrinology, Special Issue on Sexual Orientation
- otoacoustic emissions (OAEs) and auditory evoked potentials (AEPs) are described
- both OAEs and AEPs exhibit differences depending upon sex and sexual orientation
- prenatal androgens appear to weaken the cochlear amplifiers
- prenatal hormone levels apparently can contribute to determining sexual orientation
- there may be localized and nonmonotonic effects of prenatal androgen exposure
References
- 1.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum MJ, Schretlen P. Neuroendocrine effects of perinatal androgenization in the male ferret. In: Gispen WH, Van Wimersma Greidanus TB, Bohus B, de Wied D, editors. Prog. Brain Res. Vol. 42. Elsevier; Amsterdam: 1975. pp. 343–355. [DOI] [PubMed] [Google Scholar]
- 3.Baumeister RF. Gender differences in erotic plasticity: The female sex drive as socially flexible and responsive. Psych. Bull. 2000;126:347–374. doi: 10.1037/0033-2909.126.3.347. [DOI] [PubMed] [Google Scholar]
- 4.Bilger R, Matthies ML, Hammel DR, Demorest ME. Genetic implications of gender differences in the prevalence of spontaneous otoacoustic emissions. J. Speech Lang. Hear. Res. 1990;33:418–432. doi: 10.1044/jshr.3303.418. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert AF, Blanchard R. Physical development and sexual orientation in men: Height, weight and age of puberty comparisons. Pers. Indiv. Diffs. 1996;21:77–84. [Google Scholar]
- 6.Bogaert AF, Hershberger S. The relation between sexual orientation and penile size. Arch. Sex. Behav. 1999;28:213–221. doi: 10.1023/a:1018780108597. [DOI] [PubMed] [Google Scholar]
- 7.Boklage CE. Interactions between opposite-sex dizygotic fetuses and the assumptions of Weinberg difference method epidemiology. Am. J. Hum. Genet. 1985;37:591–605. [PMC free article] [PubMed] [Google Scholar]
- 8.Breedlove SM. Organizational hypothesis: Instances of the fingerpost. Endocrinol. 2010;151:4116–4122. doi: 10.1210/en.2010-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown-Grant K, Fink G, Greig F, Murray MAF. Altered sexual development in male rats after oestrogen administration during the neonatal period. J. Reprod. Fert. 1975;44:25–42. doi: 10.1530/jrf.0.0440025. [DOI] [PubMed] [Google Scholar]
- 10.Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair-cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 11.Burns EM. Long-term stability of spontaneous otoacoustic emissions. J. Acoust. Soc. Am. 2009;125:3166–3176. doi: 10.1121/1.3097768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns EM, Arehart KH, Campbell SL. Prevalence of spontaneous otoacoustic emissions in neonates. J. Acoust. Soc. Am. 1992;91:1571–1575. doi: 10.1121/1.402438. [DOI] [PubMed] [Google Scholar]
- 13.Burns EM, Campbell SL, Arehart KH. Longitudinal measurements of spontaneous otoacoustic emissions in infants. J. Acoust. Soc. Am. 1994;95:385–394. doi: 10.1121/1.408330. [DOI] [PubMed] [Google Scholar]
- 14.Clark MM, Galef BG., Jr. Effects of intrauterine position on the behavior and genital morphology of litter-bearing rodents. Dev. Neuropsychol. 1998;14:197–211. [Google Scholar]
- 15.Clark MM, Robertson RK, Galef BG., Jr. Effects of perinatal testosterone on handedness of gerbils: Support for part of the Geschwind–Galaburda hypothesis. Behav. Neurosci. 1996;110:1–5. doi: 10.1037//0735-7044.110.2.413. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 17.Cohen-Bendahan CCC. Biological roots of sex differences: A longitudinal twin study. University Medical Center; Utrecht, The Netherlands: 2005. [Google Scholar]
- 18.Collaer ML, Hines M. Human behavioral sex differences: A role for gonadal hormones during early development? Psychol. Bull. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey PJ, Townsend GC, Richards LC. Increased tooth crown size in females with twin brothers: Evidence for hormonal diffusion between human twins in utero. Am. J. Hum. Biol. 1999;11:577–586. doi: 10.1002/(SICI)1520-6300(199909/10)11:5<577::AID-AJHB1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Diamond M, Llacuna A, Wong CL. Sex behavior after neonatal progesterone, testosterone, estrogen, or antiandrogens. Horm. Behav. 1973;4:73–88. [Google Scholar]
- 21.Dohler KD, Hancke JL, Srivastava SS, Hofmann C, Shryne JE, Gorski RA. Participation of estrogens in female sexual differentiation of the brain: Neuroanatomical, neuroendocrine and behavioural evidence. Prog. Brain Res. 1984;61:99–117. doi: 10.1016/S0079-6123(08)64430-1. [DOI] [PubMed] [Google Scholar]
- 22.Elkind-Hirsch KE, Stoner WR, Stach BA, Jerger JF. Estrogen influences auditory brainstem responses during the normal menstrual cycle. Hear. Res. 1992;60:143–148. doi: 10.1016/0378-5955(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 23.Elkind-Hirsch KE, Wallace E, Malinak LR, Jerger JJ. Sex hormones regulate ABR latency. Otolaryngol. Head Neck Surg. 1994;110:46–52. doi: 10.1177/019459989411000105. [DOI] [PubMed] [Google Scholar]
- 24.Elkind-Hirsch KE, Wallace E, Stach BA, Jerger JF. Cyclic steroid replacement alters auditory brainstem responses in young women with premature ovarian failure. Hear. Res. 1992;64:93–98. doi: 10.1016/0378-5955(92)90171-i. [DOI] [PubMed] [Google Scholar]
- 25.Galis F, Ten Brock CM, van Dongen S, Wijnaedts LC. Sexual dimorphism in the prenatal digit ratio (2D:4D) Arch. Sex Behav. 2010;39:57–62. doi: 10.1007/s10508-009-9485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glinianaia SV, Magnus P, Harris JR, Tambs K. Is there a consequence for fetal growth of having an unlike-sexed cohabitant in utero? Int. J. Epidemiol. 1998;27:657–659. doi: 10.1093/ije/27.4.657. [DOI] [PubMed] [Google Scholar]
- 27.Gorga MP, Kaminski JR, Beauchaine KA, Jesteadt W. Auditory brainstem responses to tone bursts in normally hearing subjects. J. Speech Lang. Hear. Res. 1988;31:87–97. doi: 10.1044/jshr.3101.87. [DOI] [PubMed] [Google Scholar]
- 28.Grimbos T, Dawood K, Burriss RP, Zucker KJ, Puts DA. Sexual orientation and the second to fourth finger length ratio: A meta-analysis in men and women. Behav. Neurosci. 2010;124:278–287. doi: 10.1037/a0018764. [DOI] [PubMed] [Google Scholar]
- 29.Haggerty HS, Lusted HS, Morton SC. Statistical quantification of 24-hour and monthly variabilities of spontaneous otoacoustic emission frequency in humans. Hear. Res. 1993;70:31–49. doi: 10.1016/0378-5955(93)90050-b. [DOI] [PubMed] [Google Scholar]
- 30.Hall JW., III . New Handbook of Auditory Evoked Responses. Pearson Education; Boston: 2007. [Google Scholar]
- 31.Hall JAY, Kimura D. Sexual orientation and performance on sexually dimorphic motor tasks. Arch. Sex. Behav. 1995;24:395–407. doi: 10.1007/BF01541855. [DOI] [PubMed] [Google Scholar]
- 32.Henderson BA, Berenbaum SA. Sex-typed play in opposite-sex twins. Dev. Psychobiol. 1997;31:115–123. [PubMed] [Google Scholar]
- 33.Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Brit. Med. Bull. 2002;63:223–241. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- 34.Kopsida E, Stergiakouli E, Lynn PM, Wilkinson LS, Davies W. The role of the Y chromosome in brain function. Open Neuroendocrinol. J. 2009;2:20–30. doi: 10.2174/1876528900902010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalumiere ML, Blanchard R, Zucker KJ. Sexual orientation and handedness in men and women: A meta-analysis. Psychol. Bull. 2000;126:575–592. doi: 10.1037/0033-2909.126.4.575. [DOI] [PubMed] [Google Scholar]
- 36.LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 37.LeVay S. Gay, Straight, and the Reason Why. The Science of Sexual Orientation; Oxford, New York: 2010. [Google Scholar]
- 38.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–38. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippa RA. The relation between sex drive and sexual attraction to men and women: A cross-national study of heterosexual, bisexual, and homosexual men and omen. Arch. Sex. Behav. 2007;36:209–222. doi: 10.1007/s10508-006-9146-z. [DOI] [PubMed] [Google Scholar]
- 40.Loehlin JC, Martin NG. A comparison of adult female twins from opposite-sex and same-sex pairs on variables related to reproduction. Behav. Genet. 1998;28:21–27. doi: 10.1023/a:1021452630561. [DOI] [PubMed] [Google Scholar]
- 41.Loehlin JC, McFadden D. Otoacoustic emissions, auditory evoked potentials, and traits related to sex and sexual orientation. Arch. Sex. Behav. 2003;32:115–127. doi: 10.1023/a:1022496207882. [DOI] [PubMed] [Google Scholar]
- 42.Lonsbury-Martin BL, Martin GK. Otoacoustic emissions: Basic studies in mammalian models. In: Manley GA, Popper AH, Fay RR, editors. Active Processes and Otoacoustic Emissions. Vol. 14. Springer Series in Auditory Research; Springer-Verlag, New York: 2008. pp. 261–303. [Google Scholar]
- 43.Lummaa V, Pettay JE, Russell AF. Male twins reduce fitness of female co-twins in humans. Proc. Natl. Acad. Sci. USA. 2007;104:10915–10920. doi: 10.1073/pnas.0605875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malas MA, Dogan S, Evcil EH, Desdicioglu K. Fetal development of the hand, digits and digit ratio (2D:4D) Early Hum Dev. 2006;82:469–475. doi: 10.1016/j.earlhumdev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Manning JT, Stewart A, Bundred PE, Trivers RL. Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Hum. Develop. 2004;80:161–168. doi: 10.1016/j.earlhumdev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 46.McCormick CM, Witelson SF. A cognitive profile of homosexual men compared to heterosexual men and women. Psychoneuroendocrinology. 1991;16:459–473. doi: 10.1016/0306-4530(91)90030-w. [DOI] [PubMed] [Google Scholar]
- 47.McFadden D. A masculinizing effect on the auditory systems of human females having male co-twins. Proc. Natl. Acad. Sci. USA. 1993;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McFadden D. Sex differences in the auditory system. Develop. Neuropsych. 1998;14:261–298. [Google Scholar]
- 49.McFadden D. Masculinizing effects on otoacoustic emissions and auditory evoked potentials in women using oral contraceptives. Hear. Res. 2000;142:23–33. doi: 10.1016/s0378-5955(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 50.McFadden D. Masculinization effects in the auditory system. Arch. Sex. Behav. 2002;31:93–105. doi: 10.1023/a:1014087319682. [DOI] [PubMed] [Google Scholar]
- 51.McFadden D. What do sex, twins, spotted hyenas, ADHD, and sexual orientation have in common? Persp. Psychol. Sci. 2008;3:309–323. doi: 10.1111/j.1745-6924.2008.00082.x. [DOI] [PubMed] [Google Scholar]
- 52.McFadden D. Masculinization of the mammalian cochlea. Hear. Res. 2009;252:37–48. doi: 10.1016/j.heares.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McFadden D, Champlin CA. Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. J. Assoc. Res. Otolaryngol. 2000;1:89–99. doi: 10.1007/s101620010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McFadden D, Hsieh MD, Garcia-Sierra A, Champlin CA. Differences by sex, ear, and sexual orientation in the time intervals be tween successive peaks in auditory evoked potentials. Hear. Res. 2010;270:56–64. doi: 10.1016/j.heares.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McFadden D, Loehlin JC. On the heritability of spontaneous otoacoustic emissions: A twins study. Hear. Res. 1995;85:181–198. doi: 10.1016/0378-5955(95)00045-6. [DOI] [PubMed] [Google Scholar]
- 56.McFadden D, Loehlin JC, Breedlove SM, Lippa RA, Manning JT, Rahman QA. A reanalysis of five studies on sexual orientation and the relative length of the 2nd and 4th fingers (the 2D:4D ratio) Arch. Sex. Behav. 2005;34:341–356. doi: 10.1007/s10508-005-3123-9. [DOI] [PubMed] [Google Scholar]
- 57.McFadden D, Loehlin JC, Pasanen EG. Additional findings on heritability and prenatal masculinization of cochlear mechanisms: Click-evoked otoacoustic emissions. Hear. Res. 1996;97:102–119. [PubMed] [Google Scholar]
- 58.McFadden D, Martin GK, Stagner BB, Maloney MM. Sex differences in distortion-product and transient-evoked otoacoustic emissions compared. J. Acoust. Soc. Am. 2009;125:239–246. doi: 10.1121/1.3037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McFadden D, Pasanen EG. Otoacoustic emissions and quinine sulfate. J. Acoust. Soc. Am. 1994;95:3460–3474. doi: 10.1121/1.410022. [DOI] [PubMed] [Google Scholar]
- 60.McFadden D, Pasanen EG. Comparison of the auditory systems of heterosexuals and homosexuals: Click-evoked otoacoustic emissions. Proc. Natl. Acad. Sci. USA. 1998;95:2709–2713. doi: 10.1073/pnas.95.5.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McFadden D, Pasanen EG. Spontaneous otoacoustic emissions in heterosexuals, homosexuals, and bisexuals. J. Acoust. Soc. Am. 1999;105:2403–2413. doi: 10.1121/1.426845. [DOI] [PubMed] [Google Scholar]
- 62.McFadden D, Pasanen EG, Callaway NL. Changes in otoacoustic emissions in a transsexual male during treatment with estrogen. J. Acoust. Soc. Am. 1998;104:1555–1558. doi: 10.1121/1.424366. [DOI] [PubMed] [Google Scholar]
- 63.McFadden D, Pasanen EG, Raper J, Lange HS, Wallen K. Sex differences in otoacoustic emissions measured in rhesus monkeys (Macaca mulatta) Horm. Behav. 2006;50:274–284. doi: 10.1016/j.yhbeh.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 64.McFadden D, Pasanen E, Valero MD, Roberts EK, Lee TM. Effect of prenatal androgens on click-evoked otoacoustic emissions in male and female sheep (Ovis aries) Horm. Behav. 2009;55:98–105. doi: 10.1016/j.yhbeh.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McFadden D, Pasanen EG, Weldele ML, Glickman SE, Place NJ. Masculinized otoacoustic emissions in female spotted hyenas (Crocuta crocuta) Horm. Behav. 2006;50:285–292. doi: 10.1016/j.yhbeh.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 66.McFadden D, Shubel E. Relative lengths of fingers and toes in human males and females. Horm. Behav. 2002;42:492–500. doi: 10.1006/hbeh.2002.1833. [DOI] [PubMed] [Google Scholar]
- 67.McFadden D, Wightman FL. Audition: some relations between normal and pathological hearing. Ann. Rev. Psych. 1983;34:95–128. doi: 10.1146/annurev.ps.34.020183.000523. [DOI] [PubMed] [Google Scholar]
- 68.McGue M, Bouchard TJ, Jr., Iacono WG, Lykken DT. Behavioral genetics of cognitive ability: A life-span perspective. In: Plomin R, McClearn GE, editors. Nature, Nurture, and Psychology. American Psychological Association; Washington DC: 1993. pp. 59–76. [Google Scholar]
- 69.McGuire LS, Ryan KO, Omenn GS. Congenital adrenal hyperplasia. II: Cognitive and behavioral studies. Behav. Genet. 1975;5:175–188. doi: 10.1007/BF01066810. [DOI] [PubMed] [Google Scholar]
- 70.Medland SE, Loehlin JC, Martin NG. No effects of prenatal hormone transfer on digit ratio in a large sample of same- and opposite-sex dizygotic twins. Pers. Indiv. Diff. 2008;44:1225–1234. [Google Scholar]
- 71.Miller EM. Prenatal sex hormone transfer: A reason to study opposite-sex twins. Pers. Indiv. Diff. 1994;17:511–529. [Google Scholar]
- 72.Morlet T, Lapillone A, Ferber C, Duclaux R, Sann L, Putet G, Salle B, Collet L. Spontaneous otoacoustic emissions in preterm neonates: Prevalence and gender effects. Hear. Res. 1995;90:44–54. doi: 10.1016/0378-5955(95)00144-4. [DOI] [PubMed] [Google Scholar]
- 73.Morlet T, Perrin E, Durrant JD, Lapillone A, Ferber C, Duclaux R, Putet G, Collet L. Development of cochlear active mechanisms in humans differs between gender. Neurosci. Lett. 1996;220:49–52. doi: 10.1016/s0304-3940(96)13226-2. [DOI] [PubMed] [Google Scholar]
- 74.Pollak EI, Sachs BD. Masculine sexual behavior and morphology: Paradoxical effects of perinatal androgen treatment in male and female rats. Behav. Biol. 1975;13:401–411. doi: 10.1016/s0091-6773(75)90949-9. [DOI] [PubMed] [Google Scholar]
- 75.Pujol R, Lavigne-Rebillard M. Development and plasticity of the human auditory system. In: Luxon LM, Martini A, Furman JM, Stephens DDG, editors. A Textbook of Audiological Medicine: Clinical Aspects of Hearing and Balance. Dunitz; London: 2003. pp. 147–156. [Google Scholar]
- 76.Rahman Q, Wilson GD. Born gay? The psychobiology of human sexual orientation. Pers. Indiv. Diff. 2003;24:1337–1382. [Google Scholar]
- 77.Rosenthal R. The file drawer problem and tolerance for null results. Psych. Bull. 1979;86:638–641. [Google Scholar]
- 78.Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci. Biobehav. Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 79.Sachs BD, Thomas DA. Differential effects of perinatal androgen treatment on sexually dimorphic characteristics in rats. Physiol. Behav. 1985;34:735–742. doi: 10.1016/0031-9384(85)90372-5. [DOI] [PubMed] [Google Scholar]
- 80.Scamvougeras A, Witelson SF, Bronskill M, Stanchev P, Black S, Cheung G, Steiner M, Buck B. Sexual orientation and anatomy of the corpus callosum [Abstract] Soc. Neurosci. 1994;20:1425. [Google Scholar]
- 81.Shera CA, Guinan JJ., Jr. Evoked otoacoustic emissioqns arise by two fundamentally different mechanisms; A taxonomy for mammalian OAEs. J. Acoust. Soc. Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- 82.Smail PJ, Reyes FI, Winter JSD, Faiman C. The fetal hormonal environment and its effect on the morphogenesis of the genital system. In: Kogan SJ, Hafez ESE, editors. Pediatric Andrology. Martinus Nijhoff; The Hague: 1981. pp. 9–19. [Google Scholar]
- 83.Stenberg AE, Wang H, Fish J, Schrott-Fischer A, Sahlin L, Hultcrantz M. Estrogen receptors in the normal adult and developing human inner ear and in Turner's syndrome. Hear. Res. 2001;157:87–92. doi: 10.1016/s0378-5955(01)00280-5. [DOI] [PubMed] [Google Scholar]
- 84.Strickland EA, Burns EM, Tubis A. Incidence of spontaneous otoacoustic emissions in children and infants. J. Acoust. Soc. Am. 1985;78:931–935. doi: 10.1121/1.392924. [DOI] [PubMed] [Google Scholar]
- 85.Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Dobie DJ, Freedman R, Green MF, Gur RE, Light GA, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Calkins ME. Abnormal auditory N100 amplitude: A heritable endophenotype in first-degree relatives of schizophrenia probands. Biol. Psychiatry. 2008;64:1051–1059. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valero MD, Pasanen EG, McFadden D, Ratnam R. Distortion product otoacoustic emissions in the common marmoset (Callithrix jacchus): Parameter optimization. Hear. Res. 2008;243:57–68. doi: 10.1016/j.heares.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Anders SM, Vernon PA, Wilbur CJ. Finger-length ratios show evidence of prenatal hormone-transfer between opposite-sex twins. Horm. Behav. 2006;49:315–319. doi: 10.1016/j.yhbeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 88.vom Saal FS. Sexual differentiation in litter-bearing mammals: Influence of sex of adjacent fetuses in utero. J. Anim. Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- 89.Voracek M, Dressler SG. Digit ratio (2D:4D) in twins: Heritability estimates and evidence for a masculinized trait expression in women from opposite-sex pairs. Psych. Reports. 2007;100:115–126. doi: 10.2466/pr0.100.1.115-126. [DOI] [PubMed] [Google Scholar]
- 90.Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: Comparative aspects of steroid hormone action. In: Pfaff D, Arnold A, Etgen A, Farhbach S, Rubin R, et al., editors. Hormones, Brain and Behavior. Academic Press; New York: 2002. pp. 385–423. [Google Scholar]
- 91.Wegesin DJ. Event-related potentials in homosexual and heterosexual men and women: Sex-dimorphic patterns in verbal asymmetries and mental rotation. Brain Cogn. 1998;36:73–92. doi: 10.1006/brcg.1997.0964. [DOI] [PubMed] [Google Scholar]
- 92.Whitlon DS. Cochlear development: Hair cells don their wigs and get wired. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:449–454. doi: 10.1097/01.moo.0000134451.07239.66. [DOI] [PubMed] [Google Scholar]
- 93.Woodson JC, Gorski RA. Structural sex differences in the mammalian brain: Reconsidering the male/female dichotomy. In: Matsumoto A, editor. Sexual differentiation of the brain. CRC Press; Boca Raton: 1999. pp. 229–255. [Google Scholar]
- 94.Yellin MW, Stillman RD. Otoacoustic emissions in normal-cycling females. J. Am. Acad. Audiol. 1999;10:400–408. [PubMed] [Google Scholar]
- 95.Zucker KJ, Beaulieu N, Bradley SJ, Grimshaw GM, Wilcox A. Handedness in boys with gender identity disorder. J. Child Psychol. Psychiatry. 2001;42:767–776. doi: 10.1111/1469-7610.00773. [DOI] [PubMed] [Google Scholar]