Abstract
In this report, we have tested the cytotoxicity of two organotin (OT) compounds by flow cytometry on a panel of immortalized cancer cell lines of human and murine origin. Although the OT compounds exhibited varying levels of cytotoxicity, diphenylmethyltin chloride was more toxic than 1,4-bis (diphenylchlorostannyl)p-xylene on all cell lines tested. The OT compounds were found to be highly cytotoxic to lymphoma cell lines with lower toxicity toward the HeLa cervical cancer cell line. In order to discern the mechanism by which cell death was induced, additional experiments were conducted to monitor characteristic changes consistent with apoptosis and/or necrosis. Cell lines treated with the experimental compounds indicated that there was no consistent mode of cell death induction. However, both compounds induced apoptosis in the pro-B lymphocyte cell line, NFS-70. The work presented here also demonstrates that the two OT compounds possess selective cytotoxicity against distinct transformed cell lines.
Keywords: Anticancer, Apoptosis, Cell death, Flow cytometry, Necrosis, Organometallic
Introduction
Organotin (OT) compounds are mostly known for their biocidal effects and have been used for multiple applications such as wood preservatives, acaricides, disinfectants, bactericides, fungicides, molluskicides, PVC stabilizers, and marine antifouling products (Piver 1973). Over 95% of the total worldwide production of OT compounds is consumed as PVC stabilizers, agricultural use, and industrial biocides (Baggenstoss 2004). The trialkyl organotins are mainly used as biocides while mono- and dialkyl organotin chlorides are the intermediates used in the production of PVC polymers and are also the major degradation products associated with PVC plastics (Fent 1996). In 1974, it was estimated that at least one third of human race had been in contact with PVC products on a daily basis (Levinson 1974). Butyltins have been found in >80% of human blood samples analyzed at concentrations ranging from 64 to 155 ng/ml (260 to 600 ng/g, on a dry-weight basis; Whalen et al. 1999). Due to their ample distribution and usages, OTs are continuously contaminating the soil, marine, and freshwater environments and also accumulate, which in turn causes toxicity to humans and non-human organisms (Baggenstoss 2004). Therefore, a number of concerns have been raised by the public and legislators regarding the utilization of OTs (Champ 2000). The usage of OTs as antifouling in boat paints has been banned in many nations, due to poisonous effects observed on snails (Bailey and Davies 1988) and oysters (Alzieu et al. 1986; Garg and Bhosle 2005). Human exposure to high doses of trimethyltin (TMT) provokes neurological detriment manifested by memory deficits, seizures, hearing loss, disorientation, and death (Brown et al. 1979; Feldman et al. 1993; Fortemps et al. 1978; Gui-bin et al. 2000; Ross et al. 1981). In rats, the behavioral profile of a single intravenous dose of TMT hydroxide resulted in tremor syndrome, increased reactivity, and hyperactivity (Moser 1996). Also in rats, di-n-butyltin dichloride (DBT) is known to induce pancreatitis (Merkord et al. 1997) and can provoke thymus atrophy and depletion of CD4+CD8+ thymocytes suggesting a selective antiproliferative activity (Pieters et al. 1994).
One of the chemotherapeutic drugs currently in use is the platinum-based compound, cisplatin, which was the first metal-containing anticancer drug but its use in patients can lead to severe side effects and this has spurred interest to identify safer metal-based compounds (Jamieson and Lippard 1999). Since platinum and tin atoms possess common chemical properties (Gielen 2002), tin complexes have been proposed to be potential therapeutic alternatives to cisplatin and similar anticancer agents. The antitumor activity of several tin-based compounds has been previously investigated (Gielen et al. 1995; Chojnacki 2003), and as a consequence, an interest in tin compounds has been sparked. A review encompassing 5 years of research, from 2003 to 2007, describes the antiproliferative properties of 195 organotin compounds against a variety of cell lines (Hadjikakou and Hadjiliadis 2009). The most potent of these organotin compounds against HeLa cells was triphenyltin 2-phenyl-1,2,3-triazole-4-carboxylate, which exhibited an IC50 of 0.00447 µg/ml and was more active than cisplatin (Tian et al. 2005). In a recent publication, exposure of colon cancer DLD-1 cells to triphenyltin (IV) chloride carboxylate complexes (containing SnPh3+ cations) was found to provoke accumulation in the number of sub-G1 peak cells (hypodiploid), caused DNA laddering (as a consequence of nuclear DNA degradation), and increased caspase activity (Kaluderovic et al. 2010). Taken together, these results provide clear evidence that triphenyltin(IV) complexes inflicted cell death on DLD-1 cells via apoptosis (Kaluderovic et al. 2010). Moreover, triphenyltin benzimidazolethiol copper chloride (TPT-CuCl2) has also been reported to induce apoptosis in HeLa cells (Hoti et al. 2004). By analyzing cells after exposure for 12 h to 6 µM of TPT-CuCl2, approximately 50% of the cells were shown to lose viability via trypan blue exclusion or flow cytometry (Hoti et al. 2004).
Even though OTs have been incriminated in important deleterious ecological effects, it is possible that by chemical modification an OT compound can be generated with low toxic side effects and higher antitumor activity. For this reason, more stable tin-based compounds with different ligands have been synthesized and are starting to be tested as potential cancer treatments. Most importantly, it has been demonstrated in mice that dimethyl tin 4-cyclohexyl thiosemicarbazone exhibits antitumor activity and has the capacity to activate lymphocytes and increase the immune response, without any significant toxicity (Sen and Chaudhuri 2009).
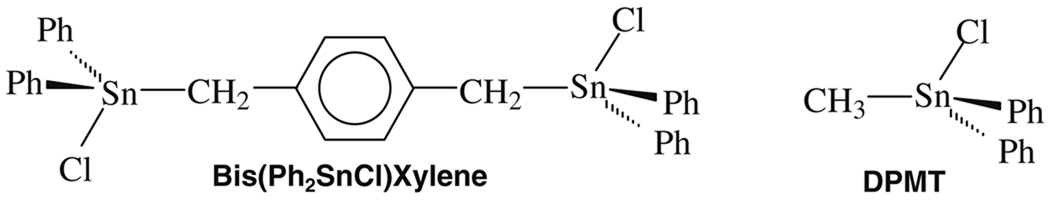
Two OT compounds were tested in this study for their capacity to cause cytotoxicity against cancer cell lines. 1,4-Bis-(diphenylchlorostannyl)xylene, also annotated as Bis(Ph2SnCl)Xylene, and diphenylmethyltin (DPMT) were chosen on the basis of a report many years ago that suggested that bis-organotins were extremely active compared to mono-organotins (Narayanan et al. 1990); however, little or no data were provided by the National Cancer Institute about their activity. Furthermore, it has been established that OT chlorides containing Sn-C(sp2) compared to Sn-C(sp3) bonds exert distinctive biological activity (Gomez et al. 2007); hence, we wished to maintain a similar set of such bonds in this study, i.e., two Sn-C (sp2) and one Sn-C(sp3).
Since each tumor cell type possesses a unique origin with specific genetic alterations (Park and Rich 2009), we have tested several tumor cell lines from both human and mouse origins to determine if there were common trends of cytotoxicity. In addition to the two species of origin (human and murine), the cells that were used in this study also represent different morphology, diverse form of growth (adherent and suspension), and tissue origin (lymphoid and cervical). Also by testing various cancer cell types, a compound could be detected that exhibits specific cytotoxicity against a particular cancer cell type but not others. An example of drug specificity is the platinum-based cisplatin derivative, SKI-2053R or heptaplatin, which exhibits cytotoxicity against several cisplatin-resistant cancer cell lines (Kim et al. 1995). Thus, indicating that some chemical compounds exhibit cytotoxic selectivity to affect cell viability. Furthermore, drug screening efforts have identified several compounds with cell type specificity (Ito et al. 2009; Yip et al. 2006).
In this study, we have employed flow cytometry to characterize the cytotoxic mechanisms and determine the CC50 values of cell death induced by OT compounds in human and mouse cell lines. Cytotoxicity was monitored using the DNA intercalating dye, propidium iodide (PI), which stains nuclear DNA once the cell becomes permeable (Jones and Senft 1985). To discriminate between necrotic and apoptotic modes of death, we subsequently used a well-established cytometry method that allows the detection of outer-membrane phosphatidylserine (PS), which is an early marker of apoptosis through the specific binding of PS by fluorescently labeled annexin V (Fadok et al. 1992). Our analysis revealed a complex pattern of selectivity for induction of apoptosis or necrosis by these compounds on various cell lines. The work presented here also demonstrates that the two OT compounds possess selective cytotoxicity against distinct transformed cell lines.
Material and methods
Organotins dilutions
The two organotin compounds studied were synthesized and purified according to a previously published procedure (for Bis(Ph2SnCl)Xylene, Thodupunoori et al. 2006; for DPMT, Kapoor et al. 2005). Both OTs were initially dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM. Stock solutions and their dilutions of experimental compounds were added directly to well containing cells in complete media and assessed in triplicate.
Cytotoxicity assay using cancer cells
Four immortalized cell lines from human and murine origin were used to perform cytotoxic assays. All cell lines were seeded at a density of 100,000 per well in 1 ml of culture media using a 24-well plate format. Cells were incubated overnight in the absence of compound, for optimal attachment for adherent lines and recovery from all the manipulation steps involved in preparing the experimental plate (trypsinization, centrifugation, etc.). HeLa human cervical cancer cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM), whereas NFS-70 and EL4 cells were cultured using RPMI media. Both DMEM and RPMI culture media were supplemented with antibiotics (100 U/ml of penicillin, 100 µg/ml of streptomycin, and 0.25 µg/ml of amphotericin B; Invitrogen, Carlsbad, CA, USA) and 10% heat-inactivated newborn calf serum (Hyclone, Logan, UT, USA). The human T cell chronic lymphocytic leukemia-derived, IL-2-dependent, Kit 225 cell line was initially established from a patient with leukemia (Hori et al. 1987) and was grown in RPMI supplemented with antibiotics, 10%fetal bovine serum, and 100 IU/ml recombinant IL-2 (Hoffmann-LaRoche, Nutley, NJ, USA). After addition of the chemical compounds, cells were incubated for 16 h and cytotoxic effects were monitored using flow cytometry as previously described (Elie et al. 2009). Prolonged incubation times result in significant cellular degradation that can generate large amount of debris which can introduce significant noise (excess cellular counts) when analyzed by flow cytometry. After compound treatment, detached HeLa were harvested in the culture supernatant and placed on ice. The remaining adherent cells were collected by using 300 µl of 0.25% trypsin solution (Invitrogen, Carlsbad, CA, USA), diluted in serum-free DMEM, and incubated for 15 min at 37°C. Cells from each individual well, including those harvested by trypsinization and the detached population, were mixed and centrifuged at 1,400 rpm for 5 min at 4°C. Collection of lymphoid tumor lines that grow in suspension, NFS-70, Kit 225, and EL4, involved a similar protocol with the exception of the trypsinization step (Shaik et al. 2009). After centrifugation, the supernatant media were removed by decantation, and the cell pellets were resuspended in 500 µl of staining solution, containing 2 µg/ml PI dissolved in FACS buffer (PBS, 0.5 mM EDTA, 2% heat-inactivated fetal bovine serum, and 0.1% sodium azide) and incubated in the dark at room temperature for 15 min. The stained cells were then analyzed by flow cytometry using Cytomic FC 500, and the data were acquired and analyzed using CXP software (Beckman Coulter, Miami, FL, USA). Approximately 10,000 events were acquired for each individual sample and the data analyzed using CXP software (Beckman Coulter). As a positive control for cytotoxicity, hydrogen peroxide (H2O2) was included at a concentration of 300 µM to provoke cell death (Miyoshi et al. 2006). Also, the OT diluent, DMSO, was tested at the same concentration (0.1%, v/v) as contained in the experimental samples, as a control for non-specific effects. The CC50 values were calculated as previously described (Elie et al. 2009). Briefly, the mean of three independent experiments of cytotoxicity annotated as a percentage was plotted alongside chemical compound concentration in a xy (scatter) chart function. The best fit regression line and its equation were employed to estimate the concentration of chemical compound necessary to disrupt the plasma membrane of 50% of the cell population, as compared to untreated cells, as previously described (Elie et al. 2009; see supplementary Figure S1). Although a range of compound concentrations was initially used to determine the nearest value to 50% cytotoxicity, the actual CC50 was determined from the two closest concentrations to the 50% mark to improve accuracy and diminishing abnormal distribution in dose–response curves. When high concentrations of OT were used, it was necessary to include both 5 and 10 µl of DMSO controls, to be used to normalize the data values, since DMSO itself could be toxic at these concentrations (0.5% and 1%, v/v).
Apoptosis/necrosis using cancer cells
All cell lines (ATCC, Manassas, VA, USA) were seeded, incubated, and collected as described above in “Cytotoxicity assay using cancer cells”, exposed to a range of concentrations of OT compound (10, 2, 0.4, 0.08, 0.016, and 0.032 µM), and incubated for 16 h at 37°C. After exposure of cells to the chemical compounds, all subsequent manipulations were performed on ice to slow down cell deterioration. Cells from each individual well were collected and washed as described above. The staining process was initiated by resuspending the cell pellet in 100 µl of binding buffer (10 mM HEPES, pH=7.4; 140 mM NaCl; 2.55 mM CaCl2) containing 1 µl of 25 µg/ml annexin V-FITC and 5 µl of 250 µg/ml PI (Beckman Coulter, Miami, FL, USA). After incubation for 15 min on ice in the dark, 400-µl ice-cold binding buffer was added to the stained cell suspensions, gently homogenized, and immediately analyzed by flow cytometry (Shaik et al. 2009). Around 10,000 events were collected for each individual sample and the data analyzed using CXP software (Beckman Coulter). The total percentage of apoptotic cells, reactive to annexin V-FITC, is expressed as the sum of both early and late stages of apoptosis. DMSO and H2O2 were used as controls (see supplementary Figures S2b, c). The H202 control was adjusted at final concentration of 300 µM and utilized to calibrate the flow cytometer acquisition protocol by allowing the detection of the four distinct cell populations in the treated samples (see Figure S2b).
Results and discussion
In this study, the cytotoxicity profile of Bis(Ph2SnCl) Xylene and DPMT (Fig. 1) was tested on several immortalized cell lines by flow cytometry. In initial tests, cell cytotoxicity was measured by PI staining after treatment with the OTs. As summarized in Table 1, Bis(Ph2SnCl)Xylene was significantly more toxic to EL4 (CC50=5.4) and NFS-70 (CC50=3.2) murine cell lines than to Kit 225 (CC50=29.9) and HeLa human cell lines (CC50=58.65). On the other hand, DPMT exhibited high toxicity against EL4 (CC50=2.4), NFS-70 (CC50=1.2), and Kit 225 (CC50=1.5) lymphocyte cell lines but was much less toxic against HeLa cell (CC50=32.4). On the basis of the CC50 measurements, DPMT was more toxic against all cells tested than Bis(Ph2SnCl)Xylene. The latter was significantly more toxic for cells from mouse origin (EL4 and NFS-70) than for human cell lines (Kit 225 and HeLa). In general, the OT compounds exhibited a comparable potent cytotoxic activity against murine cell lines with CC50 values in the range 1.5–5.38 µM. In addition, HeLa cells were the most resistant cells to both OT compounds (Table 1). The CC50 of Bis(Ph2SnCl)Xylene and DPMT against HeLa cells was 58.65 and 32.35 µM, respectively. Interestingly, in recent work, we have shown that the CC50 of cisplatin (14.9 µM) on HeLa cells (Shaik et al. 2009) was much lower that the two OT compounds tested in this study. Additionally, when HeLa cells were individually treated with seven different di- and triphenyltin(IV) complexes, containing carboxylate ligands, the IC50 values were from 0.15 to 1.57 µM (Gómez-Ruiz et al. 2008). Those OT complexes were significantly more effective in killing HeLa cells as compared to the compounds studied here. However, in those experiments, the cytotoxicity was measured using the MTT method after incubating the cells for 72 h with the test compound (Gómez-Ruiz et al. 2008) and therefore not directly comparable to our results.
Fig. 1.
1,4-Bis-(diphenylchlorostannyl) xylene, also annotated as Bis(Ph2SnCl) Xylene, and diphenylmethyltin chloride, also referred as DPMT
Table 1.
CC50 values of organotin compounds against human and murine cell lines CC50 (micromolars)
| Cell line | Bis(Ph2SnCl)Xylene | DPMT |
|---|---|---|
| EL4 | 5.38a | 2.41 |
| NFS-70 | 3.17 | 1.15 |
| Kit-225 | 29.85 | 1.5 |
| HeLa | 58.65 | 32.35 |
CC50 in micromolars is defined as the concentration of compound required to disrupt the plasma membrane of 50% of cell population compared to untreated cells after 16 h of incubation. Cells with compromised plasma membrane were monitored using PI staining and flow cytometry
Adherent cells were seeded 16–18 h prior to exposure, and it is therefore possible that they have increased in cell number, which could affect the CC50 determination when compared to other cell types. However, it has been previously reported that the doubling time of these cells is between 17 (Tamm et al. 1982) and 20 h (Ngan et al. 2001), which would mean that upon addition of chemical compound, the cells could not have yet divided and that death induction would have interfered with cell division. Furthermore, addition of the same amount of experimental compound to double the number of cells leads to minor changes in CC50 values (our unpublished observations). The majority of the non-adherent lymphocyte cell lines utilized in this study have a similar doubling time as that of HeLa (18–24 h), and thus, it is likely that these cells were tested at the original seeded concentration.
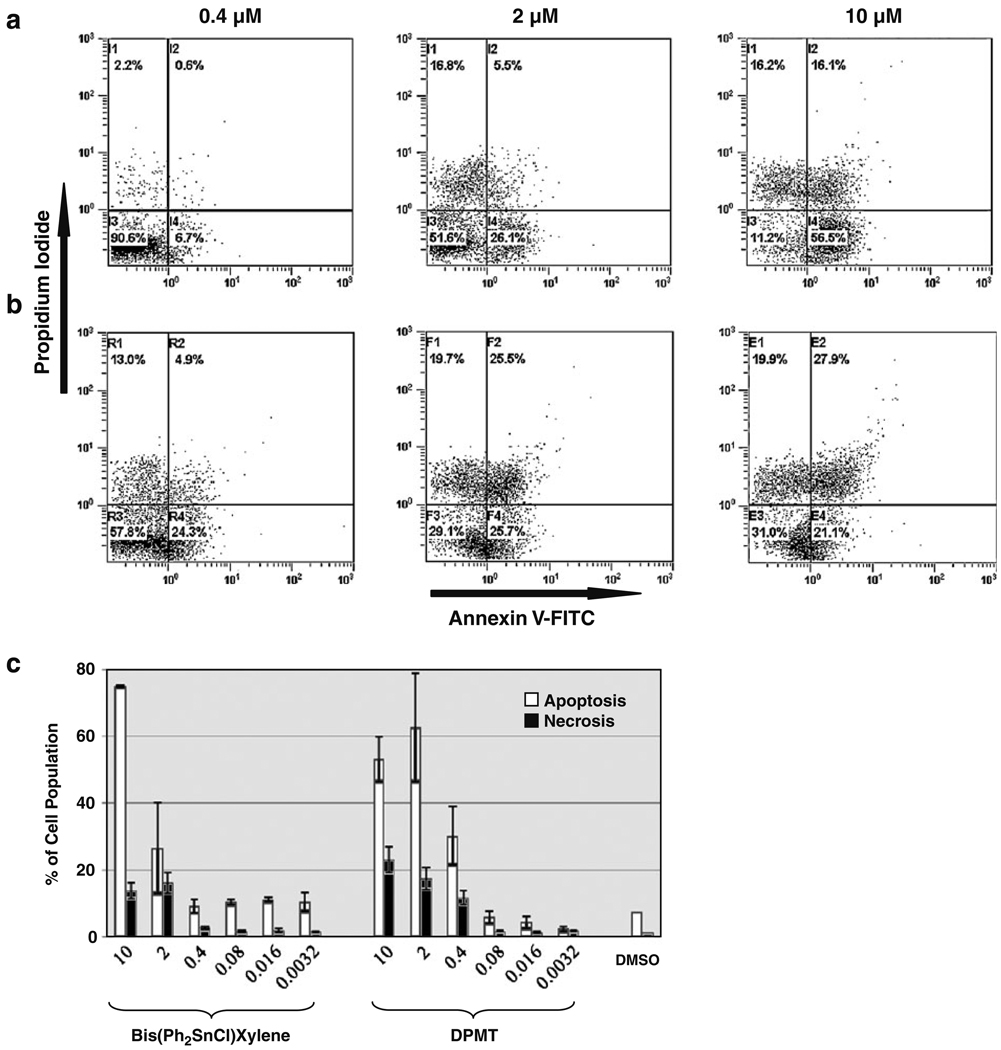
In order to determine if the OT compounds stimulated apoptotic or necrotic modes of death, we performed flow cytometry using the combination of PI and annexin V-FITC (Shaik et al. 2009). As can be seen in Fig. 2, this analysis revealed that these compounds primarily generate cell death via apoptosis on the murine pro-B lymphocyte cell line, NFS-70. Both OT compounds were tested at three concentrations, and both were found to provoke significant apoptosis on NFS-70 cells at the highest concentration tested. Bis (Ph2SnCl)Xylene was less cytotoxic against NFS-70 than DPMT, and this is particularly evident at the lowest concentration of 0.4 µM (see Fig. 2a, c). At this concentration, cell death promoted by Bis(Ph2SnCl) Xylene was negligible and resembled the results obtained with media or media plus solvent (Fig. 2c) controls. However, at the highest concentration used (10 µM), 72.6% of the cells died primarily via apoptosis (sum of early and late apoptosis) with 16.2% of the cells dying via necrosis (upper left hand quadrant; see Fig. 2a). The more cytotoxic OT compound, DPMT, produced a significant amount (42.2%) of cell death at the lowest concentration tested in this assay and showed the highest level of necrosis generated by these compounds on the NFS-70 cell line (>19% at higher concentrations). Unexpectedly, the DPMT compound induced apoptosis on a high percentage of cells (~50%) at the highest concentrations used (2 and 10 µM; see Fig. 2c). Taken together, these data demonstrate that both OTs primarily induce apoptosis in the pro-B lymphoid cells.
Fig. 2.
Cytotoxic effects of OTs on a pro-B lymphocyte cell line detected by flow cytometry. Representative flow cytometric histograms of the cytotoxic effects, apoptosis/necrosis, caused by Bis(Ph2SnCl)Xylene and DPMT on the murine NFS-70 pro-B cell lymphocyte cell line. a, b The effects of increasing concentrations of Bis(Ph2SnCl)Xylene and DPMT indicated on top of each histogram, respectively. Approximately 10,000 events were acquired and analyzed for each individual sample using CXP software (Beckman Coulter). c A graph of apoptosis and/or necrosis effects of OT compounds on NFS-70 cells. Cells were simultaneously stained with annexin V-FITC and PI, and the total percentage of apoptotic cells is expressed as the sum of both early and late stages of apoptosis (white bars). Cells permeable to PI due to the loss of plasma membrane integrity but without annexin V-FITC signal were considered as percentage of necrotic cells (black bars). The concentrations in micromolars of OTs used in these experiments are annotated below the X-axis. DMSO was added to final concentration of 0.1% (v/v). Each bar represents the average value of three measurements, and error bars represent standard deviations of the mean
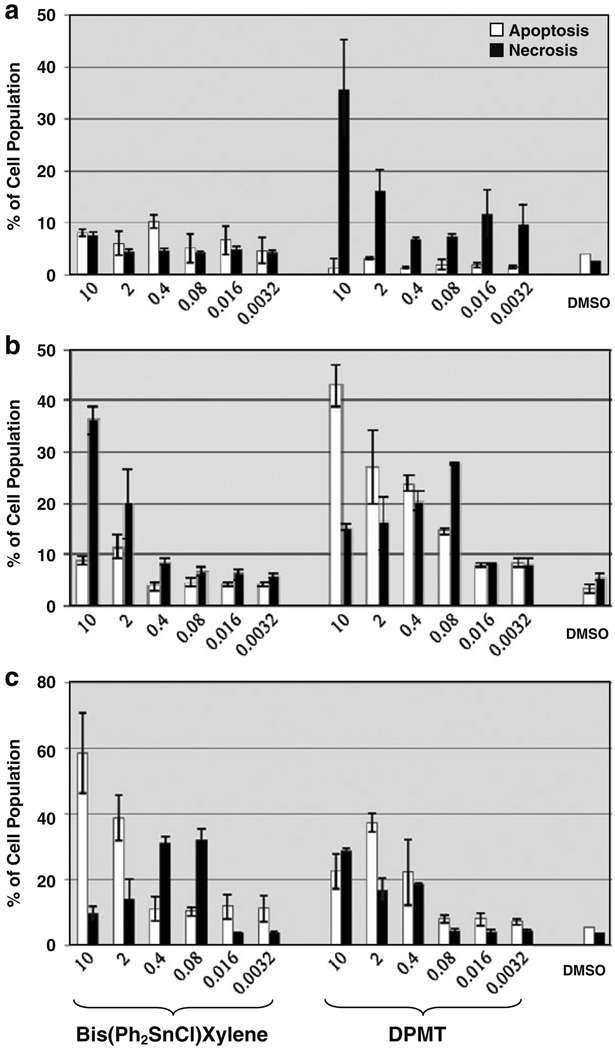
Further tests were conducted on additional human and mouse cell lines to determine how the death signal was transmitted by the OT compounds. As can be seen in Fig. 3a, treatment of HeLa with Bis(Ph2SnCl)Xylene did not result in a significant level of cell death, but treatment of these cells with DPMT resulted in a high percentage of necrosis (>40%) at the highest concentration tested and little induction of apoptosis at any concentration. Interestingly, a high level of necrosis was also detected when the human Kit 225 T cell line was treated with Bis(Ph2SnCl)Xylene (>35% at 10 µM; Fig. 3b). However, when these cells were treated with DPMT, the mode of death induced was mostly through apoptosis (>40% at 10 µM). In the present study, both OT compounds revealed much higher CC50 than TPT-CuCl2 (6 µM) against HeLa cells (Hoti et al. 2004). This difference in cytotoxicity between the two OT compounds could be attributed to the presence of CuCl2 in the TPT-CuCl2 molecule. The CuCl2 could potentiate the organotin-mediated cytotoxicity since this molecule possesses well-known biocidal properties (Gant et al. 2007). However, due to the different time of induction used in the previous report (12 vs. 16 h), a true comparison of the CC50 of these distinct compounds could not be made accurately.
Fig. 3.
Cytotoxic effects of the OT compounds on various cell lines. The following cell lines were analyzed by flow cytometry for cytotoxicity: a HeLa cervical carcinoma, b Kit 225 human T-lymphoma, and c EL4 murine T lymphoblastic cell lines. Cells were simultaneously stained with annexin V-FITC and PI (see Fig. 2 legend for details). DMSO was added to final concentration of 0.1% (v/v). Each bar represent average value of three measurements, and error bars represent standard deviations. The units corresponding to the concentration of OTs annotated below the X-axis are in micromolars
It has been previously reported that exposure to TMT for 24 h results in either apoptosis at low concentrations (0.01–0.1 µM) or to necrosis at high concentrations (10 µM) in primary rat cerebellar cells (Gunasekar et al. 2001). In contrast, apoptosis was observed at high concentrations (2 and 10 µM) of Bis(Ph2SnCl)Xylene in the EL4 cell line, and necrosis was detected at lower concentrations (0.08 and 0.4 µM; Fig. 3c). After testing hundreds of diverse compounds, this is the first time that a situation has been encountered in which a compound causes necrosis at a low concentration and apoptosis at a high concentration. In general, once a compound causes severe necrotic cell damage, it will continue to do so at higher concentrations. This is a perplexing situation that will merit further in depth analyses (caspase activation, analysis of mitochondrial involvement, and other more sophisticated assays). DPMT preferentially induced apoptosis in NFS-70 (Fig. 2b, c) and Kit 225 but only at the highest concentration tested (10 µM; Fig. 3b), caused necrosis in HeLa cells (Fig. 3a), and at experimental concentration tested here had no discernable mode of death induction on EL4 cells (Fig. 3c). On the other hand, when Bis(Ph2SnCl)Xylene was tested at 10 µM, it elicited apoptosis in NFS-70 (Fig. 2a, c) and EL4 cells (Fig. 3c), necrosis in Kit 225 cell line (Fig. 3b), and low levels of both necrosis and apoptosis in the HeLa cell line (Fig. 3a). Why there is so much variability in the death induction process with these compounds is currently unknown, but likely due to the altered properties of each transformed line (i.e., inherent levels of pro-apoptotic or survival proteins/signals). Additional cell lines will have to be tested in the future to determine if this unexpected pattern of death induction can be reproduced in other cell types.
Several previous reports have demonstrated that some OT compounds have the capacity to inflict cell death via apoptosis. Tributyltin (TBT) has been shown to promote apoptosis on normal rat thymocytes, while DBT caused death via necrosis against the same type of cells (Tomiyama et al. 2009). However, the result of DBT is controversial, since it was previously reported that this compound caused apoptosis, instead of necrosis on normal rat thymocytes (Gennari et al. 2000). Interestingly, the death signal originated by Bis(Ph2SnCl)Xylene in NFS-70 pro-B and EL4 murine T lymphoid cells resembled the apoptosis promoted by TBT on rat thymocytes (Tomiyama et al. 2009).
More recently, three new OT compounds were tested on human (A549 lung adenocarcinoma) and murine (L929 fibroblast) cell lines. Treatment with these compounds resulted in ID50 values between 1.72 and 2.51 µg/ml against both cell lines (Dylag et al. 2010). Interestingly, these novel OT compounds exhibited a comparable potent cytotoxic activity to those presented here against lymphoid cell lines.
Due to their toxicity, the use of OT complexes has led to increasing preoccupation about their biotoxic potential. However, there is the possibility that novel organometallic compounds can be generated with more restricted cytotoxic effects with antineoplastic activity and tumor selectivity, but with no or low effects on normal cells. Out of the two OT compounds studied in this report, neither induced cell death in HeLa cells via apoptosis (Fig. 3a). Both OT compounds clearly inflicted efficient apoptosis against pro-B cell line, suggesting that the mode of death induced by these compounds had selectivity. Future analyses of these compounds will have to be performed on additional murine and human pro-B cell lines to determine if this selectivity is retained across species, which could lead to the discovery of novel anti-B cell lymphoma agents.
Supplementary Material
Acknowledgments
The authors thank Dr. S. Nagy for the generous gift of the Kit 225 cell line and to the staff of the UTEP’s Cell Culture and High Throughput Screening Core Facility for services and facilities provided. This work was supported by grant 5G12RR008124 to the Border Biomedical Research Center, granted to the University of Texas at El Paso from the National Center for Research Resources of the NIH. Y.P.C. was supported by the NIH-MARC*USTAR (5T34GM008048-25) and RISE (R25 GM069621-07) programs. Also, the authors thank Drs. Julia Bader for statistic analysis and Carolina Lema for critically reading the manuscript and help with organizing the bibliography.
Abbreviations
- ATCC
American Type Culture Collection
- Bis(Ph2SnCl) Xylene
1,4-Bis (diphenylchlorostannyl)p-xylene
- CC50
Concentration that results in 50% cytotoxicity
- DBT
Di-n-butyltin chloride
- DMEM
Dulbecco’s modified Eagle medium
- DMSO
Dimethyl sulfoxide
- DPMT
Diphenylmethyltin chloride
- EDTA
Ethylenediaminetetraacetic acid
- FACS
Fluorescence-activated cell sorter
- FITC
Fluorescein isothiocyanate
- H2O2
Hydrogen peroxide
- ID50
The 50% inhibitory dose
- IL-2
Interleukin-2
- NK
Natural killer
- OT
Organotin
- PBS
Phosphate buffered saline
- PI
Propidium iodide
- PS
Phosphatidylserine
- PVC
Polyvinyl chloride
- ROS
Reactive oxygen species
- RPMI
Roswell Park Memorial Institute
- TBT
Tri-n-butyltin chloride
- TMT
Trimethyltin
- TPT
Triphenyltin
- TPT-CuCl2
Triphenyltin benzimidazolethiol copper chloride
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10565-010-9178-y) contains supplementary material, which is available to authorized users.
Contributor Information
Armando Varela-Ramirez, Email: avarela2@utep.edu, Department of Biological Sciences, Biosciences Research Building, University of Texas at El Paso, 500 West University Ave., El Paso, TX 79968-5816, USA.
Margaret Costanzo, Department of Biological Sciences, Biosciences Research Building, University of Texas at El Paso, 500 West University Ave., El Paso, TX 79968-5816, USA.
Yazmin P. Carrasco, Chemistry Department, University of Texas at El Paso, 500 West University Ave., El Paso, TX 79902-5816, USA
Keith H. Pannell, Chemistry Department, University of Texas at El Paso, 500 West University Ave., El Paso, TX 79902-5816, USA
Renato J. Aguilera, Department of Biological Sciences, Biosciences Research Building, University of Texas at El Paso, 500 West University Ave., El Paso, TX 79968-5816, USA
References
- Alzieu C, Sanjuan J, Deltreil P, Borel M. Tin contamination in Arcachon Bay: effects on oyster shell anomalies. Mar Pollut Bull. 1986;17:494. [Google Scholar]
- Baggenstoss J. Ecole Polytechnique Federale de Lausanne (EPFL) Switzerland: Lausanne; 2004. [Accessed 12 Mar 2010]. The fate of organotin compounds in a waste water treatment plant. Faculte de l’Environnement Naturel, Architectural et Construit (ENAC); Section Sciences et Ingenierie de l’Environnement (SIE); Laboratoire de chimie environnementale et ecotoxicologie (CECOTOX) http://www.sea.eawag.ch/inhalt/sites/projekte/pdf/Diploma_Thesis_JB_organotin.pdf. [Google Scholar]
- Bailey SK, Davies IM. Tributyltin contamination in the Firth of Forth (1975–87) Sci Total Environ. 1988;76:185–192. doi: 10.1016/0048-9697(88)90106-4. [DOI] [PubMed] [Google Scholar]
- Brown AW, Aldridge WN, Street BW, Verschoyle RD. The behavioral and neuropathologic sequelae of intoxication by trimethyltin compounds in the rat. Am J Pathol. 1979;97:59–82. [PMC free article] [PubMed] [Google Scholar]
- Champ MA. A review of organotin regulatory strategies, pending actions, related costs and benefits. Sci Total Environ. 2000;258:21–71. doi: 10.1016/s0048-9697(00)00506-4. [DOI] [PubMed] [Google Scholar]
- Chojnacki H. Quantum chemical studies on newly synthesized tin anticancer compounds. J Mol Struct. 2003;630:291–295. [Google Scholar]
- Dylag M, Pruchnik H, Pruchnik F, Majkowska-Skrobek G, Ulaszewski S. Antifungal activity of organotin compounds with functionalized carboxylates evaluated by the micro-dilution bioassay in vitro. Med Mycol. 2010;48:373–383. doi: 10.1080/13693780903188680. [DOI] [PubMed] [Google Scholar]
- Elie BT, Levine C, Ubarretxena-Belandia I, Varela-Ramírez A, Aguilera RJ, Ovalle R, et al. Water-soluble phosphane–gold (I) complexes. Applications as recyclable catalysts in a three-component coupling reaction and as antimicrobial and anticancer agents. Eur J Inorg Chem. 2009;23:3421–3430. doi: 10.1002/ejic.200900279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Feldman RG, White RF, Ikechukwu EI. Trimethyltin encephalopathy. Arch Neurol. 1993;50:1320–1324. doi: 10.1001/archneur.1993.00540120035010. [DOI] [PubMed] [Google Scholar]
- Fent K. Ecotoxicology of organotin compounds. Crit Rev Toxicol. 1996;26:1–117. [PubMed] [Google Scholar]
- Fortemps E, Amand G, Bomboir A, Lauwerys R, Laterre EC. Trimethyltin poisoning. Report of two cases. Int Arch Occup Environ Health. 1978;41:1–6. doi: 10.1007/BF00377794. [DOI] [PubMed] [Google Scholar]
- Gant VA, Wren MW, Rollins MS, Jeanes A, Hickok SS, Hall TJ. Three novel highly charged copper-based biocides: safety and efficacy against healthcare-associated organisms. J Antimicrob Chemother. 2007;60:294–299. doi: 10.1093/jac/dkm201. [DOI] [PubMed] [Google Scholar]
- Garg A, Bhosle NB. Butyltin compounds in the oyster, Saccostrea cucculata, from the west coast of India. Bull Environ Contam Toxicol. 2005;75:982–988. doi: 10.1007/s00128-005-0846-1. [DOI] [PubMed] [Google Scholar]
- Gennari A, Viviani B, Galli CL, Marinovich M, Pieters R, Corsini E. Organotins induce apoptosis by disturbance of [Ca2+]i and mitochondrial activity, causing oxidative stress and activation of caspases in rat thymocytes. Toxicol Appl Pharmacol. 2000;169:185–190. doi: 10.1006/taap.2000.9076. [DOI] [PubMed] [Google Scholar]
- Gielen M. Organotin compounds and their therapeutic potential: a report from the organometallic. Appl Organomet Chem. 2002;16:481–494. [Google Scholar]
- Gielen M, Bouhdid A, Willem R, Bregadze VI, Ermanson LV, Tiekink ERT. X-ray structure of the dimeric bis[(1,7-dicarba-close-dodecaborane-1-carboxylato)-di-n-butyltin] oxide. J Organomet Chem. 1995;501:277–281. doi: 10.1155/MBD.1997.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez FD, Apodaca P, Hollowa LN, Pannell KH, Whalen MM. Effect of a series of triorganotins on the immune function of human natural killer cells. Environ Toxicol Pharmacol. 2007;23:18–24. doi: 10.1016/j.etap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Gómez-Ruiz S, Kaluderović GN, Prashar S, Hey-Hawkins E, Erić A, Zizak Z, et al. Study of the cytotoxic activity of di and triphenyltin(IV) carboxylate complexes. J Inorg Biochem. 2008;102:2087–2096. doi: 10.1016/j.jinorgbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Gui-bin J, Qun-fang Z, Bin H. Tin compounds and major trace metal elements in organotin-poisoned patient’s urine and blood measured by gas chromatography-flame photometric detector and inductively coupled plasma-mass spectrometry. Bull Environ Contam Toxicol. 2000;65:277–284. doi: 10.1007/s0012800125. [DOI] [PubMed] [Google Scholar]
- Gunasekar P, Li L, Prabhakaran K, Eybl V, Borowitz JL, Isom GE. Mechanisms of the apoptotic and necrotic actions of trimethyltin in cerebellar granule cells. Toxicol Sci. 2001;64:83–89. doi: 10.1093/toxsci/64.1.83. [DOI] [PubMed] [Google Scholar]
- Hadjikakou SK, Hadjiliadis N. Antiproliferative and anti-tumor activity of organotin compounds. Coord Chem Rev. 2009;253:235–249. [Google Scholar]
- Hori T, Uchiyama T, Tsudo M, Umadome H, Ohno H, Fukuhara S, et al. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987;70:1069–1072. [PubMed] [Google Scholar]
- Hoti N, Zhu DE, Song Z, Wu Z, Tabassum S, Wu M. p53-dependent apoptotic mechanism of a new designer bimetallic compound tri-phenyl tin benzimidazolethiol copper chloride (TPT-CuCl2): in vivo studies in Wistar rats as well as in vitro studies in human cervical cancer cells. J Pharmacol Exp Ther. 2004;311:22–33. doi: 10.1124/jpet.104.069104. [DOI] [PubMed] [Google Scholar]
- Ito E, Yip KW, Katz D, Fonseca SB, Hedley DW, Chow S, et al. Potential use of cetrimonium bromide as an apoptosis-promoting anticancer agent for head and neck cancer. Mol Pharmacol. 2009;76:969–983. doi: 10.1124/mol.109.055277. [DOI] [PubMed] [Google Scholar]
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin–DNA adducts. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate–propidium iodide. J Histochem Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Kaluderovic GN, Kommera H, Hey-Hawkins E, Paschke R, Gomez-Ruiz S. Synthesis and biological applications of ionic triphenyltin(IV) chloride carboxylate complexes with exceptionally high cytotoxicity. Metallomics. 2010;2:419–428. doi: 10.1039/c0mt00007h. [DOI] [PubMed] [Google Scholar]
- Kapoor RN, Apodaca P, Montes M, Gomez FD, Pannell KH. Mixed aryl–alkyl organotin compounds, ArnMeSnCl3-n (Ar = RC6H4, R = H, ethyl, i-propyl, t-butyl; n-hexyl, n-octyl) and the effect of R upon antibiotic activity. Appl Organomet Chem. 2005;19:518–522. [Google Scholar]
- Kim DK, Kim HT, Cho YB, Tai JH, Ahn JS, Kim TS, et al. Antitumor activity of cis-malonato[(4R, 5R)-4, 5-bis (aminomethyl)-2- isopropyl-1, 3-dioxolane]platinum(II), a new platinum analogue, as an anticancer agent. Cancer Chemother Pharmacol. 1995;35:441–445. doi: 10.1007/s002800050260. [DOI] [PubMed] [Google Scholar]
- Levinson C. Vinyl chloride: a case study of the new occupational health hazard. Geneva: International Chemical Federation; 1974. p. 15. [Google Scholar]
- Merkord J, Jonas L, Weber H, Kroning G, Nizze H, Hennighausen G. Acute interstitial pancreatitis in rats induced by dibutyltin dichloride (DBTC): pathogenesis and natural course of lesions. Pancreas. 1997;15:392–401. doi: 10.1097/00006676-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Oubrahim H, Chock PB, Stadtman ER. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc Natl Acad Sci USA. 2006;103:1727–1731. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser VC. Rat strain- and gender-related differences in neurobehavioral screening: acute trimethyltin neurotoxicity. J Toxicol Environ Health. 1996;47:567–586. doi: 10.1080/009841096161546. [DOI] [PubMed] [Google Scholar]
- Narayanan VL, Nasr M, Paull K. Computer assisted structure–antileukemic activity correlations of organotin compounds and initial exploration of their potential anti-HIV activity. In: Gielen M, editor. Tin-based anti-tumor drugs. New York: Springer; 1990. pp. 200–217. [Google Scholar]
- Ngan VK, Bellman K, Hill BT, Wilson L, Jordan MA. Mechanism of mitotic block and inhibition of cell proliferation by the semisynthetic Vinca alkaloids vinorelbine and its newer derivative vinflunine. Mol Pharmacol. 2001;60:225–232. doi: 10.1124/mol.60.1.225. [DOI] [PubMed] [Google Scholar]
- Park DM, Rich JN. Biology of glioma cancer stem cells. Mol Cells. 2009;28:7–12. doi: 10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- Pieters RH, Bol M, Seinen W, Penninks AH. Cellular and molecular aspects of organotin-induced thymus atrophy. Hum Exp Toxicol. 1994;13:876–879. doi: 10.1177/096032719401301210. [DOI] [PubMed] [Google Scholar]
- Piver WT. Organotin compounds: industrial applications and biological investigation. Environ Health Perspect. 1973;4:61–79. doi: 10.1289/ehp.730461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross WD, Emmett EA, Steiner J, Tureen R. Neurotoxic effects of occupational exposure to organotins. Am J Psychiatry. 1981;138:1092–1095. doi: 10.1176/ajp.138.8.1092. [DOI] [PubMed] [Google Scholar]
- Sen A, Chaudhuri TK. Synthesis and evaluation of dimethyl tin 4-cyclohexyl thiosemicarbazone as a novel antitumor agent. Exp Oncol. 2009;31:22–26. [PubMed] [Google Scholar]
- Shaik N, Martinez A, Augustin I, Giovinazzo H, Varela-Ramirez A, Sanau M, et al. Synthesis of apoptosis-inducing iminophosphorane organogold(III) complexes and study of their interactions with biomolecular targets. Inorg Chem. 2009;48:1577–1587. doi: 10.1021/ic801925k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm I, Kikuchi T, Murphy JS. Serum enhances the cycling and survival of HeLa cells treated with 5, 6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci USA. 1982;79:2569–2573. doi: 10.1073/pnas.79.8.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodupunoori SK, Alamudun IA, Cervantes-Lee F, Gomez FDG, Carrasco YP, Pannell KH. Synthesis, structures and preliminary biological screening of bis(diphenyl)chlorotin complexes and adducts: Ph2ClSnCH2RCH2SnClPh2, R = p-C6H4, CH2CH2. J Organomet Chem. 2006;691:1790–1796. [Google Scholar]
- Tian L, Sun Y, Li H, Zheng X, Cheng Y, Liu X, et al. Synthesis, characterization and biological activity of triorganotin 2-phenyl-1, 2, 3-triazole-4-carboxylates. J Inorg Biochem. 2005;99:1646–1652. doi: 10.1016/j.jinorgbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Tomiyama K, Yamaguchi A, Kuriyama T, Arakawa Y. Analysis of mechanisms of cell death of T-lymphocytes induced by organotin agents. J Immunotoxicol. 2009;6:184–193. doi: 10.1080/15476910903100066. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environ Res. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Yip KW, Mao X, Au PY, Hedley DW, Chow S, Dalili S, et al. Benzethonium chloride: a novel anticancer agent identified by using a cell-based small-molecule screen. Clin Cancer Res. 2006;12:5557–5569. doi: 10.1158/1078-0432.CCR-06-0536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.