To the Editor:
Cathelicidins are a class of widely conserved antimicrobial peptides (AMPs) produced by essentially all mammalian species as part of the innate immune system. They have broad activity against both Gram-positive and Gram-negative bacteria and have additional effects including neutralizing lipopolysaccharide, stimulating leukocyte chemotaxis, promoting angiogenesis. There is only one known cathelicidin in humans. Although it is found in the secondary granules of neutrophils and other leukocyte populations, and a range of squamous epithelia including the skin, airways, mouth and intestine, it also circulates at high levels in the plasma.1, 2 Impairment in cathelicidin or other AMPs has been linked to increased susceptibility to and severity of infection while overexpression of cathelicidin confers protection against sepsis in animal models.3 Dialysis patients with the lowest circulating levels of cathelicidin are at a greater than two-fold risk of death due to infectious causes.4
The transcription of CAMP, the gene encoding cathelicidin in humans, is regulated by the vitamin D receptor. In vitro studies of human tissues, including epithelial cells, macrophages, and neutrophils, have demonstrated that cathelicidin levels can be increased following administration of 1,25-dihydroxyvitamin D.5 Topical and oral vitamin D analogues similarly lead to stimulation of cutaneous cathelicidin production.6 Prior studies of cathelicidin have given particular focus to its anti-tuberculosis properties, and humans given a single dose of oral vitamin D demonstrated improved mycobacterial immunity with an ex vivo assay.7 While circulating levels of cathelicidin have been most tightly linked to sepsis and mortality, little is known about the association of vitamin D status and plasma cathelicidin in healthy individuals, or of the ability of vitamin D supplementation to alter these levels.
In order to clarify the relationship between plasma cathelicidin, vitamin D status, and vitamin D supplementation, we conducted a prospective study in our outpatient clinical research center. Plasma levels of cathelicidin and 25-hydroxyvitamin D (25[OH]D) were measured in 60 healthy volunteers, along with calcium and creatinine. All subjects were free of known infection or renal disease and were not taking immunosuppressive medications. Individuals with plasma 25(OH)D levels ≤32 ng/ml (based on the laboratory reference range and previous studies 8) were treated with ergocalciferol, 50,000 IU every other day for 5 days. Post-treatment levels of 25(OH)D, cathelicidin and calcium were then measured in these subjects a median of 10 days after completing treatment. Total plasma cathelicidin (including the protein hCAP18 and its c-terminal peptide, LL37) was measured by ELISA, as described elsewhere.2 Screening 25-hydroxyvitamin D was measured via liquid chromatography-tandem mass spectrometry (LCMS). The study was approved by the institutional review boards at Massachusetts General Hospital and the Massachusetts Institute of Technology and all subjects provided written informed consent.
Estimated glomerular filtration rate (eGFR) was calculated using the widely-used Modification of Diet in Renal Disease formula. Spearman correlation was performed to assess the relationship between vitamin D and cathelicidin levels; as vitamin D associated relationships may emerge only below a threshold, levels ≤32 ng/ml and >32 ng/ml were analyzed separately. Multivariate linear spline regression (with an inflection point at 32 ng/ml) was used to confirm this approach and multiple linear regression was used to adjust for potential confounders. Stata 11.1 (StataCorp, College Station, TX) was used for all analysis.
Subject characteristics are detailed in table 1. Subjects were predominantly female and white, and all subjects had normal renal function (eGFR > 60 ml/min). Median levels of 25(OH)D and cathelicidin were 30 ng/ml and 698 ng/ml, respectively. A positive correlation between 25(OH)D and cathelicidin was evident at 25(OH)D levels ≤32 ng/ml (r=0.45, P=0.005) but not at higher levels (r=0.12, P=0.58). This relationship was verified using a piecewise polynomial (linear spline) regression with an infection point of 32 ng/ml. The linear relationship was significant at levels ≤32 ng/ml (β=27.8, P=0.003) and remained significant after adjustment for age, sex, race, serum calcium, use of supplements containing vitamin D, and season (p<0.001). There was no significant relationship with cathelicidin at 25(OH)D levels >32 ng/ml (P=0.16). The results are summarized in table E1 in the Online Repository.
Table 1.
Baseline characteristics of subjects screened (n=60). Continuous variables are reported as median (interquartile range).
| Parameter | Median or % |
|---|---|
| Age, years | 39 (29, 51) |
| Female | 77% |
| Race | |
| White | 91% |
| Black | 2% |
| Asian | 7% |
| Hispanic | 8% |
| Creatinine, mg/dl | 0.94 (0.87, 1.03) |
| eGFR, ml/min | 76 (70, 87) |
| 25-hydroxyvitamin D, ng/ml | 30 (22, 36) |
| Calcium, mg/dl | 9.3 (8.9, 9.5) |
| Cathelicidin, ng/ml | 698 (594,903) |
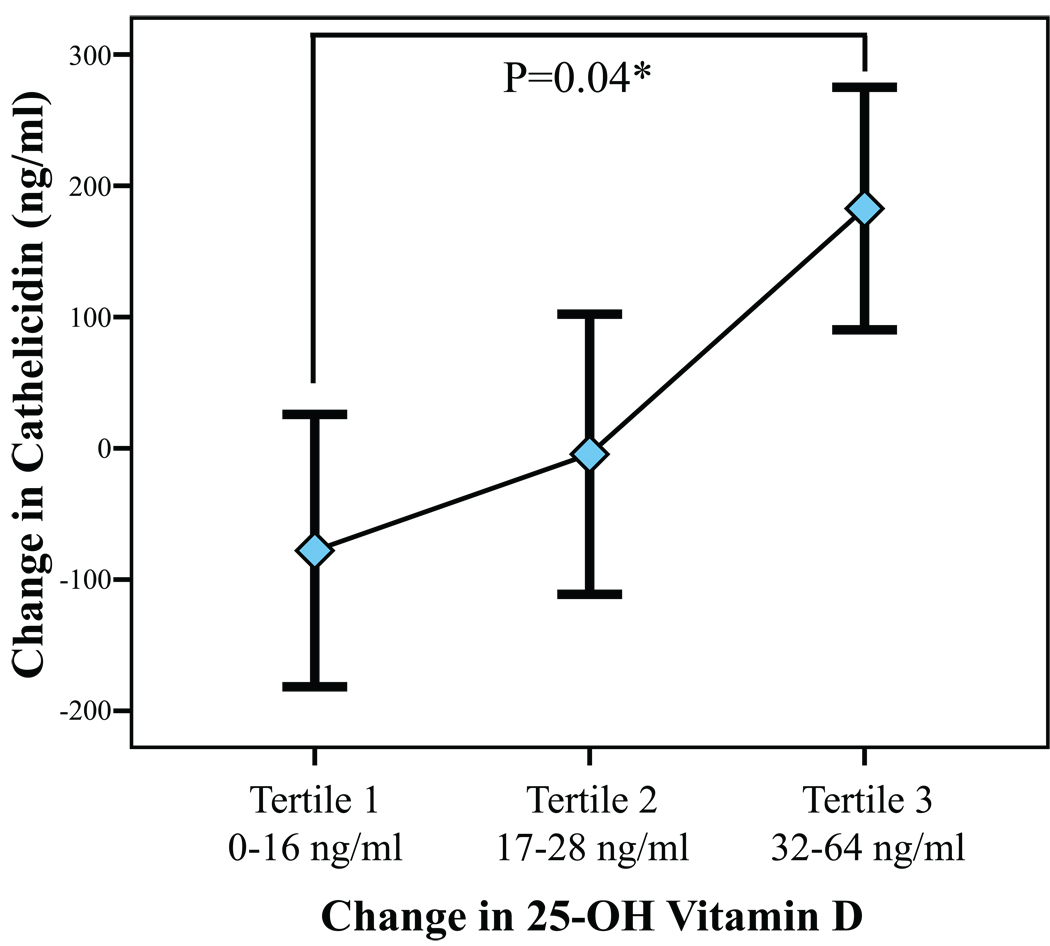
Based on the presence of a 25(OH)D level ≤ 32 ng/ml, 25 subjects had repeat testing for cathelicidin and 25(OH)D following ergocalciferol treatment. The mean increase in 25(OH)D following treatment was 25 ng/ml. The change in cathelicidin associated with treatment was analyzed by change in 25(OH)D (categorized by tertile; Figure 1). Only in the third tertile (change in 25(OH)D 32–64 ng/ml) was the change in cathelicidin significantly greater than 0 (P=0.04). After adjustment for age, sex, and change in calcium, only change in vitamin D was significantly associated with change in cathelicidin (P=0.03). Furthermore, the change in cathelicidin in tertile 3 was significantly different from that of tertile 1 (P=0.04). When grouped by tertile, subjects with the greatest increase in 25(OH)D had the lowest baseline 25(OH)D levels (p for trend, p=0.048), consistent with prior findings. However, no such relationship was observed with cathelicidin levels (p=0.82), suggesting that changes in cathelicidin levels were driven by the ergocalciferol treatment itself.
Figure 1.
Change in cathelicidin by change in 25-hydroxyvitamin D following ergocalciferol treatment (n=25). Diamonds represent group means, while black bars represent standard error. *The difference between tertile 1 and 3 was significant (P=0.04), adjusted for age, sex, and change in calcium.
In recent years, there has been increasing interest in the “non-traditional” effects of vitamin D, actions that are outside the traditional axis of mineral metabolism. With vitamin D receptors identified in many immunologically active cells, data have emerged suggesting an immunologic role for vitamin D. To date, much of this research has been limited to animal studies, in vitro, or ex vivo analysis. Our study of healthy adults demonstrates, for the first time, that 25(OH)D levels correlate with both baseline cathelicidin levels and changes in cathelicidin levels following high-dose ergocalciferol treatment. A recent study by Adams and colleagues did not show a correlation between circulating levels of 25(OH)D and cathelicidin, but this analysis was limited to 50 elderly patients with established bone disease, a group that was considerably older than the current study population.9 It is likely that additional factors confound the relationship between vitamin D and cathelicidin in older individuals. Nevertheless, these authors were able to identify vitamin D-based changes in cathelicidin mRNA production after monocyte stimulation.9 Of note, we found a relationship between baseline cathelicidin and 25(OH)D only at levels ≤32 ng/ml, a relationship analogous to that of 25(OH)D and parathyroid hormone.8
Given the previously observed associations of circulating cathelicidin levels and infection-associated mortality in human studies and animal models of sepsis,3, 4 our findings support the possibility that supplementation with vitamin D may be clinically useful in patients at high risk of infection. However, a treatment-associated increase in cathelicidin was not universal and was statistically significant only in subjects with the greatest increase in 25(OH)D. Given the limited nature of the relationship between cathelicidin and 25(OH)D and the potential for unmeasured confounding variables, randomized controlled trials studying the effect of vitamin D supplementation on clinically relevant infection outcomes (such as infection rates, severity of disease indices, and infection-related mortality) are necessary to definitively establish an immunologic role for vitamin D supplementation.
Supplementary Material
Acknowledgments
Source of funding: Dr. Bhan is supported by NIH Young Investigator Grant K23 1K23DK081677 (Bethesda, MD). Drs Camargo and Thadhani are supported, in part, by the Massachusetts General Hospital Center for D-receptor Activation Research (Boston, MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frohm Nilsson M, Sandstedt B, Søensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sørensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 3.Bals R, Weiner DJ, Moscioni AD, Meegalla RL, Wilson JM. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect Immun. 1999;67:6084–6089. doi: 10.1128/iai.67.11.6084-6089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Koeffler HP, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48:418–424. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–831. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 8.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 9.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.