Abstract
Background
Opioid-induced hyperalgesia (OIH) and tolerance are challenging maladaptations associated with opioids in managing pain. Recent genetic studies and the existing literature suggest the 5-hydroxy tryptamine type 3 (5-HT3) receptor participates in these phenomena. The location of the relevant receptor populations and the interactions between the 5-HT3 system and other systems controlling OIH and tolerance have not been explored, however. We hypothesized that 5-HT3 receptors modulate OIH and tolerance, and that this modulation involves the control of expression of multiple neurotransmitter and receptor systems.
Methods
C57BL/6 mice were exposed to a standardized 4-day morphine administration protocol. The 5-HT3 antagonist ondansetron was administered either during or after the conclusion of morphine administration. Mechanical testing was used to quantify OIH, and thermal tail flick responses were used to measure morphine tolerance. In other experiments spinal cord and dorsal root ganglion tissues were harvested for analysis of messenger RNA levels by real-time polymerase chain reaction or immunochemistry analysis.
Results
The results showed 1) Systemic or intrathecal injection of ondansetron significantly prevented and reversed OIH, but not local intraplantar injection. 2) Systemic or intrathecal injection of ondansetron prevented and reversed tolerance, and 3) Ondansetron blocked morphine induced increases of multiple genes -relevant to OIH and tolerance in dorsal root ganglion and spinal cord.
Conclusions
Morphine acts via a 5-HT3 dependent mechanism to support multiple maladaptations to the chronic administration of morphine. Furthermore, the use of 5-HT3 receptor antagonists may provide a new avenue to prevent or reverse OIH and tolerance associated with chronic opioid use.
Opioids are a mainstay of treatment for acute and chronic pain. However, repeated or chronic administration of these medications is accompanied by various maladaptations. Tolerance (the reduction of opioid analgesic potency), hyperalgesia (opioid-induced hyperalgesia [OIH], the sensitization to noxious or painful stimuli) and physical dependence (the requirement to continue opioid administration to avoid a withdrawal state) are all challenging problems associated with the utilization of opiates in managing pain. However, the relationships between and mechanisms of these maladaptations are complex and not fully understood.
Genetic strategies provide a novel approach to understanding the molecular basis underlying these phenomena.1–5 For example, our group used a murine haplotypic mapping approach to identify several target genes associated with specific maladaptations: the β2-adrenergic receptor (β2-AR) with mechanical OIH6, the P-glycoprotien drug transporter (Abcb1b) with thermal OIH3 and the 5-hydroxytryptamine receptor subunit type 3A (5-HT3A) with physical dependence.7 Furthermore, correlative genetic analysis of the strain-specific data and limited pharmacologic analyses done in the course of these studies suggested that these three principal opioid maladaptations may share common mechanistic underpinnings.8–9 Not well established, however, is the location of the relevant populations of receptors such as 5-HT3 controlling OIH and tolerance.
The 5-HT3 receptor, the focus of the present studies, is a pentameric ligand-gated ion channel consisting of five monomers which form a structure centrally permeable to cations.10–12 The receptor subunits are expressed in brain, spinal cord and dorsal root ganglia (DRG) tissue.13–18 The 5-HT3 receptor has multiple functions including those involved in nausea and vomiting, pain processing, the drug reward system and anxiety. A few studies concluded that 5-HT3 receptor antagonists can reduce various opioid maladaptations.7,19–23 However, these studies involved limited behavioral assessments, and efforts to determine site of action as well as effects on gene expression or other mechanisms of chronic adaptation are largely lacking.
In light of the confirmed genetic finding of 5-HT3 receptor regulation of physical dependence and existing evidence supporting the hypothesis that 5-HT3 receptor might mediate opioid tolerance and OIH, we conducted a series of experiments to define the role of this receptor in opioid tolerance and OIH through pharmacology and molecular analysis. In an attempt to define the mechanism of this modulation we evaluated the location of the relevant 5-HT3 receptor expression and the ability of 5-HT3 receptor to control the expression of other genes established to participate in OIH and tolerance.
Materials and Methods
Animals
All animal experiments were done after approval of protocols by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, California) and complied with the Guide for the Care and Use of Laboratory Animals available through the National Academy of Sciences. Male C57BL/6J mice were obtained from Jackson Laboratory (JAX, Bar Harbor, ME) at 7–8 weeks of age. Mice were kept a further 7–10 days from the date of arrival in our animal care facility before use to allow for acclimation. Mice were housed 4–6 per cage under pathogen-free conditions with soft bedding and were provided food and water ad libitum with a 12:12 light:dark cycle.
Chronic Morphine Administration
After baseline nociceptive testing, morphine (σ Chemical, St. Louis, MO) was subcutaneously administered to mice 10 mg/kg twice per day on day 1, 20 mg/kg twice per day on days 2–3 and 40 mg/kg twice per day on day 4 in 50–100 μl volumes of 0.9% NaCl similar to our previous protocols for OIH and tolerance.3,6,8–9
Ondansetron Administration
Ondansetron (σ Chemical) was administered acutely and chronically via systemic application (subcutaneous and intrathecal injection) or local hind paw site application. For systemic administration, ondansetron was injected subcutaneously in a 100 μl volume in 0.9% NaCl to some groups of mice. The drug was either given at a dose of 1 mg/kg along with each dose of morphine during the chronic dosing paradigm, or given once at a dose of 2 mg/kg 30 min before tolerance or nociceptive testing. The procedure for intrathecal drug administration was based on the technique described by Hylden and Wilcox.24 Hylden and Wilcox.24 Briefly, the intervertebral space between L5 and L6 was punctured directly using a 28-gauge needle attached to a microsyringe. Mice were lightly anesthetized with isoflurane during these procedures. A tail flick was used as an indication that the needle had penetrated the dura. Once inserted, 5 μl of injectate was slowly administered using a microsyringe, and the animals were used within 20 min of the injection.
Behavioral Measurement
Opioid-induced hyperalgesia: Mechanical allodynia was assessed using nylon von Frey filaments according to the “up-down” algorithm described by Chaplan et al.25 as previously described.6,26 In these experiments, mice were placed on wire mesh platforms in clear cylindrical plastic cylinders. After 15 min of acclimation, fibers of sequentially increasing stiffness were applied to the plantar surface of one hind paw, and left in place 5 s. Withdrawal of the hind paw from the fiber was scored as a response. When no response was obtained, the next stiffest fiber in the series was applied to the same paw; if a response was obtained a less stiff fiber was applied. Testing proceeded in this manner until four fibers had been applied after the first one causing a withdrawal response allowing the estimation of the mechanical withdrawal threshold.27 This data-fitting algorithm allowed the use of parametric statistics for analysis.
Morphine dose-response
Cumulative morphine dose-response curves were constructed using the tail flick assay and methods similar to those we described previously.8–9 For these measurements mice were gently restrained within a cone shaped tube made of cotton toweling. Using a tail-flick analgesic apparatus (Columbus Instruments, Columbus, OH), tail flick latency was measured with 0.1 s precision. A 10-s cutoff time was used to prevent permanent tissue damage. Two measurements were made per mouse with the light beam focused on two different points 1 cm apart on the tail. The lamp intensity was the same for all animals which resulted in baseline tail flick measurements of 3–4 s. For the assessment of tolerance, these dose-response experiments followed 18 h after the final dose of morphine given as part of the chronic morphine administration protocol. The cumulative doses of morphine used were 0.1, 2, 4, 8, 16, and 32 mg/kg. When administered acutely, ondansetron (2 mg/kg) was injected with the first cumulative morphine injection. Tail flick latency was determined 25 min after morphine injection as previous experiments established 25 min to be the time at which peak morphine effect was achieved. The percent maximal possible effect (%) was determined according to the following formula:
Expression Studies
Messenger RNA expression: Mice were sacrificed at specific time points by carbon dioxide asphyxiation. Spinal cord lumbar segments were harvested by extrusion and rapid dissection on a prechilled surface. The DRG were dissected using low power binocular magnification. Tissue was then quick frozen in liquid nitrogen and stored at −80°C until use. For synthesis of Complementary DNA and real-time polymerase chain reaction (PCR), total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, its purity and concentration were determined spectrophotometrically as described previously for spinal cord and DRG samples.28 Complementary DNA was synthesized from total RNA using random hexamer priming and a first strand synthesis system (Invitrogen, Carlsbad, CA). Briefly, 1 μg of total RNA was mixed with 4 μl of 10× reverse transcription buffer, 8 μl of 25 mM MgCl2, 4 μl 0.1 M dithiothreitol, 1 μl RNasin, 2 μl Super Script II reverse transcriptase (50 u/μl), 5 μl hexomers and RNase-free water to 40 μl. Incubation was then carried out at 42°C for 60 min followed by heat inactivation at 70°C. Finally 1 μl RNase H was added to each reaction and incubated at 37°C for 20 min to degrade the RNA. For real-time quantitative PCR, reactions were conducted in a volume of 4 μl using the Sybr Green I master kit (PE Applied Biosystems, Foster City, CA). Briefly, 2 μl of a mixture of 2× sybr green and target gene primers (See table 1) was loaded with 2 μl diluted Complementary DNA template in each well. Following this, 8 μl mineral oil was loaded in each well to prevent loss of solution. Using an ABI prism 7900 HT system (Applied Biosystems, Foster City, CA), PCR was carried out using the parameters 52°C, 5 min→ 95, 10 min then [95°C, 30s→ 60°C, 60s] for 40 cycles. Samples were analyzed in triplicate. Melting curves were performed to document single product formation. 18s RNA was used as an internal control. The 18s primers were purchased from Ambion (Ambion, Austin, TX). Quantification was accomplished according to the standard curve method as described previously.9 In order to achieve the same PCR efficiency for each analyte, serial dilution of Complementary DNA was used to construct standard curve for the target genes. The R2 values for the standard curves of the test genes approached 1.0 suggesting the same amplification efficiency in the PCR reactions under these conditions. The expression level of specific genes was normalized to the level of 18S expression in each sample.
Table 1.
Primer Sequences for Target Genes
| Name | Forward (5′→3′) | Reverse (5′→3′) | Product Size (bp) | Genbank Accession |
|---|---|---|---|---|
| α-CGRP | tgacaggagctaaagctaagtgc | aacatcaacaggatggtttatgg | 142 | NM_001033954 |
| β-AR | caactctgccttcaatcctctta | ctagagtagccgttcccataggt | 117 | NM_007420 |
| 5HT3A | catgtatgccatcctcaacg | ccacgtccacaaactcattg | 188 | BC14479 |
| NOS1 | ctggaggaagtagccaagaaaat | ctcattctccatgtgtttgatga | 177 | NM_008712 |
| NR1 | gtagctgggatcttcctcatttt | gatagccctaaatgtggctttct | 177 | NM_008169 |
| PDP | ttctgaaggctgaagtgataagg | actcgactactgaagagcacagc | 188 | AF026537 |
| PPT-A | atgcagaactacgaaagaagacg | ttcctcatagcgcacattttatt | 110 | NM_009311 |
| TRPV1 | ggctgtcttcatcatcctgttac | ctcttgtgcaatcttgttgacag | 109 | NM_001001445 |
| 18s | aagacgatcagataccgtcgtag | tccgtcaattcctttaagtttca | 160 | NR_003278 |
The information of primer sequences of target genes tested, including gene access number in GenBank, polymerase chain reaction product size (bp). αCGRP: Alpha-calcitonin gene-related peptide; β-AR: β2-adrenergic receptor; 5-HT3A: 5-hydroxytryptamine receptor subunit type 3A; NOS1: Nitric oxide synthase-1; NR1: N-methyl d-aspartate receptor-1; PDP: Prodynorphin; PPT-A: Preprotachykinin-A; TRPV1: transient receptor potential vanilloid-1; 18S: 18S ribosomal RNA gene.
Immunohistochemistry
The localization of expression of 5-HT3A and substance P in DRG was tested by utilizing fluorescence confocal microscopy as we have described in detail previously.29 Mice used in these experiments were first asphyxiated using by carbon dioxide asphyxiation and perfused by intracardiac injection of 10 ml of 0.9% NaCl. This was followed by perfusion with 20 ml of 4% papaformaldehyde in 0.1 M phosphate-buffered saline. The DRG was then dissected under low power magnification and fixed in 4% paraformaldehyde for 4 h at room temperature follow by overnight incubation in 30% sucrose at 4°C. The tissues were then embedded in optimal cutting temperature medium, and 8 μm sections made on a cryostat with subsequent processing on slides. Blocking took place overnight at 4°C in tris buffered saline containing 5% dry milk, followed by exposure to the primary antibodies: polyclonal anti 5-HT3A, 1: 500 dilution (Abcam, Cambridge, MA); goat polyclonal anti substance P, 1:500, (Santa Cruz Bitechnology, Santa Cruz, CA). The anti 5-HT3A antibody is against a synthetic peptide corresponding to selective portion of the first extracellular loop of mouse 5HT3A. This peptide is located within amino acid sequence of 40–140 of mouse protein. For the specificity of 5-HT3A and substance P antibody the preabsorption of the antibody with specific blocking peptide was conducted before adding to the section. Sections were then be rinsed and transferred to milk-tris buffered saline containing either CY3 conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) or Texas Red conjugated secondary antibody (Vector Labs, Burlingame, CA) and incubated for another 1 h. After washing, coverslips were applied. Confocal laser-scanning microscopy was carried out using a Zeiss LSM/510 META microscope (Thornwood, NY). Control experiments included incubation of slices in primary or secondary antibody-free solutions. Both conditions lead to low intensity nonspecific staining patterns in preliminary experiments.
Statistical analysis
All data are expressed as the means ± SEM (SEM) unless otherwise noted. The data of mechanical sensitivity, tail flick response, gene expression in DRG and spinal cord were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test for multiple comparisons. Simple comparisons of two groups involved unpaired t-testing with two tail P values. Dose-response data were fitted using a sigmoidal function with variable slope as shown in a four-parameter logistic equation below and the top of the curve set at 100% to determine 50% analgesic effective dose (ED50) values and 95% confident intervals (Prism 5, GraphPad Software, La Jolla, CA). Pairs of dose-response curves were compared using the F test.
Results
The effects of systemic 5-HT3 receptor blockade on OIH
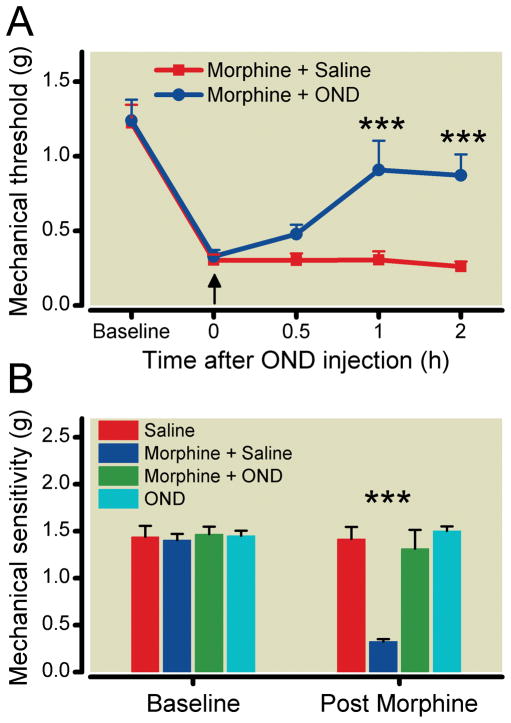
We first hypothesized that if OIH was supported by the 5-HT3 receptor, the administration of a selective receptor antagonist should reduce sensitization after chronic opioid administration. Figure 1A shows data demonstrating that after 4 days of escalating dose morphine administration mice display allodynia to mechanical stimuli. In this setting of established OIH, administration of the selective 5-HT3 receptor antagonist ondansetron (2 mg/kg, subcutaneously) significantly reversed OIH over the first few hours in comparison with control group of OIH nice which were treated with saline.
Figure 1.
The pharmacological reversal of the mechanical manifestations of Opioid-induced hyperalgesia (OIH) using the selective 5-HT3 antagonist ondansetron (OND). Mice of the C57BL/6J strain were used after chronic morphine treatment (see Methods). In Panel A, data representing the measurement of mechanical withdrawal thresholds after a single subcutaneous administration (↑) of OND (2 mg/kg) or saline in morphine treated mice are presented. In Panel B, mice were subcutaneously administered saline or ondansetron (1 mg/kg) at the time of each of the bi-daily morphine injections during the 4-day morphine treatment period. Data are presented as the means +/− SEM; *** P < 0.001. Six mice were used in each group.
While the acute systemic administration of ondansetron reversed OIH, we conducted a series of experiments to determine if administration of ondansetron during the morphine treatment phase could prevent the development of OIH. To accomplish this, morphine and ondansetron were coinjected daily. The data presented in figure 1B show that chronic administration of ondansetron fully prevented OIH development normally induced by chronic morphine treatment. Chronic ondansetron treatment alone did not induce any change of mechanical sensitivity in comparison with the control group.
Localization of ondansetron’s site of action in reversing OIH
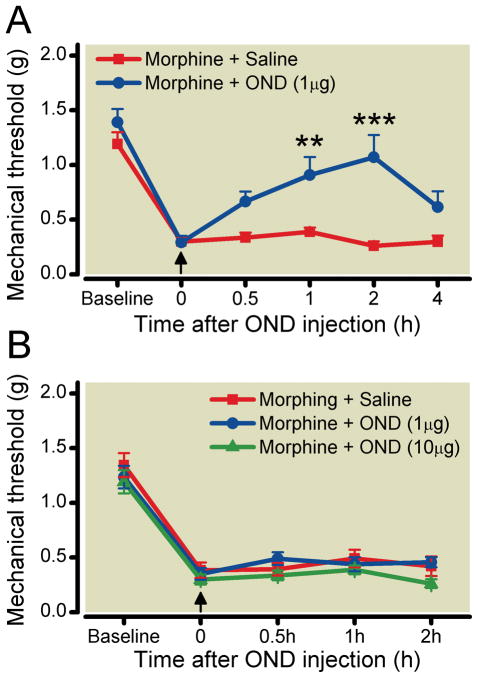
Next we determined if ondansetron influenced OIH by acting on central 5-HT3 receptors versus those in the periphery. Intrathecal injection of ondansetron (1 μg/2 μl) dramatically reduced opioid-induced mechanical sensitivity as displayed in figure 2A. Similar to systemic administration, the effect of ondansetron after Intrathecal injection was maximal 2 h after administration. However, peripheral hind paw administration of ondansetron at the same and a 10-fold higher dose did not change the morphine-induced sensitization. These data are presented in figure 2B.
Figure 2.
The differential effects of ondansetron (OND) on Opioid-induced hyperalgesia(OIH) when administered via intrathecal or intraplantar injection. Mice were used after chronic morphine treatment (see Methods). The assessment mechanical sensitivity at baseline was conducted before morphine treatment (baseline), after morphine treatment and after subsequent OND administration (↑). Panel A displays the effect of intrathical injection of OND (1 μg) or saline in morphine treated mice. Panel B displays the effect of intraplantar injection of OND (1 and 10 μg) or saline in morphine treated mice. Data are presented as the means +/− SEM; ** < 0.01; *** P < 0.001. Six mice were used in each group.
The effects of 5-HT3 receptor blockade on morphine antinociception in morphine tolerant mice
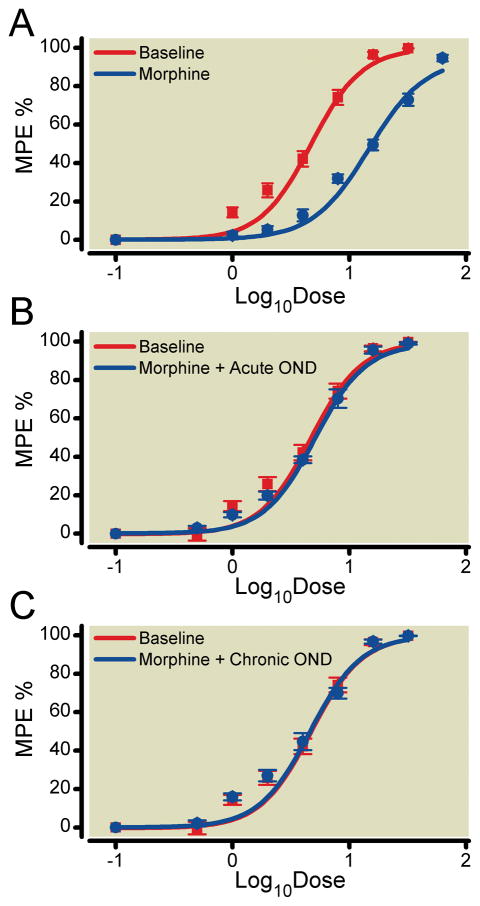
Next we treated the mice with morphine for four consecutive days in a chronic morphine dosing paradigm. Figure 3A shows the large rightward shift in the morphine dose-response relationship after this treatment protocol. Also provided are data for a separate group of tolerant mice in which ondansetron was administered immediately before dose-response testing (fig. 3B). These data demonstrate that the acute administration of ondansetron to morphine tolerant mice completely reverses morphine tolerance. Figure 3C demonstrates that if ondansetron was administered along with morphine during the chronic phase of treatment tolerance was prevented. Table 2 provides the calculated ED50 values for each of these curves. Additional control experiments established that ondansetron administration alone did not change baseline tail flick latency (data not shown).
Figure 3.
The effect of ondansetron (OND) on morphine tolerance. Morphine dose-response relationships were assessed using the tail flick assay. MPE%: The percent maximal possible effect (%). Panel A presents the morphine dose-response relationships before (baseline) and after 4 days of morphine treatment. Panel B presents the effects of OND (2mg/kg, subcutaneous injection) on morphine analgesic tolerance when administered immediately prior to dose-response testing. Panel C presents the effects of OND administration (1 mg/kg, subcutaneous injection) on morphine analgesic tolerance when administered at the time of each of the bi-daily morphine injections during the 4-day morphine treatment period. Data are presented as the means +/− SEM. Six mice were used in each group.
Table 2.
Effect of OND on Morphine Analgesic Potency
| Treatment | Analgesic ED50 (mg/kg) | 95% Confidence Interval |
|---|---|---|
| Baseline | 4.31 | 3.961 – 5.146 |
| Chronic morphine | 15.08* | 13.56 – 16.78 |
| Acute OND+Chronic Morphine | 5.02 | 4.614 – 5.462 |
| Chronic OND+Chronic Morphine | 4.28 | 3.8000 – 4.817 |
50% morphine analgesic effective dose (ED50)values in animals treated with or without OND were determined by using the sigmoidal fitting-curve method (See statistical analysis).
p < 0.05 in comparison of chronic morphine alone with other groups.
OND = Ondansetron
The effects of spinal 5-HT3 receptor blockade on tolerance
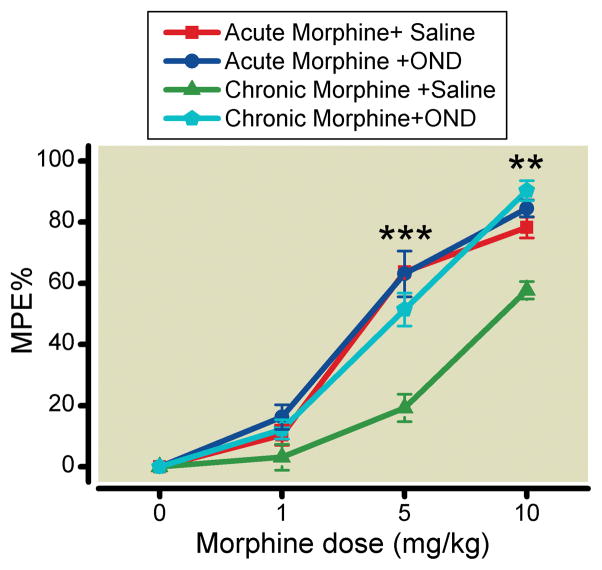
In the OIH experiments we had confirmed that intrathecal injection of a 5-HT3 receptor antagonist reversed morphine-induced nociceptive sensitization. Therefore, we hypothesized that blockade of spinal 5-HT3 receptors would reverse tolerance as well. Figure 4 shows that intrathecal injection of ondansetron reverses opioid tolerance in previously morphine treated mice, but does not affect the morphine dose-response relationship if the drugs are coadministered.
Figure 4.
Intrathecal injection of ondansetron (OND) reverses morphine tolerance in mice. Morphine analgesic responses were assessed after the intrathecal administration of OND (1 μg) or saline either before or after chronic morphine treatment. MPE%: The percent maximal possible effect (%). Data are presented as the means +/− SEM; ** p < 0.01 and *** p < 0.001) (Chronic Morphine vs. Chronic Morphine + OND). Six mice were used in acute morphine + Saline or chronic morphine + Saline group, eight mice were in acute morphine + OND or chronic morphine + OND group.
The effects of 5-HT3 receptor blockade on pain and tolerance-related gene expression in DRG and spinal cord tissue
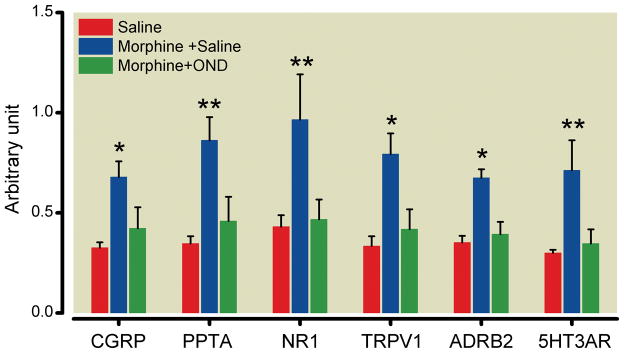
The behavioral data demonstrate that 5-HT3 receptor blockade prevented the development of OIH and tolerance. Given the present results and the genetic association of the 5-HT3 with several opioid maladaptations (OIH, tolerance and physical dependence), we hypothesized that the morphine-induced up-regulation of multiple pain and tolerance-related genes in the spinal cord and DRG tissue would be prevented by 5-HT3 receptor blockade during opioid treatment. The intrathecal administration of ondansetron could be hypothesized to act on either DRG or spinal cord tissue. For the analysis of DRG tissue we measured the expression of messenger RNA species coding for α-calcitonin gene-related peptide, the substance P precursor preprotachykinin-A, the N-methyl d-aspartate receptor-1, the transient receptor potential vanilloid-1, the β2-adrenergic receptor and the 5-HT3A. The sensory neuron expression of all of these have been linked to OIH and/or tolerance.9,30–33 The data in figure 5 show that 5-HT3 blockade reduced the morphine-induced up-regulation of all members of this panel of genes.
Figure 5.
Ondansetron (OND) down-regulates morphine augmented messenger RNA expression in dorsal root ganglia. Mice were treated with saline, morphine or morphine plus OND during the 4-day treatment protocol. αCGRP: alpha-calcitonin gene-related peptide; PPTA: preprotachykinin-A; NR1: N-methyl d-aspartate receptor-1; TRPV1: transient receptor potential vanilloid-1; β2-AR: β2-adrenergic receptor; 5-HT3A: 5-hydroxytryptamine receptor subunit type 3A. Data are presented as the means +/− SEM. * p < 0.05 and ** < 0.01. Five mice were used in each group.
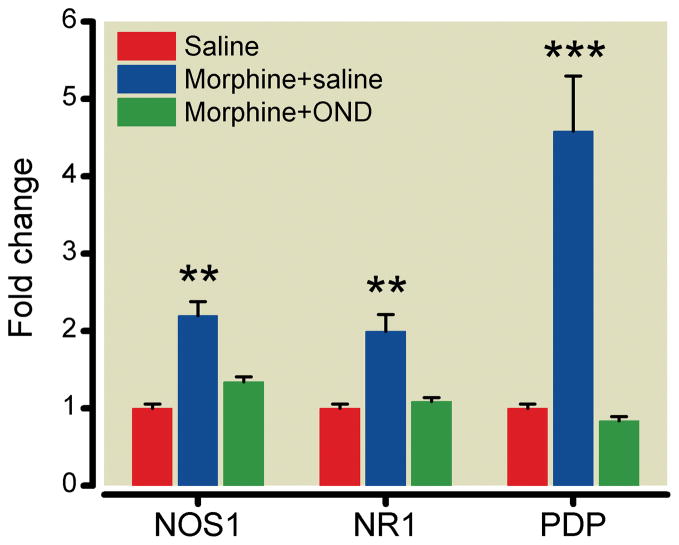
To determine the role of the 5-HT3 receptor in controlling morphine-induced changes in spinal cord tissue, we chose to analyze the expression of nitric oxide synthase 1, the N-methyl d-aspartate receptor-1, and prodynorphin, all of which, again, have been linked to OIH and/or tolerance.34–35 The data in figure 6 demonstrate that 5-HT3 receptor blockade suppressed the increases of these genes driven by chronic morphine treatment. In additional experiments we found that expression of the 5-HT3A was not significantly enhanced by in spinal cord tissue by chronic morphine (P > 0.05)treatment.
Figure 6.
Ondansetron (OND) down-regulates morphine augmented messenger RNA expression in spinal cord tissue induced by chronic morphine treatment. Mice were treated with saline, morphine or morphine plus OND during the 4-day treatment protocol. NOS1: nitric oxide synthase; NR1: N-methyl d-aspartate receptor-1; PDP: prodynorphin. Data are presented as the means +/− SEM. ** p < 0.01 and *** < 0.001. Five mice were used in each group.
Protein expression of 5-HT3 receptor and substance P in DRG
Our messenger RNA results indicated that the population of 5-HT3A most strongly linked to OIH and tolerance may be those expressed on sensory nerves. Figure 7 demonstrates that the 5-HT3A is strongly expressed on DRG peptidergic neurons stained with substance P. The immunostaining signal was abolished when the sections were incubated with antibody preabsorbed with the block peptide of 5-HT3A or substance P. It demonstrates that such immounoreactive signal is specific for 5-HT3A or substance P. The coexpression of these targets may not have been carefully studied before this time although other reports have confirmed existence of the 5-HT3A on DRG neurons in rat.18,36
Figure 7.
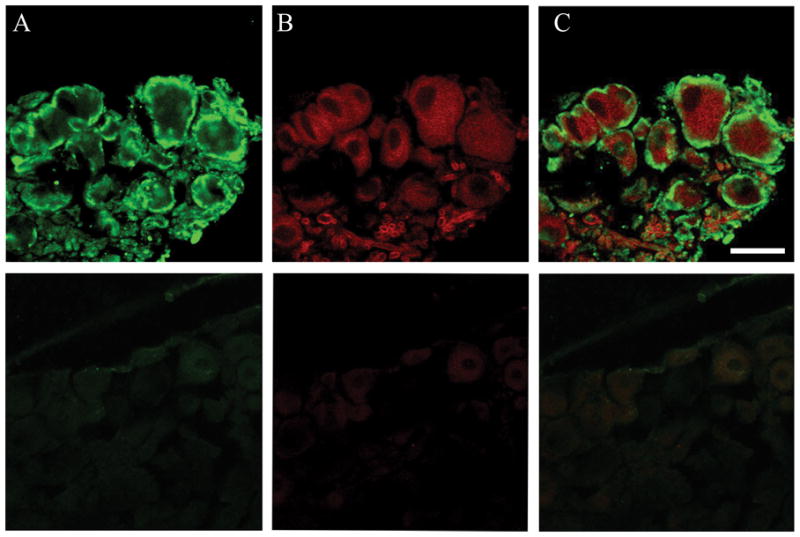
Immunohistochemical analysis of 5-hydroxytryptamine receptor subunit type 3A(5-HT3A) and substance-P expression in DRG. Panel A represents 5-HT3A staining, Panel B represents substance P staining, and Panel C presents the double staining. The top section is straining with specific primary antibody. The bottom section is preabsorbed primary antibody staining with specific block peptide as control. Scale bar is 50 μm.
Discussion
In this series of studies we addressed the ability of the 5-HT3 receptor to limit and reverse key opioid maladaptations observed after chronic administration, namely OIH and tolerance. Such ability was suggested by earlier pharmacologic studies and by genetic investigations7,20. Lacking from the literature, however, was information from systematic studies on the location of relevant 5-HT3 receptor populations, and careful exploration of the prevention versus reversal of established adaptations. Moreover, little information before this time was available to address the mechanisms by which the 5-HT3 receptor might regulate opioid adaptations such as by controlling gene expression in specific tissues involved in nociception and analgesia. Using a well characterized protocol for sustained morphine treatment in a strain of mice known to display robust adaptations to morphine exposure3,6–8 we observed, 1) that both systemic and intrathecal but not local administration of the selective 5-HT3 antagonist ondansetron could prevent and reverse OIH, 2) that systemic and intrathecal injection of ondansetron prevent and reverse tolerance to morphine, and 3) that the concomitant treatment of mice with ondansetron prevents adaptive effects on gene expression in spinal cord and DRG tissues. Importantly, the genes studied were all 1 s for which functional roles in OIH and tolerance have been demonstrated.
Tolerance and hyperalgesia have recently been recognized as maladaptations complicating acute and chronic pain management. Studies using both methadone maintained opioid addicts and opioid maintained chronic pain patients have revealed enhanced pain sensitivity to various stimuli including ice water immersion of a limb and the injection of lidocaine37–39 Studies addressing the topic universally demonstrate increased opioid requirements postoperatively in chronic opioid consuming patients, and often increased pain scores as well.40–42 Difficulties in managing these patients have lead to the publication of several reviews on the perioperative management of chronic opioid consuming patients.43–46 Even the acute administration of relatively high dose opioids during surgery elevates postoperative opioid requirements.47–49 Despite these observations, we currently lack proven techniques for limiting the development of OIH or tolerance in clinical populations. With the possible exception of ketamine infusion used postoperatively, we also lack therapies which can reverse OIH or tolerance to a clinically useful degree once established.
One of our key observations was that intrathecal ondansetron administration was effective in reversing tolerance and OIH whereas injection of this selective antagonist in at the same or 10-fold higher dose into the paw at the site of noxious stimulation failed to reduce OIH. As intrathecally administered drugs have access to spinal cord tissue and at least some portion of the DRG, the relevant 5-HT3 receptor populations may be expressed in either spinal neurons or afferent sensory fibers. Primary sensory neurons are known to express 5-HT3 receptors,18,50 and functional receptors capable of causing excitation and neurotransmitter release have been identified on both the sensory neuron cell bodies and on presynaptic terminals within the dorsal horn of the spinal cord51–52. Many of these receptors are expressed on capsaicin sensitive small afferent fibers consistent with our observations of 5-HT3 regulation of substance P, α-calcitonin gene-related peptide and transient receptor potential vanilloid-1.51 Data are sparse demonstrating the expression of 5-HT3 receptors on distal primary afferent nerve terminals. Though 5-HT3 itself is an important inflammatory mediator, and the injection of 5-HT itself in distal peripheral tissues causes hyperalgesia sensitive to selective 5-HT3 blockade, this is postulated to occur via an indirect action of serotonin on afferent neurons requiring local noepinephrine release and the activation of β2-adrenergic receptors53. Thus although mechanisms of OIH involving peripheral nerve terminals and the enhanced production of inflammatory mediators in skin tissue have been demonstrated,54 we failed to find evidence for the participation of distally expressed 5-HT3 receptors in the present studies.
On the other hand, there exists abundant evidence for the participation of 5-HT3 receptors in multiple aspects of nociceptive signal transmission in the spinal cord. Immunohistochemical studies have revealed the dense expression of 5-HT3 receptors in the superficial dorsal horn,13 an area which receives nociceptive input. Available reports indicate that 5-HT3 action can be either inhibitory to nociceptive signal transmission or facilitory with the later action more consistent with OIH and tolerance. For example, the intrathecal injection of the selective 5-HT3 agonist 2-Me-5-HT has analgesic activity in some models. This may be the result of 5-HT3 mediated enhancement of GABAergic inhibitory signaling.55 Other evidence, however, demonstrates a clear role for descending serotonergic facilitory mechanisms in supporting hyperalgesia and facilitating nociceptive signal transmission. For example electrophysiological, pharmacological and behavioral evidence support roles for descending neurons from the rostral ventromedial medulla in facilitating nociceptive signaling in models of cancer-induced bone pain,56 inflammatory pain,57 and neuropathic pain.58 Importantly, facilitory descending circuitry from the rostral ventromedial medulla to the dorsal horn of the spinal cord also supports both opioid tolerance and OIH,59 and it has been suggested that tolerance and OIH are essentially different manifestations of a common set of alterations in spinal cord neurophysiology.32 One report using single acute intrathecal ondansetron injections reported reversal of morphine tolerance and OIH in rats.23 In the present studies we demonstrated the ability of ondansetron to prevent as well as reverse tolerance and OIH, and further explored the effects of systemic, intrathecal and local peripheral administration of the 5-HT3 blocker.
While our observations regarding the acute administration of ondansetron confirm and extend earlier reports regarding the facilitory activity of 5-HT3 receptors in pain and opioid adaptations, we also addressed the ability of 5-HT3 blockade to prevent opioid adaptations from occurring. The approach involved ondansetron-morphine coadministration paradigms in conjunction with behavioral and gene expression studies. For both the OIH and tolerance testing, nociceptive measurements were made at a point more than 6 half-lives after the final dose of ondansetron was delivered making ongoing ondansetron blockade unlikely.60 Our gene expression experiments provide a basis for explaining these observations. When we studied the morphine induced expression of a group of genes in the DRG, which had been implicated in previous reports as being functionally involved in OIH and tolerance. These genes includes α-calcitonin gene-related peptide, preprotachykinin A, the N-methyl d-aspartate receptor-1, the transient receptor potential vanilloid-1 and the β2-Adrenergic receptor9,30,61–63. They are mainly synthesized in primary afferent neurons. Chronic morphine treatment induces the increases of messenger RNA synthesis encoding these targets mostly in DRG as our group and other groups reported. Blockade of these targets with inhibitors or antagonists reduces opioid tolerance and OIH development In the current investigation, we demonstrated that 5-HT3 receptor antagonist, ondansetron prevented morphine enhanced expression in all instances. Likewise, when a second group of genes expressed on spinal cord neurons with proven involvement in OIH and tolerance was studied (Nitric oxide synthase1, N-methyl d-aspartate receptor-1, and prodynorphin)30,35,61,64 again all genes showed morphine enhanced expression which was completely prevented by the coadministration of ondansetron. The widespread prevention of gene expression adaptations to chronic morphine administration in DRG and spinal cord tissue provides a rational basis for the ability of ondansetron to prevent OIH and tolerance.
Though the effects of 5-HT3 antgonists were not tested in clinical models of OIH or tolerance, it is notable that 5-HT3 antagonists have a remarkable record of safety even when used in high doses.65 Furthermore, in some clinical settings such as surgery and cancer care, 5-HT3 blockers are commonly employed for their antiemetic effects along with opioids. The present findings might therefore be translated to clinical studies in which populations vulnerable to poor pain relief because of tolerance or OIH might be provided 5-HT antagonists to determine if opioid use declines or the quality of analgesia improves. In the United States, several selective 5-HT3 drugs would be available for such trials including ondansetron, granisetron, tropisetron, palonosetron and dolasetron.
In summary, the 5-HT3 system has been explored in humans and animals as controlling addiction, anxiety, depression, irritable bowel syndrome, pain, and nausea/vomiting. Our studies combined with others suggest that the 5-HT3 system may participate in the acquisition and maintenance of two clinically key opioid maladaptations, OIH and tolerance. In this regard, it seems most likely 5-HT3 receptors expressed on the soma or the central terminals of afferent neurons are most important. Acutely, 5-HT3 blockade may reduce tolerance and OIH by blocking descending serotonergic facilitation, while the blockade of changes in gene expression may be more central to the preventative effects of the antagonists. The long safety record for the use of these drugs and the availability of several well characterized agents speaks to the potential translation of our rodent observations into humans.
Acknowledgments
Support: This work was supported by National Institute of Health (Bethesda, Maryland): RO1 GMO79126 to J. David Clark in Department of Anesthesiology, Veterans Affair Palo Alto Health Care System, Palo Alto, California.
This work is attributed to Department of Anesthesiology, Veterans Affairs Palo Alto Health Care System, Palo Alto, California
References
- 1.Eidelberg E, Erspamer R, Kreinick CJ, Harris J. Genetically determined differences in the effects of morphine on mice. Eur J Pharmacol. 1975;32:329–36. doi: 10.1016/0014-2999(75)90300-3. [DOI] [PubMed] [Google Scholar]
- 2.Korostynski M, Kaminska-Chowaniec D, Piechota M, Przewlocki R. Gene expression profiling in the striatum of inbred mouse strains with distinct opioid-related phenotypes. BMC Genomics. 2006;7:146. doi: 10.1186/1471-2164-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang DY, Liao G, Lighthall GK, Peltz G, Clark DJ. Genetic variants of the P-glycoprotein gene Abcb1b modulate opioid-induced hyperalgesia, tolerance and dependence. Pharmacogenet Genomics. 2006;16:825–35. doi: 10.1097/01.fpc.0000236321.94271.f8. [DOI] [PubMed] [Google Scholar]
- 4.Somogyi AA, Barratt DT, Coller JK. Pharmacogenetics of opioids. Clin Pharmacol Ther. 2007;81:429–44. doi: 10.1038/sj.clpt.6100095. [DOI] [PubMed] [Google Scholar]
- 5.Tapocik JD, Letwin N, Mayo CL, Frank B, Luu T, Achinike O, House C, Williams R, Elmer GI, Lee NH. Identification of candidate genes and gene networks specifically associated with analgesic tolerance to morphine. J Neurosci. 2009;29:5295–307. doi: 10.1523/JNEUROSCI.4020-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang DY, Liao G, Wang J, Usuka J, Guo Y, Peltz G, Clark JD. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006;104:1054–62. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu LF, Liang DY, Li X, Sahbaie P, D’Arcy N, Liao G, Peltz G, David Clark J. From mouse to man: The 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics. 2009;19:193–205. doi: 10.1097/FPC.0b013e328322e73d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–40. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Liang DY, Shi X, Li X, Li J, Clark JD. The β2 adrenergic receptor regulates morphine tolerance and physical dependence. Behav Brain Res. 2007;181:118–26. doi: 10.1016/j.bbr.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor–the relationship between structure and function. Neuropharmacology. 2009;56:273–84. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher S, Barnes NM. Desperately seeking subunits: Are native 5-HT3 receptors really homomeric complexes? Trends Pharmacol Sci. 1998;19:212–5. doi: 10.1016/s0165-6147(98)01210-3. [DOI] [PubMed] [Google Scholar]
- 12.Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research–evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Kia HK, Miquel MC, McKernan RM, Laporte AM, Lombard MC, Bourgoin S, Hamon M, Verge D. Localization of 5-HT3 receptors in the rat spinal cord: Immunohistochemistry and in situ hybridization. Neuroreport. 1995;6:257–61. doi: 10.1097/00001756-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Kidd EJ, Laporte AM, Langlois X, Fattaccini CM, Doyen C, Lombard MC, Gozlan H, Hamon M. 5-HT3 receptors in the rat central nervous system are mainly located on nerve fibres and terminals. Brain Res. 1993;612:289–98. doi: 10.1016/0006-8993(93)91674-h. [DOI] [PubMed] [Google Scholar]
- 15.Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, Doucet E, Hamon M, Verge D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci. 2002;15:449–57. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- 16.Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- 17.Morales M, Battenberg E, de Lecea L, Sanna PP, Bloom FE. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Brain Res Mol Brain Res. 1996;36:251–60. doi: 10.1016/0169-328x(96)88406-3. [DOI] [PubMed] [Google Scholar]
- 18.Morales M, McCollum N, Kirkness EF. 5-HT(3)-receptor subunits A and B are co-expressed in neurons of the dorsal root ganglion. J Comp Neurol. 2001;438:163–72. doi: 10.1002/cne.1307. [DOI] [PubMed] [Google Scholar]
- 19.Roychoudhury M, Kulkarni SK. Prevention of morphine discontinuation phenomenon in mice by ondansetron, a selective 5-HT3 antagonist. Methods Find Exp Clin Pharmacol. 1996;18:677–83. [PubMed] [Google Scholar]
- 20.Hui SC, Sevilla EL, Ogle CW. Prevention by the 5-HT3 receptor antagonist, ondansetron, of morphine-dependence and tolerance in the rat. Br J Pharmacol. 1996;118:1044–50. doi: 10.1111/j.1476-5381.1996.tb15504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins GA, Nguyen P, Joharchi N, Sellers EM. Effects of 5-HT3 receptor antagonists on behavioural measures of naloxone-precipitated opioid withdrawal. Psychopharmacology (Berl) 1991;105:322–8. doi: 10.1007/BF02244425. [DOI] [PubMed] [Google Scholar]
- 22.Hui SC, Sevilla EL, Ogle CW. 5-HT3 antagonists reduce morphine self-administration in rats. Br J Pharmacol. 1993;110:1341–6. doi: 10.1111/j.1476-5381.1993.tb13966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vera-Portocarrero LP, Zhang ET, King T, Ossipov MH, Vanderah TW, Lai J, Porreca F. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2007;129:35–45. doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 25.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 2001;93:204–9. doi: 10.1097/00000539-200107000-00040. [DOI] [PubMed] [Google Scholar]
- 27.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: A possible role for peripheral α2-adrenoceptors. Anesth Analg. 1998;87:941–8. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Lighthall G, Liang DY, Clark JD. Alterations in spinal cord gene expression after hindpaw formalin injection. J Neurosci Res. 2004;78:533–41. doi: 10.1002/jnr.20274. [DOI] [PubMed] [Google Scholar]
- 29.Liang D, Li X, Clark JD. Increased expression of Ca2+/calmodulin-dependent protein kinase II α during chronic morphine exposure. Neuroscience. 2004;123:769–75. doi: 10.1016/j.neuroscience.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Chen SR, Prunean A, Pan HM, Welker KL, Pan HL. Resistance to morphine analgesic tolerance in rats with deleted transient receptor potential vanilloid type 1-expressing sensory neurons. Neuroscience. 2007;145:676–85. doi: 10.1016/j.neuroscience.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: Involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci. 2008;28:5836–45. doi: 10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Malan TP, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–88. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trang T, Quirion R, Jhamandas K. The spinal basis of opioid tolerance and physical dependence: Involvement of calcitonin gene-related peptide, substance P, and arachidonic acid-derived metabolites. Peptides. 2005;26:1346–55. doi: 10.1016/j.peptides.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Clark JD. Spinal cord nitric oxide synthase and heme oxygenase limit morphine induced analgesia. Brain Res Mol Brain Res. 2001;95:96–102. doi: 10.1016/s0169-328x(01)00251-0. [DOI] [PubMed] [Google Scholar]
- 35.Liang D, Li X, Lighthall G, Clark JD. Heme oxygenase type 2 modulates behavioral and molecular changes during chronic exposure to morphine. Neuroscience. 2003;121:999–1005. doi: 10.1016/s0306-4522(03)00483-4. [DOI] [PubMed] [Google Scholar]
- 36.Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–41. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen SP, Christo PJ, Wang S, Chen L, Stojanovic MP, Shields CH, Brummett C, Mao J. The effect of opioid dose and treatment duration on the perception of a painful standardized clinical stimulus. Reg Anesth Pain Med. 2008;33:199–206. doi: 10.1016/j.rapm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: Effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–46. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 39.Compton P, Kehoe P, Sinha K, Torrington MA, Ling W. Gabapentin improves cold-pressor pain responses in methadone-maintained patients. Drug Alcohol Depend. 109:213–9. doi: 10.1016/j.drugalcdep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Leon-Casasola OA, Lema MJ. Epidural bupivacaine/sufentanil therapy for postoperative pain control in patients tolerant to opioid and unresponsive to epidural bupivacaine/morphine. Anesthesiology. 1994;80:303–9. doi: 10.1097/00000542-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Patanwala AE, Jarzyna DL, Miller MD, Erstad BL. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naive patients after total knee arthroplasty. Pharmacotherapy. 2008;28:1453–60. doi: 10.1592/phco.28.12.1453. [DOI] [PubMed] [Google Scholar]
- 42.Rapp SE, Ready LB, Nessly ML. Acute pain management in patients with prior opioid consumption: A case-controlled retrospective review. Pain. 1995;61:195–201. doi: 10.1016/0304-3959(94)00168-E. [DOI] [PubMed] [Google Scholar]
- 43.Carroll IR, Angst MS, Clark JD. Management of perioperative pain in patients chronically consuming opioids. Reg Anesth Pain Med. 2004;29:576–91. doi: 10.1016/j.rapm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 45.Richebe P, Beaulieu P. Perioperative pain management in the patient treated with opioids: Continuing professional development. Can J Anaesth. 2009;56:969–81. doi: 10.1007/s12630-009-9202-y. [DOI] [PubMed] [Google Scholar]
- 46.Rozen D, DeGaetano NP. Perioperative management of opioid-tolerant chronic pain patients. J Opioid Manag. 2006;2:353–63. doi: 10.5055/jom.2006.0052. [DOI] [PubMed] [Google Scholar]
- 47.Hansen EG, Duedahl TH, Romsing J, Hilsted KL, Dahl JB. Intra-operative remifentanil might influence pain levels in the immediate post-operative period after major abdominal surgery. Acta Anaesthesiol Scand. 2005;49:1464–70. doi: 10.1111/j.1399-6576.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt S, Bethge C, Forster MH, Schafer M. Enhanced postoperative sensitivity to painful pressure stimulation after intraoperative high dose remifentanil in patients without significant surgical site pain. Clin J Pain. 2007;23:605–11. doi: 10.1097/AJP.0b013e318122d1e4. [DOI] [PubMed] [Google Scholar]
- 49.Xuerong Y, Yuguang H, Xia J, Hailan W. Ketamine and lornoxicam for preventing a fentanyl-induced increase in postoperative morphine requirement. Anesth Analg. 2008;107:2032–7. doi: 10.1213/ane.0b013e3181888061. [DOI] [PubMed] [Google Scholar]
- 50.Smith GM, Berry RL, Yang J, Tanelian D. Electrophysiological analysis of dorsal root ganglion neurons pre- and post-coexpression of green fluorescent protein and functional 5-HT3 receptor. J Neurophysiol. 1997;77:3115–21. doi: 10.1152/jn.1997.77.6.3115. [DOI] [PubMed] [Google Scholar]
- 51.Hamon M, Gallissot MC, Menard F, Gozlan H, Bourgoin S, Verge D. 5-HT3 receptor binding sites are on capsaicin-sensitive fibres in the rat spinal cord. Eur J Pharmacol. 1989;164:315–22. doi: 10.1016/0014-2999(89)90472-x. [DOI] [PubMed] [Google Scholar]
- 52.Todorovic SM, Scroggs RS, Anderson EG. Cationic modulation of 5-HT2 and 5-HT3 receptors in rat sensory neurons: The role of K+, Ca2+ and Mg2+ Brain Res. 1997;765:291–300. doi: 10.1016/s0006-8993(97)00574-x. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira MC, Pelegrini-da-Silva A, Parada CA, Tambeli CH. 5-HT acts on nociceptive primary afferents through an indirect mechanism to induce hyperalgesia in the subcutaneous tissue. Neuroscience. 2007;145:708–14. doi: 10.1016/j.neuroscience.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 54.Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. J Neurosci. 1995;15:8031–8. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukushima T, Ohtsubo T, Tsuda M, Yanagawa Y, Hori Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J Neurophysiol. 2009;102:1459–71. doi: 10.1152/jn.91160.2008. [DOI] [PubMed] [Google Scholar]
- 56.Donovan-Rodriguez T, Urch CE, Dickenson AH. Evidence of a role for descending serotonergic facilitation in a rat model of cancer-induced bone pain. Neurosci Lett. 2006;393:237–42. doi: 10.1016/j.neulet.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 57.Svensson CI, Tran TK, Fitzsimmons B, Yaksh TL, Hua XY. Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lett. 2006;580:6629–34. doi: 10.1016/j.febslet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–9. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–86. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang SH, Lee MG. Dose-independent pharmacokinetics of ondansetron in rats: Contribution of hepatic and intestinal first-pass effects to low bioavailability. Biopharm Drug Dispos. 2008;29:414–26. doi: 10.1002/bdd.628. [DOI] [PubMed] [Google Scholar]
- 61.Liu JB, Yao YX, Jiang W. Inhibitory effects of Group I metabotropic glutamate receptors antagonists on the expression of NMDA receptor NR1 subunit in morphine tolerant rats. Neurosci Lett. 2009;452:268–72. doi: 10.1016/j.neulet.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 62.Menard DP, van Rossum D, Kar S, St Pierre S, Sutak M, Jhamandas K, Quirion R. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16:2342–51. doi: 10.1523/JNEUROSCI.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gu G, Kondo I, Hua XY, Yaksh TL. Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: Parallels with tolerance and dependence. J Pharmacol Exp Ther. 2005;314:1362–9. doi: 10.1124/jpet.105.087718. [DOI] [PubMed] [Google Scholar]
- 64.Santamarta I, Perez-Redondo R, Lorenzana LM, Martin JF, Liras P. Different proteins bind to the butyrolactone receptor protein are sequence located upstream of the regulatory ccaR gene of Streptomyces clavuligerus. Mol Microbiol. 2005;56:824–35. doi: 10.1111/j.1365-2958.2005.04581.x. [DOI] [PubMed] [Google Scholar]
- 65.McNulty R. Are all 5-HT3 receptor antagonists the same? J Natl Compr Canc Netw. 2007;5:35–43. doi: 10.6004/jnccn.2007.0005. [DOI] [PubMed] [Google Scholar]