Abstract
Efficacy and safety of medications used for the treatment of astronauts in space may be compromised by altered stability in space. We compared physical and chemical changes with time in 35 formulations contained in identical pharmaceutical kits stowed on the International Space Station (ISS) and on Earth. Active pharmaceutical content (API) was determined by ultra- and high-performance liquid chromatography after returning to Earth. After stowage for 28 months in space, six medications aboard the ISS and two of matching ground controls exhibited changes in physical variables; nine medications from the ISS and 17 from the ground met the United States Pharmacopeia (USP) acceptance criteria for API content after 28 months of storage. A higher percentage of medications from each flight kit had lower API content than the respective ground controls. The number of medications failing API requirement increased as a function of time in space, independent of expiration date. The rate of degradation was faster in space than on the ground for many of the medications, and most solid dosage forms met USP standard for dissolution after storage in space. Cumulative radiation dose was higher and increased with time in space, whereas temperature and humidity remained similar to those on the ground. Exposure to the chronic low dose of ionizing radiation aboard the spacecraft as well as repackaging of solid dosage forms in flight-specific dispensers may adversely affect stability of pharmaceuticals. Characterization of degradation profiles of unstable formulations and identification of chemical attributes of stability in space analog environments on Earth will facilitate development of space-hardy medications.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-011-9270-0) contains supplementary material, which is available to authorized users.
KEY WORDS: chromatography, dissolution, pharmaceutical stability, potency, space radiation
INTRODUCTION
Pharmaceutical stability is a key determinant of therapeutic efficacy and toxicity of medications. The objective of stability testing is to provide evidence as to how the quality of a drug product varies as a function of time and storage conditions such as temperature, humidity, and light, which allows determination of shelf life (expiration date) for a drug product. Stability testing is also used to determine if the container closure system or packaging is suitable (1). Stability testing provides evidence that the quality of a drug substance or drug product under the influence of various environmental factors changes with time (2). The information obtained from stability studies can subsequently be used to provide guidelines on handling and storage, and provide information to guide formulation stabilization strategies (3). Shelf life is defined as the time a product, stored under certain conditions, is expected to remain stable or retain, in most cases, at least 90% of its labeled potency (4). The Food and Drug Administration (FDA) requires that drug companies determine a time limit to which they can guarantee the full potency and safety of medications. This time limit or expiration date is typically set at 1–2 years from the manufacture date for medications stored in the original, unopened containers under recommended conditions.
Efficacious pharmaceuticals with adequate shelf life are essential for successful space medical operations. Therefore, stability of pharmaceuticals is of paramount importance to ensure health and wellness of astronauts on future space exploration missions. Unique physical and environmental factors of space missions such as radiation, excessive vibration, microgravity, and an enclosed and CO2-rich environment, in addition to humidity and temperature variations, may contribute to instability of pharmaceutical dosage forms. Alterations in physical and/or chemical stability of a formulation can result in reduced potency (5). Degradation of pharmaceuticals can result in inadequate efficacy and untoward toxic effects that could compromise astronaut safety and health. Over the life of NASA’s human spaceflight program, duration of missions has increased, resulting in a concomitant increase in demand for pharmaceuticals during flights. The use of pharmaceuticals during space shuttle missions is common with crews taking more than 500 individual doses of 31 different medications during the first 33 flights (6). Primarily, medications taken during shuttle flights have been orally administered, although ocular, topical, rectal, and parenteral formulations are included in the onboard operational medical kits.
Stability characteristics and industry standards for shelf life are well established for terrestrial environments. For example, photolytic degradation of drugs by exposure to the low energy of visible light (5–11) and fluorescent light (12–14) is a well-documented phenomenon. Light is destructive to many drug classes, making amber bottles standard for dispensing most pharmaceuticals. Although amber-colored bottles are effective in protecting drugs from destructive exposure to components of visible light, they do not protect drugs from other forms of radiation that may affect drug stability in space.
On Earth, stability and shelf life of commercial pharmaceutical preparations is ensured by designing protective packaging and dispensing practices, for example, humidity and temperature conditions for storage are specified on the basis of results from accelerated stability studies required before the product is released to the marketplace (13,15). A comprehensive stability study includes characterization of hygroscopicity, dehydration, physical and chemical degradation, drug release pattern, hardness, photosensitivity as a function of relative humidity and temperature, gas liberation tendency, product packaging material interaction, and dimensional aspects (16). Contrary to standard dispensing practices on Earth, pharmaceuticals are packed and dispensed in special flight-certified containers and stored in compactly packed kits, and this packaging could affect stability and shelf life of pharmaceuticals in space. This investigation aims to evaluate the effect of prolonged exposure to the space craft environment on the stability of pharmaceuticals. The objective was to identify differences in variables that indicate physical and chemical stability of medications between ground controls and spaceflight; chemical degradation profiles of formulations and relevant environmental variables of temperature, humidity, and cumulative radiation dose were compared for the two conditions.
MATERIALS AND METHODS
Experimental payload kits were fabricated to match the design and materials used for operational medication package flown aboard the International Space Station (ISS) (Fig. 1). Medications in this study represent 18% of the formulary flown on spaceflights and include those commonly used by astronauts during flight, candidates from therapeutic classes contained in the ISS and shuttle medical kits, different dosage forms, and formulations in unique commercial dispensers. Table S I of the supplementary material (SM) is a list of formulations selected for the study. A sufficient quantity of each medication from the same lot was purchased from a local pharmacy vendor and packed in these specially designed identical kits that were used as ground control and flight medication payload kits. All kits were equipped with passive radiation dosimeters (provided by the NASA Space Radiation Analysis Group) and with temperature and relative humidity (RH) recorders (HOBO® U12). Twenty-two of the 24 solid medications (tablets and capsules) were packed in custom-manufactured polypropylene flight medication bottles analogous to those used in operational medical kits for spaceflight (Wyle Laboratories, NASA Johnson Space Center, Houston, TX, USA). The remaining medications were flown in their original commercial packaging.
Fig. 1.
Experimental payload and ISS operational medications kits
Four kits were stowed on board a Space Transportation System (STS) flight for delivery to the ISS; four matching kits were stored in temperature- and humidity-controlled environmental chambers at the Johnson Space Center (JSC) Pharmacotherapeutics Laboratory to serve as time- and lot-matched ground controls. When the shuttle orbiter docked with the ISS, three of the four kits aboard the STS were transferred to the ISS for stowage, to be returned to Earth at predesignated time intervals aboard assigned STS flights; the remaining kit on the STS was returned to Earth after 13 days aboard the same STS flight representing the short duration spaceflight environment. Figure 2 shows the schedule of sample return from the ISS aboard the STS flights.
Fig. 2.
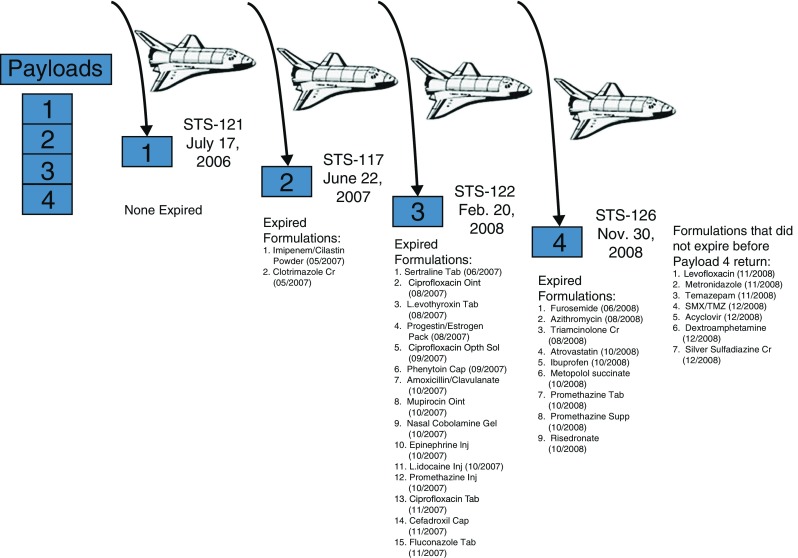
Flight payload kit manifestation and return schedule. Each kit contained 33 dosage forms as 22 solid, seven semisolid, and four liquid formulations
Sample Analysis
All physical and chemical analyses were conducted simultaneously for ground and flight samples to minimize analytical variability. The dispensing details, sample size, and physical variables measured for each formulation type are presented in Table I. Once on the ground, flight kits were stored in the same environmental chamber as the control kits until they were analyzed. All physical and chemical analyses were completed within 6–8 weeks after the flight kits were returned to Earth.
Table I.
Formulation Type and Physical Variables Assessed
| Formulation type | Number in each kit | Number repackaged | Physical variables | Number of unit doses tested |
|---|---|---|---|---|
| Solids (tablet and capsule) | 24 | 22 | Weight, appearance, color, odor, caking, clumping, hardness, and friability | 3–6 |
| Semisolids (gel, cream, ointment, and suppository) | 7 | 0 | Cracking, melting, drying, and phase separation | 1–3 |
| Liquids (ophthalmic and injectable) | 4 | 0 | Microbial count and pH | 1 |
Temperature and RH data were retrieved from HOBO® U12 Temp/RH Data Loggers after the return of each flight kit along with the data from the time-matched control kit. The cumulative radiation dose for the shuttle and ISS was estimated from the dosimeter in each flight kit and reported by the Space Radiation Analysis Group at the JSC. Radiation on the ground was monitored using passive dosimeters placed in the environmental chamber; dosimeters and radiation reports were provided by contract vendors.
Procedures for the assessment of stability-indicating physical attributes, chemical potency, and dosage form performance (physical and chemical) were obtained from the most current version of the United States Pharmacopeia (USP).
Physical Variables
Most physical variables were assessed by visual observation and by using standard instrumentation for verification of compliance with pharmaceutical stability. Weight, physical appearance, color, odor, and texture of solid dosage forms (capsule, tablet) were examined for physical characterization. Tablet hardness and friability were determined for compressed and coated tablets, whereas caking, clumping, and drying of content were assessed for capsules. For semisolid (gel, cream, ointment, and suppository) formulations, physical characterization also included cracking, liquefaction, drying, and phase separation. Liquid and injectable formulations were examined for presence of particulates, microbial contamination according to standard operating procedures of the NASA microbiology laboratory, and pH.
Chemical Content Analysis
The chemical content of each formulation was determined by high-performance or ultrahigh-performance liquid chromatography (HPLC, UPLC) according to standard USP methods for 28 formulations and from published literature for five formulations. The two multiple vitamin preparations were not analyzed since the vitamin content of nutritional supplements before and after stowage in space were reported elsewhere (17). All assay methods were validated using commercial reference standards before analyzing experimental samples. All active standards were purchased from USP (Rockville, MD) and analytical grade reagents for HPLC were procured from Sigma (St. Louis, MO) or Fisher (Pittsburgh, PA). Deionized water from Milli-Q Plus, Billerica, MA ultrapure water system was used for all standards, sample preparation, and analyses.
Assay samples were prepared by a modified USP method as follows. Six tablets were weighed individually and pulverized together; three separate pulverized powder samples of equivalent mean tablet weight to represent three tablets were used for content analysis, each of the three samples were assayed in duplicate using the UPLC/HPLC system to determine chemical content.
Dissolution Performance Test
A dissolution test was performed according to standard USP methods for two capsule and ten tablet dosage forms using a Distek dissolution apparatus. Three tablets/capsules of each formulation were assessed, and triplicate samples at each dissolution time point were analyzed to determine active pharmaceutical ingredient (API) content.
Data Analysis
The acceptance criteria for shelf life published by the USP for each drug product in accordance with FDA-adopted Guidance ICH Q1A (R2) were examined, and the total number of formulations that met or failed stability-indicating physical and chemical parameters after each storage period was compared between space and ground control conditions. Degradation profiles for API were compiled using the percentage of label content remaining at the end of each experimental time interval (Fig. 2) and were compared between the two conditions using linear regression analysis. The following acceptance criteria were used for determining product viability:
USP acceptance criteria for chemical potency or ≤10% decrease in percent content from label strength, where USP criteria are unavailable
Acceptance criteria for appearance and physical attributes (e.g., discoloration, phase separation) and physical performance test of hardness and friability
Acceptance criterion for pH (liquid formulations)
USP limits for microbiological contamination (sterile liquid formulations)
USP acceptance criteria for API dissolution performance (dissolution percentage at USP tolerance time interval, Q)
Values of stability-indicating variables of formulations in the matching ground-based kits served as the control or standard condition to which results from flight were compared.
RESULTS
Physical Variables
No changes in physical appearance were observed in ground control samples of formulations from payloads 1–3 while physical changes in some of the solid and semisolid formulations after flight were observed with samples from all the four payloads. However, discoloration of amoxicillin/clavulanate (Augmentin®) tablets and liquefaction of ciprofloxacin ophthalmic ointment were noticed also in control samples from payload 4. A list of formulations with physical changes in flight samples is presented in Table II. While the most frequently observed physical change in flight samples was discoloration, samples of clotrimazole cream and mupirocin ointment from payload 4 exhibited phase separation. Ciprofloxacin ophthalmic ointment was the only semisolid formulation with liquefaction in both control and flight samples from payload 4; however, the storage period for this payload (880 days) was beyond the labeled expiration date for this formulation. An interesting observation is that the number of formulations with physical changes was higher in flight samples from all payloads than in controls but variable between payloads. Six formulations from payload 2 flight samples had discoloration, and the number but not the formulations were the same in later payloads as well (3 and 4). All of the six formulations were within labeled expiration date during payload 2 time period while five of them expired by the time of the return of payload 4.
Table II.
Formulations with Physical Changes after Spaceflight
| Payload 1 | Payload 2 | Payload 3 | Payload 4 |
|---|---|---|---|
| Promethazine suppository | Acyclovir tablet | Dextroamphetamine tablet | Amoxicillin/clavulanate tableta |
| Triamcinolone cream | Amoxicillin/clavulanate tablet | Imipenem/cilastatin powdera | Ciprofloxacin ophthalmic ointmenta |
| Ciprofloxacin tablet | Levothyroxine tableta | Fluconazole tableta | |
| Ciprofloxacin ophthalmic ointment | Promethazine suppositorya | Imipenem/cilastatin powdera | |
| Dextroamphetamine tablet | Silver sulfadiazine cream | Silver sulfadiazine creama | |
| Levothyroxine tablet | Triamcinolone cream | Sulfamethoxazole/trimethoprim tablet | |
| Metronidazole tablet |
aReturned after expiration date
Chemical Content
The data presented in Table III suggest that the number of formulations that did not meet content requirement of API was higher in flight kits compared to the corresponding control kits from all four payloads. This difference in the number of unstable formulations between flight and control increased with the length of storage time in space and consistently fewer formulations from the flight kit met acceptance criteria for API content than from the respective control kit at the end of each payload period. After 880 days of storage in flight (payload 4), only 27% of solid formulations met the acceptance criteria for content. After 596 days (payload 3) in flight, fewer than half of the solid dosage forms in the flight payload kit met content acceptance criteria. A list of medications from control and flight payload kits with percentage of chemical content less than the USP requirement or less than 10% of the label claim is presented in Table S II of SM. A list of stable formulations after each payload is presented in Table S III A of SM. Of these formulations listed, acyclovir, the only antiviral formulation in this study and metronidazole, an antibiotic, had expiration dates beyond the study period; the other seven formulations were stable, both on the ground and in space, beyond their expiration date.
Table III.
Formulations Failing Chemical Potency Requirement
| Payload 1 | Payload 2 | Payload 3 | Payload 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Flight | Control | Flight | Control | Flight | Control | Flight | |
| Number of failed formulations | 0 | 1 | 2 | 11 | 8 | 17 | 16 | 24 |
| Number of expired formulations | 0 | 2 | 18 | 26 | ||||
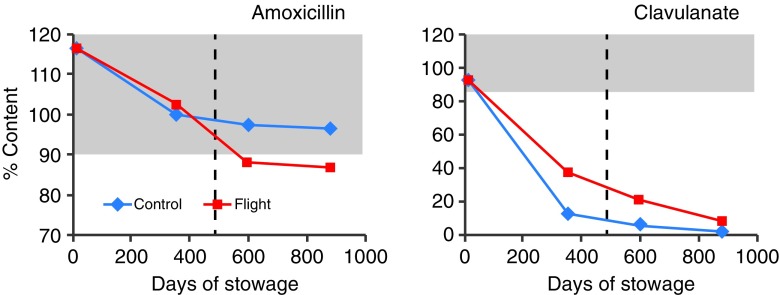
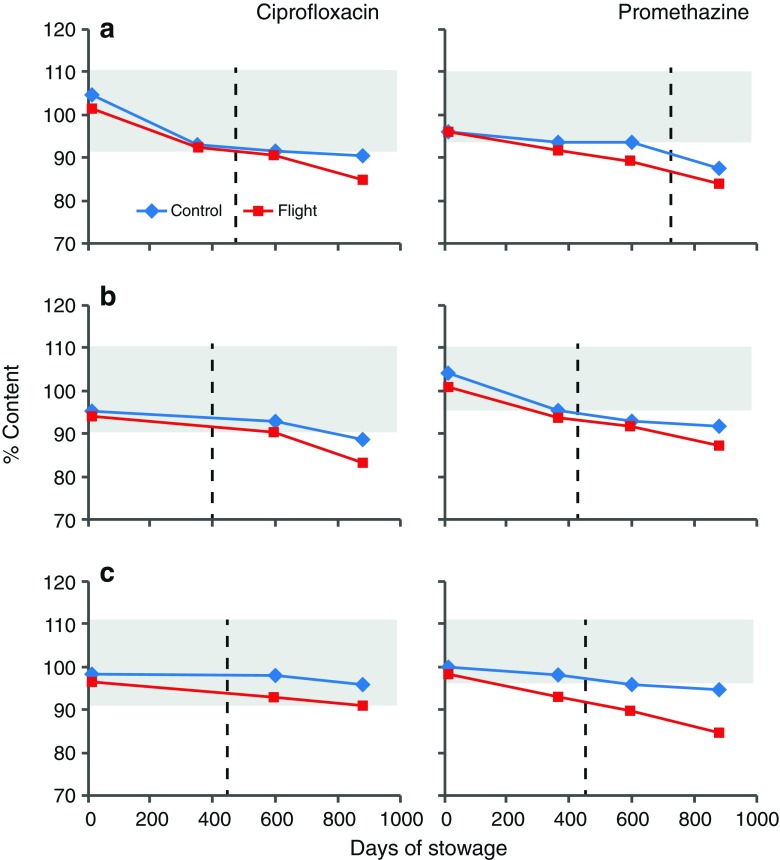
The percent content of amoxicillin and clavulanate after each payload period, presented in Fig. 3, shows a faster degradation of clavulanate on the ground than in flight. These data indicate that clavulanate may be inherently unstable and is more susceptible to environmental factors on the ground. Degradation profiles for ciprofloxacin and promethazine (PMZ), the two medications that were flown in three different formulations, are presented in Fig. 4. The API content for the formulations of both medications is comparable between flight and ground. Ciprofloxacin ophthalmic solution from flight and control kits had acceptable API content beyond labeled expiration date while PMZ injection degraded faster during flight, reaching less than acceptable potency before the expiration date. The potency of PMZ tablets from both control and flight was below acceptable limit by payload 2 period onwards prior to expiration date. Degradation profiles for other antibiotic tablet formulations are presented in Fig. 5. As can be seen, another combination antibiotic formulation, trimethoprim/sulfamethoxazole had lower API content in flight samples than in corresponding controls while all other antibiotics had comparable API content between flight and control.
Fig. 3.
Degradation of amoxicillin and clavulanate in augmentin tablets. Each data point represents one of four payloads. Shaded area represents USP range for label claim; dashed line indicates labeled expiration date
Fig. 4.
Degradation of ciprofloxacin and promethazine dosage forms. a Solid, b semisolid, c liquid. Each data point represents one of four payloads. Shaded area represents USP range for label claim; dashed lines indicate labeled expiration date
Fig. 5.
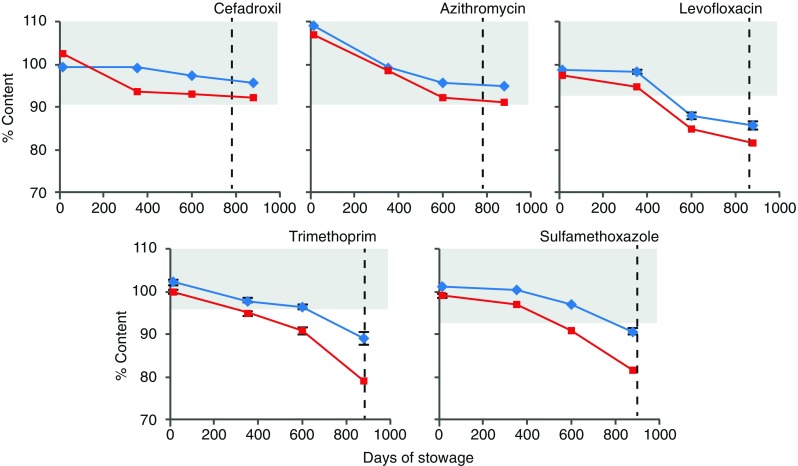
Degradation of antibiotic tablets. Each data point represents one of four payloads. Shaded area represents USP range for label claim; dashed lines indicate labeled expiration date
Linear regression analysis of percent API content as a function of duration of stowage was performed for comparing degradation of formulations between control and spaceflight. These data are presented in Tables III S A and III S B of SM. While the correlation coefficient values (r2) of linear regression for most of the formulations from spaceflight and control indicate acceptable fit (0.62–0.99), it is apparent from the low r2 values observed for both control and flight degradation profiles of cilastatin in imipenem/cilastatin formulation that linear regression analysis may not adequately describe degradation of this API in this formulation. Interestingly, degradation appears to be marginal for this API under both conditions. In contrary, imipenem in the same formulation in addition to ibuprofen tablets had low r2 values for control but not for flight samples suggesting that degradation profiles may be different between control and flight for these two APIs. Atorvastatin, cefadroxil, and PMZ liquid formulation degraded more than two times faster in flight than on the ground (Table S III B).
It is apparent that clavulanate in amoxicillin/clavulanate (Augmentin®) was the most unstable API. The rates of degradation of clavulanate on the ground and in spaceflight were 0.1005% and 0.0936%/100 days, with a predicted loss of potency close to 50% occurring by 489 and 534 days, respectively, much sooner than the expiration date. In general, while degradation was faster in space than on the ground for most of the APIs, loss of API content was <20% of label claim except for levothyroxine and trimethoprim which were 28% and 21%, respectively.
The percentage of dissolution at USP tolerance time interval (Q) from control and flight samples for 12 solid dosage forms after each payload period are presented in Table IV and compared between the two conditions. These results indicate that most formulations from the control and spaceflight met USP dissolution standards. The dissolution of clavulanate from amoxicillin/clavulanate tablets did not meet USP tolerance standard and was very low due to its chemical degradation in both control and flight samples from all four payloads. In contrast, the Q values for amoxicillin from the same formulation met USP standard for all samples. The dissolution of temazepam tablets and sulfamethoxazole in sulfamethoxazole/trimethoprim tablets from payload 4 did not meet USP tolerance standard (Table IV) for both control and flight.
Table IV.
Dissolution Percent of API at USP Tolerance Time Period (Q) for Solid Formulations
| Payload | Payload 1 | Payload 2 | Payload 3 | Payload 4 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Label | USP | Control | Flight | Control | Flight | Control | Flight | Control | Flight | ||||||||
| Active Substance | (mg) | Q (% in min) | Q (%) | SD | Q (%) | SD | Q (%) | SD | Q (%) | SD | Q | SD | Q | SD | Q (%) | SD | Q (%) | SD |
| Acyclovir | 400 | 80 in 45 | 99.8 | 0.27 | 99.1 | 0.51 | 97.5 | 1.76 | 98.8 | 0.23 | 98.6 | 0.37 | 95.7 | 0.80 | 94.3 | 0.73 | 91.8 | 1.06 |
| Amoxicillin/ | 875 | 85 in 30 | 118.1 | 1.99 | 113.9 | 0.96 | 100.6 | 1.14 | 99.7 | 0.66 | 98.1 | 0.68 | 88.4 | 2.54 | 85.6 | 0.65 | 86.1 | 0.17 |
| Clavulanate | 125 | 80 in 30 | 31.6 | 4.51 | 44.4 | 1.55 | 11.1 | 0.05 | 32.8 | 0.13 | 6.2 | 0.22 | 20.3 | 1.28 | 4.4 | 0.84 | 8.7 | 1.81 |
| Cefadroxil | 500 | 80 in 30 | 100.4 | 0.44 | 92.4 | 2.51 | 98.8 | 1.63 | 97 | 3.94 | 97.5 | 3.40 | 91.9 | 4.92 | 95.2 | 0.90 | 91.0 | 2.43 |
| Ciprofloxacin (T) | 500 | 80 in 30 | 94.3 | 1.28 | 92.2 | 3.02 | 88.3 | 4.07 | 89.0 | 0.09 | 90.7 | 1.02 | 89.8 | 0.47 | 84.7 | 0.48 | 82.1 | 0.19 |
| Dextroamphetamine | 5 | 75 in 45 | 93.5 | 0.38 | 95.2 | 3.93 | 104.1 | 5.03 | 94.8 | 3.05 | 92.7 | 5.23 | 86.6 | 5.14 | 83.0 | 1.79 | 79.1 | 4.16 |
| Furosemide | 20 | 80 in 60 | 98.6 | 2.55 | 98.5 | 1.09 | 97.0 | 0.11 | 97.9 | 1.17 | 85.2 | 0.11 | 81.2 | 1.17 | 80.4 | 0.39 | 79.8 | 2.74 |
| Ibuprofen | 400 | 80 in 60 | 97.4 | 2.93 | 99.7 | 0.28 | 99.2 | 0.97 | 98.4 | 2.01 | 97.5 | 0.31 | 93.5 | 1.32 | 94.6 | 0.30 | 91.6 | 2.29 |
| Levothyroxine | 0.025 | 70 in 45 | 83.4 | 1.77 | 81.6 | 0.62 | 90.7 | 1.52 | 92.9 | 9.09 | 82.8 | 6.92 | 71.2 | 4.61 | 81.0 | 11.83 | 82.9 | 2.14 |
| Metronidazole | 250 | 85 in 60 | 99.9 | 0.09 | 100.0 | 0.07 | 99.9 | 0.00 | 99.9 | 0.00 | 92.9 | 0.70 | 91.1 | 0.93 | 91.5 | 0.28 | 88.6 | 0.68 |
| Promethazine (T) | 25 | 75 in 45 | 100.0 | 0.00 | 99.8 | 0.28 | 99.8 | 0.23 | 100.0 | 0.04 | 100.0 | 0.00 | 100.0 | 0.00 | 85.1 | 0.12 | 80.9 | 0.13 |
| Temazepam | 15 | 80 in 30 | 85.8 | 3.04 | 104.8 | 4.25 | 91.4 | 1.17 | 89.4 | 3.23 | 87.9 | 0.96 | 86.4 | 1.30 | 69.4 | 1.37 | 64.5 | 6.37 |
| Sulfamethoxazole/ | 800 | 75 in 60 | 90.5 | 4.24 | 88.0 | 0.06 | 97.5 | 0.14 | 97.0 | 0.76 | 81.1 | 1.12 | 80.0 | 1.19 | 72.7 | 0.76 | 69.4 | 0.08 |
| Trimethoprim | 160 | 75 in 60 | 95.9 | 0.43 | 93.1 | 5.38 | 95.7 | 0.80 | 94.1 | 0.96 | 95.1 | 0.69 | 92.7 | 0.49 | 80.7 | 0.04 | 78.7 | 0.75 |
Numbers in bold indicate failure to meet USP standard
SD standard deviation of mean
Environmental Factors
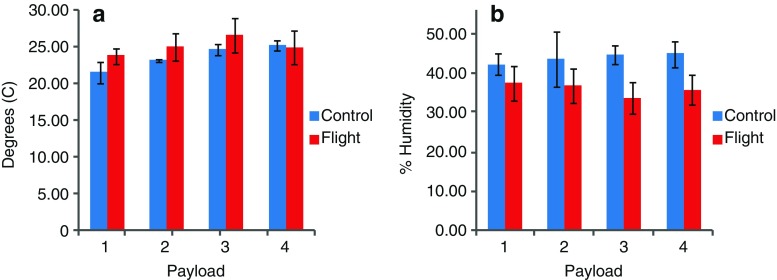
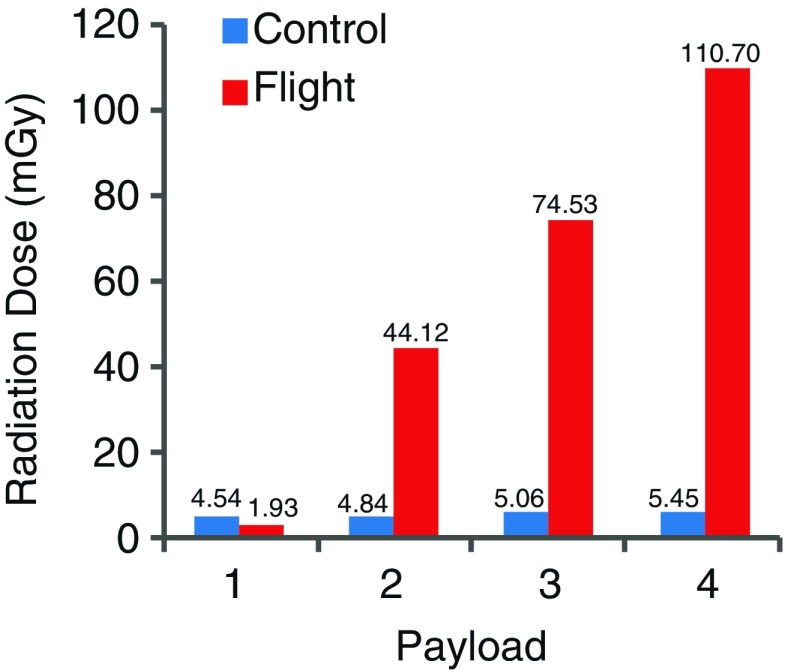
The mean temperature and relative humidity data for control and flight for all four payloads are presented in Fig. 6. Temperature and humidity were similar for ground and flight conditions, and they remained within the USP recommended range. Although the average percent humidity for all payloads remained well within the USP-defined range during flight, there were isolated incidents of RH values falling below the recommended minimal level (30%). As expected, the cumulative radiation dose for control conditions for all four payloads (5.45 mGy) was negligible and substantially lower than the cumulative radiation dose during flight (110.7 mGy), and the accumulation rate appears to be linear (Fig. 7).
Fig. 6.
Comparison of mean temperature (a) and relative humidity (b) conditions between ground and spaceflight
Fig. 7.
Comparison of cumulative radiation dose between ground and spaceflight
DISCUSSION
Results of this investigation constitute a preliminary but a systematic evaluation for the first time, of changes in physical and chemical variables of pharmaceuticals stored on space missions. In general, a number of formulations tested had a lower potency or percent content of API after storage in space with a consistently higher number of formulations failing USP potency requirement after each storage period interval in space than on Earth (Table III). This reduction in potency of flight samples occurred sooner than the labeled expiration date for many formulations suggesting that storage conditions unique to the spacecraft environment may influence stability of pharmaceuticals in space.
On Earth, drug potency is known to vary with storage conditions, especially temperature and humidity. Many drugs stored under standard conditions retain 90% of their potency for at least 5 years after the expiration date on the label and sometimes for even longer periods (18). However, most stability studies and guidance pertain to pharmaceutical products dispensed in original commercial containers in contrast to the ones used in this study which are special custom-manufactured polypropylene containers that match those used for dispensing solid dosage forms in operational medical kits; such off-nominal packaging may compromise stability of formulations. Additionally, susceptibility of formulations to spacecraft environment appears to depend also on the specific physical–chemical characteristics of formulations tested.
Susceptibility of API
Certain APIs such as levothyroxine, dextroamphetamine, promethazine, trimethoprim, sulfamethoxazole, and clavulanate appear to be more susceptible to spaceflight environmental conditions than others in the study. Levothyroxine was the only API that fell below potency level in flight samples from all four payloads while remained stable on ground until after the first two payload intervals (332 days). Additionally, levothyroxine had a higher percent degradation than others with more than 20% loss of API after 880 days of storage in space (Table S III B). Levothyroxine formulation is known to be unstable on the ground (19), and these results suggest that this formulation may not be suitable for spaceflight.
Promethazine and dextroamphetamine, the two light-sensitive stereoisomer compounds (20), also appear to be more susceptible to spaceflight conditions failing potency requirement after storage for 353 days in space which was earlier than expiration date. Similarly, ciprofloxacin, another light-sensitive compound (11) also appears to have degraded faster in space than on the ground (Fig. 3) suggesting that the rate of degradation for light-sensitive pharmaceuticals may be accelerated during spaceflight. Interestingly, the degradation rate of promethazine liquid in flight samples was more than 200% faster than in controls. A contributing factor for the observed faster degradation in flight with light-sensitive APIs could come from the radiation-rich environment of the spacecraft.
In contrast to the results with light-sensitive APIs, the percentage of degradation of clavulanate was higher on the ground than in spaceflight, probably resulting from the higher RH (36–44%) recorded on the ground than in flight (23–25%) since clavulanate is very sensitive to RH differences. Amoxicillin, the other API in this combination antibiotic formulation was stable with acceptable potency on the ground and in space for over 353 days, consistent with reports of lower susceptibility of this compound to environmental conditions (9,21,22).
Susceptibility of Dosage Form
To assess relative susceptibility of dosage forms to spaceflights environment, solid, semisolid, and liquid formulations of PMZ and ciprofloxacin, included in the ISS medical kits, were examined. The rate of degradation for all three formulations for both drugs was slightly faster in flight than on the ground as indicated by the difference in degradation profiles of API between the two conditions; this difference was greater with PMZ than with ciprofloxacin. Interestingly, the liquid formulation of ciprofloxacin in sealed commercial vials, remained potent beyond its expiration date in contrast to PMZ which degraded faster during flight. It is noteworthy that for light-sensitive APIs like promethazine, commercial dispensers may not provide adequate protection against radiation-rich spacecraft environment and might warrant the need for the development of space-hardy dispensing technologies.
However, consistent with the general consensus on the ground that stability issues are less frequent with solid than with liquid or semisolid formulations (18), seven of nine formulations that remained potent by the end of the study period (880 days) were solid dosages in spite of being repackaged in flight dispensers, and the other two stable formulations, triamcinolone cream and ciprofloxacin ointment, were flown in commercial dispensers.
Susceptibility of Anti-Infective Formulations
Treatment efficacy of infections in space is critical for mission success and astronaut safety for which potency is a contributing factor. For this reason, 13 anti-infectives consisting of ten antibiotic, two antifungal, and one antiviral compounds were included in the study, and ten of the formulations were solid dosage forms. The two combination antibiotic formulations, sulfamethoxazole/trimethoprim and amoxicillin/clavulanate degraded faster in space than on the ground with amoxicillin/clavulanate combination being the least stable preparation both on the ground and in space. Imipenem/cilastatin, another combination antibiotic, was flown in its original commercial vial and appeared to be relatively stable both on the ground and in flight. Two formulations, metronidazole and acyclovir antiviral formulation were stable in space, concurrent with reports of their sustained stability on the ground (23,24).
In a related ground-based study conducted by the FDA under the Shelf Life Extension Program (SLEP), stability of medications stored in their original containers under ideal storage conditions (cool, dry, and dark conditions) beyond their label expiration date was examined in an attempt to extend shelf life of expensive formulations such as the antibiotics. Results of this study indicated that ciprofloxacin stored unopened in commercial containers was stable for more than 10 years, with a shelf life extension up to 13 years. Results also indicated that 88% of the 3,005 lots of 122 medications tested were stable and remained potent for an average 66 months after their expiration date (9). However, while the SLEP study aims to extend shelf life of stock pharmaceuticals in sealed commercial containers, in the present investigation; we examined potency of repackaged antibiotics exposed to the unique environmental conditions of spaceflight. Our results indicate that 18 of the 33 medications even in the control kits on the ground were not stable by the end of the study period (28 months) representing less than half of the average shelf life observed for medications in the SLEP study. This disparity in potency and shelf life of medications between the two studies may be attributed to the dispensing and storage conditions, especially with the solid dosage forms kept in unopened commercially sealed containers for the SLEP formulations as opposed to those repackaged in polypropylene vials in our study. This difference in dispensing conditions offers compelling evidence in favor of the observation that repackaging solid dosage forms in containers used in space medical kits may compromise stability and shelf life of medications and increases susceptibility to adverse environmental conditions in space as well as on the ground. For this reason, FDA guidance limits shelf life of pharmaceuticals to 1 year from the date of dispensing.
At present, medication lots contained in the ISS operational formulary that are within 6 months of labeled expiration date are replaced as needed in compliance with FDA guidelines. However, this will not be possible for exploration missions planned for the future which warrants the need for research and development of space-hardy formulations as well as pharmaceutical dispensing technologies. Additionally, identification of those formulations that remain stable in space beyond their expiration date, especially with respect to expensive antibiotics like ciprofloxacin, could provide valuable information on evidence-based cost saving procedures for ISS formulary operations in the future.
Results on dissolution performance of solid dosage forms indicate that no major differences exist between control and flight samples with most formulations meeting USP dissolution tolerance standards in both conditions. These results might suggest that environmental conditions unique to spaceflight may affect chemical degradation but not dissolution performance of most solid dosage forms during prolonged storage in space.
Radiation Environment of Spaceflight
It is noteworthy that in contrast to contributing environmental factors of stability on the ground, results from passive radiation dosimetry demonstrate that the cumulative radiation dose, although at a low level, was much higher in space than on Earth; this radiation enrichment in space is composed of ionizing radiation of protons and heavy ions. The concept of radiation-induced chemical degradation has been suggested in numerous studies in the literature (25–28), but little systematic research has been done in this area. Although gamma radiation is known to be used to sterilize pharmaceuticals in commercial environments, space radiation consists of mixed fluences of high- and low-intensity radiation (29,30) with a lower dose rate and longer duration of accumulation in contrast to exposure for pharmaceutical sterilization that typically last for a few minutes. In our study, the rate of accumulation of radiation dose was consistent through the time intervals between the two ISS payload time intervals, 31and 36 mGy accumulated by the time of return of payloads 3 and 4 with 12.5% and 12.7% accumulation per day, respectively.
Studies of radiation effects on pharmaceuticals on the Earth are not aimed to understand or estimate the effect of fragmentation ions on stability, thus, the extent of damage caused by different daughter ions to each class of medication is unknown. With respect to medications, in addition to the concern about radiation effects that can induce significant changes in the API levels or potency of the medication, it is rather important to determine structure and biological activity of degradation products and establish toxicity limits of active degradants in a formulation. This may pose a greater risk than efficacy for the assessment of therapeutic index of a formulation degraded in space. As such, information on differences in the degradation products formed during ground versus flight conditions will aid the assessment of therapeutic index of formulations packed in operational medical kits on board long duration space missions. To this end, accelerated stability studies in a simulated spaceflight environment that include variables such as vibration, gravity fluctuations, and ionizing proton and heavy ion radiation on the ground with the unstable formulations identified in this study will allow characterization of degradation profiles of APIs and facilitate the assessment of safety of medications in space. Additionally, research on new and emerging formulation and packaging technologies may enhance and ensure adequate shelf life of medications in space.
Finally, constraints associated with conducting research in space limit the opportunity for robust experimental design and adequate statistical rigor. However, this study attempts to examine whether or not the issue of pharmaceutical stability exists in space. To this end, results presented here clearly demonstrate that while certain formulations like levothyroxine and amoxicillin/clavulanate are inherently unstable and may not be suitable for space formulary, physical–chemical characteristics of others that appear to be unstable in space are due, at least in part, to the effect of unique environmental factors other than temperature and humidity unlike on Earth. As such, it is important to characterize space-specific degradation products and toxicity limits using ground-based analog environments of space that include proton and heavy ion radiation, vibration, and multiple gravity conditions. This information can facilitate research for the development of space-hardy pharmaceuticals and packaging technologies.
CONCLUSION
A spaceflight presents unique environmental challenges that include radiation enrichment, excessive vibration, and off-nominal gravity environments which might affect pharmaceutical stability in space. Results from this preliminary investigation designed to examine changes in chemical content and physical attributes of dosage forms stored aboard space missions suggest that there may be differences with respect to potency and rate of degradation of formulations stored in space compared to those on the ground. Some APIs and formulations may be more sensitive than others, and specific chemical and formulation characteristics may influence the stability of medications in space. Cumulative low-dose radiation and dispensers used for solid dosages in space appear to influence stability of pharmaceuticals in space.
ELECTRONIC SUPPLEMENTARY MATERIALS
Below is the link to the electronic supplementary material.
(DOC 51 kb)
(DOC 63 kb)
(DOC 66 kb)
(DOC 131 kb)
ACKNOWLEDGMENTS
This research was funded by an NRA research grant from the National Aeronautics and Space Administration (NASA).
The authors acknowledge and appreciate the technical support provided by team members of the NASA Non-exercise Physiological Countermeasures Project and the International Space Station Medical Project. We thank Dr. Ramona Garza, a scientist from Universities Space Research Association, for providing reports of radiation dosimetry data and for her guidance on space radiation results for this study.
REFERENCES
- 1.ICH. ICH quality guidelines. 2010. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm065005.htm.
- 2.Lucas T, Bishara R, Seevers RH. A stability program for the distribution of drug products. Pharm Technol. 2004;2:68–73. [Google Scholar]
- 3.Chen XQ, Antman MD, Gesenberg C, Gudmundsson OS. Discovery pharmaceutics—challenges and opportunities. AAPS J. 2006;8(2):E402–E408. doi: 10.1007/BF02854912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carstensen JT. Stability of solids and solid dosage forms. J Pharm Sci. 1974;63(1):1–14. doi: 10.1002/jps.2600630103. [DOI] [PubMed] [Google Scholar]
- 5.Putcha L, Berens KL, Marshburn TH, Ortega HJ, Billica RD. Pharmaceutical use by U.S. astronauts on space shuttle missions. Aviat Space Environ Med. 1999;70(7):705–708. [PubMed] [Google Scholar]
- 6.Tietze KJ, Putcha L. Factors affecting drug bioavailability in space. J Clin Pharmacol. 1994;34(6):671–676. doi: 10.1002/j.1552-4604.1994.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 7.Cosa G, Scaiano JC. Laser techniques in the study of drug photochemistry. Photochem Photobiol. 2004;80(2):159–174. doi: 10.1562/2004-04-24-IR-153.1. [DOI] [PubMed] [Google Scholar]
- 8.Langner MD, Maibach HI. Many common drugs in dermatology are light, temperature, or moisture-sensitive. Skin Therapy Lett. 2009;14(1):3–5. [PubMed] [Google Scholar]
- 9.Lyon RC, Taylor JS, Porter DA, Prasanna HR, Hussain AS. Stability profiles of drug products extended beyond labeled expiration dates. J Pharm Sci. 2006;95(7):1549–1560. doi: 10.1002/jps.20636. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Kojima K, Nagano H, Matsubara S, Yokota T. Photostability and biological activity of fluoroquinolones substituted at the 8 position after UV irradiation. Antimicrob Agents Chemother. 1992;36(8):1715–1719. doi: 10.1128/aac.36.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips G, Johnson BE, Ferguson J. The loss of antibiotic activity of ciprofloxacin by photodegradation. J Antimicrob Chemother. 1990;26(6):783–789. doi: 10.1093/jac/26.6.783. [DOI] [PubMed] [Google Scholar]
- 12.Okeke CC, Srinivasan VS. Expiration and beyond-use dates. Am J Health Syst Pharm. 1998;55(5):433–434. doi: 10.1093/ajhp/55.5.433. [DOI] [PubMed] [Google Scholar]
- 13.Okeke CC, Bailey L, Medwick T, Grady LT. Revised USP standards for product dating, packaging, and temperature monitoring. Am J Health Syst Pharm. 2000;57(15):1441–1445. doi: 10.1093/ajhp/57.15.1441. [DOI] [PubMed] [Google Scholar]
- 14.Parks OW. Screening tests for sulfa drugs and/or dinitrobenzamide coccidiostats and their monoamino metabolites in chicken livers. J Assoc Off Anal Chem. 1985;68(1):20–23. [PubMed] [Google Scholar]
- 15.Allinson JG, Dansereau RJ, Sakr A. The effects of packaging on the stability of a moisture sensitive compound. Int J Pharm. 2001;221(1–2):49–56. doi: 10.1016/S0378-5173(01)00670-6. [DOI] [PubMed] [Google Scholar]
- 16.Van Weeren R, Sashidharan A. Sensitivity profiling of solid oral doses. Pharm Process. Apr 22 2008; 1–5.
- 17.Zwart SR, Kloeris VL, Perchonok MH, Braby L, Smith SM. Assessment of nutrient stability in foods from the space food system after long-duration space flight on the ISS. Food Sci. 2009;74(7):209–217. doi: 10.1111/j.1750-3841.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 18.Abramowicz M. Drugs past their expiration date. Med Lett. 2002;44(W1142B):93–94. [PubMed] [Google Scholar]
- 19.Patel H, Stalcup A, Dansereau R, Sakr A. The effect of excipients on the stability of levothyroxine sodium pentahydrate tablets. Int J Pharm. 2003;264(1–2):35–43. doi: 10.1016/S0378-5173(03)00387-9. [DOI] [PubMed] [Google Scholar]
- 20.Bosakova Z, Klouckova I, Tesarova E. Study of the stability of promethazine enantiomers by liquid chromatography using a vancomycin-bonded chiral stationary phase. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;770(1–2):63–69. doi: 10.1016/S0378-4347(01)00559-X. [DOI] [PubMed] [Google Scholar]
- 21.Moore TD, Horton R, Utrup LJ, Miller LA, Poupard JA. Stability of amoxicillin-clavulanate in BACTEC medium determined by high-performance liquid chromatography and bioassay. J Clin Microbiol. 1996;34(5):1321–1322. doi: 10.1128/jcm.34.5.1321-1322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vahdat L, Sunderland VB. Kinetics of amoxicillin and clavulanate degradation alone and in combination in aqueous solution under frozen conditions. Int J Pharm. 2007;342(1–2):95–104. doi: 10.1016/j.ijpharm.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Scholtissek C, Webster RG. Long-term stability of the anti-influenza A compounds—amantadine and rimantadine. Antiviral Res. 1998;38(3):213–215. doi: 10.1016/S0166-3542(98)00015-1. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Fassihi R. Stability of metronidazole, tetracycline HCl and famotidine alone and in combination. Int J Pharm. 2005;290(1–2):1–13. doi: 10.1016/j.ijpharm.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Barbarina N, Tilquin B, de Hoffmann E. Radiosterilization of cefotaxime: investigation of potential degradation compounds by liquid chromatography-electrospray mass spectrometry. J Chromatogr A. 2001;929(1–2):51–61. doi: 10.1016/S0021-9673(01)01175-X. [DOI] [PubMed] [Google Scholar]
- 26.Maggi L, Segale L, Ochoa ME, Buttafava A, Faucitano A, Conte U. Chemical and physical stability of hydroxypropylmethylcellulose matrices containing diltiazem hydrochloride after gamma irradiation. J Pharm Sci. 2003;92(1):131–141. doi: 10.1002/jps.10271. [DOI] [PubMed] [Google Scholar]
- 27.Kane MP, Tsuji K. Radiolytic degradation scheme for 60Co-irradiated corticosteroids. J Pharm Sci. 1983;72(1):30–35. doi: 10.1002/jps.2600720108. [DOI] [PubMed] [Google Scholar]
- 28.Ahrabi SF, Sande SA, Waaler T, Graffner C. Effects of thermal neutron irradiation on some potential excipients for colonic delivery systems. Drug Dev Ind Pharm. 1999;25(4):453–462. doi: 10.1081/DDC-100102195. [DOI] [PubMed] [Google Scholar]
- 29.Benton ER, Benton EV. Space radiation dosimetry in low-Earth orbit and beyond. Nucl Instrum Methods Phys Res B. 2001;184(1–2):255–294. doi: 10.1016/S0168-583X(01)00748-0. [DOI] [PubMed] [Google Scholar]
- 30.National Council on Radiation Protection and Measurements (NCRP) Guidance on radiation received in space activities: report No. 98. Bethesda: NCRP; 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 51 kb)
(DOC 63 kb)
(DOC 66 kb)
(DOC 131 kb)