Abstract
Recent clinical evidence indicates that the non-eosinophilic subtype of severe asthma is characterized by fixed airway obstruction, which may be related to emphysema. Transgenic studies have demonstrated that high levels of IFN-γ in the airways induce emphysema. Fibroblast growth factor 2 (FGF2), which is the downstream mediator of TGF-β, is important in wound healing. We investigated the role of FGF2 in IFN-γ-induced emphysema and the therapeutic effects of recombinant FGF2 in the prevention of emphysema in a severe non-eosinophilic asthma model. To evaluate the role of FGF2 in IFN-γ-induced emphysema, lung targeted IFN-γ transgenic mice were cross-bred with FGF2-deficient mice. A severe non-eosinophilic asthma model was generated by airway application of LPS-containing allergens twice a week for 4 weeks. To evaluate protective effects of FGF2, recombinant FGF2 (10 µg) was injected subcutaneously during allergen challenge in the severe asthma model. We found that non-eosinophilic inflammation and emphysema induced by transgenic overexpression of IFN-γ in the airways were aggravated by the absence of FGF2. Airway challenge with LPS-containing allergens induced more inflammation in mice sensitized with LPS-containing allergens compared to challenge with allergens alone. In addition, LPS-induced lung inflammation and emphysema depended on IFN-γ but not on IL-13. Interestingly, emphysema in the severe asthma model was significantly inhibited by treatment with recombinant FGF2 during allergen challenge, whereas lung inflammation was unaffected. Therefore, our present data suggest that FGF2 may help protect against IFN-γ-induced emphysema, and that recombinant FGF2 may help lessen the severity of emphysema.
Keywords: asthma, emphysema, fibroblast growth factor 2, interferon-γ, pulmonary eosinophilia
Introduction
Asthma is a complex disease, which has various subtypes according to fixed airflow obstruction, the presence of airway hyperresponsiveness (AHR), and eosinophilic inflammationand (Busse and Lemanske, 2001). Depending on severity, asthma is classified into mild, moderate, and severe subtypes. Mild and moderate asthma are related to eosinophilic inflammation, whereas severe asthma is associated with neutrophilic (or non-eosinophilic) inflammation (Busse and Lemanske, 2001; Bateman et al., 2008). In terms of the immunopathogenesis of asthma, eosinophilic asthma represents IL-4 and IL-13-dependent Th2 inflammation, whereas neutrophilic asthma is related to IFN-γ-dependent Th1 inflammation (Kim et al., 2007b).
Cluster analysis to identify asthma subtypes has shown that non-eosinophilic or neutrophilic severe asthma is characterized by fixed airway obstruction, whereas the eosinophilic subtype is characterized by reversible lung function (Haldar et al., 2008; Moore et al., 2010). Emphysema is defined as airspace enlargement of the lungs (Filley, 1967). Most animal models of emphysema are provoked by cigarette smoke, and IFN-γ is the critical mediator of cigarette-induced emphysema (Kang et al., 2006; Taraseviciene-Stewart and Voelkel, 2008). IFN-γ is a pleiotropic cytokine that plays an essential role in both the innate and adaptive immune response. It is a representative cytokine of Th1 inflammation and plays a crucial role in the development of severe non-eosinophilic asthma (Jeon et al., 2007b) (Kim et al., 2007b). In addition, transgenic (TG) studies have shown that high levels of IFN-γ in the airways induce neutrophilic inflammation associated with emphysema (Wang et al., 2000).
In the pathogenesis of severe asthma, lung tissue is injured by inflammatory processes followed by wound healing. During the healing process, TGF-β1 serves as a key mediator by inhibiting proteases that degrade extracellular matrix (ECM), up-regulating the synthesis of protease inhibitors, and stimulating the differentiation of fibroblasts to myofibroblasts, which participate in lung fibrosis by producing collagen (O'Kane and Ferguson, 1997; Morishima et al., 2001). Basic fibroblast growth factor, also known as fibroblast growth factor-2 (FGF2), is a member of the large FGF family, which includes several isoforms that differ in their N-terminal extensions, subcellular distribution, and function (Chlebova et al., 2009). FGF2 is a pleiotypic growth factor that induces proliferation of vascular endothelial and smooth muscle cells during angiogenesis, triggers the migration and proliferation of fibroblasts, reverses myofibroblast phenotypes, and leads the migration, proliferation, and regeneration of airway epithelial cells during wound healing (Nugent and Iozzo, 2000; Maltseva et al., 2001). FGF receptors belong to the receptor tyrosine kinase (RTK) family; four FGF receptors (FGFR1-4) are known.(Kannan and Givol, 2000). Epithelial cells in the large airways express FGFR1, 2, and 4, and alveolar cells express FGFR2, 3, and 4 (Davies et al., 2003). Animal experiments have shown that prenatal inhibition of FGFR signaling induces emphysematous phenotype (Hokuto et al., 2003). In addition, knock out (KO) studies have shown that FGFR3 and FGFR4 cooperatively promoted direct alveogenesis in the murine lung (Weinstein et al., 1998).
In the present study, we hypothesized that FGF2 helps protect the development of emphysema induced by IFN-γ, a key mediator of severe non-eosinophilic asthma. To test this, we evaluated the role of FGF2 by cross-breeding IFN-γ and FGF2 KO mice. We also evaluated the therapeutic effects of recombinant FGF2 on the development of severe neutrophilic asthma induced by airway challenge with LPS-containing allergens.
Results
Role of FGF2 in the development of lung inflammation induced by IFN-γ
Previously, we reported that FGF2 mRNA expression in the lung was enhanced in TGF-β1 TG mice (Jeon et al., 2007a). In the present study, we evaluated the production of FGF2 from mouse fibroblasts after stimulation with recombinant TGF-β1 (10 µg/ml). We found that protein levels of FGF2 peaked 24 h and were resolved 48 h after treatment (Figure 1A).
Figure 1.
Lung inflammation in the context of spontaneous IFN-γ production is aggravated by the absence of FGF2. (A) The level of secreted FGF2 from fibroblasts (NIH3T3) stimulated by TGF-β1 (10 µg/ml). (B) BAL cellularity of WT, FGF2-deficient mice, non-dox-treated IFN-γ transgenic mice, and IFN-γ transgenic mice crossed with FGF2-deficient mice (n=5). (C) Numbers of CD4+ and CD8+ T cells in the BAL fluid. D. IP-10 and TGF-β1 were assayed in BAL fluid. * and **P <0.05; * denotes a significant difference compared to WT and FGF2-deficient mice and ** denotes a significant difference compared to IFN-γ transgenic mice.
To investigate the effect that FGF2 may have on the development of IFN-γ-induced lung inflammation, we compared the reactions of sex-matched WT, FGF2-deficient, IFN-γ TG, and IFN-γ TG+FGF2-deficient mice to inflammation. All mice were 8 weeks old. BAL cellularity showed that there were significantly more inflammatory cells, especially neutrophils, in IFN-γ TG+FGF2-deficient mice than in IFN-γ TG mice, but similar numbers between WT and FGF2-deficient mice (Figure 1B). In addition, there were significantly more CD4+ and CD8+ T cells in IFN-γ TG+FGF2-deficient mice than in IFN-γ TG mice (Figure 1C). Moreover, TGF-β1 levels in BAL fluids were higher in the former group than the latter group, whereas IP-10 (CXCL10) levels were similar in the two groups (Figure 1D). Taken together, these data suggest that IFN-γ-induced lung inflammation was aggravated by the absence of FGF2.
Role of FGF2 in the development of emphysema induced by IFN-γ
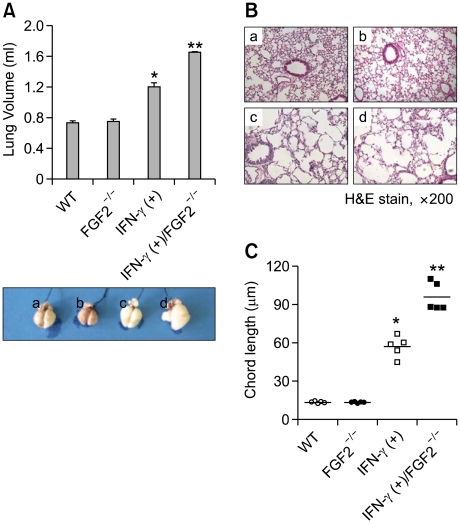
We also evaluated the effect that FGF2 may have on the development of IFN-γ-induced emphysema. Lung volume, an index of emphysema, was significantly higher in IFN-γ TG+FGF2-deficient mice than in IFN-γ TG mice, and higher in the latter group than in WT mice, but similar between WT and FGF2-deficient mice (Figure 2A). Lung histology revealed remarkable emphysematous changes in the lungs of both IFN-γ TG and IFN-γ TG+FGF2-deficient mice (Figure 2B). In addition, chord length, another index of emphysema, was significantly longer in IFN-γ TG+FGF2-deficient mice than in IFN-γ TG mice, and longer in the latter group than in WT mice, but similar between WT and FGF2-deficient mice (Figure 2C). Taken together, these data suggest that FGF2 helps protect against the development of IFN-γ-induced emphysema.
Figure 2.
Emphysema in the context of spontaneous IFN-γ production is aggravated by the absence of FGF2. (A) Lung volume of each group: (a) WT, (b) FGF2-/-, (c) IFN-γ transgenic, and (d) IFN-γ transgenic crossed with FGF2-/- mice (n=5). (B) Representative H&E-stained lung sections: (a) WT, (b) FGF2-/-, (c) IFN-γ transgenic, and (d) IFN-γ transgenic crossed with FGF2-/- mice. (C) Chord length of each group. * and **P <0.05; * denotes a significant difference compared to WT and FGF2-deficient mice and ** denotes a significant difference compared to IFN-γ transgenic mice.
Generation of severe non-eosinophilic asthma by repeated airway challenge with LPS-containing allergens
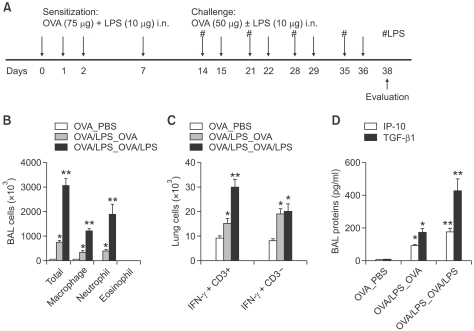
Emphysema mouse models can be broadly categorized as genetic and exposure models. Genetic models can be generated by over-expressed TG or gene-targeted KO mice, whereas exposure models can be generated by exposing mice to cigarette smoke or via elastase instillation (Taraseviciene-Stewart and Voelkel, 2008). Here, we generated a severe non-eosinophilic asthma model with emphysema by repeated LPS-containing allergen challenge, as shown in Figure 3A. BAL cellularity showed that there were significantly more non-eosinophil inflammatory cells in OVA/LPS-sensitized, OVA/LPS-challenged mice than in OVA/LPS-sensitized, OVA-challenged mice, and more in the latter group than in OVAsensitized, PBS-challenged mice (Figure 3B). In addition, IFN-γ expression was significantly enhanced in T cells from OVA/LPS-sensitized, OVA/LPS-challenged mice compared to OVA/LPS-sensitized, OVA-challenged mice (Figure 3C). In terms of downstream mediator production, the levels of IP-10 and TGF-β1 in BAL fluids were significantly higher in OVA/LPS-sensitized, OVA/LPS-challenged mice than in OVA/LPS-sensitized, OVA-challenged mice (Figure 3D). Taken together, these data suggest that airway challenge with LPS-containing allergens induces severe non-eosinophilic asthma phenotypes, which are related to an enhanced Th1 cell response.
Figure 3.
Repeated airway challenge with LPS-containing allergens induces severe lung inflammation. (A) Experimental protocol of the mouse model. (B) Comparison of BAL cellularity on day 38 among PBS-, OVA-, and OVA/LPS-challenged mice (n=5 each). (C) IFN-γ producing CD3+ T cells and CD3- non-T cells. (D) IP-10 and TGF-β1 were assayed in BAL fluid. * and **P <0.05; * denotes a significant difference compared to PBS-treated mice and ** denotes a significant difference compared to OVA-treated mice.
Role of IL-13 in the development of severe non-eosinophilic asthma phenotypes
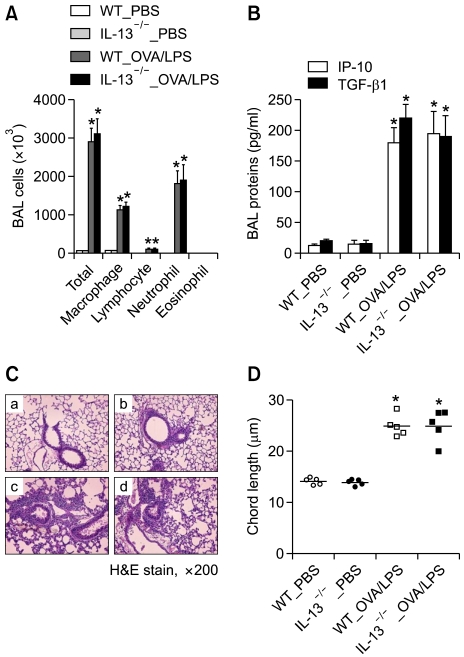
IL-13 is a key mediator in the development of Th2-type inflammation, which induces lung remodeling such as fibrosis and emphysema (Elias et al., 2003). To evaluate the relationship between IL-13 and the development of severe non-eosinophilic asthma phenotypes, the severe asthma model was applied to IL-13-deficient and WT mice. BAL cellularity showed that OVA/LPS-challenged IL-13-deficient and WT mice had similar numbers of inflammatory cells (Figure 4A). The levels of IP-10 and TGF-β1 in BAL fluids were also similar in these two groups (Figure 4B). Lung histology showed that OVA/LPS challenge induced similar parenchymal changes between IL-13-deficient and WT mice (Figure 4C). Finally, the degree of emphysema, assessed by chord length, was similar in the two groups (Figure 4D). Taken together, these findings suggest that severe non-eosinophilic asthma phenotypes, such as lung inflammation and emphysema, induced by airway challenge with LPS-containing allergens do not depend on IL-13.
Figure 4.
Lung inflammation and emphysema induced by LPS-containing allergens do not depend on IL-13. (A) BAL cellularity of WT and IL-13-deficient mice on day 38 (n=5 each). (B) IP-10 and TGF-β1 were assayed in BAL fluid. (C) Representative H&E-stained lung sections from WT and IL-13-deficient mice. (a) WT_PBS, (b) IL-13-/-_PBS, (c) WT_OVA/LPS, and (d) IL-13-/-_OVA/LPS-treated mice. (D) Chord length of WT and IL-13-deficient mice with or without OVA/LPS treatment. *P <0.05, compared to PBS-treated mice.
Role of IFN-γ in the development of severe non-eosinophilic asthma phenotypes
IFN-γ is a key mediator in the development of non-eosinophilic inflammation induced by LPS-containing allergens (Kim et al., 2007b) and emphysema (Wang et al., 2000). To evaluate the relationship between IFN-γ and the development of severe non-eosinophilic asthma phenotypes, the severe asthma model was applied to IFN-γ-deficient and WT mice. BAL cellularity showed that there were significantly fewer inflammatory cells in OVA/LPS-challenged IFN-γ-deficient than in OVA/LPS-challenged WT mice (Figure 5A). In addition, the production of IP-10 was significantly lower in the former group than in the latter group, whereas TGF-β1 production was higher in the former group than in the latter group (Figure 5B). Lung histology showed that the alveolar destruction induced by OVA/LPS challenge was lower in IFN-γ-deficient mice compared to WT mice (Figure 4C). In addition, chord length was significantly smaller in OVA/LPS-challenged IFN-γ-deficient mice than in OVA/LPS-challenged WT mice (Figure 5D). Taken together, these data suggest that severe non-eosinophilic asthma phenotypes, such as lung inflammation and emphysema, induced by airway challenge with LPS-containing allergens depend in part on IFN-γ.
Figure 5.
Lung inflammation and emphysema induced by LPS-containing allergens partly depend on IFN-γ. (A) BAL cellularity of WT and IFN-γ-deficient mice on day 38 (n=5 each). (B) IP-10 and TGF-β1 were assayed in BAL fluid. (C) Representative H&E-stained lung sections from WT and IFN-γ-deficient mice. (a) WT_PBS, (b) IFN-γ-/-_PBS, (c) WT_OVA/LPS, and (d) IFN-γ-/-_OVA/LPS-treated mice. (D) Chord length of WT and IFN-γ-/- mice with or without OVA/LPS treatment. * and **P <0.05, * denotes a significant difference compared to PBS-treated mice and ** denotes a significant difference compared to IFN-γ-/-_OVA/LPS-treated mice.
Therapeutic effects of recombinant FGF2 on the development of non-eosinophilic asthma phenotypes in the experimental severe asthma model
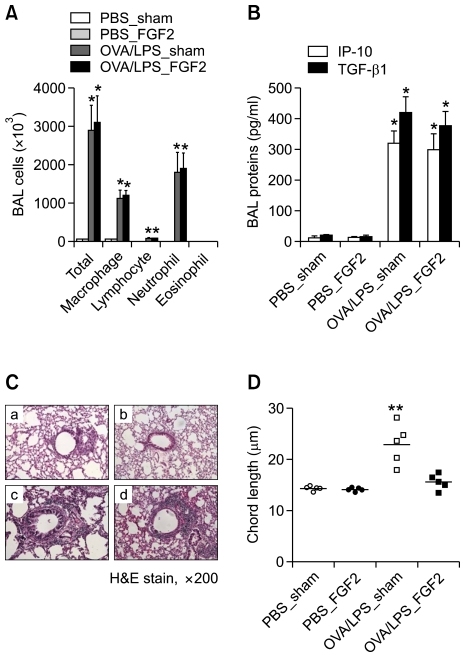
Finally, we evaluated the potential therapeutic effects of FGF2 on the development of IFN-γ-dependent, severe asthma phenotypes. We injected 10 µg recombinant FGF2 (rFGF2) subcutaneously into mice challenged with OVA/LPS or OVA. The two groups had similar levels of inflammatory cells (Figure 6A) and pro-inflammatory mediators such as IP-10 and TGF-β1 (Figure 6B). Lung histology showed that the alveolar destruction induced by OVA/LPS challenge was reduced by treatment with rFGF2 compared to the control (Figure 6C). Based on chord length, rFGF2 treatment significantly inhibited the degree of OVA/LPS-induced emphysema (Figure 6D). Taken together, these findings suggest that rFGF2 has a protective effect against emphysema but no effect on the development of lung inflammation.
Figure 6.
Treatment with rFGF2 protects against the development of emphysema, but not lung inflammation, induced by LPS-containing allergens. (A) BAL cellularity of PBS_control, PBS_FGF2 (10 µg), OVA/LPS_control, and OVA/LPS/FGF2-treated mice (n=5). (B) IP-10 and TGF-β1 were assayed in BAL fluid. (C) Representative H&E-stained lung sections: (a) PBS_control, (b) PBS_FGF2 (10 µg), (c) OVA/LPS_control, and (d) OVA/LPS/FGF2-treated mice. (D) Chord length of each group. * and **P <0.05, * denotes a significant difference compared to PBS-treated mice and ** denotes a significant difference compared to OVA/LPS_ FGF2-treated mice.
Discussion
We investigated the potential role of FGF2 in the development of IFN-γ-induced emphysema using two emphysema models: a lung-targeted IFN-γ TG model and a severe non-eosinophilic asthma model induced by airway exposure to LPS-containing allergens. Cross-breeding with IFN-γ TG and FGF2 KO mice showed that IFN-γ-induced emphysema was aggravated by the absence of FGF2. In addition, treatment with rFGF2 prevented the development of emphysema in the severe asthma model, which depends in part on IFN-γ.
Asthma can be divided into two inflammatory subtypes: eosinophilic and non-eosinophilic (Wenzel et al., 1999; Lemiere et al., 2006) and the most consistent cellular finding in severe asthmatics is the high number and percentage of neutrophils in induced sputum, BAL, and biopsy specimens (Wenzel et al., 1997; Elias, 2004; Kim et al., 2007b). Our previous clinical studies showed that the expression of IFN-γ, but not IL-4, was enhanced in severe asthma patients compared to patients with mild and moderate asthma (Kim et al., 2007b). In addition, asthma animal models using allergen inhalation and lung-targeted IFN-γ transgenes have demonstrated that IFN-γ is a key mediator in the development of non-eosinophilic asthma phenotypes (Kim et al., 2007a, 2007b). Furthermore, the present data showed that non-eosinophilic inflammation induced by LPS-containing allergens depended on IFN-γ. Taken together, these findings suggest that IFN-γ is a key mediator in the development of severe non-eosinophilic asthma.
While on average and in the extremes of presentation, asthma and chronic obstructive pulmonary disease (COPD) appear to be easily distinguishable, significant proportions of patients are indistinguishable on the basis of structural and functional evaluations (Sciurba, 2004). Asthma patients with persistent maximum expiratory airflow limitations, despite optimal poly-therapy, have a marked loss of lung elastic recoil, which is related to emphysema in COPD (Gelb et al., 2008). In terms of alveolar remodeling, asthma is generally considered a disease with normal alveoli; in contrast, alveolar destruction with secondary alveolar enlargement is a characteristic feature of emphysema (Elias, 2004). Studies of emphysematous human tissues have highlighted alterations that heighten tissue proteolysis, such as increased elastin degradation and enhanced expression of proteases (Ohnishi et al., 1998; Takeyabu et al., 1998). However, these changes have also been documented in severe asthma patients, leading to COPD-like alterations in lung compliance (Bousquet et al., 1996; Gelb and Zamel, 2000). These findings suggest that alveolar remodeling (such as that which is associated with emphysema) in severe asthma patients can lead to fixed or irreversible airway obstruction.
When IL-13 is over-expressed in the murine lung, both asthma phenotypes (e.g., eosinophilic inflammation, mucous metaplasia, and AHR) and COPD phenotypes (e.g., bronchiolar fibrosis and alveolar enlargement) are obvious (Zhu et al., 1999). In contrast, the TG expression of IFN-γ in the murine lung causes neutrophilic inflammation and impressive pulmonary emphysema without fibrosis (Wang et al., 2000). In the present study, repeated airway exposure to LPS-containing allergens induced non-eosinophilic inflammation and alveolar remodeling, as in emphysema. Furthermore, these phenotypes induced by LPS-containing allergens depended on IFN-γ but not IL-13. These findings suggest that IFN-γ is a key mediator in the development of the fixed airway obstruction seen in patients with severe non-eosinophilic asthma.
Cigarette smoke-induced emphysema animal models were used to elucidate the pathogenesis of emphysema and to discover therapeutic candidate drugs because of the strong correlation between cigarette smoking and emphysema (Turner and Grose, 2010). However, not all emphysemas can be explained by the cigarette smoke emphysema model (Joos et al., 2002). The emphysema seen in the LPS-containing allergen-induced severe asthma model in the present study may be an alternative model to back up the shortcomings of the cigarette smoking models. The so-called Dutch hypothesis suggests that asthma and COPD are not distinct entities, and that similar pathogenic mechanisms may be involved in the pathogenesis of asthma and COPD in some individuals (Sluiter et al., 1991; Elias, 2004). The present severe asthma model supports this idea in that the progression of severe asthma leads to emphysema or COPD.
IFN-γ in the lungs induces pulmonary destruction through the production of various proteases (Zheng et al., 2005) and apoptosis of lung epithelial cells (Lesur et al., 2004). The chronic inflammatory process causes tissue injury followed by healing (Lee et al., 2002). It is generally accepted that the modulation of fibroblastic cells toward the myofibroblastic phenotype, in combination with the acquisition of specialized contractile features and the enhanced production of extracellular matrix proteins, is essential for connective tissue remodeling during wound healing (Holgate et al., 2000, 2002). TGFβ1 is a key mediator in wound healing and in the fibroblast-to-myofibroblast phenotypic change, (Morishima et al., 2001) and a key antagonist of IFN-γ (Lin et al., 2005). High levels of TGFβ1 in the lungs cause prominent pulmonary fibrosis rather than destruction (Lee et al., 2006). These data suggest that IFN-γ-induced emphysema can be inhibited by TGFβ1.
FGF2 is a downstream mediator of TGFβ1 (Jeon et al., 2007a). The present data also showed that TGFβ1 induced in vitro production of FGF2 from fibroblasts. During lung branching morphogenesis (or lung modeling) or remodeling, the local production of cytokines and growth factors by epithelial and mesenchymal cells effectively creates an epithelial-mesenchymal trophic unit (Holgate et al., 2000). Interactions between the FGF family and their receptors can mediate mesenchymal-epithelial cell interactions during lung modeling and remodeling (Sekine et al., 1999). FGF2 plays a role in wound healing by stimulating fibroblast differentiation and the regeneration of epithelial cells from endogenous or exogenous stem/progenitor cells (Warburton et al., 2008). Because lung inflammation and emphysema induced by transgenic IFN-γ in the lung was aggravated by the absence of FGF2, we hypothesized that rFGF2 treatment inhibits severe asthma phenotypes, such as lung inflammation and emphysema induced by LPS-containing allergens. The present data showed that rFGF2 treatment significantly inhibited the development of emphysema but not lung inflammation. Taking into consideration the induction of apoptosis in lung epithelial cells by IFN-γ, these data suggest that the protective effects of FGF2 against the development of emphysema are related to the enhanced regeneration of epithelial cells.
In summary, the present study suggests that IFN-γ is a key mediator in the development of emphysema seen in severe non-eosinophilic asthma, and FGF2 helps to protect against the development of IFN-γ-induced emphysema. In terms of clinical application, recombinant FGF2 may be a promising therapeutic agent for the treatment of emphysema progression seen in severe asthma and COPD.
Methods
Animals and recombinant FGF2
C57BL/6, IFN-γ-deficient, IL-13-deficient, and wild type (WT) control mice were purchased from Jackson Laboratory (Bar Harbor, Maine). The generation and breeding of lung-targeted IFN-γ TG (C57BL/6) mice was previously described.(Wang et al., 2000) FGF2-deficient mice (mixed background) were purchased from the Jackson Laboratory and their genetic background was changed to a C57BL/6 background as previously described.(Jeon et al., 2007a) Lung-specific IFN-γ TG (+) and FGF2-deficient mice (both C57BL/6 background) were then cross-bred to obtain IFN-γ (-)/FGF2+/+, IFN-γ (+)/ FGF2+/+, IFN-γ (-)/FGF2-/-, and IFN-γ (+)/FGF2-/- mice. Animal study protocols were approved by the Institutional Animal Care and Use Committee of POSTECH and of Yale University. Recombinant FGF2 (rFGF2) was generously donated by the Donga Pharmaceutical Company (Suwon, Korea).
Generation of a severe non-eosinophilic asthma mouse model and pharmacological intervention
LPS-depleted OVA, as an allergen, was prepared as previously described (Kim et al., 2007b). To generate a severe non-eosinophilic asthma mouse model, 6-week-old mice were sensitized intranasally four times with 75 µg OVA and 10 µg LPS on days 0, 1, 2 and 7. As an allergen challenge, OVA (50 µg) was administered intransally on days 14, 15, 21, 22, 28, 29, 35, and 36. To obtain the severe asthma model, LPS (10 µg) was co-administered with OVA on days 14, 21, 28, and 35. Severe asthma phenotypes, such as inflammation and emphysema, and immunologic parameters were evaluated 48 h after the last allergen challenge. In addition, to evaluate the therapeutic effects of rFGF2 on the development of severe asthma phenotypes, recombinant FGF2 (10 µg) was subcutaneously injected on days 14, 15, 21, 22, 28, 29, 35, and 36.
Cellularity of bronchoalveolar lavage (BAL) fluids
BAL cellularity was analyzed as previously described (Kim et al., 2007b), after diluting the cell pellets with 100 µl PBS. Inflammatory cells were classified as macrophages, lymphocytes, neutrophils, or eosinophils using Diff-quick staining solution.
Histological evaluation of lung tissue
Lung sections were stained with hematoxylin and eosin (H&E) after pressure fixation with Streck solution (Streck Laboratories, La Vista, NE). All sample slides were compared at the same magnification. Chord length was measured using the analySIS® LS Research software provided by Olympus.
Lung volume assay
Lung volume was measured as previously described (Zheng et al., 2000). Briefly, mice were anesthetized, their trachea were annulated, and their lungs and heart were removed en bloc. The lung tissue was inflated with PBS by gravity force and the inflated volume was calculated.
Fluorescent-activated cell sorting (FACS) analysis and intracellular cytokine staining
To identify T cells recruited into the lung and BAL fluid, FACS analysis was performed using antibodies for T-cell surface markers, including anti-CD3, anti-CD4, and anti-CD8. After preparation, single-cell-suspended 1×106 isolated cells were aliquoted into tubes and stained with FACS antibodies (BD Biosciences Pharmingen, San Diego, CA). To determine the intracellular cytokine levels, isolated T cells were incubated at 37℃ for 3 h in RPMI media containing 10% fetal bovine serum (FBS) and 2 µg/ml brefeldin A (Sigma-Aldrich). Then the cells were washed in PBS containing 3% FBS and 0.1% NaN3, followed by fixation in PBS containing 4% formaldehyde for 20 min. After washing, the cells were permeabilized with 0.5% saponin (Sigma-Aldrich) in PBS for 10 min, centrifuged, re-suspended in 50 µl of the same solution, and stained with anti-IFN-γ antibody for 30 min. The cells were analyzed using the FACS Calibur system (BD Biosciences, Franklin Lakes, NJ), and the results were processed using CellQuest software (BD Biosciences). The number of each type in the lung was determined by multiplying the total number of lung cells by the percentage of CD4+ or CD8+ T cells.
In vitro production of FGF2
To elucidate FGF2 production from mouse fibroblasts (NIH3T3), TGF-β1 (10 µg/ml) was added and the cells were incubated, and the supernatants were collected at different time points after incubation.
ELISA assay to measure levels of mediators
Levels of FGF2, IP-10, and TGF-β1 were measured by ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical analysis
Significant differences between the treatments were assessed using Student's t-test, ANOVA, or Wilcoxon's rank-sums test. For multiple comparisons, ANOVA was initially used, and when significant differences were found, individual t-tests or Wilcoxon's rank-sums tests for pairs of groups were performed.
Acknowledgements
We thank the Donga Phamaceutical Company (Suwon, Korea) for the kind donation of recombinant FGF2, Jee-In Lim for providing secretarial assistance, and members of the POSTECH animal facility for their help with animal care. This study was supported by grants from the Korean Ministry of Health and Welfare, Republic of Korea (A080711), and the National Research Foundation of Korea (NRF-2009-352-E00020).
Abbreviations
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- FGF2
fibroblast growth factor 2
- OVA
ovalbumin
References
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention : GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Lacoste JY, Chanez P, Vic P, Godard P, Michel FB. Bronchial elastic fibers in normal subjects and asthmatic patients. Am J Respir Crit Care Med. 1996;153:1648–1654. doi: 10.1164/ajrccm.153.5.8630616. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 4.Chlebova K, Bryja V, Dvorak P, Kozubik A, Wilcox WR, Krejci P. High molecular weight FGF2 : the biology of a nuclear growth factor. Cell Mol Life Sci. 2009;66:225–235. doi: 10.1007/s00018-008-8440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma : New insights. J Allergy Clin Immunol. 2003;111:215–225. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 6.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insight into the pathogesesis of asthma. J Clin Invest. 2003;111:291–297. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elias JA. The relationship between asthma and COPD. Lessons from transgenic mice. Chest. 2004;126:111S–116S. doi: 10.1378/chest.126.2_suppl_1.111S. [DOI] [PubMed] [Google Scholar]
- 8.Filley GF. Emphysema and chronic bronchitis: clinical manifestations and their physiologic significance. Med Clin North Am. 1967;51:283–292. doi: 10.1016/s0025-7125(16)33056-5. [DOI] [PubMed] [Google Scholar]
- 9.Gelb AF, Zamel N. Unsuspected pseudophysiologic emphysema in chronic persistent asthma. Am J Respir Crit Care Med. 2000;162:1778–1782. doi: 10.1164/ajrccm.162.5.2001037. [DOI] [PubMed] [Google Scholar]
- 10.Gelb AF, Zamel N, Krishnan A. Physiologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14:24–30. doi: 10.1097/MCP.0b013e3282f197df. [DOI] [PubMed] [Google Scholar]
- 11.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hokuto I, Perl AK, Whitsett JA. Prenatal, but not postnatal, inhibition of fibroblast growth factor receptor signaling causes emphysema. J Biol Chem. 2003;278:415–421. doi: 10.1074/jbc.M208328200. [DOI] [PubMed] [Google Scholar]
- 13.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 14.Holgate ST. Airway inflammation and remodeling in asthma: current concepts. Mol Biotechnol. 2002;22:179–189. doi: 10.1385/MB:22:2:179. [DOI] [PubMed] [Google Scholar]
- 15.Jeon SG, Lee CG, Oh MH, Chun EY, Gho YS, Cho SH, Kim JH, Min KU, Kim YY, Kim YK, Elias JA. Recombinant basic fibroblast growth factor inhibits the airway hyperresponsiveness, mucus production, and lung inflammation induced by an allergen challenge. J Allergy Clin Immunol. 2007a;119:831–837. doi: 10.1016/j.jaci.2006.12.653. [DOI] [PubMed] [Google Scholar]
- 16.Jeon SG, Oh SY, Park HK, Kim YS, Shim EJ, Lee HS, Oh MH, Bang B, Chun EY, Kim SH, Gho YS, Zhu Z, Kim YY, Kim YK. TH2 and TH1 lung inflammation induced by airway allergen sensitization with low and high doses of double-stranded RNA. J Allergy Clin Immunol. 2007b;120:803–812. doi: 10.1016/j.jaci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Joos L, Pare PD, Sandford AJ. Genetic risk factors. In: Voelkel N, Macnee W, editors. Chronic obstructive lung diseases. London, United Kingdom: B.C. Decker; 2002. pp. 56–64. [Google Scholar]
- 18.Kang MJ, Lee CG, Cho SJ, Homer RJ, Elias JA. IFN-gamma-dependent DNA injury and/or apoptosis are critical in cigarette smoke-induced murine emphysema. Proc Am Thorac Soc. 2006;3:517–518. doi: 10.1513/pats.200603-075MS. [DOI] [PubMed] [Google Scholar]
- 19.Kannan K, Givol D. FGF receptor mutations: dimerization syndromes, cell growth suppression, and animal models. IUBMB Life. 2000;49:197–205. doi: 10.1080/713803609. [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, Ko HM, Kang NI, Song CH, Zhang X, Chung WC, Kim JH, Choi IH, Park YM, Kim GY, Im SY, Lee HK. Mast cells play a key role in the development of late airway hyperresponsiveness through TNF-alpha in a murine model of asthma. Eur J Immunol. 2007a;37:1107–1115. doi: 10.1002/eji.200636612. [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, Kim JH, Gho YS, Cho SH, Min KU, Kim YY, Zhu Z. Airway exposure levels of lipopolysaccharide determine type 1 versus type 2 experimental asthma. J Immunol. 2007b;178:5375–5382. doi: 10.4049/jimmunol.178.8.5375. [DOI] [PubMed] [Google Scholar]
- 22.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, Graham BS, Johnson TR, Elias JA. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia,tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002;277:35466–35474. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 23.Lee CG, Cho S, Homer RJ, Elias JA. Genetic control of transforming growth factor-beta1-induced emphysema and fibrosis in the murine lung. Proc Am Thorac Soc. 2006;3:476–477. doi: 10.1513/pats.200603-040MS. [DOI] [PubMed] [Google Scholar]
- 24.Lemiere C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, Martin JG, Hamid Q. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006;118:1033–1039. doi: 10.1016/j.jaci.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Lesur O, Brisebois M, Thibodeau A, Chagnon F, Lane D, Füllöp T. Role of IFN-gamma and IL-2 in rat lung epithelial cell migration and apoptosis after oxidant injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L4–L14. doi: 10.1152/ajplung.00367.2002. [DOI] [PubMed] [Google Scholar]
- 26.Lin JT, Martin SL, Xia L, Gorham JD. TGF-beta 1 uses distinct mechanisms to inhibit IFN-gamma expression in CD4+ T cells at priming and at recall: differential involvement of Stat4 and T-bet. J Immunol. 2005;174:5950–5958. doi: 10.4049/jimmunol.174.10.5950. [DOI] [PubMed] [Google Scholar]
- 27.Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- 28.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morishima Y, Nomura A, Uchida Y, Noguchi Y, Sakamoto T, Ishii Y, Goto Y, Masuyama K, Zhang MJ, Hirano K, Mochizuki M, Ohtsuka M, Sekizawa K. Triggering the induction of myofibroblast and fibrogenesis by airway epithelial shedding. Am J Respir Cell Mol Biol. 2001;24:1–11. doi: 10.1165/ajrcmb.24.1.4040. [DOI] [PubMed] [Google Scholar]
- 30.Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 31.O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 32.Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest. 1998;78:1077–1087. [PubMed] [Google Scholar]
- 33.Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest. 2004;126:117S–124S. doi: 10.1378/chest.126.2_suppl_1.117S. discussion 159S-61S. [DOI] [PubMed] [Google Scholar]
- 34.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 35.Sluiter HJ, Koeter GH, de Monchy JG, Postma DS, de Vries K, Orie NG. The Dutch hypothesis (chronic non-specific lung disease) revisited. Eur Respir J. 1991;4:479–489. [PubMed] [Google Scholar]
- 36.Takeyabu K, Betsuyaku T, Nishimura M, Yoshioka A, Tanino M, Miyamoto K, Kawakami Y. Cysteine proteinases and cystatin C in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Eur Respir J. 1998;12:1033–1039. doi: 10.1183/09031936.98.12051033. [DOI] [PubMed] [Google Scholar]
- 37.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest. 2008;118:394–402. doi: 10.1172/JCI31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner N, Grose R. Fibroblast growth factor signalling : from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B. Stem/Progenitor cells in lung development, injury, repair, and regeneration. Proc Am Thorac Soc. 2008;5:703–706. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156:737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 43.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 44.Zheng T, Kang MJ, Crothers K, Zhu Z, Liu W, Lee CG, Rabach LA, Chapman HA, Homer RJ, Aldous D, De Sanctis GT, Underwood S, Graupe M, Flavell RA, Schmidt JA, Elias JA. Role of cathepsin S-dependent epithelial cell apoptosis in IFN-gamma-induced alveolar remodeling and pulmonary emphysema. J Immunol. 2005;174:8106–8115. doi: 10.4049/jimmunol.174.12.8106. [DOI] [PubMed] [Google Scholar]
- 45.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Jr, Chapman HA, Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–1093. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]