Abstract
Parkinson's disease (PD) is characterized by selective and progressive degeneration of dopamine (DA)-producing neurons in the substantia nigra pars compacta (SNpc) and by abnormal aggregation of α-synuclein. Previous studies have suggested that DA can interact with α-synuclein, thus modulating the aggregation process of this protein; this interaction may account for the selective vulnerability of DA neurons in patients with PD. However, the relationship between DA and α-synuclein, and the role in progressive degeneration of DA neurons remains elusive. We have shown that in the presence of DA, recombinant human α-synuclein produces non-fibrillar, SDS-resistant oligomers, while β-sheet-rich fibril formation is inhibited. Pharmacologic elevation of the cytoplasmic DA level increased the formation of SDS-resistant oligomers in DA-producing neuronal cells. DA promoted α-synuclein oligomerization in intracellular vesicles, but not in the cytosol. Furthermore, elevation of DA levels increased secretion of α-synuclein oligomers to the extracellular space, but the secretion of monomers was not changed. DA-induced secretion of α-synuclein oligomers may contribute to the progressive loss of the dopaminergic neuronal population and the pronounced neuroinflammation observed in the SNpc in patients with PD.
Keywords: alpha-synuclein, amyloid, dopamine, Parkinson's disease, protein aggregation, secretion
Introduction
Parkinson's disease (PD) is an age-related, progressive neurodegenerative disorder characterized by motor abnormalities, such as resting tremors, bradykinesia, and rigidity (Fahn and Sulzer, 2004). Such parkinsonian motor symptoms are attributed to selective loss of pigmented, dopaminergic neurons in the substantia nigra pars compacta (SNpc) in the midbrain (Fahn and Sulzer, 2004). Some of the surviving neurons in this region contain cytoplasmic inclusion bodies known as Lewy bodies (LBs). One of the major protein components of LBs is the amyloid fibril form of α-synuclein, a presynaptic protein that modulates synapse function (Goedert, 2001). It has been reported that α-synuclein is a native, unfolded protein that spontaneously forms fibrils in a nucleation-dependent manner (Uversky, 2007). In the process of forming fibrils, α-synuclein generates various forms of nonfibrillar oligomeric intermediates, or protofibrils (Volles and Lansbury, 2003). Critical evidence linking α-synuclein aggregation to PD has accumulated from the finding of missense mutations and the multiplication of the α-synuclein gene in rare forms of early-onset PD (Farrer, 2006). All of these mutations result in accelerated formation of one or more forms of aggregates in the process of fibrillation (Kim and Lee, 2008).
Despite being a cytosolic protein, a small amount of α-synuclein is released from neuronal cells via unconventional exocytosis (Lee et al., 2005; Jang et al., 2010). The release of α-synuclein is increased under cellular stress conditions, and more aggregated forms are present in the released proteins (Jang et al., 2010). It has been suggested that this release of α-synuclein is an important step in the mechanism underlying the spread of LBs during disease progression, and also provides an extracellular trigger of neuroinflammation (Kim and Joh, 2006; Lee, 2008; Desplats et al., 2009; Lee et al., 2010).
In an effort to explain selective vulnerability of dopaminergic neurons in patients with PD, it has been hypothesized that high concentrations of free cytosolic dopamine (DA) and the subsequent increase in reactive oxygen species (ROS) levels promote oxidative stress, mitochondrial dysfunction, and protein modifications, leading to neuronal death. We have shown that elevation of the DA concentration increases the formation of non-fibrillar α-synuclein aggregates both in vitro and in a dopaminergic neuronal cell model. In neuronal cells, by increasing the DA concentration, α-synuclein aggregation takes place in intracellular vesicles, and the release of aggregates, but not monomers, is increased. These results may explain the mechanism underlying the progressive and selective loss of DA neurons in patients with PD.
Results
DA-induced α-synuclein oligomer formation in vitro
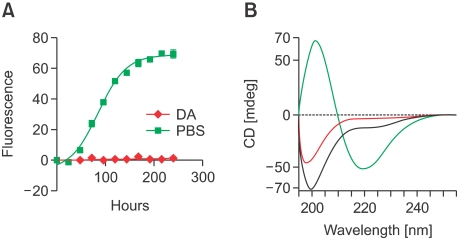
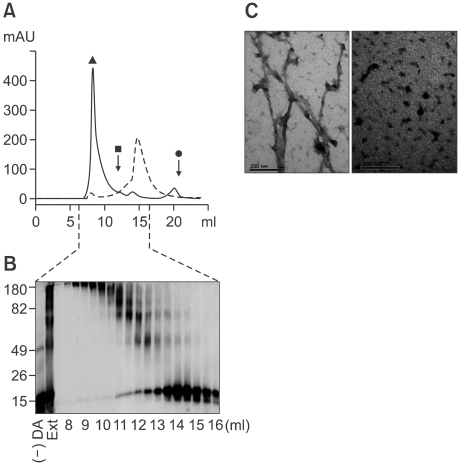
To determine the effect of DA on the α-synuclein aggregation process in vitro, we incubated recombinant human α-synuclein with 20 times molar excess of DA and analyzed the aggregation kinetics and protein structure. The Thioflavin T fluorescence assay showed that fibril formation was significantly slowed by DA (Figure 1A). Similarly, the CD spectra showed that β-sheet formation was drastically reduced in the presence of DA (Figure 1B). In contrast, size exclusion chromatography (SEC) revealed that the formation of non-fibrillar oligomeric aggregates was increased in the presence of DA (Figure 2A). Oligomeric assemblage formed in the presence of DA was maintained in the denaturing gel containing sodium dodesylsulfate (SDS), perhaps indicating that the oligomers were either covalently cross-linked or non-covalently associated in a highly organized manner (Figure 2B). Confirming these results, electron microscopy showed that incubation with DA resulted only in non-fibrillar aggregates with heterogeneous shapes and sizes (Figure 2C). Thus, in agreement with previous reports (Conway et al., 2001; Cappai et al., 2005), our results suggest that DA leads to the accumulation of non-fibrillar, non-β-sheet-rich, oligomeric aggregates, thus preventing the formation of fibrils.
Figure 1.
DA-induced oligomerization of α-synuclein in vitro. (A) Thioflavin T binding assay. Recombinant α-synuclein was incubated in the presence (red) or absence (green) of DA. (B) CD analysis of α-synuclein monomer (blue), fibrils (green), and oligomer-monomer mixture prepared in the presence of DA (red).
Figure 2.
Analysis of DA-induced α-synuclein oligomers Size exclusion chromatography (A) and Western blotting (B) of α-synuclein proteins incubated with DA. (Dotted line: α-synuclein monomer, black line: DA-induced α-synuclein oligomer profiles. △: blue-dextran [2,000 kDa], █: albumin [66,000 Da], ●: cytochrome c [12,400 Da]) (C) Electron microscopy images of control (left) and DA-induced oligomers (right) (Scale bar: 0.2 µm).
Increased α-synuclein aggregation in neuronal cells by elevation of DA concentration
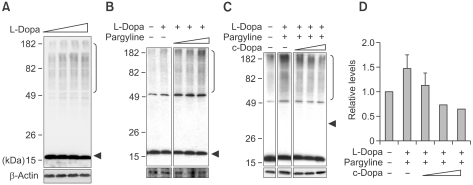
To determine the effect of DA on α-synuclein aggregation in neuronal cells, we analyzed α-synuclein aggregation in differentiated SH-SY5Y cells, which produce catecholamines, including DA, and express dopaminergic markers, such as tyrosine hydroxylase, DA transporter, and vesicular monoamine transporter-2 (VMAT-2) (Presgraves et al., 2004). First, we examined the extent of α-synuclein aggregation when the cells were treated with L-DOPA, the precursor of DA. After L-DOPA treatment, α-synuclein aggregation was increased in a dose-dependent manner (Figure 3A). Treatment with L-DOPA did not cause significant cell toxicity as shown in Supplementary Figure 1. The effect of L-DOPA was further augmented when the metabolic turnover of DA was blocked with pargyline, a selective inhibitor of monoamine oxidase B (MAOB) (Figure 3B). The increase in α-synuclein aggregation in L-DOPA-treated cells was due to increased levels of DA rather than the effect of L-DOPA itself because the L-DOPA effect was eliminated when the L-DOPA-to-DA conversion was blocked with carbi-DOPA, an inhibitor of L-aromatic amino acid decarboxylase (Figures 3C, D).
Figure 3.
DA-induced α-synuclein aggregation and vesicular localization in SH-SY5Y cells. (A) L-dopa treatment increased α-synuclein aggregation in differentiated SH-SY5Y cells in a dose-dependent manner. Differentiated SH-SY5Y neuronal cells overexpressing α-synuclein were treated with L-dopa at final concentrations of 0, 50, 150, and 400 µM for 48 hours. (B) Inhibition of MAO-B by pargyline (0, 1, 5, and 20 µM) further elevated α-synuclein aggregate formation in L-dopa-treated cells (1 mM). The data were obtained from the same blot. (C) Addition of c-dopa (1, 4, and 10 mM) inhibited DA-induced α-synuclein aggregation (L-dopa [1 mM)], pargyline [5 µM]). All the data shown here were originated from the same blot. (D) Quantitation of high MW α-synuclein aggregates in blot (C). β-actin was shown to demonstrate equal loading of proteins on all blots.
DA-induced aggregation in vesicles and secretion of α-synuclein aggregates
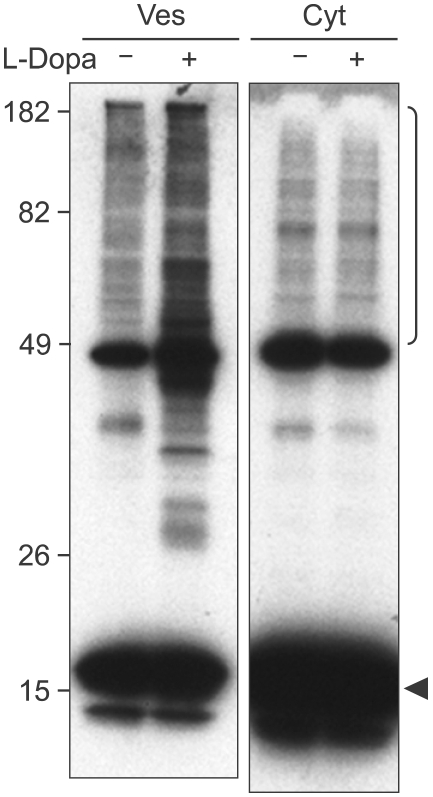
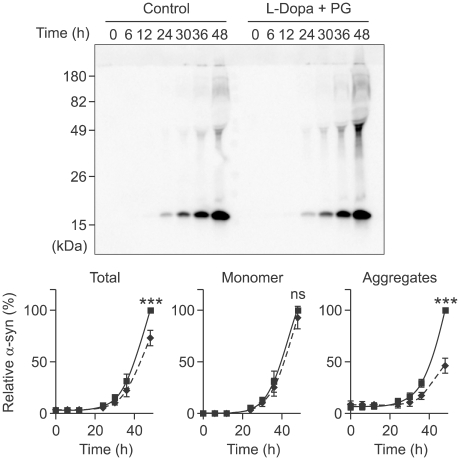
Previous studies have shown that α-synuclein aggregation occurs within vesicles and that these aggregates can be secreted from cells (Lee et al., 2005). Consistent with these findings, the DA-induced increase in α-synuclein aggregation was exclusively found in the vesicle fraction, but not in the cytosol (Figure 4). The purity of cytosolic and vesicle fractions were tested by blotting against cytosolic marker Hsp27 and vesicle marker synaptotagmin (Supplementary Figure 2). In order to determine the effect of DA on α-synuclein secretion, we measured the levels of α-synuclein in the culture media over time in the presence or absence of L-DOPA and pargyline (Figure 5). Both monomeric and aggregated forms of α-synuclein were detected in the culture media, and the overall levels of secreted α-synuclein were increased in the presence of DA. When the monomers and aggregates were quantified separately, we found that only the aggregates were increased in the media, while monomer levels were not changed significantly. Therefore, elevation of cytoplasmic DA levels promotes α-synuclein aggregation in intracellular vesicles and specifically increases the release of aggregated forms of α-synuclein into the extracellular space.
Figure 4.
Localization of DA-induced α-synuclein aggregates in vesicle fractions. Differentiated SH-SY5Y neuronal cells overexpressing α-synuclein were treated with 1 mM L-dopa and the extract was separated by density gradient centrifugation to obtain vesicle (Ves) or cytosolic (Cyt) fractions. Note the increased α-synuclein aggregates (side bar) in vesicle fraction of L-dopa treated cells.
Figure 5.
DA-induced secretion of α-synuclein aggregates from SH-SY5Y cells. Differentiated SH-SY5Y cells were treated with 1 mM L-dopa and 10 µM pargyline for indicated times and the conditioned media were collected and analyzed for α-synuclein release. Note that the monomer levels do not differ but the amount of α-synuclein aggregates increased significantly in L-dopa- and pargyline-treated cells (***P < 0.001).
Discussion
Primary symptoms of PD are attributed to the selective loss of DA neurons in the SNpc of the ventral midbrain. We hypothesized that the interaction of DA with α-synuclein contributes to the selective vulnerability of DA neurons in PD. In support of this hypothesis, it has been shown that high concentrations of cytosolic DA and Ca2+ in α-synuclein-overexpressing DA neurons of SNpc evoked selective death of these neurons (Mosharov et al., 2009). In the present study, we determined the effects of DA on aggregation of α-synuclein in test tubes and in a DA neuronal model. The presence of DA inhibited fibril formation, and produced a heterogeneous mixture of non-fibrillar aggregates. These aggregates have little β-sheet conformation, lack thioflavin dye-binding ability, and have various non-fibrillar morphologies under EM. This is in good agreement with previous studies reported by a number of groups (Conway et al., 2001; Li et al., 2004; Cappai et al., 2005). When the cellular DA concentration was elevated pharmacologically, α-synuclein aggregation was increased. This DA-induced aggregation was specifically increased in vesicle fractions, and subsequently led to the increased release of α-synuclein aggregates into the medium.
In the presence of oxygen molecules at normal pH, DA auto-oxidizes into dopamine-quinone (DAQ) species through a series of oxidation steps (Bisaglia et al., 2007). Studies suggest that these nonenzymatic reactive intermediates of DA oxidation, rather than DA itself, interact with α-synuclein and modulate the aggregation process of this protein. In the presence of dopaminochrome (Norris et al., 2003) or more stable derivatives, such as 5,6-dihydroxyindole (Pham et al., 2009) and indole-5,6-quinone (Bisaglia et al., 2007), α-synuclein forms SDS-resistant soluble oligomers that are indistinguishable to those formed in the presence of DA (Cappai et al., 2005). DA can also undergo an enzymatic oxidation reaction, the first step of which is catalyzed by MAO to produce 3,4-dihydroxyphenylacetaldehyde (DOPAL) (Galvin, 2006). DOPAL is a highly reactive aldehyde which can induce α-synuclein aggregation into oligomers in vitro (Burke et al., 2008). However, the present study suggests that the production of DOPAL may not be necessary for increased α-synuclein aggregation and secretion; inhibition of DOPAL production by pargyline, a MAO inhibitor, further increased L-DOPA-induced α-synuclein aggregation (Figure 3). Therefore, DAQ species may be responsible for the increased α-synuclein aggregation and secretion shown in the present study. Several mechanisms have been suggested regarding how DA metabolites modulate a-synuclein aggregation. These include α-synuclein-DAQ adduct formation (Conway, et al., 2001; Norris et al., 2003; Li et al., 2004; 2005), non-covalent interaction of DAQ with the C-terminal region of α-synuclein (Norris et al., 2003), and methionine oxidation (Leong et al., 2009). Elucidation of the mechanism may provide us with the strategy to modulate α-synuclein aggregation in DA neurons, thereby protecting these neurons.
One of the common features of neurodegenerative diseases is the progressive development of clinical symptoms. This progression of disease is accompanied by spreading of pathologic protein aggregates. In the brains of patients with PD, LBs first appear in the dorsal motor nucleus of the vagus nerve in the lower brain stem, spread through the midbrain and mesocortex, and finally affect large areas in the neocortex (Braak et al., 2003). As a model explaining the progressive spreading of LBs, a prion-like, direct cell-to-cell transmission of α-synuclein aggregates has recently been suggested (Desplats et al., 2009). The mechanism of transmission is not fully understood. However, results from recent studies demonstrate that neurons can release α-synuclein aggregates via unconventional exocytosis (Lee et al., 2005; Jang et al., 2010), and the released α-synuclein aggregates can internalize into neighboring neurons via endocytosis (Lee et al., 2008; Desplats et al., 2009). Therefore, understanding the modulators of neuronal α-synuclein release will provide critical insight into the mechanism of cell-to-cell aggregate transmission. Release of α-synuclein is not a robust event; only a small fraction of cytosolic α-synuclein is released from neuronal cells (Lee et al., 2005). Thus, there must be a mechanism by which the protein to be released is selected. We have recently shown that the release of α-synuclein is increased under conditions in which misfolding of this protein is promoted and the released protein is oxidatively-modified more extensively than the cellular protein (Jang et al., 2010). Consistent with this finding, we have shown in the present study that the increased concentration of cytosolic DA promotes α-synuclein aggregation and exocytosis of these aggregates into the extracellular space. Increased release of α-synuclein aggregates from DA neurons may accelerate the cell-to-cell transmission and may thus explain why the SNpc is more severely affected than other regions of the brain. Since extracellular α-synuclein aggregates can activate microglia, DA-induced α-synuclein release may also explain extensive microglia activation in the SNpc of brains from patients with PD. Therefore, investigations into the transmissibility, neurotoxicity, and ability to induce neuroinflammation of DA-modified α-synuclein will enhance our understanding of the mechanism underlying progressive loss of the SNpc DA neurons and associated neuroinflammation.
Methods
Materials
Isopropyl-1-thio-β-D-galactopyranoside (IPTG), dopamine hydrochloride, pargyline, levo-DOPA (L-dopa), carbi-DOPA (c-DOPA), glycine, thioflavin T, and protease inhibitor cocktail were purchased from Sigma (St. Louis, MO). Anti-α-synuclein and synaptotagmin antibodies were obtained from BD Biosciences (San Jose, CA). Hsp27 antibody was from Stressgen (British Columbia, Canada).
Expression and purification of α-synuclein
Plasmid containing α-synuclein (α-syn/pDualGC) was expressed in E. coli BL21(DE3) strain (RBC Korea, Seoul, Korea); protein expression was induced with 0.1 mM IPTG for 3 hours at 37℃. Cells were sonicated in 0.1 M sodium phosphate buffer (pH 7.6), then boiled for 15 min before centrifugation at 10,000 g for 10 min. The supernatant was subjected to anion-exchange chromatography and Sephacryl S-200 gel filtration column chromatography for further purification.
Preparation of dopamine-induced α-synuclein oligomers
Dopamine hydrochloride was dissolved in a 20 mM Tris (pH 7.4)/0.1 M NaCl solution to a final concentration of 100 mM. The dopamine solution was immediately mixed with purified α-synuclein to obtain a 100 µM α-synuclein/2 mM dopamine solution and incubated at 37℃ with 290 RPM for 110 hours. The mixture was spun at 16,000 g for 10 minutes and the supernatant was filtered through a 0.2 µM filter.
Thioflavin T binding assay
Protein samples were mixed with 10 µM Thioflavin T solution (1:1) in 10 µM glycine buffer (pH 8.5) and the emission fluorescence was recorded at 490 nm with excitation at 450 nm using a SpectraMax GEMINI EM device (Molecular Probes, Eugene, OR).
Circular dichroism (CD)
The CD spectra of protein samples (0.5 mg/ml) were recorded in 0.01 cm cells from 190-260 nm with a J-810 spectropolarimeter (Jasco Inc., Easton, MD). Ten scans were obtained for each spectrum and the mean value was obtained.
Electron microscopy
Protein samples were applied to formvard-coated copper grids and negatively stained with 1% (w/v) uranyl acetate and washed with deionized water. Transmission electron microscopy images were collected at 100 kV on a Philips CM electron microscope (Philips, Eindhoven, The Netherlands).
Cell culture and α-synuclein expression
Maintenance and differentiation of the human neuroblastoma cell line, SH-SY5Y, have been previously described (Lee et al., 2004). For α-synuclein expression in differentiated SH-SY5Y cells, a recombinant adenoviral vector containing human α-synuclein cDNA (adeno/α-syn) was used, as described (Lee et al., 2004). Briefly, differentiated SH-SY5Y cells were infected with recombinant adenoviral vector 'adeno/α-syn' at a m.o.i. of 33 in 1/2 volume of fresh medium. Following a 90 min incubation, the remaining 1/2 volume of fresh medium was added and incubated overnight. The next day, the medium was replaced with fresh medium and incubated further before treatment.
Treatment of L-dopa and pargyline
Differentiated SH-SY5Y cells infected with adeno/α-syn were treated with 10 µM pargyline and 1 mM L-dopa on day 3 of infection for 48 hours, or as indicated. To prevent oxidation of L-dopa in the culture medium, 5 mM glutathione was added.
Preparation of cell extract and conditioned medium
The conditioned medium was centrifuged at 10,000 g for 20 min to remove cell debris and stored with protease inhibitor cocktail at -80℃ until further analysis. Protein extracts were obtained in the manner previously described (Lee et al., 2005). Briefly, cells were washed twice in ice-cold PBS, followed by the addition of extraction buffer (PBS/1% Triton X-100/protease inhibitor cocktail). The extract was then spun at 16,000 g for 10 min to obtain triton-soluble (supernatant) and -insoluble (pellet) fractions. The amount of protein was quantified by the BCA protein assay (Pierce, Rockford, IL).
Total vesicle preparation
The procedure for vesicle preparation has been described previously (Lee et al., 2005). Briefly, buffer M (10 mM HEPES [pH 7.2], 10 mM KCl, 1 mM EGTA, and 250 mM sucrose) with a protease inhibitor cocktail was used for cell harvesting. Cells were disrupted using a N2 cell disruption bomb (Parr Instrument, Moline, IL) or a Dounce homogenizer. The supernatant from a 1,000 g centrifugation was mixed with OptiPrep density gradient medium (Sigma) to obtain a final concentration of 35%; the mixture was layered under 30% and 5% step-gradient layers. After centrifugation at 200,000 g for 2 h, total vesicles were collected at the interphase between 5% and 30%, and the cytosolic fraction was collected from the 35% bottom fraction.
Western blotting
Western blotting was performed as previously described (Lee et al., 2002). Chemiluminescence detection was performed using a FUJIFILM Luminescent Image Analyzer LAS-3000 and Multi Gauge (v3.0) software (FUJIFILM, Tyoko, Japan).
Cell viability assay
Cells treated with 1 mM L-dopa or dH2O for 48 hours were trypsinized and transferred to two tubes. Detergent was added to one of the tubes to permeabilize cells. Propidium iodide was then added to both tubes to label total cells (detergent added cells) and damaged cells (no detergent). Cells from both tubes were counted by ADAM cell counter (NanoEnTek, Seoul, Korea).
Statistical analysis
All experiments were blind-coded and repeated three-to-four times. The values in the figures are expressed as the mean ± SEM. Differences were considered significant if P values were < 0.05. The graphs were drawn with Prism 5 software (Graphpad Software Inc., La Jolla, CA). For determination of statistical significance, values were compared by one-way ANOVA with Tukey's post-test using InStat (version 3.05) software (Graphpad Software Inc.).
Supplemental data
Supplemental data include two figures and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-43-4-06.pdf.
Acknowledgements
This work was supported by the Mid-career Research Program (2010-0015188), Diseases Network Research Program (2010-0020610) through a NRF grant and the Korea Science and Engineering Foundation (KOSEF) grant (2009-0083737) funded by the MEST, and the National Research Foundation of Korea Grant funded by the Korean Government (2009-0073566).
Abbreviations
- DA
dopamine
- LB
Lewy body
- PD
Parkinson's disease
- ROS
reactive oxygen species
- DAQ
dopamine-quinone
- SNpc
Substantia Nigra pars compacta
Supplementary Material
Supplemental Data
References
- 1.Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with {alpha}- synuclein. J Biol Chem. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 3.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O'Dell M, Li SW, Pan Y, Chung HD, Galvin JE. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 4.Cappai R, Leck SL, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, Culvenor JG, Bottomley SP, Masters CL, Barnham KJ, Hill AF. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- 5.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 6.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 9.Galvin JE. Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson's disease: a case for the selective vulnerability of the substantia nigra. Acta Neuropathol. 2006;112:115–126. doi: 10.1007/s00401-006-0096-2. [DOI] [PubMed] [Google Scholar]
- 10.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 11.Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–1274. doi: 10.1111/j.1471-4159.2010.06695.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Lee SJ. Controlling the mass action of alpha-synuclein in Parkinson's disease. J Neurochem. 2008;107:303–316. doi: 10.1111/j.1471-4159.2008.05612.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Shin SY, Choi C, Lee YH, Lee SJ. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 20.Leong SL, Pham CL, Galatis D, Fodero-Tavoletti MT, Perez K, Hill AF, Masters CL, Ali FE, Barnham KJ, Cappai R. Formation of dopamine-mediated alpha-synuclein-soluble oligomers requires methionine oxidation. Free Radic Biol Med. 2009;46:1328–1337. doi: 10.1016/j.freeradbiomed.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Li HT, Lin DH, Luo XY, Zhang F, Ji LN, Du HN, Song GQ, Hu J, Zhou JW, Hu HY. Inhibition of alpha-synuclein fibrillization by dopamine analogs via reaction with the amino groups of alpha-synuclein. Implication for dopaminergic neurodegeneration. FEBS J. 2005;272:3661–3672. doi: 10.1111/j.1742-4658.2005.04792.x. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL. Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson's and Alzheimer's disease. Faseb J. 2004;18:962–964. doi: 10.1096/fj.03-0770fje. [DOI] [PubMed] [Google Scholar]
- 23.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norris EH, Giasson BI, Ischiropoulos H, Lee VM. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- 25.Pham CL, Leong SL, Ali FE, Kenche VB, Hill AF, Gras SL, Barnham KJ, Cappai R. Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J Mol Biol. 2009;387:771–785. doi: 10.1016/j.jmb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Presgraves SP, Ahmed T, Borwege S, Joyce JN. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res. 2004;5:579–598. doi: 10.1007/BF03033178. [DOI] [PubMed] [Google Scholar]
- 27.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 28.Volles MJ, Lansbury PT., Jr Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in parkinson's disease. Biochemistry. 2003;42:7871–7878. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data