Abstract
The “acute phase” is clinically characterized by homeostatic alterations such as somnolence, adinamia, fever, muscular weakness, and leukocytosis. Dramatic changes in iron metabolism are observed under acute-phase conditions. Rats were administered turpentine oil (TO) intramuscularly to induce a sterile abscess and killed at various time points. Tissue iron content in the liver and brain increased progressively after TO administration. Immunohistology revealed an abundant expression of transferrin receptor-1 (TfR1) in the membrane and cytoplasm of the liver cells, in contrast to almost only nuclear expression of TfR1 in brain tissue. The expression of TfR1 increased at the protein and RNA levels in both organs. Gene expression of hepcidin, ferritin-H, iron-regulatory protein-1, and heme oxygenase-1 was also upregulated, whereas that of hemojuvelin, ferroportin-1, and the hemochromatosis gene was significantly downregulated at the same time points in both the brain and the liver at the RNA level. However, in contrast to observations in the liver, gene expression of the main acute-phase cytokine (interleukin-6) in the brain was significantly upregulated. In vitro experiments revealed TfR1 membranous protein expression in the liver cells, whereas nuclear and cytoplasmic TfR1 protein was detectable in brain cells. During the non-bacterial acute phase, iron content in the liver and brain increased together with the expression of TfR1. The iron metabolism proteins were regulated in a way similar to that observed in the liver, possibly by locally produced acute-phase cytokines. The significance of the presence of TfR1 in the nucleus of the brain cells has to be clarified.
Keywords: Acute phase; Iron regulation; Transferrin receptor 1; Cytokines; Liver and brain; Rat (male, Wistar)
Introduction
The acute-phase response (APR) is a major physiological defence reaction of the body to tissue injury and aims to eliminate the injuring noxae and to re-establish homeostasis. Clinically, it is characterized by fever, somnolence, weakness, muscular joint pain, and adinamia. This reaction is mediated by both interleukin (IL)-1-like cytokines (IL-1, tumor necrosis factor-alpha [TNF-α]) and IL-6-like cytokines (IL-6, oncostatin M, and others), through the activation of the transcription factors nuclear factor kappa B, activator protein 1, signal transducer and activator of transcription 3/5, or CCAAT/enhancing-binding protein β. These signaling cascades result in an increase in the plasma levels of a number of positive acute-phase proteins (APPs), including clotting proteins, transport proteins, antiproteases, and complement factors, with a parallel decrease in negative APPs, such as albumin or transferrin (Ramadori and Christ 1999). Furthermore, the decrease of the iron serum level is also a hallmark of APR (Sheikh et al. 2007).
Iron is an important co-factor for oxygen transport, heme and nonheme iron proteins, electron transfer, neurotransmitter synthesis, myelin production energy metabolism, and mitochondrial function in organs (Camaschella 2005; Hentze et al. 2004; Napier et al. 2005; Stankiewicz et al. 2007). Iron homeostasis is controlled by a large group of iron-regulatory proteins. Transferrin (Tf) carries iron to the reticuloendothelial system, to liver parenchymal cells, and to all proliferating cells in the body. Interaction of diferric Tf with the Tf receptor 1 (TfR1) and internalization of the complex by receptor-mediated endocytosis leads to iron uptake in the cells (Conner and Schmid 2003; Frazer and Anderson 2005; Jandl et al. 1959; Morgan and Appleton 1969). The synthesis of TfR1 is generally known to be regulated at the mRNA level through iron-responsive elements (IRE) present in the untranslated region of the mRNA. If the cellular iron level is low, these IREs interact with iron-regulatory proteins to protect mRNA from cleavage and degradation and thereby increase protein synthesis (Casey et al. 1989; Rouault 2006). Several other genes involved in iron homeostasis have been characterized including ferroportin 1 (Fpn1; McKie et al. 2000), transferrin receptor 2 (TfR2; Chen et al. 2007), hepcidin (Pigeon et al. 2001), and hemojuvelin (Hjv; Lanzara et al. 2004). Hepcidin is also found to control iron levels by directly interacting with Fpn1, leading to internalization and degradation of Fpn1 when iron levels are high and consequently blocking the release of iron from storage sites, hepatocytes, and macrophages (Nemeth et al. 2004).
In addition, iron absorption in the intestine is considered an important processes in iron metabolism. Duodenal cytochrome b (Dcytb), divalent-metal transporter 1 (DMT1), Fpn1, and hephaestin (Heph) are major proteins involved in iron absorption (Gunshin et al. 1997; McKie et al. 2001; Vulpe et al. 1999).
Although the liver is the main target of acute-phase cytokines during acute-phase conditions and regulates the body iron by dramatic changes in the gene expression of iron-regulatory proteins, the changes in the local expression of these proteins have been observed in several extrahepatic tissues (Sheikh et al. 2007). The brain has a high requirement of iron as it is the organ that has the highest oxidative metabolism (Wrigglesworth and Baum 1988). Previous reports have also shown the expression of some iron-regulatory proteins in the murine central nervous system (CNS), including TfR1 (Moos 1996), iron-regulatory protein (Leibold et al. 2001), ferritin (Moos 1996), neogenin (Rodriguez et al. 2007), and hepcidin (Zechel et al. 2006), but their regulation in the brain under acute-phase conditions has not been investigated so far. Expression of the acute-phase cytokines IL-1β, IL-6, and TNFα in normal CNS has also been reported, but their behavior under acute-phase conditions has not been studied (Zhao and Schwartz 1998).
The mechanism of iron uptake is partially known in other organs but is poorly understood in the brain, as the CNS is not directly in contact with the plasma iron pool, because it resides behind the blood/brain barrier. Hence, the TfR-mediated uptake of iron by brain capillary endothelial cells followed by further transport into the brain is the only known mechanism by which iron is transported into the brain (Crowe and Morgan 1992). However, the recent spark of interest in research concerning the molecular links among the nervous, endocrine, and immune systems has caused an explosion of new knowledge concerning the fine mechanisms of iron uptake. One report suggests that iron overload, secondary to end-stage liver disease, might result in the deposition of iron in other organs including the CNS, even in the absence of hemochromatosis (Eng et al. 2005). As the brain “suffers” under acute-phase conditions in which the liver is centrally involved, we have investigated the effects of the intra-muscular administration of turpentine oil (TO) on iron tissue content and on the gene expression of TfR1, and the effects of various proteins involved in iron metabolism and acute-phase cytokines in the brain.
Our data provide evidence that the intra-muscular administration of TO can induce changes in the expression of iron-regulatory proteins and acute-phase cytokines at the mRNA and protein levels, not only in peripheral organs such as the liver, but also in the brain. Furthermore, iron content increases in the brain, as has also been observed in the liver under acute-phase conditions. However, whereas changes observed in the liver are induced by acute-phase cytokines delivered by the blood, the local production of the cytokines seems to take place in the brain. Interestingly, although TfR1 is mainly detectable in the plasma membrane of the major liver cells, it is mainly localized in the nucleus of the brain cells. The functional consequences of this difference need to be investigated.
Materials and methods
Animals
Male Wistar rats of about 170-200 g body weight were purchased from Harlan-Winkelmann (Brochen, Germany). The rats were kept under standard conditions with 12-h light/dark cycles and ad libitum access to fresh water and food pellets. All animals were cared for according to the University’s guidelines, German regulations for the protection of animals, and NIH guidelines.
Materials
All chemicals used were of analytical grade and purchased from commercial sources as follows: real-time polymerase chain reaction (PCR) primers, primers for Northern blot, M-MLV reverse transcriptase, reverse transcription buffer, 0.1 M dithiothreitol (DTT), Platinum Sybr green qPCR-UDG mix were from(Invitrogen (Darmstadt Germany); dNTPs, Protector RNase inhibitor, Klenow enzyme, primer oligo (DT)15 for complementary DNA (cDNA) synthesis, and Salmon sperm DNA were from Roche (Mannheim, Germany); [α-32P]-labeled deoxy-cytidine triphosphate (specific activity: 3000ci/mmol), NICK TM columns, and Hybond N nylon membranes were from Amersham Pharmacia Biotech (Freiburg, Germany); hybridization solution QuickHyb was from Stratagene (Germany); iron ferrozine was from Rolf Greiner BioChemica (Flacht, Germany). All other reagents and chemicals were from Sigma-Aldrich (Steinheim, Germany) or Merck (Darmstadt, Germany).
Induction of acute phase and removal of liver and brain
APR was induced by injecting TO at a dose of 5 ml/kg body weight; 0.5 ml TO was injected into each of the right and left hind limb gluteal muscles of ether-anesthetized rats. Control animals for each time point received a saline injection. All animals were killed at time points ranging from 0.5 to 48 h after TO administration under pentobarbital anesthesia (Tron et al. 2005). The skull was opened from the mid-dorsal side by using sharp pointed scissors, and the brain was removed, rinsed with physiological saline, frozen in liquid nitrogen, and stored at −80°C until used. The liver was taken from the abdomen, frozen in liquid nitrogen, and stored at −80°C.
Isolation and culture of rat liver cells
Hepatocytes
Hepatocytes were isolated from normal animals according to a protocol described previously (Ramadori et al. 1990). The purity of the isolated cell populations was determined by phase-contrast microscopy and by immunocytochemistry by using antibodies against laminin or glial fibrillary acidic protein to identify stellate cells (both Sigma, Deisenhofen, Germany) or against ED1 and ED2 (gift from C. Dijkstra) for macrophages. Dulbecco’s modified Eagle’s medium (DMEM; Biochrom, Berlin, Germany) was supplemented with 10% fetal calf serum (FCS; PAA, Cölbe, Germany), 1 nM insulin (Roche), and 100 nM dexamethasone (Sigma, Munich, Germany).
Macrophages (Kupffer cells)
Kupffer cells were isolated and cultured according to the method described by Tello et al. (2008). Briefly, liver macrophages were plated by using 200,000 cells per ml culture medium supplemented with 10% FCS. The purity of the cell isolation was determined by ED1/ED2 staining.
Rat liver hepatic stellate cells and (myo)fibroblasts
Rat liver hepatic stellate cells (HSC) and (myo)fibroblasts (LMF) were isolated and cultured as described previously (Dudas et al. 2007). In brief, cells were cultured in DMEM supplemented with 15% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% L-glutamine.
Neural stem cells
Neural stem cells from adult rat hippocampus were purchased from Chemicon (USA). Cells were cultured in DMEM/F12 medium with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Human cell lines
The human hepatoma cell line (HepG2 cells) was purchased from the American Tissue Culture Collection (ATCC, Manassas, Va., USA). HepG2 cells were cultured in RPMI supplemented with 10% FCS, 2% glutamine, and 1% sodium pyruvate. The glioblastoma cell line (U373MG) was obtained from the Tumor Bank of the German Cancer Research Center in Heidelberg, cultured in DMEM and supplemented with 10% FCS, 1% glutamine, 1% penicillin/streptomycin. All cells were maintained at 37°C in a 5% CO2 atmosphere at 100% humidity.
Preparation of tissue and cell lysate
About 50 mg frozen tissue or 2×105 cells per dish was homogenized with an Ultra-turrax TP 18/10, three times for 10 s each, in 10 vol 50 mM TRIS-HCl buffer, pH 7.4, containing 150 mM sodium chloride, 1 mM EDTA, 1% Triton X-100, 1 mM phenylmethane sulfonyl-fluoride (PMSF), 1 mM benzamidine, 1 mg/ml leupeptin, 10 mM chymostatin, 1 mg/ml antipain, and 1 mg/ml pepstatin A. The entire procedure was carried out at 4°C. Crude homogenates were passed five times through a 22-G needle attached to a syringe and centrifuged for 5 min at 10,000g, 4°C. The protein concentration was determined in supernatants by using the BCA (bicinchoninic acid) protein assay reagent kit (Pierce, Bonn, Germany). Aliquots of the homogenates were stored at −20°C until further used for Western blot analysis and measurement of the iron levels by a colorimetric ferrozine-based assay (Riemer et al. 2004).
Cellular fractionation for protein isolation
Nuclear protein and cytoplasmic fractions were isolated as previously described (Budick-Harmelin et al. 2008). For membrane and cytosolic extracts, 2×105 HepG2 cells per dish were homogenized in membrane buffer containing 5 M NaCl, 1 M TRIS-HCl pH 7.5, 0.5 M EDTA, 10 mg/ml PMSF, aprotinin, and leupeptin. After homogenization, the cell lysate was centrifuged at 1000 rpm for 10 min. The supernatant was collected as a cytosolic extract, and the pellet (membrane extract) was resuspended in lysis buffer (prepared as given above for the whole lysate) and stored at −20°C for future use.
Tissue iron level
The iron content of tissue was measured by the colorimetric ferrozine-based assay (Riemer et al. 2004). Briefly, the iron bound to transferrin (Tf) was released in an acidic medium as ferric iron and then reduced to ferrous iron in the presence of ascorbic acid.
Immunohistochemistry and immunocytology
Liver and brain sections were cut in a cryostat at a thickness of 5 μm, air-dried, fixed with acetone (−20°C, 10 min), and stored at −20°C. Cells (Labtek) were fixed with methanol (−20°C, 10 min) and acetone (−20°C, 10 sec) and also stored at −20°C before being used. An anti-ED1 antibody (Serotec Düsseldorf, Germany) and a mouse monoclonal antibody specific for TfR1 (IgG1, specific to residues 3–28 of the TfR1 tail, clone H68.4; catalog no. 13–6800; Invitrogen) were used. Immunoflorescence was performed according to a protocol described previously (Malik et al. 2010). Briefly, cryostat sections (~5 μm) were fixed in acetone for 10 min, incubated in the primary antibodies for TfR1 (1:200) and mouse monoclonal anti-ED1 primary antibodies (1:50) overnight at 4°C, rinsed in phosphate-buffered saline (PBS), incubated in Alexa Fluor and rodamine-conjugated anti-mouse secondary antibody (1:200; Molecular Probes, Germany) at room temperature for 1 h, and washed three times for 5 min in PBS. Finally, the nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI), and the sections were washed and mounted.
Western blot analysis
Samples of 50 μg tissue protein and 20 μg cell protein were applied per well and subjected to polyacrylamide gel electrophoresis using NuPAGE (4%-12% Bis-Tris Gel; Invitrogen) under reducing conditions (Laemmli 1970). After electrophoresis, the proteins were transferred to Hybond-ECL (enhanced chemiluminescence) nitrocellulose membranes (Towbin et al. 1979). Immunodetection was performed according to the ECL Western blotting protocol. The mouse anti-TfR1 antibody, the monoclonal antibody against transferrin receptor (Invitrogen), and β-actin (Santa Cruz Biotechnology, Heidelberg, Germany) were used at a dilution of 1 μg/ml, 1:1000, and 1:5000, respectively.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from liver and brain by means of guanidine isothiocyanate extraction, caesium chloride density-gradient ultracentrifugation, and ethanol precipitation according to a previously described method (Chirgwin et al. 1979), with some modifications as described elsewhere (Ramadori et al. 1985). The cDNA was generated by reverse transcription of 1 μg total RNA by using 100 nM dNTPs, 50 pM primer oligo(dT)15, 200 U moloney murine leukemia virus reverse transcriptase, 16 U protector RNase inhibitor, 1× RT buffer, and 2.5 μl 0.1 M DTT. Real-time PCR was performed on an ABI prism 7000 sequence detection system as described elsewhere (Malik et al. 2010). β-Actin and ubiquitin C were used as housekeeping genes. The primer sequences used are given in Table 1. The results were normalized to the housekeeping genes, and the fold change in expression was calculated by using threshold cycle values.
Table 1.
Sequences of the primers (Fpn1 ferroportin 1, DMT1 divalent metal transporter 1, Dcytb duodenal cytochrome B reductase, Hjv hemojuvelin,HFE hemochromatosis gene, Heph hephaestin, Tf transferrin, TfR1, TfR2 Tf receptors 1 and 2, IRP1, IRP2 iron-responsive element binding proteins 1 and 2, IL-1β, IL-6 interleukins 1β and 6, TNF-α tumor necrosis factor alpha, UBC ubiquitin C) used for the real-time polymerase chain reaction (PCR)
| Primers | 5----3 | 5----3 |
|---|---|---|
| Forward | Reverse | |
| Hepcidin | GAA GGC AAG ATG GCA CTA AGC A | TCT CGT CTG TTG CCG GAG ATA G |
| Ferritin H | GCC CTG AAG AAC TTT GCC AAA T | TGC AGG AAG ATT CGT CCA CCT |
| Fpn1 | TTC CGC ACT TTT CGA GAT GG | TAC AGT CGA AGC CCA GGA CTG T |
| DMT1 | GCT GAG CGA AGA TAC CAG CG | TGT GCA ACG GCA CAT ACT TG |
| Dcytb | TCC TGA GAG CGA TTG TGT TG | TTA ATG GGG CAT AGC CAG AG |
| Hjv | ATG CCG TGT CCA AGG AGC TT | TCC ACC TCA GCC TGG TAG AC |
| HFE | ATC AGC CTC TCA CTG CCA CT | CAA GTG TGT CCC CTC CAA GT |
| Heph | CAC ATT TTT CCA GCC ACC TT | TGA CGA ACT TTG CCT GTG AG |
| Tf | GGC ATC AGA CTC CAG CAT CA | GCA GGC CCA TAG GGA TGT T |
| TfR1 | ATA CGT TCC CCG TTG TTG AGG | GGC GGA AAC TGA GTA TGG TTG A |
| TfR2 | AGC TGG GAC GGA GGT GAC TT | TCC AGG CTC ACG TAC ACA ACA G |
| IRP1 | GAG TCA TGC CTT ACC TGT CCC A | TGA TAG CCT CCA CCA CAG GTT C |
| IRP2 | CTG CAT CCC AGC CTA TTG AAA A | GCA CTG CTC CTA GCA ATG CTT C |
| IL-6 | GTC AAC TCC ATC TGC CCT TCA G | GGC AGT GGC TGT CAA CAA CAT |
| TNF-α | ACA AGG CTG CCC CGA CTA T | CTC CTG GTA TGA AGT GGC AAA TC |
| IL-1β | TAC CTA TGT CTT GCC CGT GGA G | ATC ATC CCA CGA GTC ACA GAG G |
| β-Actin | TGT CAC CAA CTG GGA CGA TA | AAC ACA GCC TGG ATG GCT AC |
| UBC | CACCAAGAAGGTCAAACAGGAA | AAGACACCTCCCCATCAAACC |
Southern blot analysis of PCR products
In order to confirm the specificity of the primers, hepcidin gene expression was also analyzed in the brain by PCR in a volume of 10 μl. PCR products were separated electrophoretically in a 1.2% agarose gel and blotted onto a nylon membrane as described previously. The blot was hybridized with a probe synthesized by PCR with cDNA obtained from rat liver, and the hybridization was followed by washing steps as described elsewhere (Ramadori et al. 2010). β-Actin was used as an internal control to check equal loading.
Statistical analysis
The data were analyzed by using Prism Graph pad 4 software (San Diego, USA). All experimental errors are shown as SEM. Statistical significance was calculated by one-way analysis of variance (ANOVA) and the Dunnett post hoc test. Significance was accepted at P<0.05.
Results
Tissue iron level
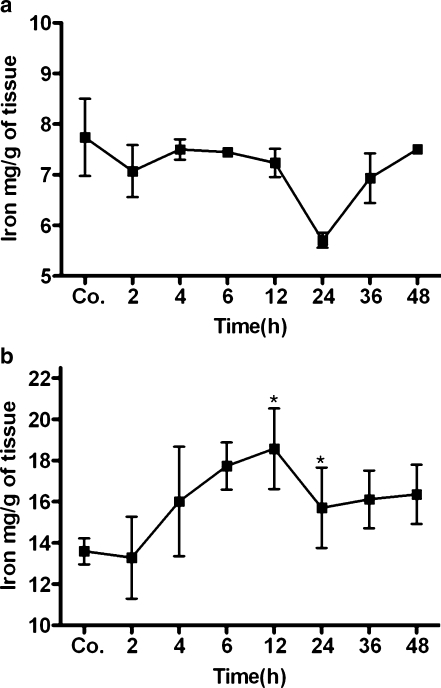
Tissue iron level was measured in the brain and liver tissue lysates. Compared with control animals (6.8±0.5 mg/g tissue), the iron level of brain tissue increased with a maximum at 2 h (7.6±0.3 mg/g tissue) and remained above control levels until 6 h after TO injection, but changes were statistically non-significant (Fig. 1a). However, compared with control animals (13.6±0.6 mg/g tissue), a statistically significant increase in the iron content was observed in the liver tissue of TO-treated animals, with a maximum at 24 h (17.8±2.1 mg/g tissue; Fig. 1b).
Fig. 1.
Changes of tissue iron concentration during acute-phase response (APR). Brain (a) and liver tissue (b) iron levels were determined by ferrozine-based assay (Co. control). Results represent mean values±SEM (*P<0.05 analyzed by one-way ANOVA; n=3)
TfR1 protein expression in rat liver, brain, and serum
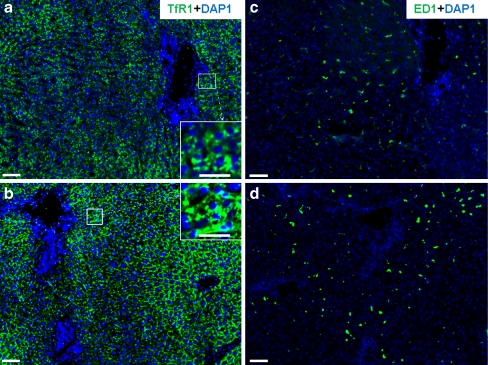
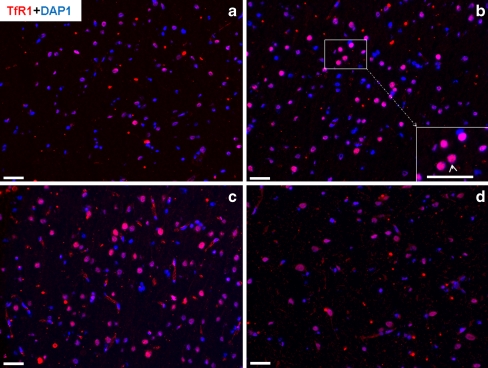
By using the monoclonal antibody against TfR1, the immunoreactivity of TfR1 was detected by immunofluorescent staining in normal rat liver and brain taken at various time points after intramuscular TO administration. In serial sections of liver tissue, strong membranous expression of TfR1 was observed in hepatocytes and in sinusoidal cells (Fig. 2a-d). Interestingly, when the same monoclonal antibody was used on sections of the brain, TfR1 protein expression was found mainly in the nucleus (Fig. 3a-d).
Fig. 2.
Immunofluorescence detection of transferrin receptor 1 (TfR1, green) in rat liver. TfR1- and ED-1-positive cells in serial sections of rat liver tissue at various time points after turpentine oil (TO) administration (DAP1 DAPI nuclear staining [blue]). a Control TfR1. b TfR1 at 6 h after TO treatment. c Control ED-1. d ED-1 at 6 h after TO injection. Insets Higher magnification of boxed areas in a, b showing TfR1-positive cells in liver tissue. Results are representative of three animals. Original magnification: ×100. Bar 100 μm
Fig. 3.
Immunofluorescence detection of TfR1 (pink) on cryostat sections of rat brain. TfR1-positive cells in rat brain at various time points after TO administration (blue DAPI nuclear staining). a Control TfR1. b At 4 h after TO treatment. Inset bottom right Higher magnification of boxed area in b. TfR1-positive cells (white arrow) in the brain. c TfR1 at 6 h after TO treatment. d TfR1 at 24 h after TO treatment. Results are representative of three animals. Original magnification: ×200. Bar 50 μm
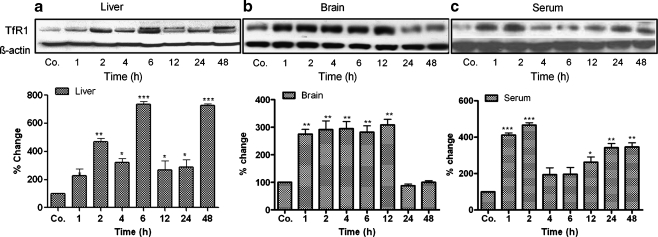
Western blot analysis detected a protein of the same molecular weight in liver and brain tissue and in serum. Furthermore, an increase in protein expression of TfR1 was observed in both organs and in serum; the protein expression remained upregulated until the last time points (Fig. 4a-c).
Fig. 4.
Identification of TfR1 in rat liver, brain, and serum at various time points after TO administration (Co. control). Western blot analysis of TfR1 (95 kDa) from total protein of rat liver, brain, and serum. β-Actin (43 kDa) was used as equal-loading control. Densitometry analysis of Western blots was also performed to show the changes in the protein expression of TfR1. Results represent mean values±SEM (***P<0.0001 analyzed by one-way ANOVA; n=3)
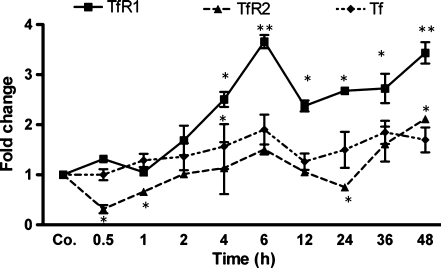
Quantitative changes of TfR1, TfR2, and Tf transcripts in brain and liver
In brain tissue, TfR1 mRNA started increasing 1 h after the onset of APR with a maximum (3.7±0.13-fold) at the 6-h time point (P<0.0001 vs. control). On the other hand, changes in TfR2 mRNA were mild but significant. Tf gene expression was upregulated in the brain (two-fold) after the onset of APR (P<0.0005 vs. control; Fig. 5). In the liver, TfR1 and TfR2 mRNA was slightly upregulated. In addition, a slight down-regulation of Tf mRNA was observed after a slight up-regulation in rat liver at 2 h after TO administration (Table 2).
Fig. 5.
Changes in amount of TfR1, TfR2, and Tf transcript in brain tissue during APR. Fold change in mRNA expression of transferrin (Tf), transferrin receptor 1 (TfR1), and transferrin receptor 2 (TfR2) was normalized to β-actin and ubiquitin C as housekeeping genes in the brain at various time points after intramuscular TO injection as revealed by real-time polymerase chain reaction (PCR). Results represent mean values±SEM (**P<0.001, analyzed by one-way ANOVA; n=3)
Table 2.
PCR analysis of total RNA from liver and brain in turpentine oil (TO)-treated animals. Expression of transferrin (Tf), Tf receptors 1 and 2 (TfR1, TfR2), hepcidin, ferroportin 1 (Fpn1), and hemojuvelin (Hjv) in the rat liver and brain at various time points after intramuscular TO injection in comparison with that of control animals. Data show the results of three animals (mean values±SEM)
| Time (h) | Tf | TfR-1 | TfR-2 | Hepcidin | Fpn1 | Hjv | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Brain | Liver | Brain | Liver | Brain | Liver | Brain | Liver | Brain | Liver | Brain | |
| Control | 1±0.00 | 1±0.00 | 1±0.01 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.01 | 1±0.00 |
| 0.5 | 0.7±0.02 | 1.3±0.5 | 1.7±0.24 | 1.3±0.0 | 0.9±0.2 | 0.3±0.0 | 0.6±0.04 | 0.7±0.1 | 1.1±0.2 | 0.6±0.1 | 0.8±0.1 | 0.3±0.01 |
| 1 | 0.7±0.2 | 1.4±0.1 | 1.5±0.1 | 1.1±0.1 | 0.8±0.1 | 0.7±0.0 | 1.0±0.02 | 0.9±0.2 | 0.8±0.2 | 0.4±0.1 | 0.8±0.1 | 0.8±0.2 |
| 2 | 1.5±0.1 | 0.9±0.3 | 1.4±0.1 | 1.7±0.3 | 0.7±0.1 | 1.0±0.3 | 1.5±0.3 | 1.8±0.4 | 0.7±0.2 | 0.5±0.1 | 0.6±0.1 | 0.3±0.04 |
| 4 | 1.2±0.1 | 0.7±0.3 | 0.9±0.1 | 2.5±0.2 | 1.3±0.1 | 0.9±0.4 | 3.1±0.3 | 2.8±0.3 | 0.9±0.01 | 0.5±0.01 | 0.6±0.02 | 0.6±0.2 |
| 6 | 1.3±0.1 | 1.8±0.1 | 1.5±0.4 | 3.7±0.1 | 1.8±0.1 | 1.5±0.01 | 7.2±0.7 | 2.9±0.3 | 0.9±0.03 | 0.9±0.1 | 0.2±0.01 | 0.2±0.01 |
| 12 | 0.5±0.1 | 1.3±0.1 | 0.9±0.2 | 2.4±0.1 | 0.9±0.03 | 1.1±0.1 | 4.4±0.4 | 2.2±0.3 | 0.5±0.2 | 0.7±0.01 | 0.2±0.1 | 0.3±0.1 |
| 24 | 0.7±0.1 | 1.1±0.1 | 1.3±0.3 | 2.3±0.1 | 0.9±0.02 | 0.8±0.01 | 1.2±0.1 | 2.2±0.3 | 0.4±0.1 | 0.6±0.1 | 0.8±0.1 | 0.3±0.04 |
| 36 | 0.9±0.2 | 2.1±0.11 | 1.2±0.3 | 2.4±0.3 | 0.9±0.2 | 1.5±0.1 | 0.4±0.02 | 2.3±0.6 | 0.7±0.4 | 0.6±0.1 | 0.9±0.1 | 0.6±0.3 |
| 48 | 1.4±0.2 | 1.7±0.01 | 0.6±0.1 | 2.4±0.2 | 1.2±0.1 | 2.1±0.01 | 2.5±0.01 | 2.2±0.5 | 0.9±0.3 | 1.3±0.1 | 1.0±0.1 | 0.8±0.2 |
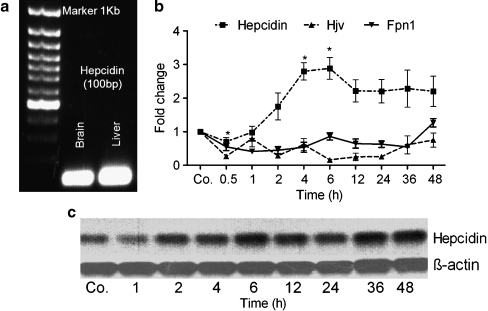
Kinetics of hepcidin, HJV, and Fpn1 gene expression in liver and brain during APR
We quantified cDNA by real-time PCR with rat hepcidin-specific primers to demonstrate the expression of the hepcidin gene in the brain. Hepcidin cDNA was amplified, confirming the presence of hepcidin in the brain, as in the liver (Fig. 6a). Real-time PCR and Southern blot analysis indicated a steep increase in the expression of the hepcidin gene (approximately three-fold) in the brain at 4-6 h after TO injection; this increase remained significantly above control levels until 48 h (P<0.005 vs. control; Fig. 6b, c). In contrast, the expression of the genes for Hjv and Fpn1 behaved inversely to that of the hepcidin gene. Hjv gene expression was significantly downregulated (0.2±0.014-fold) with a minimum level at the 6-h time point, when hepcidin gene expression was at its maximum. Similarly, Fpn1 gene expression was downregulated after TO injection (P<0.0001 vs. control; Fig. 6b). On comparing rat liver with brain, similar changes in the gene expression of hepcidin, Hjv, and Fpn1 (but with a difference in the order of magnitude) were found in rat liver after the onset of APR (Table 2).
Fig. 6.
Changes in the amount of hepcidin, hemojuvelin (Hjv), and ferroportin 1 (Fpn1) transcripts in brain tissue during APR. a Ultraviolet image of agarose gel (1%) demonstrating the expression of the hepcidin gene (amplicon size 100 bp) in the brain and liver. b mRNA expression of hepcidin, Hjv, and Fpn1 analyzed by real-time PCR and normalized by using β-actin and ubiquitin C as housekeeping genes in the brain at various time points after intramuscular injection of TO. c Change in gene expression of hepcidin at the transcriptional level as shown by Southern blot analysis with β-actin as the loading control (Co. saline-treated control). Results represent mean values±SEM (*P<0.05, analyzed by one-way ANOVA; n=3)
Comparison of gene-expression of other iron-regulatory proteins in rat liver and brain
A mild and time-related increase in the gene expression of ferritin-H was observed in brain tissue. In contrast, the expression of the genes for DMT1, HFE, and Dcytb was downregulated after the onset of APR. Hephaestin (heph) gene expression was significantly upregulated (4.8±0.41-fold) in the brain at 24 h after TO injection. Similarly, IRP1 and IRP2 (iron-responsive element binding protein 1 and 2) gene expression was induced with a maximum at 4 h and 12 h, respectively. In liver, gene expression for DMT1 and ferritin-H expression was significantly increased, whereas heph gene expression was reduced after TO injection. The expression of the genes for HFE, Dcytb, and IRPs behaved in the liver in a manner similar to that in the brain during APR (Table 3).
Table 3.
PCR analysis of total RNA from liver and brain in TO-treated animals. Comparison of ferritin-H, divalent metal transporter 1 (DMT1), hephaestin (Heph), hemochromatosis gene (HFE), duodenal cytochrome B reductase (Dcytb), and iron-responsive element binding proteins 1 and 2 (IRP1, IRP2) in the rat liver and brain at various time points after intramuscular TO injection in comparison with control animals. Data show the results of three animals (mean values±SEM)
| Time (h) | Ferritin-H | DMT1 | Heph | HFE | Dcyt B | IRP1 | IRP2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Brain | Liver | Brain | Liver | Brain | Liver | Brain | Liver | Brain | Liver | Brain | Liver | Brain | |
| Control | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.00 | 1±0.002 | 1±0.002 | 1±0.002 | 1±0.00 | 1±0.00 |
| 0.5 | 0.9±0.1 | 1.0±0.5 | 1.0±0.04 | 0.5±0.1 | 0.9±0.2 | 0.5±0.01 | 0.7±0.1 | 0.9±0.01 | 0.8±0.09 | 0.6±0.11 | 1.2±0.2 | 0.4±0.01 | 0.6±0.2 | 0.6±0.03 |
| 1 | 0.9±0.1 | 0.9±0.3 | 1.2±0.03 | 0.4±0.01 | 0.7±0.1 | 0.7±0.1 | 0.7±0.2 | 0.4±0.01 | 0.5±0.1 | 0.3±0.03 | 1.1±0.1 | 0.5±0.01 | 0.4±0.3 | 1.1±0.3 |
| 2 | 0.97±0.1 | 1.1±0.1 | 1.2±0.2 | 0.6±0.02 | 0.8±0.11 | 1.3±0.13 | 0.6±0.2 | 0.6±0.02 | 0.8±0.04 | 0.6±0.1 | 1.1±0.02 | 1.0±0.1 | 0.7±0.1 | 1.2±0.3 |
| 4 | 0.9±0.1 | 1.9±0.01 | 1.8±0.3 | 0.8±0.2 | 0.7±0.1 | 3.8±0.5 | 0.5±0.01 | 0.5±0.03 | 0.5±0.1 | 0.9±0.1 | 1.3±0.02 | 2.9±0.5 | 0.9±0.2 | 0.9±0.2 |
| 6 | 1.5±0.1 | 1.5±0.01 | 1.9±0.4 | 0.5±0.1 | 1.2±0.1 | 3.2±0.4 | 0.5±0.01 | 0.6±0.02 | 0.9±0.03 | 0.7±0.1 | 1.7±0.1 | 1.1±0.1 | 1.8±0.3 | 1.3±0.2 |
| 12 | 0.8±0.1 | 1.6±0.01 | 2.9±0.3 | 0.7±0.001 | 0.6±0.1 | 3.4±0.02 | 0.2±0.03 | 0.3±0.01 | 0.3±0.1 | 0.6±0.03 | 0.6±0.2 | 0.9±0.1 | 0.8±0.2 | 1.5±0.2 |
| 24 | 0.8±0.1 | 1.8±0.01 | 1.9±0.8 | 0.6±0.01 | 0.7±0.1 | 4.8±0.4 | 0.3±0.03 | 0.6±0.001 | 0.3±0.02 | 0.7±0.13 | 1.1±0.2 | 0.9±0.06 | 0.3±0.01 | 1.2±0.1 |
| 36 | 0.8±0.2 | 1.6±0.02 | 1.9±0.3 | 0.4±0.11 | 0.4±0.1 | 2.7±0.5 | 0.3±0.1 | 0.6±0.01 | 0.2±0.02 | 0.8±0.13 | 1.2±0.01 | 1.1±0.09 | 0.4±0.01 | 1.1±0.1 |
| 48 | 0.8±0.1 | 1.7±0.01 | 1.3±0.12 | 0.9±0.16 | 0.4±0.1 | 2.9±0.5 | 0.4±0.1 | 0.7±0.01 | 0.2±0.03 | 0.9±0.06 | 1.4±0.4 | 1.2±0.2 | 0.6±0.2 | 1.3±0.03 |
mRNA expression of acute-phase cytokines in brain as compared with that of liver and injured muscle
A mild change in the gene expression of acute-phase cytokines (IL-6, IL-1β, and TNF-α) was observed in the rat brain during APR. Gene expression for IL-6 and TNF-α was upregulated (2.3±0.4-fold) by 6 h (P < 0.05) and (2.31±0.11-fold) by 12 h compared with the saline-treated controls. In contrast, IL-1β gene expression was significantly downregulated.
In comparison with their levels in the brain, IL-1β and TNF-α were significantly upregulated in the rat liver with a maximum expression at 6 h. In contrast, no significant change of IL-6 gene expression was observed in the liver after the onset of APR (P<0.01).
Acute-phase cytokine gene expression in injured muscle demonstrated a highly significant increase with a maximum expression of IL-6 at 6 h. An early (2 h) and constant (until 6 h) significant up-regulation of IL-1β was detected. TNF-α was also significantly induced with a maximum at 48 h, but at an order of magnitude lower that of IL-1β and IL-6 (P<0.01; Table 4).
Table 4.
PCR analysis of total RNA from liver, brain, and injured muscle of TO-treated animals. Comparison of acute-phase cytokines (IL1β, IL6 and, TNF-α) in the rat liver, brain, and injured muscle at various time points after intramuscular TO injection in comparison with control animals. Data show the results of three animals (mean values±SEM)
| Time (h) | IL-1β mRNA expression | IL-6 mRNA expression | TNF-α mRNA expression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Injured muscle | Liver | Brain | Injured muscle | Liver | Brain | Injured muscle | Liver | Brain | |
| Control | 1.0±0.00 | 1.0±0.00 | 1.0±0.00 | 1.0±0.1 | 1.0±0.3 | 1.0±0.3 | 1.0±0 | 1.0±0.3 | 1.0±0.3 |
| 0.5 | 1.4±0.3 | 1.6±0.01 | 0.5±0.01 | 0.8±0.3 | 1.2±0.5 | 0.5±0.1 | 0.7±0.3 | 0.8±0.1 | 0.3±0.01 |
| 1 | 1.1±0.2 | 1.8±0.1 | 0.4±0.1 | 0.2±0.1 | 0.5±0.1 | 0.8±0.1 | 0.3±0.1 | 1.0±0.2 | 0.9±0.2 |
| 2 | 28±6 | 1.1±0.01 | 0.3±0.01 | 147±582 | 0.3±0.1 | 1.2±0.2 | 1.1±0.4 | 1.2±0.1 | 0.4±0.1 |
| 4 | 355±80 | 3±0.4 | 0.5±0.2 | 1055±414 | 0.5±0.1 | 1.9±0.4 | 3.6±1.5 | 2.4±0.2 | 0.2±0.01 |
| 6 | 425±95 | 4.5±0.6 | 0.5±0.1 | 1982±777 | 0.4±0.1 | 2.3±0.4 | 2.2±0.9 | 4.0±1.0 | 1.3±0.3 |
| 12 | 299±67 | 3.1±0.4 | 0.8±0.1 | 371±61 | 1±0.2 | 0.4±0.01 | 4.0±1.1 | 2.1±0.6 | 2.3±0.1 |
| 24 | 53±12 | 0.8±0.1 | 0.6±0.4 | 27±19 | 1.7±0.4 | 0.7±0.04 | 3.2±2.6 | 1.2±0.04 | 0.8±0.1 |
| 36 | 23±5 | 1.8±0.3 | 0.4±0.2 | 109±43 | 0.4±0.1 | 0.4±0.01 | 1.4±0.6 | 1.4±0.01 | 0.8±0.01 |
| 48 | 80±18 | 1.4±0.4 | 0.9±0.2 | 75±30 | 1.2±0.3 | 0.6±0.02 | 3.6±3.0 | 1.4±0.02 | 0.9±0.2 |
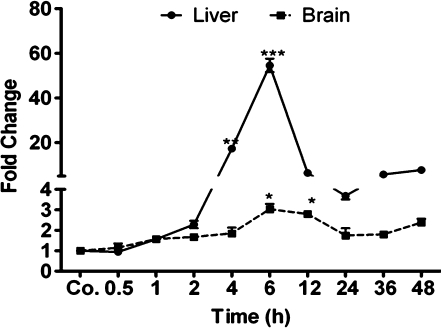
Change in gene expression of heme oxygenase-1 in liver and brain
Heme oxygenase (HO) is an enzyme that catalyzes the degradation of heme (Tenhunen et al. 1968). HO-1, an isoform of this enzyme, is known to be induced during localized inflammation (Tron et al. 2005).
Gene expression of HO-1 started to increase 1 h after TO injection, with strong upregulation in the liver at 6 h (54.59±2.97-fold). In the brain, the kinetics of HO-1 expression were similar to those observed in liver, although upregulation started slightly later, and the order of magnitude of the increase was lower with a maximum at 6 h after treatment (3.03±0.25-fold; P<0.05 vs. control; Fig. 7).
Fig. 7.
Changes in amount of heme oxygenase-1 (HO-1) transcript in the liver and brain tissue during APR. Fold change in the mRNA expression of HO-1 in the liver and in the brain after intramuscular TO injection (Co. saline-injected control). qRT-PCR was normalised by using two housekeeping genes: β-actin and ubiquitin C. Results represent mean values±SEM (*P<0.05, analyzed by one-way ANOVA; n=3)
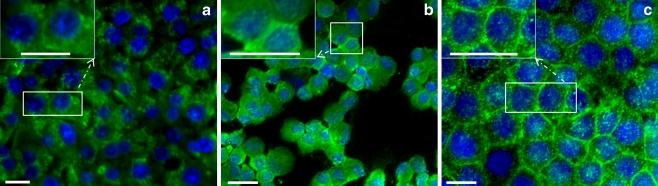
Detection of TfR1 in liver and brain cells
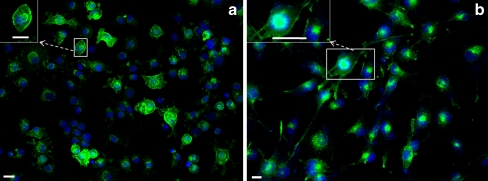
By using a monoclonal antibody against TfR1 for immunoflourscence staining, TfR1 was observed in the membrane of primary cultured rat hepatocytes and Kupffer cells and in HepG2 cells (human hepatoma cell line; Fig. 8a-c).
Fig. 8.
Immunofluorescence detection of TfR1 (green) in isolated rat hepatocytes (a), Kupffer cells (b), and human hepatoma cell line HepG2 (c). Insets Higher magnification images of the relevant boxed areas. Results are representative of three experiments for each cell type (blue DAPI nuclear staining). Original magnification: ×400. Bar 20 μm
In contrast to liver cells and in agreement with the immunohistochemistry findings for the brain tissue, the same monoclonal antibody showed strong nuclear positivity in brain neural stem cells; however, a weak cytoplasmic and membranous expression was also detected (Fig. 9a). A human glioblastoma cell line (U373MG) also showed strong nuclear positivity for TfR1 (Fig. 9b).
Fig. 9.
Immunofluorescence detection of TfR1 (green) in rat neural stem cells (a) and human glioblastoma cell line U373MG (b). Insets Higher magnification images of the relevant boxed areas. Results are representative of three experiments for each cell type (blue DAPI nuclear staining, turquoise nuclear TfR1/DAPI staining). Original magnification: ×200. Bar 10 μm
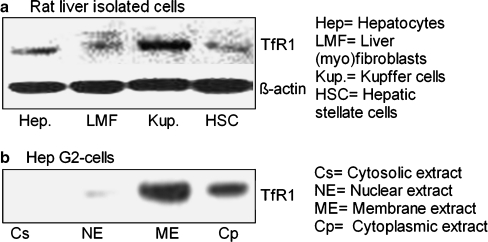
By using the monoclonal antibody against TfR1 for Western blot analysis, we were able to demonstrate positivity for TfR1 (MW: 95 kDa) in the total lysate from isolated liver cells (Fig. 10a). The protein intensity of TfR1 was the strongest in the Kupffer cells followed by the hepatocytes. LMF and HSC showed the weakest detection of TfR1 at protein level (Fig. 10a).
Fig. 10.
a Western blot analysis of TfR1 (95 kDa) in isolated cells rat liver; β-actin (43 kDa) was used as a loading control. b Western blot analysis of TfR1 in protein extracted from various fractions of hepatoma cell line Hep G2. Results represent one of three experiments
In human hepatoma cell line HepG2, TfR1 was detected only in the membrane and cytoplasmic fractions (Fig. 10b). Cyclooxygenase-2 was used as a positive control for membrane protein (data not shown).
Discussion
This study has compared the changes in gene expression of TfR1 and other iron-regulatory proteins in the liver and brain in a rat model of APR. Our results show an increase of iron content in the brain at the same order of magnitude as that observed in the liver during a TO-induced acute-phase condition (intramuscular sterile abscess).
In addition, we report the nuclear expression of TfR1 in brain tissue and in brain cells, as compared with the liver in which a membranous and cytoplasmic expression has been observed in Kupffer cells, hepatocytes, and human hepatoma cells (HepG2). By immunohistology, strong expression of TfR1 has been observed in the liver and brain; this has been further confirmed at the protein level by means of Western blot, and at RNA level by PCR. By means of the last two methods, an increase in TfR1 gene expression has been detected after TO administration. Minor changes in Tf gene expression have also been detected. Moreover, we show not only that hepcidin is upregulated in the brain at 4-6 h after TO administration, but also that the gene expression for hemojuvelin and ferroportin-1 is simultaneously downregulated, as we have previously shown in the liver (Sheikh et al. 2007). A similar pattern of an increase of ferritin-H and IRP1 has additionally been observed. Similar to the in vivo data, liver cells exhibit strong membranous positivity for TfR1, in contrast to brain cells in which abundant nuclear detection has been observed. Furthermore, isolated Kupffer cells show the strongest protein expression for TfR1 among the major liver cell types.
Although the gene expression of some iron-regulatory proteins has been described in the murine CNS (Zechel et al. 2006), this is the first attempt, to our knowledge, to determine a relationship between inflammation and the regulation of iron metabolism genes in the rat liver and brain. The induction of a sterile abscess by intra-muscular injection can indeed modulate the gene expression of iron-regulatory proteins at the mRNA and protein levels, not only in peripheral organs such as the liver, but also in the brain. Additionally, the pattern of change in the major iron-regulatory proteins is similar in both organs, although it can show differences in magnitude.
Transferrin binding maintains iron in a soluble form and serves as a major vehicle of plasma iron delivery into cells via TfR1. Diferric Tf has a high affinity for TfR1, and this has important physiological implications in terms of the mechanism of Tf uptake by cells (Conner and Schmid 2003; Frazer and Anderson 2005; Herbison et al. 2009; Levy et al. 1999; Trowbridge and Omary 1981). A recent study of a hepatoma cell line has shown that an increased expression of TfR1 has significant effects on transferrin-bound iron uptake at low transferrin concentrations, suggesting that TfR1 is the major Tf receptor responsible for iron transport (Herbison et al. 2009).
Although TfR1 is thought to be inversely regulated by cellular iron status via the posttranscriptional IRE-iron-regulatory protein mechanism (Levy et al. 1999), we have detected an early increase in TfR1 protein expression parallel to an increase in tissue iron level; this upregulation of TfR1 might be attributable to either the activation of IRP-1 (Caltagirone et al. 2001) or hypoxia-inducible factor 1α (HIF-1α), which binds to a conserved binding site within the TfR1 promoter (Tacchini et al. 1999), as the induction of hepatic HIF-1α has also been observed in our model (Ramadori et al. 2010). This suggests that acute-phase mediators counteract the downregulating effect of iron on TfR1.
Despite previous reports showing decreased TfR1 mRNA expression in the liver and hepatocytes of HFE-knockout mice (Chua et al. 2008; Ludwiczek et al. 2005), the downregulation of this gene in brain and liver during APR is not accompanied by a downregulation of TfR1 suggesting an independent regulation at least in this rat model. A recent study has identified TfR1 as being a receptor of ferritin-H and mediating most of ferritin-H binding in human cells (Li et al. 2010).
The presence of DMT1 in brain/cerebrospinal fluid barriers is functionally important with regard to the necessity of the stable homeostasis of essential elements in brain extracellular fluids for normal brain function (Wang et al. 2006; Rouault and Cooperman 2006). Heph is a ferroxidase associated with iron export by interacting with the Fpn1, and its expression has been reported in the brain. Heph mRNA expression is influenced by the tissue iron status and is probably regulated at the transcriptional level by the metal (Qian et al. 2007).
IRPs are critical determinants of the post-transcriptional regulation of TfR expression. In addition to ferritin and TfR1, DMT1 and Fpn1 mRNA contain IRE-like sequences, suggesting that IRPs are involved in the regulation of their mRNA expression (Gunshin et al. 1997; McKie et al. 2000). Their role is still unclear, and further investigation is required.
To demonstrate that acute-phase changes of the gene expression in the brain take place under the experimental conditions used here, we have been able to show that the behaviour of the HO-1 gene is similar to that observed in liver tissue. HO-1 is a positive APP, and its expression is significantly increased in the liver (Tron et al. 2005) during localized inflammation, as is the case for hepcidin (Sheikh et al. 2007). Kartikasari et al. (2009) have observed low hepcidin levels in HO-1-deficient patients, suggesting a direct regulatory effect of HO-1 on hepcidin gene expression. However, they have also demonstrated that HO-1 activity has no effect on hepcidin gene expression in human hepatoma cells. This indicates that IL-6 (a main mediator in TO-induced APR) released into the blood from the site of inflammation (skeletal muscle) independently upregulates HO-1 and hepcidin gene expression in liver (and isolated hepatocytes) and in brain.
Moreover, another remarkable finding of the current study is the upregulation of IL-6 and TNF-α gene expression in the brain, whereas IL-1β and TNF-α (but not IL-6) gene expression is upregulated in the liver. The induction of cytokines in the brain might be attributable to the production of reactive oxygen species generated by oxidative stress as a result of increased iron concentration, as has been observed in other neurodegenerative disorders (Mancuso et al. 2006). On the other hand, a positive feedback mechanism might exist that is specific for the brain, at least for IL-6 gene expression. This also suggests that the concentration of IL-6 reaching the brain might not be enough to modulate gene expression under acute-phase conditions.
In a recent case, a reduction in iron overload was reported in the liver but not in the brain when an iron chelator was used to treat a neurodegenerative disorder; this was attributable to the incomplete penetration of the chelator through the blood/brain barrier (Finkenstedt et al. 2010). This suggests that some differences exist in iron handling between the liver and brain.
In summary, an increased concentration of iron has been found in rat brain and liver tissue during APR. Although this is supposed to be a mechanism of iron sequestration to reduce iron availability to pathogens (Chlosta et al. 2006; Nairz et al. 2007; Wessling-Resnick 2010), our data obtained in a model of sterile inflammation suggest an increased need of iron supply to satisfy the increased metabolic work under acute-phase conditions, as is known for liver (Ramadori and Christ 1999). Furthermore, we have demonstrated that TfR1 and other iron-regulatory proteins known to be expressed in the liver are also expressed in the rat brain. Moreover, we have shown that the changes in gene expression of several iron regulating proteins observed in the brain tissue during APR are similar to those observed in the liver, including an accumulation of iron. In addition to events in the liver, the changes observed in the brain might be attributable to the local production of acute-phase cytokines. Our findings have significant implications for the further understanding of the importance of iron metabolism not only in the liver, but also in the brain.
Acknowledgements
We thank Mrs. Anke Herbst and Christin Hoffmann for their kind and skilful technical assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Glossary
Abbreviations
- APP
Acute-phase proteins
- APR
Acute-phase response
- cDNA
Complementary DNA
- CNS
Central nervous system
- Dcytb
Duodenal cytochrome B reductase
- DMEM
Dulbecco’s modified Eagle’s medium
- DMT1
Divalent metal transporter 1
- DTT
Dithiothreitol
- FCS
Fetal calf serum
- Fpn1
Ferroportin 1
- Ferritin-H
Ferritin heavy chain
- Hepc
Hepcidin
- Heph
Hephaestin
- HFE
Hemochromatosis gene
- HIF-1α
Hypoxia-inducible factor 1α
- Hjv
Hemojuvelin
- HO-1
Heme oxygenase-1
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- IRE
Iron-responsive elements
- IRP1
Iron-responsive element binding protein 1
- IRP2
Iron-responsive element binding protein 2
- PBS
Phosphate-buffered saline
- PCR
Polymerase chain reaction
- PMSF
Phenylmethane sulfonyl-fluoride
- SEM
Standard error of the mean
- Tf
Transferrin
- TfR1
Transferrin receptor 1
- TfR2
Transferrin receptor 2
- TNF-α
Tumor necrosis factor alpha
- TO
Turpentine oil
Footnotes
Ihtzaz Ahmed Malik and Naila Naz contributed equally to this work.
Contributor Information
Ihtzaz Ahmed Malik, Email: i.malik@med.uni-goettingen.de.
Naila Naz, Email: Naila.Naz@stud.uni-goettingen.de.
Nadeem Sheikh, Email: s_nadeem77@yahoo.com.
Sajjad Khan, Email: sajjad183@yahoo.com.
Federico Moriconi, Email: fmoriconi@med.uni-goettingen.de.
Martina Blaschke, Email: mblasch1@gwdg.de.
Giuliano Ramadori, Phone: +49-551-396301, FAX: +49-551-396921, Email: gramado@med.uni-goettingen.de.
References
- Budick-Harmelin N, Dudas J, Demuth J, Madar Z, Ramadori G, Tirosh O. Triglycerides potentiate the inflammatory response in rat Kupffer cells. Antioxid Redox Signal. 2008;10:2009–2022. doi: 10.1089/ars.2007.1876. [DOI] [PubMed] [Google Scholar]
- Caltagirone A, Weiss G, Pantopoulos K. Modulation of cellular iron metabolism by hydrogen peroxide. Effects of H2O2 on the expression and function of iron-responsive element-containing mRNAs in B6 fibroblasts. J Biol Chem. 2001;276:19738–19745. doi: 10.1074/jbc.M100245200. [DOI] [PubMed] [Google Scholar]
- Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106:3710–3717. doi: 10.1182/blood-2005-05-1857. [DOI] [PubMed] [Google Scholar]
- Casey JL, Koeller DM, Ramin VC, Klausner RD, Harford JB. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989;8:3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chloupkova M, Gao J, Chapman-Arvedson TL, Enns CA. HFE modulates transferrin receptor 2 levels in hepatoma cells via interactions that differ from transferrin receptor 1-HFE interactions. J Biol Chem. 2007;282:36862–36870. doi: 10.1074/jbc.M706720200. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chlosta S, Fishman DS, Harrington L, Johnson EE, Knutson MD, Wessling-Resnick M, Cherayil BJ. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun. 2006;74:3065–3067. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AC, Herbison CE, Drake SF, Graham RM, Olynyk JK, Trinder D. The role of Hfe in transferrin-bound iron uptake by hepatocytes. Hepatology. 2008;47:1737–1744. doi: 10.1002/hep.22180. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Differential requirements for AP-2 in clathrin-mediated endocytosis. J Cell Biol. 2003;162:773–779. doi: 10.1083/jcb.200304069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A, Morgan EH. Iron and transferrin uptake by brain and cerebrospinal fluid in the rat. Brain Res. 1992;592:8–16. doi: 10.1016/0006-8993(92)91652-U. [DOI] [PubMed] [Google Scholar]
- Dudas J, Mansuroglu T, Batusic D, Saile B, Ramadori G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007;329:503–514. doi: 10.1007/s00441-007-0437-z. [DOI] [PubMed] [Google Scholar]
- Eng SC, Taylor SL, Reyes V, Raaka S, Berger J, Kowdley KV. Hepatic iron overload in alcoholic end-stage liver disease is associated with iron deposition in other organs in the absence of HFE-1 hemochromatosis. Liver Int. 2005;25:513–517. doi: 10.1111/j.1478-3231.2005.01004.x. [DOI] [PubMed] [Google Scholar]
- Finkenstedt A, Wolf E, Hofner E, Gasser BI, Bosch S, Bakry R, Creus M, Kremser C, Schocke M, Theurl M, Moser P, Schranz M, Bonn G, Poewe W, Vogel W, Janecke AR, Zoller H. Hepatic but not brain iron is rapidly chelated by deferasirox in aceruloplasminemia due to a novel gene mutation. J Hepatol. 2010;53:1101–1107. doi: 10.1016/j.jhep.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer DM, Anderson GJ. Iron imports. I. Intestinal iron absorption and its regulation. Am J Physiol Gastrointest Liver Physiol. 2005;289:G631–G635. doi: 10.1152/ajpgi.00220.2005. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/S0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Herbison CE, Thorstensen K, Chua AC, Graham RM, Leedman P, Olynyk JK, Trinder D. The role of transferrin receptor 1 and 2 in transferrin-bound iron uptake in human hepatoma cells. Am J Physiol Cell Physiol. 2009;297:C1567–C1575. doi: 10.1152/ajpcell.00649.2008. [DOI] [PubMed] [Google Scholar]
- Jandl JH, Inman JK, Simmons RL, Allen DW. Transfer of iron from serum iron-binding protein to human reticulocytes. J Clin Invest. 1959;38:161–185. doi: 10.1172/JCI103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartikasari AE, Wagener FA, Yachie A, Wiegerinck ET, Kemna EH, Winkels DW. Hepcidin suppression and defective iron recycling account for dysregulation of iron homeostasis in heme oxygenase-1 deficiency. J Cell Mol Med. 2009;13:3091–3102. doi: 10.1111/j.1582-4934.2008.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzara C, Roetto A, Daraio F, Rivard S, Ficarella R, Simard H, Cox TM, Cazzola M, Piperno A, Gimenez-Roqueplo AP, Grammatico P, Volinia S, Gasparini P, Camaschella C. Spectrum of hemojuvelin gene mutations in 1q-linked juvenile hemochromatosis. Blood. 2004;103:4317–4321. doi: 10.1182/blood-2004-01-0192. [DOI] [PubMed] [Google Scholar]
- Leibold EA, Gahring LC, Rogers SW. Immunolocalization of iron regulatory protein expression in the murine central nervous system. Histochem Cell Biol. 2001;115:195–203. doi: 10.1007/s004180000246. [DOI] [PubMed] [Google Scholar]
- Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, Arase H, Torti FM, Torti SV, Nakamura MC, Seaman WE. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci USA. 2010;107:3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwiczek S, Theurl I, Bahram S, Schumann K, Weiss G. Regulatory networks for the control of body iron homeostasis and their dysregulation in HFE mediated hemochromatosis. J Cell Physiol. 2005;204:489–499. doi: 10.1002/jcp.20315. [DOI] [PubMed] [Google Scholar]
- Malik IA, Moriconi F, Sheikh N, Naz N, Khan S, Dudas J, Mansuroglu T, Hess CF, Rave-Frank M, Christiansen H, Ramadori G. Single-dose gamma-irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am J Pathol. 2010;176:1801–1815. doi: 10.2353/ajpath.2010.090505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis. 2006;10:59–73. doi: 10.3233/jad-2006-10110. [DOI] [PubMed] [Google Scholar]
- McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/S1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- Moos T. Immunohistochemical localization of intraneuronal transferrin receptor immunoreactivity in the adult mouse central nervous system. J Comp Neurol. 1996;375:675–692. doi: 10.1002/(SICI)1096-9861(19961125)375:4<675::AID-CNE8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Morgan EH, Appleton TC. Autoradiographic localization of 125-I-labelled transferrin in rabbit reticulocytes. Nature. 1969;223:1371–1372. doi: 10.1038/2231371a0. [DOI] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- Napier I, Ponka P, Richardson DR. Iron trafficking in the mitochondrion: novel pathways revealed by disease. Blood. 2005;105:1867–1874. doi: 10.1182/blood-2004-10-3856. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- Qian ZM, Chang YZ, Zhu L, Yang L, Du JR, Ho KP, Wang Q, Li LZ, Wang CY, Ge X, Jing NL, Li L, Ke Y. Development and iron-dependent expression of hephaestin in different brain regions of rats. J Cell Biochem. 2007;102:1225–1233. doi: 10.1002/jcb.21352. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis. 1999;19:141–155. doi: 10.1055/s-2007-1007106. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Moebius U, Dienes HP, Meuer S, Meyer Zum Buschenfelde KH. Lymphocytes from hepatic inflammatory infiltrate kill rat hepatocytes in primary culture. Comparison with peripheral blood lymphocytes. Virchows Arch [B] 1990;59:263–270. doi: 10.1007/BF02899413. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Sipe JD, Dinarello CA, Mizel SB, Colten HR. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985;162:930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori P, Sheikh N, Ahmad G, Dudas J, Ramadori G. Hepatic changes of erythropoietin gene expression in a rat model of acute-phase response. Liver Int. 2010;30:55–64. doi: 10.1111/j.1478-3231.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem. 2004;331:370–375. doi: 10.1016/j.ab.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Pan P, Parkkila S. Expression studies of neogenin and its ligand hemojuvelin in mouse tissues. J Histochem Cytochem. 2007;55:85–96. doi: 10.1369/jhc.6A7031.2006. [DOI] [PubMed] [Google Scholar]
- Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S. Brain iron metabolism. Semin Pediatr Neurol. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Sheikh N, Dudas J, Ramadori G. Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab Invest. 2007;87:713–725. doi: 10.1038/labinvest.3700553. [DOI] [PubMed] [Google Scholar]
- Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem. 1999;274:24142–24146. doi: 10.1074/jbc.274.34.24142. [DOI] [PubMed] [Google Scholar]
- Tello K, Christiansen H, Gurleyen H, Dudas J, Rave-Frank M, Hess CF, Ramadori G, Saile B. Irradiation leads to apoptosis of Kupffer cells by a Hsp27-dependent pathway followed by release of TNF-alpha. Radiat Environ Biophys. 2008;47:389–397. doi: 10.1007/s00411-008-0170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R (1968) The enzymatic conversion of heme to bilirubin by microsomal heme. Proc Natl Acad Sci 61:748–755 [DOI] [PMC free article] [PubMed]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron K, Novosyadlyy R, Dudas J, Samoylenko A, Kietzmann T, Ramadori G. Upregulation of heme oxygenase-1 gene by turpentine oil-induced localized inflammation: involvement of interleukin-6. Lab Invest. 2005;85:376–387. doi: 10.1038/labinvest.3700228. [DOI] [PubMed] [Google Scholar]
- Trowbridge IS, Omary MB. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci USA. 1981;78:3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- Wang X, Li GJ, Zheng W. Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res. 2006;1097:1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–122. doi: 10.1146/annurev.nutr.012809.104804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigglesworth JM, Baum H. Iron dependent enzymes in the brain. In: Youdium MBH, editor. Brain iron: neurochemical and behavioral aspects. New York: Taylor and Francis; 1988. pp. 25–66. [Google Scholar]
- Zechel S, Huber-Wittmer K, von Bohlen und Halbach O. Distribution of the iron-regulating protein hepcidin in the murine central nervous system. J Neurosci Res. 2006;84:790–800. doi: 10.1002/jnr.20991. [DOI] [PubMed] [Google Scholar]
- Zhao B, Schwartz JP. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J Neurosci Res. 1998;52:7–16. doi: 10.1002/(SICI)1097-4547(19980401)52:1<7::AID-JNR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]