Abstract
During acute rejection of cardiac transplants endothelial cell–leukocyte interaction fuelled by co-stimulatory molecules like CD40/CD154 may ultimately lead to graft loss. One key player in up-regulating the expression of such pro-inflammatory gene products is the interferon-γ-dependent transcription factor STAT-1. Hence down-regulating interferon-γ-stimulated pro-inflammatory gene expression in the graft endothelial cells by employing a decoy oligodeoxynucleotide (dODN) neutralising STAT-1 may protect the graft. To verify this hypothesis, heterotopic mouse heart transplantation was performed in the allogeneic B10.A(2R) to C57BL/6 and syngeneic C57BL/6 to C57BL/6 strain combination without immunosuppression. Graft vessels were pre-treated with STAT-1 dODN, mutant control ODN (10 μM each) or vehicle (Ringer solution). Cellular rejection (vascular and interstitial component) was graded histologically and CD40, ICAM-1, VCAM-1, MCP-1, E-selectin and RANTES expression in the graft monitored by real time PCR 24 h and 9 days post-transplantation. Nine days after transplantation both rejection scores were significantly diminished by 85 and 70%, respectively, in STAT-1 dODN-treated allografts as compared to mutant control ODN-treated allografts. According to immunohistochemistry analysis, this was accompanied by a reduced infiltration of monocyte/macrophages and T cells into the graft myocardium. In addition, pro-inflammatory gene expression was strongly impaired by more than 80% in STAT-1 dODN-treated allografts 24 h post-transplantation but not in mutant control ODN or vehicle-treated allografts. This inhibitory effect on pro-inflammatory gene expression was no longer detectable 9 days post-transplantation. Single periprocedural treatment with a STAT-1 dODN thus effectively reduces cellular rejection in mouse heart allografts. This effect is associated both with an early decline in pro-inflammatory gene expression and a later drop in mononuclear cell infiltration.
Keywords: STAT-1, CD40, Decoy oligodeoxynucleotide, Heart transplantation, Rejection
Introduction
Acute cellular rejection jeopardises the short- and long-term outcomes after heart transplantation. Host immune cells are recruited from the circulation and directed to the graft by mechanisms starting with the presentation of allogeneic peptides by antigen-presenting cells to T-helper cells, the activation of which is further enhanced by co-stimulatory molecules like CD40/CD154 [4]. As a consequence, the activated T-helper cells elicit the expression of adhesion molecules on the endothelial cell surface, thus facilitating monocyte recruitment to the graft and ultimately resulting in graft damage or failure [4]. One important cytokine produced by activated T-helper cells is interferon-γ (IFN-γ). Through activation of the transcription factor signal transducer and activator of transcription-1 (STAT-1), IFN-γ up-regulates the expression of a variety of gene products in the donor endothelial cells, which are important for transplant rejection such as, e.g. CCL5 or RANTES (regulated upon activation, normal T cell expressed, and secreted), vascular cell adhesion molecule-1 (VCAM-1) or CD40 [6, 9, 13, 31]. Increased expression of VCAM-1 for example enhances endothelial cell–leukocyte interaction [6], a hallmark of acute cellular rejection.
The role of CD40/CD154 co-stimulation in transplant rejection has been the subject of extensive research [4, 20], and histological examination of human cardiac allograft biopsies has revealed a significant correlation between acute cellular rejection and the increased expression of these molecules on both infiltrating leukocytes and microvascular endothelial cells [25]. Moreover, anti-CD40 monoclonal antibody treatment in cynomolgus monkeys has been shown to elicit a potent immunosuppressive effect, thereby prolonging allograft survival [15]. CD40 may thus be considered as a pivotal target molecule in preventing allograft rejection.
Previous in vitro and in vivo experiments have revealed that STAT-1 is involved in transcriptional regulation of both the CD40 and VCAM-1 gene which could effectively be diminished by employing a decoy oligodeoxynucleotide (dODN) targeting STAT-1 [23, 31]. Decoy ODNs are short double-stranded DNA molecules that mimic the consensus binding site of their target transcription factor in the genome, thereby blocking its activity [21]. Their uptake into cells is accomplished without the need for a transfection reagent, most probably through a carrier-based transport mechanism. By local delivery of an appropriate dODN, the activities of STAT-1 and STAT-1-dependent gene expression can be effectively suppressed.
To study the impact of this new therapeutic concept on graft rejection, we employed an improved STAT-1 dODN in a heterotopic mouse cardiac transplant model without immunosuppression. We assessed the effects of a decrease in STAT-1 activity on vascular and interstitial rejection, leukocyte infiltration and the expression of different pro-inflammatory molecules including CD40 in the graft.
Methods
Animals
Heterotopic heart transplantation was performed in the B10.A(2R) to C57BL/6 allogeneic mouse model. Syngeneic C57BL/6 to C57BL/6 transplants served as controls. All male mice (body weight 25–30 g) were purchased from Charles River (Sulzfeld, Germany) and had unrestricted access to water and food. They were kept according to the German legislation on the protection of animals, and fed standard laboratory chow. The transplantation model used herein consisting of B10.A(2R) donor (H-2h2; Kk, Db, IAk, IEk) and C57BL/6 acceptor mice (H-2b, Kb, Db, IAb, IEbetab) is a full MHC mismatched strain combination, except for the class I MHC allele D. In this strain combination with full H-2 histoincompatibility, C57BL/6 recipients reject B10.A(2R) allografts after 10–13 days which is slightly later than in the BALB/C to C57BL/6 strain combination where donor hearts are rejected approximately 10 days post-transplantation. To appropriately evaluate the effect of the STAT-1 dODN on acute cellular rejection, it was necessary to establish an intermediate degree of rejection. Maximal tissue destruction would perhaps have overridden its protective effect since no additional immunosuppressive therapy was employed.
To this end, a pilot study was conducted in which control allografts were analysed histologically 7, 9 and 12 days after transplantation, respectively (n = 3 each, data not shown). In addition, beating of the grafts, an established method to assess graft viability was examined by daily palpation. After 7 days cellular rejection appeared to be mild to moderate, after 9 days there was significant damage to the hearts due to acute cellular rejection and after 12 days grafts were almost fully necrotic. Interestingly, palpation of heart beats revealed palpable pulses in all grafts. While these were due to myocardial activity 7 and 9 days post-transplantation, the palpable pulses 12 days post-transplantation solely reflected atrial but not ventricular contractions. Therefore, a 9-day follow-up period was employed in all subsequent experiments to ensure a good quality histology not affected by autolytic processes following complete graft failure.
Decoy oligodeoxynucleotide (ODN) technique
Double-stranded ODNs were prepared from complementary single-stranded phosphorothioate-bonded ODNs obtained from Eurogentec (Cologne, Germany) by melting at 95°C for 5 min, followed by a cool-down phase of 3–4 h at ambient temperature. The efficiency of the hybridization reaction was verified with 2.5% agarose gel electrophoresis and usually found to exceed 95%. The sequences of the forward strands were as follows (underlined letters denote phosphorothioate-bonded bases; mutated bases are printed in bold and italics).
STAT-1 decoy ODN (dODN): 5′-TGTGAATTACCGGAAGTG-3′
Mutant control ODN: 5′-TGTGGACCGTAGGAAGTG-3′
Fluorescent dye-labelled decoy ODN
Consensus STAT-1 dODNs were coupled with fluorescent Alexa594 (IBA, Göttingen, Germany) and used at the same final concentration to verify in vivo uptake of the dODN by the coronary endothelial cells. Analysis was performed on frozen sections of the mouse hearts. Cells that internalised the nucleic acid were visualised by fluorescence microscopy with a MicroMax 1300Y CCD camera (Princeton Instruments, Trenton, NJ, USA) fitted to an Axiovert S100-TV microscope equipped with a 40x Plan-Neofluar objective (Carl Zeiss, Jena, Germany). Excitation was performed with a xenon lamp and a monochromator (TILL-Photonics, Munich, Germany) set to 580 nm.
Microsurgical technique
Inhalation anaesthesia was performed with isoflurane. Details of the transplantation procedure have been described elsewhere [5]. In brief, animals were systemically heparinised with 50 units of heparin dissolved in 2 ml Ringer solution intraperitoneally. Hearts were excised after ligation of the superior and inferior vena cava, the pulmonary veins and the azygos vein. The ascending aorta was cut below the brachiocephalic trunk and the pulmonary artery was cut at the bifurcation of the right and left pulmonary artery. Then the coronary arteries were perfused gently by hand either with STAT-1 dODN, mutant control ODN or Ringer solution only (600 μl each) at room temperature. Hearts were placed in Ringer solution and kept at 4°C until implantation (cold ischemia time 10 min). Implantation of the heart was accomplished by end-to-side anastomosis of the donor aorta and pulmonary artery to the infrarenal recipient aorta and infrarenal vena cava, respectively (warm ischemia time 15 min). A wash out period to prevent any spill over of the ODNs into the systemic circulation followed the completion of the venous anastomosis before complete declamping. To this end, the distal venous clamp was partially released to avoid extensive blood loss and a volume of 2–3 heart beats was collected over the venous anastomosis in a swamp placed directly at the venous anastomosis. The suture was then finally secured.
Experimental design
To investigate the protective effect of a single (and selective) pre-treatment of the donor heart endothelium with the STAT-1 dODN, coronary arteries of allogeneic transplants were perfused with the naked nucleic acid dissolved in 600 μl Ringer solution (10 μM final concentration) over the aortic root (n = 11) directly after explantation. Hearts perfused with mutant control ODN served as nucleic acid controls (n = 11). Hearts were excised after 9 days, blotted free from blood and processed for standard histology. To assess short-term effects of the ODNs on mRNA expression of CD40, E-selectin, ICAM-1, MCP-1, RANTES and VCAM-1 in the allografts, these were monitored 24 h post-transplantation by real time PCR (n = 4 for each ODN). In a separate series of experiments, longitudinal analysis of vascular and interstitial rejection and of changes in pro-inflammatory gene expression after STAT-1 dODN administration to the graft versus vehicle were analysed histologically, immunohistochemically and by real time PCR 24 h and 9 days post-transplantation in both syngeneic and allogeneic grafts (n = 3 per group).
Histological evaluation
For histological evaluation, the Banff classification for renal allograft pathology [24] was adopted to the situation in cardiac allografts according to the score proposed by Billingham et al. [1]. Light microscopy was performed on 3 μm thin whole heart cross-sections stained with haematoxylin and eosin (HE).
Vascular rejection was assessed by an experienced pathologist blinded towards the treatment regime as follows: 0 no injury; 0.5 sticking of mononuclear cells to the endothelium; 1 subendothelial location of mononuclear cells; 2 inflammation of the media, including transmural infiltration (without necrosis); 3 fibrinoid necrosis of the vessel wall and/or thrombosis of the vessel in addition to the inflammatory reaction. The acute vascular rejection score was calculated as the sum of all specific vascular injury indices, whereby the index of vessels with degree 0.5 was multiplied by 0.5, that of degree 1 by 1, that of degree 2 by 2 and that of degree 3 by 3, respectively. Vascular injury was judged in 20 high power fields (40× magnification) of the individual whole heart sections.
Interstitial rejection was evaluated as follows: 1 mild mononuclear cell infiltrate (focal), without myocyte injury; 2 moderately dense infiltrate of mononuclear cells, with or without myocyte injury; 3 diffuse dense monocytic infiltrate, with or without interstitial oedema, focal interstitial haemorrhage and/or obvious myocyte injury. It was also judged in 20 high-power fields (40× magnification) of individual whole heart sections and calculated as described for the vascular injury score.
Immunohistochemistry analysis
Immunohistochemical staining was performed on 3-μm sections of zinc fixed tissue, using rat anti-mouse monoclonal antibodies against CD3 (T cells in general; BD Biosciences Pharmingen, San Diego, USA), Ly6 (granulocytes; BD Biosciences Pharmingen) and F4/80 (monocyte/macrophages; Serotec, Oxford, UK). Interstitial positive cells were counted in 10 high-power fields (40× magnification) of individual myocardial sections (left and right ventricle) and recorded as mean per high-power field.
PCR analysis
Real time PCR was performed in a LightCycler (Roche Diagnostics, Mannheim, Germany). The QuantiTect SYBR green RT-PCR kit (Qiagen, Hilden, Germany) was used for real-time quantification of target RNA followed by cDNA synthesis with Sensiscript reverse transcriptase (Qiagen) and equal amounts of total RNA. Primer sequences used for PCR amplification were: CD40 (GenBank accession No. X67878, position 488–620, 132 bp fragment, 53°C annealing temperature) 5′-TGAGCAACAACTTGGTAACCCTGG-3′ (sense) and 5-CTGGCTATAAATGGAGCT-TGACTCG-3′ (antisense); VCAM-1 (U12880, 224–288, 64 bp, 60°C) 5′-CCCCAAGGATCCAGAGATTCA-3′ (sense) and 5′-ACTTGACCGTGACCGGCTT-3′ (antisense); ICAM-1 (X52264, 600–665, 65 bp, 60°C) 5′-ATCTCAGGCCGCAAGGG-3′ (sense) and 5′-CGAAAGTCCGGAGGCTCC-3′ (antisense); MCP-1 (NM_011333, 76–139, 63 bp, 60°C) 5′-TTCCTCCACCACCATGCAG-3′ (sense) and 5′-CCAGCCGGCAACTGTGA-3′ (antisense); E-selectin (M87862, 1,200–1,336, 136 bp, 55°C) 5′-AGTGACACCACAAATCCCAGTCT-3′ (sense) and 5′-TTCGCAGGAGAACTCACAACTGGA-3′ (antisense); RANTES (NM_013653, 256–412, 157 bp, 62°C) 5′-GTGCCAACCCAGAGAAGAAG-3′ (sense) and 5′-GTAGGGGATTACTGGAGTGGC-3′ (antisense); GAPDH (BC020308, 563–700, 137 bp, 60°C) 5′-GACCAC-AGTCCATGCCATCACTGC-3′ (sense) and 5′-ATGACCTTGCCCACAGCCTTGG-3′ (antisense). Fluorescence was monitored (excitation at 470 nm and emission at 530 nm) at the end of the annealing phase (the LightCycler F1 channel). Except for RANTES recombinant standards were synthesised in vitro from the cloned PCR fragments in plasmid DNA (TOPO TA Cloning kit, Invitrogen). Data derived from the quantitative real-time PCR analysis (except for RANTES in comparison with that of the truncated recombinant standard) were normalised by using the mRNA level of the house keeping gene GAPDH as an internal reference and expressed as the number of copies per ng RNA (copies/ng). For RANTES the relative expression ratio was calculated from the real-time PCR efficiency and the crossing point deviation of an unknown sample versus a control as described by Pfaffl [22].
Statistical analysis
Results presented in graphs are expressed as means ± standard error of the mean (SEM) in each group. Statistical differences between groups were calculated by using the non-parametric Mann–Whitney U test with a P value <0.05 considered significant.
Results
In vivo up-take of the STAT-1 decoy oligodeoxynucleotide
Administration of the Alexa594-labelled STAT-1 dODN under the same conditions as the non-labelled nucleic acid revealed an almost exclusive uptake by the coronary endothelial cells of the graft (Fig. 1), confirming them as the primary target cells of the dODN effect on pro-inflammatory gene expression.
Fig. 1.
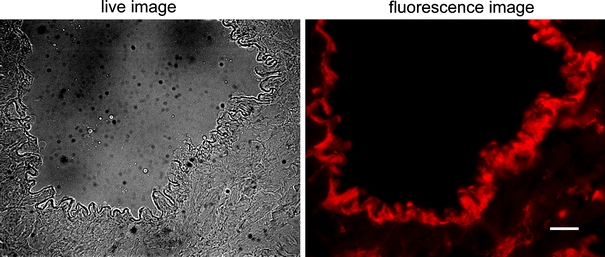
In vivo uptake of the Alexa594-labelled STAT-1 dODN (10 μM) by the coronary endothelial cells of the mouse heart. Cells that had internalised the dODN were visualised by fluorescence microscopy with emission set to 580 nm. Scale bar represents 50 μm
Vascular rejection
Twenty-four hours post-transplantation there was no appreciable cellular rejection in the vasculature of the syngeneic grafts regardless of the treatment regime (STAT-1 dODN or vehicle) whereas the allografts revealed a modest increase in the vascular injury with no significant difference between STAT-1 dODN application or vehicle at this point (Fig. 2a). Nine days post-transplantation, isografts also presented with a modest increase in vascular injury with no significant difference between treatment groups (Fig. 2a). Control allografts that had received no ODN treatment showed a marked rise in vascular rejection. This was reduced by about 75% in allografts that had been treated once with the STAT-1 dODN at the time of transplantation (Fig. 2a). Histologically, single treatment of the allograft with the STAT-1 dODN prior to implantation significantly reduced leukocyte adherence to the graft endothelium, transmural infiltration and thrombosis of capillary vessels when compared to mutant control ODN-treated hearts (Fig. 3). When compared to the mutant control ODN group, vascular injury was reduced even further by approximately 85% in the STAT-1 dODN-treated allografts (Fig. 2a).
Fig. 2.
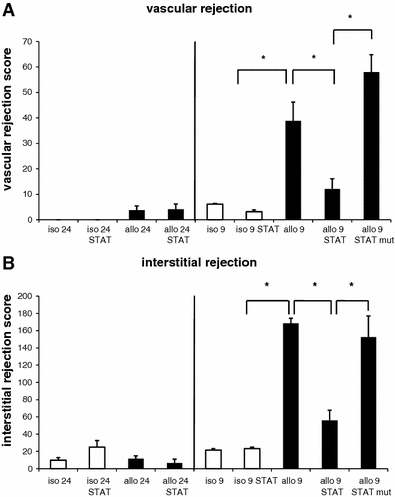
Statistical summary of a the vascular and b the interstitial rejection score in allografts pre-treated with the STAT-1 dODN (STAT), allografts pre-treated with the mutant control ODN (STAT mut), allografts pre-treated with vehicle (allo), isografts pre-treated with the STAT-1 dODN or vehicle (iso) 24 h (24; n = 3) and 9 days (9; n = 14) post-transplantation, respectively (*P < 0.05 as indicated)
Fig. 3.
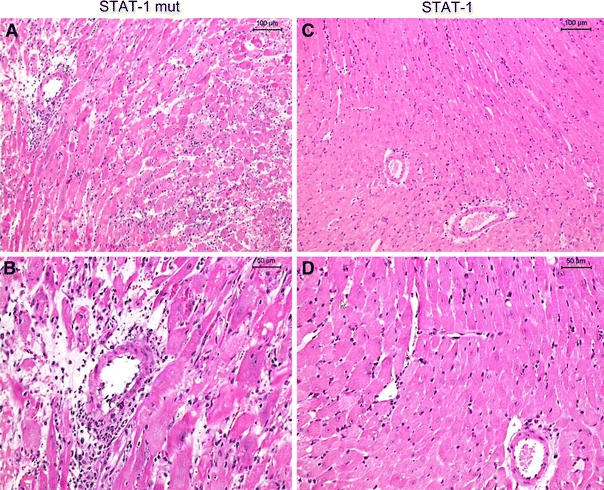
Representative histological sections 9 days post-transplantation in mutant control ODN-treated allografts (STAT-1 mut) and STAT-1-dODN-treated allografts (STAT-1). Allografts treated with the mutant control ODN showed a dense infiltrate with mononuclear cells and a scattered, loosened appearance of the myocardium (a, b). In contrast, myocardial architecture was still preserved with a much reduced infiltrate of mononuclear cells in STAT-1 dODN-treated allografts (c, d). Scale bar represents 100 μm in a and c, and 50 μm in b and d
Interstitial rejection
Twenty-four hours post-transplantation there was a modest increase in interstitial rejection in both isografts and allografts with no significant difference between treatment groups (Fig. 2b). Nine days post-transplantation the score had not worsened in the isografts irrespective of the treatment regime. However, interstitial rejection was markedly enhanced in the vehicle-treated allografts at this point, an effect that was prevented by approximately 67% in STAT-1 dODN-treated allografts (Fig. 2b). Histologically, there was a significant decline in interstitial leukocyte infiltration and damage to the cardiomyocytes in STAT-1 dODN-treated allografts as compared to mutant control ODN-treated hearts (Fig. 3). In line therewith, interstitial injury was also significantly reduced by approximately 64% in the verum group as compared to the control group (Fig. 2b).
Immunohistochemical quantification of the interstitial infiltrate at 24 h and 9 days post-transplantation revealed no alterations in any of the groups with respect to the number of granulocytes (Fig. 4a, b). In contrast, there was a clear-cut rise both in T cell (Fig. 4c, d) and monocyte/macrophage cell counts (Fig. 4e, f) 9 days post-transplantation in the control allografts but not in the isografts. No such changes were observed at 24 h. Peri-procedural application of the STAT1 dODN significantly reduced the number of infiltrating T cells in the allografts 9 days post-transplantation and also had an even more pronounced effect on the number of monocyte/macrophages which however did not gain statistical significance.
Fig. 4.
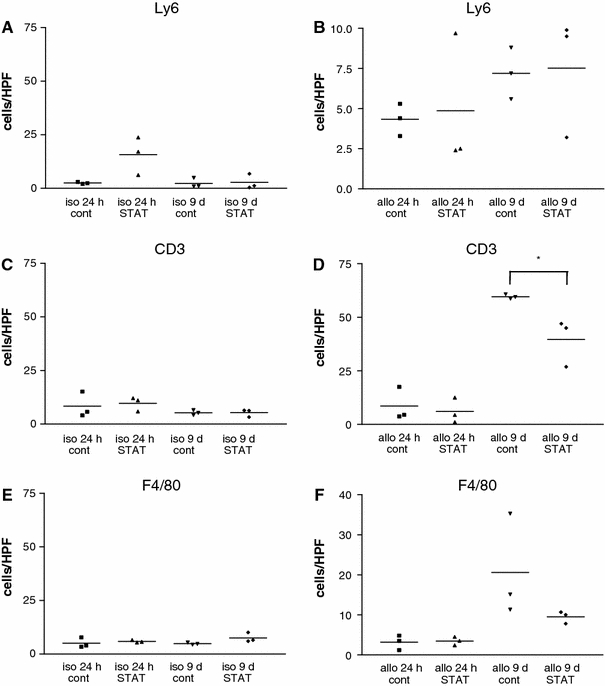
Immunohistochemical analysis of myocardial infiltration by granulocytes (Ly6), T cells (CD3) or monocyte/macrophages (F4/80) in allografts (allo) or isografts (iso) pre-treated with the STAT-1 dODN (STAT) or vehicle (cont) 24 h and 9 days post-transplantation, respectively (n = 3, *P < 0.05 as indicated)
Changes in gene expression
Twenty-four hours post-transplantation CD40 mRNA levels in whole heart tissue specimens were significantly reduced by approximately 80% in STAT-1 dODN-treated allografts as compared to mutant control ODN-treated transplants (Fig. 5a). The same was true for VCAM-1 and MCP-1 expression (Fig. 5b, c). Although reduced to approximately 40% of the level in mutant control ODN-treated transplants, the STAT-1 dODN effect on ICAM-1 and E-selectin expression did not gain statistical significance (Fig. 5d, e).
Fig. 5.
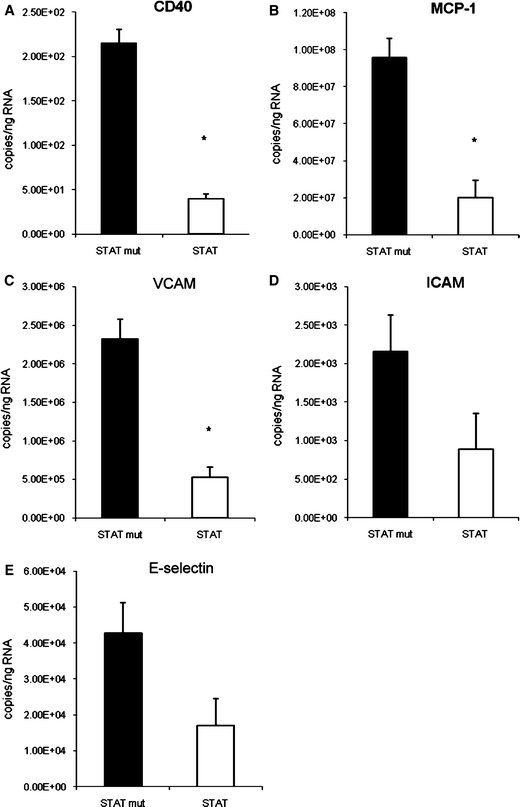
Real time PCR analysis of CD40, E-selectin, ICAM-1, MCP-1 and VCAM-1 mRNA abundance in the allografts treated with the STAT-1 dODN (STAT) or the mutant control ODN (STAT mut) 24 h post-transplantation (n = 4, *P < 0.05 vs. mutant control ODN)
In a separate series of experiments, mRNA abundance of the aforementioned pro-inflammatory gene products was examined in isografts and allografts following STAT-1 dODN or vehicle treatment 24 h and 9 days post-transplantation. At the early point, expression of all gene products except RANTES (Fig. 6f) was strongly up-regulated in the vehicle-treated allografts as compared to the respective isograft controls, only E-selectin expression in the syngeneic controls attained comparatively high levels. Treatment with the STAT-1 dODN had a profound inhibitory effect on the expression of all gene products (Fig. 6), the level of which was even lower than in the control isografts. In contrast, long-term changes in pro-inflammatory gene expression were not different between allografts and isografts, irrespective of the treatment regime with two exceptions. MCP-1 mRNA abundance remained significantly elevated in the allografts as compared to the isografts on day 9 after the transplantation but at one-third of the expression level in vehicle-treated allografts 24 h post-transplantation (Fig. 6b). RANTES mRNA abundance on the other hand was markedly increased in the control allografts on day 9 as compared to 24 h after the transplantation (Fig. 6b), compatible with its role as a late-reacting cytokine. Note that even though Figs. 5 and 6 display essentially the same data for the 24-h period these are derived from different sets of experiments so that the absolute copy numbers cannot be lumped together.
Fig. 6.
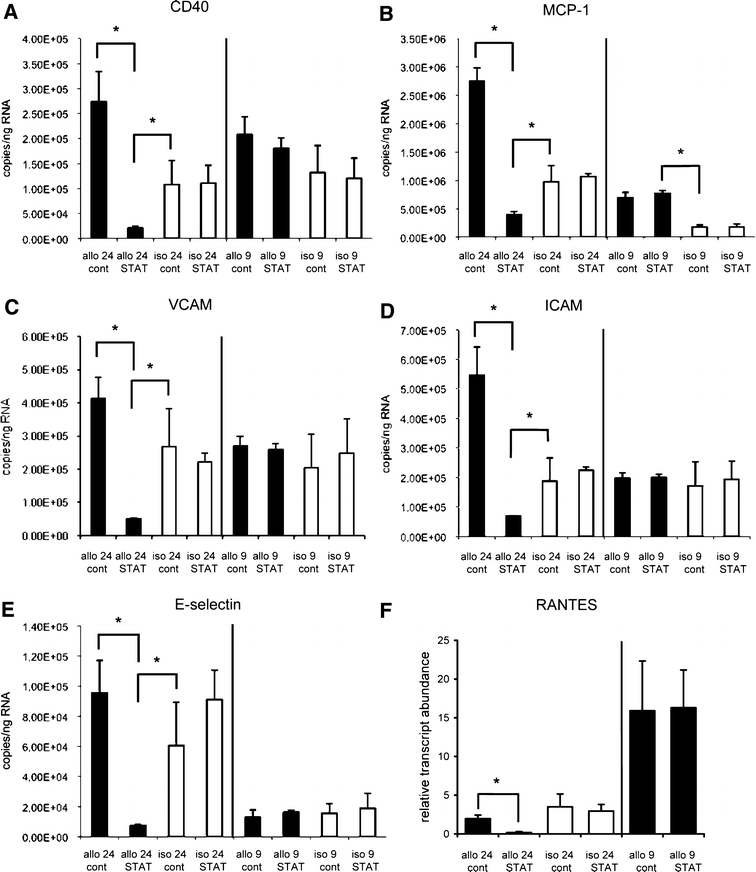
Real time PCR analysis of CD40, E-selectin, MCP-1, ICAM-1, RANTES and VCAM-1 mRNA abundance in allografts (allo) or isografts (iso) pre-treated with the STAT-1 dODN (STAT) or vehicle (cont) 24 h and 9 days post-transplantation, respectively (n = 3, *P < 0.05 as indicated)
Discussion
Despite new pharmacological therapies and the introduction of ventricular assist devices, heart transplantation remains the gold standard in the therapy of end-stage heart failure in selected patients [7, 8]. Irrespective of the availability of powerful immunosuppressants, acute rejection episodes continue to pose a major problem in heart transplantation.
During acute rejection, recipient T cell activation fuelled by graft co-stimulatory molecules such as CD40/CD154 up-regulates expression of IFN-γ [6, 32], interleukins 1, 6, 8 and 12 as well as TNF-α and MIP-1α in dendritic cells [3] and that of E-selectin, VCAM-1 and ICAM-1 in the endothelial cells of the graft blood vessels [16]. Furthermore, MCP-1/CCL2 and RANTES/CCL5, potent chemotactic factors for monocytes, CD4+ and CD8+ positive T cells, basophils, and eosinophils [26] have been shown, albeit with different kinetics, to be up-regulated in cardiac allografts [9, 13, 27], an effect that may also have important consequences for chronic graft rejection, i.e. transplant arteriosclerosis [33]. As a result, recruitment and invasion of mononuclear cells, namely monocytes and T cells, into the transplant is augmented with the ensuing acute inflammatory response presumably leading to graft loss.
In the present series of experiments, we targeted CD40/CD154-mediated endothelial cell/T-helper cell co-stimulation by employing an optimised dODN that neutralises the transcription factor STAT-1 and thus down-regulates expression of most IFN-γ-dependent pro-inflammatory genes including CD40. To prove that the graft endothelial cells are in fact the primary target cells of the dODN, a fluorescence dye-labelled ODN was administered intraluminally in the same way as the non-labelled nucleic acid and revealed that the endothelial cells of the donor heart in fact take up the bulk of the labelled dODN. It is likely, therefore, that this route of application restricts the effects of the dODN on gene expression to the graft endothelium.
Single application of the STAT-1 dODN but not that of a mutant control ODN to coronary vessels of the donor heart prior to implantation resulted in a pronounced down-regulation not only of CD40 but also of E-selectin, ICAM-1, MCP-1, RANTES and VCAM-1 expression in the allografts 24 h post-transplantation. The level of expression of these pro-inflammatory gene products at this point following STAT1 dODN treatment was much lower yet than in control isografts which irrespective of the treatment regime did not reveal this early change in pro-inflammatory gene expression. Only E-selectin mRNA levels appeared to be elevated about threefold in control isografts at 24 h as compared to 9 days post-transplantation, indicative of a modest surgical or ischemia/reperfusion injury-induced trauma. Expression of the aforementioned gene products in the allograft most likely is IFN-γ-dependent and governed by activation of the transcription factor STAT-1 [2, 6, 31]. Moreover, some of these pro-inflammatory gene products as, e.g. MCP-1 have been shown to up-regulate the expression of additional pro-inflammatory gene products [28] so that STAT-1 dODN blockade of IFN-γ-induced MCP-1 expression may have affected a much broader pattern of pro-inflammatory genes. In this context, it should also be emphasised that the dODN employed most likely binds with similar affinity to STAT-1 and STAT-3 [14]. It cannot be excluded, therefore, that down-regulation of STAT-3-dependent genes may have contributed to the potent anti-inflammatory effect of the STAT1 dODN in our mouse cardiac allograft model.
Apart from MCP-1 (and possibly RANTES) mRNA abundance which was still significantly up-regulated in the allografts as compared to the isografts 9 days post-transplantation, there were no long-term changes in pro-inflammatory gene expression in the donor hearts. Moreover, STAT-1 dODN inhibition of pro-inflammatory gene expression was no longer evident at this point. The most likely reason for this apparent lack of effect is the relative instability of the nucleic acid in the target tissue over such a long period (cf. [12]). Nonetheless, its effect on early changes in gene expression had a profound influence on the histological appearance of the allografts. Thus, there was a significant and robust reduction both in vascular and interstitial rejection. In contrast, there were no appreciable changes in the histological appearance of the isografts at this point, irrespective of the treatment regime. This finding is in line also with the immunohistochemical quantification of the number of infiltrating leukocytes in the graft myocardium which was essentially unaltered in the isografts at any time. No increase in leukocyte cell counts was likewise noted in the allografts 24 h post-transplantation whereas both monocyte/macrophage and especially T cell numbers were clearly up-regulated at the end of the 9-day follow-up period. Periprocedural administration of the STAT-1 dODN reduced the infiltration of both cell types at this point underlining not only its inhibitory effect on cellular rejection per se but also that early rather than late changes in pro-inflammatory gene expression are decisive for this to occur.
A note of caution may be appropriate though with regard to the possibility of a dissociation between pro-inflammatory gene expression and mononuclear cell infiltration in the course of rejection. Even if up-regulation of pro-inflammatory gene expression in the allograft 24 h post-transplantation is observed rather frequently [12, 29], this does not mean that the maximum level of expression is already attained at this point. On the other hand, maximum infiltration of mononuclear cells into the allograft typically occurs several days later [12, 30] so that temporally both parameters seem to be clearly segregated. Moreover, the possibility that the early rise in pro-inflammatory gene expression in the cardiac allografts is attributable in part to perioperative ischemic injury triggering an innate response rather than a graft-specific alloresponse cannot be disregarded.
The finding that periprocedural STAT-1 dODN treatment inhibited pro-inflammatory gene expression in the cardiac allografts 24 h but not 9 days post-transplantation likewise must not support the aforementioned hypothesis of a temporal division between pro-inflammatory gene expression and mononuclear cell infiltration in the course of rejection. Temporally similar effects on pro-inflammatory gene expression (near maximum inhibition at 24 h, modest inhibition at 3 days post-transplantation) have previously been observed with the STAT-1 dODN in a rat cardiac allograft model [12], suggesting that its duration of action is associated with its intracellular stability rather than the underlying pathogenetic mechanism. In fact, from all animal experimental in vivo models with single application of these nucleic acids conducted thus far a rapid onset with an average duration of action of up to 3 or 4 days may be inferred. However, to avoid any uncertainty in correctly interpreting the present data it would have been desirable to include an additional 3–5-day time point in our mouse cardiac transplant model. Regrettably, this was precluded by the limited availability of the B10.A(2R) donor mice.
Notwithstanding this the aforementioned findings may be interpreted as follows: (1) STAT-1 dODN treatment attenuates the immune response of the host against the graft, (2) IFN-γ-mediated up-regulation of pro-inflammatory gene expression including but not exclusive to that of CD40 preferentially in the donor endothelium plays a major role in exacerbating the cellular rejection process. These conclusions are supported by previous findings with anti-CD154 antibodies that prolong allograft survival both in rodent and primate transplantation models [10, 11, 18, 32] and, more recently, with anti-CD40 antibodies that prolong kidney allograft survival in monkeys [15]. However, despite these promising pre-clinical data, clinical application of the anti-CD154 antibodies has been wrought with difficulty primarily due to serious thromboembolic complications [17, 19].
In conclusion, the aforementioned findings establish a novel therapeutic principle to reduce acute and possibly also chronic graft rejection [29]. By neutralising the transcription factor STAT-1, decoy ODNs exert a powerful long-lasting anti-inflammatory effect. These nucleic acid-based drugs offer the advantage of a rapid uptake by their target cells; here the endothelial cells lining the coronary arteries of the donor heart, without any transfection reagent. They may therefore represent a novel periprocedural therapeutic perspective for the adjunct protection (in combination with standard immunosuppressants) of difficult transplants such as the heart.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, Collaborative Research Center 405 (projects B10 and B17).
Footnotes
T. Stojanovic and A. H. Wagner contributed equally to this work.
References
- 1.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 2.Burysek L, Syrovets T, Simmet T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J Biol Chem. 2002;277:33509–33517. doi: 10.1074/jbc.M201941200. [DOI] [PubMed] [Google Scholar]
- 3.Caux C, Massacrier C, Vanbervliet B, Dubois B, Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho CS, Hamawy MM, Knechtle SJ. CD40:CD154 interactions and allograft rejection. Mechanics of rejection. Curr Opin Organ Transplant. 2000;5:10–15. doi: 10.1097/00075200-200003000-00003. [DOI] [Google Scholar]
- 5.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2 K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Caterina R, Bourcier T, Laufs U, La Fata V, Lazzerini G, Neish AS, Libby P, Liao JK. Induction of endothelial-leukocyte interaction by interferon-γ requires coactivation of nuclear factor-κB. Arterioscler Thromb Vasc Biol. 2001;21:227–232. doi: 10.1161/01.atv.21.2.227. [DOI] [PubMed] [Google Scholar]
- 7.Deng MC. Orthotopic heart transplantation: highlights and limitations. Surg Clin North Am. 2004;84:243–255. doi: 10.1016/S0039-6109(03)00212-3. [DOI] [PubMed] [Google Scholar]
- 8.Feldman D, Menachemi DM, Abraham WT, Wexler RK. Management strategies for stage-D patients with acute heart failure. Clin Cardiol. 2008;31:297–301. doi: 10.1002/clc.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gröne HJ, Weber C, Weber KS, Gröne EF, Rabelink T, Klier CM, Wells TN, Proudfood AE, Schlöndorff D, Nelson PJ. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 1999;13:1371–1383. [PubMed] [Google Scholar]
- 10.Gudmundsdottir H, Turka LA. T cell costimulatory blockade: new therapies for transplant rejection. J Am Soc Nephrol. 1999;10:1356–1365. doi: 10.1681/ASN.V1061356. [DOI] [PubMed] [Google Scholar]
- 11.Hancock WW. Current trends in transplant immunology. Curr Opin Nephrol Hypertens. 1999;8:317–324. doi: 10.1097/00041552-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hölschermann H, Stadlbauer THW, Wagner AH, Fingerhuth H, Muth H, Rong S, Güler F, Tillmanns H, Hecker M. STAT-1 and AP-1 decoy oligonucleotide therapy delays acute rejection and prolongs cardiac allograft survival. Cardiovasc Res. 2006;71:527–536. doi: 10.1016/j.cardiores.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Horuk R, Clayberger C, Krensky AM, Wang Z, Grone HJ, Weber C, Weber KS, Nelson PJ, May K, Rosser M, Dunning L, Liang M, Buckman B, Ghannam A, Ng HP, Islam I, Bauman JG, Wei GP, Monahan S, Xu W, Snider RM, Morrissey MM, Hesselgesser J, Perez HD. A non-peptide functional antagonist of the CCR1 chemokine receptor is effective in rat heart transplant rejection. J Biol Chem. 2001;276:4199–4204. doi: 10.1074/jbc.M007457200. [DOI] [PubMed] [Google Scholar]
- 14.Hückel M, Schurigt U, Wagner AH, Stöckigt R, Petrow PK, Thoss K, Gajda M, Henzgen S, Hecker M, Bräuer R. Attenuation of murine antigen-induced arthritis by treatment with a decoy oligodeoxynucleotide inhibiting signal transducer and activator of transcription-1 (STAT-1) Arthritis Res. 2006;8:R17. doi: 10.1186/ar1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai A, Suzuki T, Sugitani A, Itoh T, Ueki S, Aoyagi T, Yamashita K, Taniguchi M, Takahashi N, Miura T, Shimamura T, Furukawa H, Todo S. A novel fully human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys. Transplantation. 2007;84:1020–1028. doi: 10.1097/01.tp.0000286058.79448.c7. [DOI] [PubMed] [Google Scholar]
- 16.Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc Natl Acad Sci USA. 1995;92:4342–4356. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 18.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH, Jr, Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 19.Koyama I, Kawai T, Andrews D, Boskovic S, Nadazdin O, Wee SL, Sogawa H, Wu DL, Smith RN, Colvin RB, Sachs DH, Cosimi AB. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;77:460–462. doi: 10.1097/01.TP.0000110291.29370.C0. [DOI] [PubMed] [Google Scholar]
- 20.Larsen C, Pearson T. The CD40 pathway in allograft rejection, acceptance and tolerance. Curr Opin Immunol. 1997;9:641–647. doi: 10.1016/S0952-7915(97)80043-X. [DOI] [PubMed] [Google Scholar]
- 21.Mann MJ, Dzau VJ. Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest. 2000;106:1071–1075. doi: 10.1172/JCI11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quarcoo D, Weixler S, Groneberg D, Joachim R, Ahrens B, Wagner AH, Hecker M, Hamelmann E. Inhibition of signal transducer and activator of transcription 1 attenuates allergen-induced airway inflammation and hyperreactivity. J Allergy Clin Immunol. 2004;114:288–295. doi: 10.1016/j.jaci.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Reul RM, Fang JC, Denton MD, Geehan C, Long C, Mitchell RN, Ganz P, Briscoe DM. CD40 and CD40 ligand (CD154) are coexpressed on microvessels in vivo in human cardiac allograft rejection. Transplantation. 1997;64:1765–1774. doi: 10.1097/00007890-199712270-00025. [DOI] [PubMed] [Google Scholar]
- 26.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 27.Russell ME, Adams DH, Wyner LR, Yamashita Y, Halnon NJ, Karnovsky MJ. Early and persistent induction of monocyte chemoattractant protein 1 in rat cardiac allografts. Proc Natl Acad Sci USA. 1993;90:6086–6090. doi: 10.1073/pnas.90.13.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiura A, Sata M, Hiasa K, Kitamoto S, Washida M, Egashira K, Nagai R, Makuuchi M. Antimonocyte chemoattractant protein-1 gene therapy attenuates graft vasculopathy. Arterioscler Thromb Vasc Biol. 2004;24:1886–1890. doi: 10.1161/01.ATV.0000141045.49616.6f. [DOI] [PubMed] [Google Scholar]
- 29.Stadlbauer THW, Wagner AH, Hölschermann H, Fiedel S, Fingerhuth H, Tillmanns H, Bohle RM, Hecker M. AP-1 and STAT-1 decoy oligodeoxynucleotides attenuate transplant vasculopathy in rat cardiac allografts. Cardiovasc Res. 2008;79:698–705. doi: 10.1093/cvr/cvn135. [DOI] [PubMed] [Google Scholar]
- 30.Stojanovic T, Bedke J, Gröne HJ, Proudfoot AEI, Becker H, Markus P, Hecker M. Met-RANTES inhibition of mucosal perfusion failure in acute intestinal transplant rejection—role of endothelial cell-leukocyte interaction. J Vasc Res. 2002;39:51–58. doi: 10.1159/000048993. [DOI] [PubMed] [Google Scholar]
- 31.Wagner AH, Gebauer M, Pollok-Kopp B, Hecker M. Cytokine-inducible CD40 expression in human endothelial cells is mediated by interferon regulatory factor-1. Blood. 2002;99:520–525. doi: 10.1182/blood.V99.2.520. [DOI] [PubMed] [Google Scholar]
- 32.Waldmann H. Transplantation tolerance—where do we stand? Nat Med. 1999;5:1245–1248. doi: 10.1038/15197. [DOI] [PubMed] [Google Scholar]
- 33.Yun JJ, Fischbein MP, Laks H, Fishbein MC, Espejo ML, Ebrahimi K, Irie Y, Berliner J, Ardehali A. Early and late chemokine production correlates with cellular recruitment in cardiac allograft vasculopathy. Transplantation. 2000;69:2515–2524. doi: 10.1097/00007890-200006270-00009. [DOI] [PubMed] [Google Scholar]


