Abstract
Subtle structural brain abnormalities are an established finding in first-episode psychosis. Nevertheless their relationship to the clinical course of schizophrenia is controversially discussed. In a multicentre study 45 first-episode schizophrenia patients (FE-SZ) underwent standardized MRI scanning and were followed up to 1 year. In 32 FE-SZ volumetric measurement of three regions of interests (ROIs) potentially associated with disease course, hippocampus, lateral ventricle and the anterior limb of the internal capsule (ALIC) could be performed. The subgroups of FE-SZ with good (12 patients) and poor outcome (11 patients), defined by a clinically relevant change of the PANSS score, were compared with regard to these volumetric measures. Multivariate analysis of covariance revealed a significant reduced maximal cross sectional area of the left ALIC in FE-SZ with clinically relevant deterioration compared to those with stable psychopathology. There were no differences in the other selected ROIs between the two subgroups. In conclusion, reduced maximal area of ALIC, which can be interpreted as a disturbance of fronto-thalamic connectivity, is associated with poor outcome during the 1 year course of first-episode schizophrenia.
Keywords: first-episode schizophrenia, magnetic resonance imaging, disease course, outcome
Introduction
Structural brain abnormalities have been consistently described in schizophrenic patients compared to healthy controls [19, 27]. In a recent meta-analysis, volumetric deficits at diagnosis were seen in total brain volume, in the hippocampus, in cortical grey matter, in Heschl’s gyrus, in the planum temporal and in temporal grey matter, and in longitudinal studies continued volumetric loss over time could be demonstrated in these structures [22]. In addition, the lateral ventricles were significantly larger than normal at onset of the illness and the ventricular volume tended to increase significantly in the disease course [22].
Only few studies focused on the predictive value of brain morphology for the course of schizophrenia in first-episode patients and reported inconsistent results [4, 5, 13, 16–18, 23]. It was observed that hippocampal volume was associated with a higher risk of relapse [16], greater ventricular expansion was related to an unremitting course and poor outcome [4, 5, 11], smaller temporal gray matter volume was associated with persistence of hallucinations, and more normal cerebral asymmetry was associated with adequate social/vocational functioning and full recovery [18]. Two other investigations could not demonstrate a correlation between volumetric measurement and treatment response [17] or 2-year outcome [23].
The aim of the presented study was to investigate if there is a difference in brain morphology focusing on structures suggested in previous studies to be relevant for the clinical course (hippocampus, lateral ventricles) in a sample consisting of first-episode patients well characterized for the outcome over 1 year. Based on previous findings of reduced internal capsule volume and cross sectional area in families affected with schizophrenia [25] and in first-episode patients [10], we decided to include volumetric measurement of the anterior limb of the internal capsule (ALIC).
Method
Subjects
The first-episode study program [6] of the German Research Network on Schizophrenia (GRNS) [26] consisted of an 8-week acute treatment phase and a consecutive 2-year long-term treatment phase (registered in ClinicalTrials.gov: NCT00159081). Between 2000 and 2004, 158 first-episode patients, aged 18–55 years and treated in inpatients departments of the participating centres, were randomly assigned to double-blind, low-dose haloperidol or risperidone. Drugs could be increased (up to 8 mg/day) or lowered (minimum 1 mg/day) depending on symptoms or side effects as indicated by the respective clinical global impressions (CGI) scales. Concomitant medications were permitted throughout the trial, except for additional antipsychotics or mood stabilizers. The results of the clinical study have been published recently [7]. Patients were diagnosed with schizophrenia according to DSM-IV by experienced clinical psychiatrists using a standardized clinical interview (SCID; German Version) [24]. Before entering the long-term treatment study the diagnosis of schizophrenia was confirmed by independent experienced clinical psychiatrists.
A total of 75 patients of this long-term treatment phase first-episode sample participated in the MR-imaging study and were recruited from the Psychiatry Departments of the Universities of Bonn, Cologne, Düsseldorf, Duisburg-Essen, Jena, Mainz, Munich, and Tübingen, as well as from the Central Institute of Mental Health in Mannheim. Out of this sample only 45 patients received standardized rating of psychopathology with the positive and negative syndrome scale (PANSS) [9] at baseline and during follow-up, and underwent magnetic resonance imaging (MRI) at baseline due to a defined protocol fulfilling the quality-criteria. Exclusion criteria for the first-episode long-term study and the MRI study part were any psychiatric comorbidity (DSM-IV), neurological diseases, contraindication for antipsychotic treatment, suicidal behavior in previous history, mental retardation, pregnancy, substance dependence, disorders which affect cerebral metabolism, in addition to the usual exclusion criteria for MRI (e.g. metal implants, cardiac pace makers). After a complete description of the study, written informed consent was obtained from each patient. The local ethics committees approved the protocol, which is in accordance with the Declaration of Helsinki.
From the MRI study sample we extracted 39 first-episode patients, who received follow-up visits covering a period of at least 3 months up to 1 year (mean 46.6 ± 10.7 weeks). In this MR sample volumetric measurement of all regions of interest could be performed in 32 patients. We divided this patient sample in three subgroups according to PANSS rating during the 1-year follow-up: 12 patients with stable psychopathology (good outcome; increase of PANSS total score below 10% of baseline score, approximately 5 points), 9 patients with intermediate outcome (moderate worsening), and 11 patients with clinically relevant deterioration (bad outcome; increase of PANSS total score above 40% of baseline score, approximately 20 points).
The subgroup with good outcome did not show any clinically relevant worsening of symptoms, presenting a stable course (change of mean PANSS total score less than 4 points), while the subgroup with bad outcome demonstrated a clinically relevant increase of symptoms at least in one visit during the 1-year course, predominantly in negative symptoms and general psychopathology, (change of mean PANSS total score more than 40 points) (see Table 1).
Table 1.
Clinical and sociodemographic data in the first-episode subgroups
| FE-SZ stable (n = 12) | FE-SZ bad outcome (n = 11) | ANOVA | ||||
|---|---|---|---|---|---|---|
| m | SD | m | SD | F | P | |
| Age (years) | 30.40 | 9.42 | 33.18 | 12.87 | 0.31 | 0.58 |
| Education (years) | 11.60 | 3.03 | 12.11 | 2.62 | 0.15 | 0.70 |
| Education of parents (years) | 12.67 | 3.46 | 13.29 | 4.50 | 0.10 | 0.76 |
| Baseline PANSS positive score | 10.83 | 3.93 | 8.82 | 2.52 | 2.10 | 0.16 |
| Baseline PANSS negative core | 15.33 | 6.11 | 11.09 | 3.18 | 4.24 | 0.052 |
| Baseline PANSS gen. score | 27.92 | 7.38 | 23.09 | 5.72 | 3.03 | 0.096 |
| Maximal PANSS positive score | 11.33 | 3.87 | 13.73 | 5.10 | 1.63 | 0.22 |
| Maximal PANSS negative score | 16.58 | 6.32 | 21.64 | 3.93 | 5.19 | 0.033 |
| Maximal PANSS gen. sum score | 28.42 | 6.91 | 48.91 | 12.84 | 23.28 | <0.0005 |
| Diff. PANSS positive score | 0.50 | 1.24 | 4.91 | 4.97 | 8.88 | 0.007 |
| Diff. PANSS negative score | 1.25 | 1.60 | 10.55 | 3.08 | 84.68 | <0.0005 |
| Diff. PANSS gen. score | 0.50 | 1.00 | 25.82 | 11.11 | 62.07 | <0.0005 |
| FE-SZ stable | FE-SZ bad outcome | χ² | P | |||
| Gender (male/female), n | 6/6 | 6/5 | 0.05 | 0.83 | ||
FE-SZ first-episode schizophrenic patients, Diff. difference, PANSS positive and negative syndrome scale, gen. general psychopathology, n sample size, m mean, SD standard deviation, ANOVA analysis of variance, FF-statistics, P probability, χ2 Chi-Square-test
We compared the subgroups of good and bad outcome with regard to volumetric measurement of three distinct brain regions: hippocampus, lateral ventricles and the ALIC.
Magnetic resonance imaging and measurements
Magnetic resonance imaging (MRI) was performed on Siemens and Phillips 1.5 Tesla MR scanners with T1-weighted 3D data (MPRAGE) sequences providing a spatial resolution of 1 × 1 × 1 mm3 (repetition time = 11.4 ms, echo time = 4.4 ms, flip angle = 15°).
Manual area measurements for lateral ventricles, hippocampus and ALIC were obtained using the “region of interest” tool implemented in the software Analyze (version 3.0). Volumes of ROIs were calculated by multiplying the outlined areas with slice thickness, and relative volumes were calculated to adjust for differences in total brain volumes. Intra- and interrater reliability was measured in a subset of ten subjects. Intraclass correlation was sufficiently high (ICC >0.7) [20]. The total brain volume, gray and white matter volumes were determined using an automatic algorithm programmed in MATLAB and SPM99 (Statistical Parametric Mapping) [2].
The areas of the lateral ventricles were traced for each side separately in coronal slices, and hippocampal contours were drawn in the sagittal view following the borders described in the literature [19]. The correctness of the outlines was controlled in the two other views.
The measurement of ALIC was performed as described previously [25] dividing the internal capsule into an anterior and posterior part by a line bisecting the angle of internal capsule on horizontal slices. ALIC was defined medially by the caudate and laterally by the lentiform nucleus. The ventral boundary was determined by a line connecting the lateral edge of ventral part of the caudate and the medial edge of the ventral part of the lentiform nucleus. The first slice included was the most inferior one comprising a detectable amount of white matter belonging to the internal capsule. The last slice was the one inferior to the slice which comprised the merging of internal capsule and the lamina pallidi externa. The method of manual delineation required individual optimization of contrast and brightness in each slice. ALIC identification was controlled for each slice in coronal and sagittal orientation. Additionally, ALIC length and maximal cross sectional area were measured, length as the sum of slices containing ALIC, maximal cross section as maximal area of one particular slice among all slices (see Fig. 1). In all cases the slice with maximal cross sectional area was identical to the most superior slice directly beneath the delta of merging internal capsule and lamina pallidi externa.
Fig. 1.
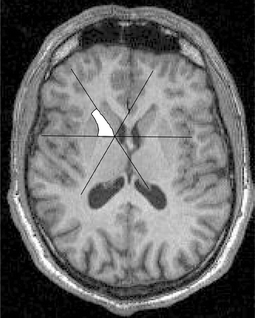
Tracing of ALIC T1-weighted MR-image. The internal capsule is divided into an anterior (ALIC) and posterior part (PLIC) by a line bisecting the angle of internal capsule on horizontal slices. The right anterior limb of the internal capsule (ALIC) is marked
Statistics
Statistical analyses were performed with SPSS 10 for Windows. All tests were two-tailed. Dependent variables were bilateral volumes of the hippocampus and the ventricular system, and bilateral volumes, maximal cross sectional area and length of the ALIC. Independent variable was the outcome of the patients. Only the extreme groups with good or bad outcome were included into the analysis.
Boxplots were examined to identify extreme values. Kolmogorov–Smirnov tests were applied to test the assumption of normal distribution. Since there were no significant deviations from the normality assumption, the general linear model (GLM) was performed using a multivariate approach for left and right side measurements separately for the blocks of dependent variables. Independent factors outcome (good/bad) and gender and covariate age were included into the model. Thus, all analyses were adjusted for intervening influences of age and gender.
Results
The patient subgroups with good and poor outcome did not differ in sociodemographic data (see Table 1). First-episode patients who subsequently worsened during the first year of the illness revealed a significantly smaller maximal cross sectional area of the ALIC compared to those patients with stable psychopathology (GLM; multivariate: F = 4.5, df = 2, 17, P = 0.028). Subsequent univariate tests showed that this reduction was significant on the left side (−19%, F = 8.2, df = 1, 18, P = 0.010; see Fig. 2). Other measurements of ALIC, as well as hippocampal and lateral ventricular volumes were not significantly reduced in patients with poor outcome compared to the stable group (see Table 2).
Fig. 2.
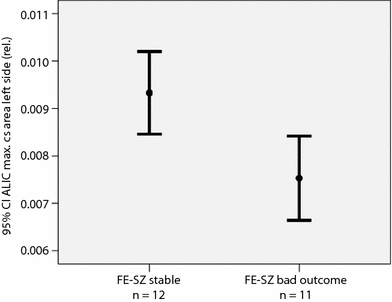
Relative left ALIC maximal cross sectional area in first-episode schizophrenia patients. ALIC, anterior limb of the internal capsule; FE-SZ, first-episode schizophrenia patients; CI, confidence interval; max. cs, maximal cross sectional; rel., relative
Table 2.
Results of volumetric measurements
| Region of interest | FE-SZ stable (n = 12) | FE-SZ bad outcome (n = 11) | Diff (%) | MANCOVA | ||||
|---|---|---|---|---|---|---|---|---|
| m | SD | m | SD | F | df | P | ||
| Hippocampus volume left side (rel.) | 0.00328 | 0.00044 | 0.00313 | 0.00034 | −4.4 | 0.4 | 1, 17 | 0.23 |
| Hippocampus volume right side (rel.) | 0.00315 | 0.00055 | 0.00307 | 0.00037 | −2.5 | 0.4 | 1, 17 | 0.45 |
| Lateral ventricle volume left side (rel.) | 0.01139 | 0.00467 | 0.00977 | 0.00528 | −14.2 | 0.8 | 1, 18 | 0.38 |
| Lateral ventricle volume right side (rel.) | 0.01038 | 0.00466 | 0.00946 | 0.00507 | −8.8 | 0.2 | 1, 18 | 0.64 |
| ALIC volume left side(rel.) | 0.00056 | 0.00011 | 0.00055 | 0.00010 | −1.7 | 0.0 | 1, 18 | 1.00 |
| ALIC volume right side(rel.) | 0.00059 | 0.00010 | 0.00051 | 0.00010 | −12.3 | 2.2 | 1, 18 | 0.15 |
| ALIC max. cs. area left side (rel.) | 0.0093 | 0.0014 | 0.0075 | 0.0013 | −19.3 | 8.2 | 1, 18 | 0.010 |
| ALIC max. cs. area right side (rel.) | 0.0085 | 0.0015 | 0.0079 | 0.0013 | −6.9 | 0.8 | 1, 18 | 0.38 |
| ALIC length left side (rel.) | 0.0923 | 0.0128 | 0.0945 | 0.0116 | 2.5 | 0.6 | 1, 18 | 0.44 |
| ALIC length right side (rel.) | 0.0930 | 0.0108 | 0.0955 | 0.0120 | 2.7 | 0.8 | 1, 18 | 0.39 |
| Total brain volume (absolute, cm³) | 1136.5 | 116.6 | 1133.1 | 103.2 | 1.4 | 0.1 | 1, 18 | 0.73 |
FE-SZ first-episode schizophrenic patients, Diff. difference, n sample size, m mean, SD standard deviation, FF-statistics, df degrees of freedom, P probability, rel. relative, max. cs. maximal cross sectional, MANCOVA multivariate analysis of covariance, F, P for factor subgroup
We found no significant correlations between the cumulative doses of risperidone or haloperidol and the ALIC maximal cross sectional area in the total group of patients and in the subgroups of schizophrenic patients with stable psychopathology or clinically relevant deterioration in the 1-year course. The cumulative doses of risperidone and haloperidol were not significantly different in both subgroups (df = 1, F = 0.001; P = 0.98, oneway-ANOVA), and the daily doses of risperidone and haloperidol in the subgroups were comparable (FE-SZ stable psychopathology: risperidone 4.3 ± 2.0 mg/day, haloperidol 3.0 ± 1.5 mg/day; FE-SZ poor outcome: risperidone 3.8 ± 1.3 mg/day, haloperidol 3.1 ± 1.6 mg/day).
While the educational level may have influenced out results we repeated the analysis with education as covariate. The results were similar, first-episode patients with deterioration during the first year of the illness still presented with a significantly smaller maximal cross sectional area of the ALIC compared to those patients with stable psychopathology (MANCOVA; P = 0.020).
Discussion
The main result of our study assessing the relationship of brain morphology and clinical outcome in first-episode schizophrenia is the significant reduced maximal cross sectional area of the anterior limb of the internal capsule (ALIC) in the subgroup of patients with clinical deterioration during the 1-year course compared to patients with stable psychopathology. There was no volumetric difference between the two subgroups with regard to the other selected regions of interest, hippocampus and lateral ventricles.
While most prominent replicable brain abnormalities in schizophrenia are volume changes of the grey matter (e.g. 19, 22, 27), some studies using voxel-based morphometry revealed evidence for white matter abnormalities, e.g. bilaterally in the frontal lobe [14] or in the left temporal and frontal lobe of schizophrenic patients with predominant negative symptoms [21]. In addition, significant decreases in white matter density were detected in connecting structures like the genu and truncus of the corpus callosum, the right ALIC and the right anterior commissure compared to healthy subjects [8].
Only a few studies assessed white matter changes in schizophrenia that were hypothesis-driven using the region of interest (ROI) approach. In the present study we morphometrically assessed the hippocampus and the lateral ventricles, whose volumetric alterations are most consistently associated with the course of the illness, as well as the anterior limb of the internal capsule, a connecting white matter structure, which has been found to exhibit decreased volumes both in schizophrenia [28] and in unaffected first-degree relatives of schizophrenic patients [25].
Out of the three mentioned structures, in our study only the left maximal cross sectional area of the anterior limb of the internal capsule was associated with the clinical course in first-episode schizophrenia. In this sample of first-episode patients those with poor outcome (high increase of positive symptoms) showed a reduced maximal cross sectional area of the ALIC on the left side compared to those with good outcome. However, when left and right ALIC maximal cross sectional areas of both subgroups are compared, there is an inverse relationship. In the good outcome group the mean left ALIC maximal cross sectional area is larger than the right one, and in the bad outcome group it is the other way round. This inverse relationship may be at least in part responsible for the significant difference between the subgroups. This may point towards a different asymmetry or shape of this structure in the subgroups. Nevertheless we can not rule out the possibility that the left ALIC maximal cross sectional area in the patients with good outcome group may be disproportionately large in our sample.
The ALIC is a very important connecting structure linking the frontal cortex to subcortical structures through the medial [15] and the basolateral limbic circuit [12]. Abnormalities in the ALIC can thus be taken as measure for disturbances in the frontothalamic connectivity that is discussed to be relevant for the pathophysiology of schizophrenia [1].
Interestingly, a recent study shows that a reduced area of the ALIC in dorsal MRI slices was associated with poor outcome based on longitudinal analysis of self-care deficits in chronic schizophrenia [3]. The authors suggest disruption of internal capsule fibers in poor-outcome patients with schizophrenia, which is supported by the results of our study. The reduced maximal cross sectional area measured in our investigation corresponds to the reduced ALIC area in dorsal MRI slices of the cited study. Additionally, our results indicate that reduced white fiber structures in this area might increase the risk not only for a poor outcome in chronic schizophrenic patients, but also increase the vulnerability for the onset of schizophrenic psychosis and an unfavourable course in first-episode schizophrenia. To our knowledge this study is the first one investigating the relevance of white matter structures, especially the ALIC, for the course of first-episode schizophrenia.
There are several limitations of our study. We were not able to control for the influence of possible confounding factors determining poor outcome, e.g. psychosocial circumstances or neurocognitive function. Secondly, the follow-up observational period without any intermediate anchor point may be too long to document the disease course adequately. Medication could serve as a confounding factor in structural neuroimaging. While the cumulative and daily doses of risperidone and haloperidol were low and similar distributed in both subgroups we do not assume a relevant influence of antipsychotic medication on our volumetric results or on clinical outcome, for instance by the induction of secondary negative symptoms.
In conclusion our finding of reduced internal capsule size in patients with bad outcome supports the dysconnectivity concept of schizophrenia [28] and points towards disturbances in the striato-thalamo-cortical neuronal circuit and abnormalities in related structures.
Disclosures and acknowledgments
This study is part of the German Research Network on Schizophrenia and was funded by the German Federal Ministry for Education and Research BMBF (grants 01 GI 9932 and 01 GI 0232).
Dr. Wobrock has participated in speaker bureaus for AstraZeneca, Bristol-Myers Squibb, Janssen-Cilag, Eli Lilly, Organon, Pfizer, and Sanofi-Synthelabo/Aventis, and received grant/research support from Astra Zeneca.
Dr. Gaebel has participated in speakers bureaus for AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen-Cilag, Eli Lilly, Lundbeck, and Sanofi-Synthelabo/Aventis; has been a member of advisory boards for Eli Lilly, Lundbeck, Novartis, and Wyeth; and has received grant/research support from Bristol-Myers Squibb, Eli Lilly, Wyeth, Janssen-Cilag, and Lundbeck.
Dr. Möller has participated in speakers bureaus for AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen Cilag, Eli Lilly, Lundbeck, Pfizer, and Sanofi- Aventis; has been a member of advisory boards for AstraZeneca, Bristol- Myers Squibb, GlaxoSmithKline, Janssen Cilag, Eli Lilly, Lundbeck, Pfizer, Servier, and Wyeth; and has received grant/research support from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen Cilag, Eli Lilly, Lundbeck, Merck, Novartis, Pfizer, Sanofi-Aventis, Servier, and Wyeth.
Dr. Klosterkötter has participated in speakers bureaus for AstraZeneca, Bristol-Myers Squibb, Janssen Cilag, Lundbeck, Sanofi-Synthelabo/Aventis; has been a member of advisory boards for AstraZeneca, Bristol- Myers Squibb, Janssen Cilag, and has received grant/research support from AstraZeneca, Bristol-Myers Squibb, Janssen Cilag, Sanofi-Aventis.
Dr. Falkai is a member of a speakers’ bureau for AstraZeneca, Eli Lilly, Janssen-Cilag and Lundbeck, and has accepted paid speaking engagements in industry-sponsored symposia from AstraZeneca, Bristol-Myers-Squibb, Eli-Lilly, Janssen-Cilag, Lundbeck and Pfizer, and travel or hospitality not related to a speaking engagement from Astra Zeneca, Bristol-Myers-Squibb, Eli Lilly, Janssen Cilag, Lundbeck and Sanofi-Synthelabo, and received a research grant from Astra Zeneca.
Drs. Wölwer, Gruber, Bender, Schlösser, Schmitt, Buchkremer, Maier, Schneider, Mr. Riesbeck and Mr. Schneider-Axmann report no additional financial or other relationships relevant to the subject of this article.
References
- 1.Andreasen NC, O′Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner J, Friston K. Multimodal image coregistration and partitioning—a unified framework. Neuroimage. 1997;6(3):209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 3.Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, Mitelman SA, Hazlett EA, Lincoln SJ, Newmark RE, Shilabuddin L. Internal capsule size in good-outcome and poor-outcome schizophrenia. J Neuropsychiatry Clin Neurosci. 2006;18(3):364–376. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- 4.Cahn W, Hulshoff Pol HE, Lems EB, Haren NE, Schnack HG, Linden JA, Schothorst PF, Engeland H, Kahn RS. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 5.Cahn W, Haren NE, Pol HE, Schnack HG, Caspers E, Laponder DA, Kahn RS. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. Br J Psychiatry. 2006;189:381–382. doi: 10.1192/bjp.bp.105.015701. [DOI] [PubMed] [Google Scholar]
- 6.Gaebel W, Möller HJ, Buchkremer G, Ohmann C, Riesbeck M, Wölwer W, Wilmsdorff M, Bottlender R, Klingberg S. Pharmacological long-term treatment strategies in first episode schizophrenia—study design and preliminary results of an ongoing RCT within the German Research Network on Schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(2):129–140. doi: 10.1007/s00406-004-0509-y. [DOI] [PubMed] [Google Scholar]
- 7.Gaebel W, Riesbeck M, Wölwer W, Klimke A, Eickhoff M, Wilmsdorff M, Jockers-Scherübl MC, Kühn K, Lemke M, Bechdolf A, Bender S, Degner D, Schlösser R, Schmidt LG, Schmitt A, Jäger M, Buchkremer G, Falkai P, Klingberg S, Köpcke W, Maier W, Häfner H, Ohmann C, Salize HJ, Schneider F, Möller HJ. Maintenance treatment with risperidone or low-dose haloperidol in first-episode schizophrenia. One-year results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry. 2007;68:1763–1774. doi: 10.4088/JCP.v68n1116. [DOI] [PubMed] [Google Scholar]
- 8.Hulshoff Pol HE, Schnack HG, Mandl RC, Cahn W, Collins DL, Evans AC, Kahn RS. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. Neuroimage. 2004;21(1):27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 10.Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, Smith GN, Honer WG. Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr Res. 2006;87(1–3):89–99. doi: 10.1016/j.schres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49(6):487–499. doi: 10.1016/S0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 12.Livingston KE, Escobar A. Anatomical bias of the limbic system concept. A proposed reorientation. Arch Neurol. 1971;24:17–21. doi: 10.1001/archneur.1971.00480310045003. [DOI] [PubMed] [Google Scholar]
- 13.Milev P, Ho BC, Arndt S, Nopoulos P, Andreasen NC. Initial magnetic resonance imaging volumetric brain measurements and outcome in schizophrenia: a prospective longitudinal study with 5-year follow-up. Biol Psychiatry. 2003;54(6):608–615. doi: 10.1016/S0006-3223(03)00293-2. [DOI] [PubMed] [Google Scholar]
- 14.Paillere-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, Recasens C, Attar-Levy D, Martinot JL. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. 2001;50(1–2):19–26. doi: 10.1016/S0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- 15.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–743. [Google Scholar]
- 16.Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DG, Woerner MG, Alvir JM, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Bilder R, Goldman R, Lieberman JA. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156:544–549. doi: 10.1176/ajp.156.4.544. [DOI] [PubMed] [Google Scholar]
- 18.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161:473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 19.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/S0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 21.Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158(2):234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- 22.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia. Systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 23.Haren NE, Cahn W, Hulshoff Pol HE, Schnack HG, Caspers E, Lemstra A, Sitskoorn MM, Wiersma D, Bosch RJ, Dingemans PM, Schene AH, Kahn RS. Brain volumes as predictor of outcome in recent-onset schizophrenia: a multi-center MRI study. Schizophr Res. 2003;64(1):41–52. doi: 10.1016/S0920-9964(03)00018-5. [DOI] [PubMed] [Google Scholar]
- 24.Wittchen HU, Schramm E, Zaudig M, Unland H (1991) Strukturiertes Klinisches Interview für DSM-III-R. Eine deutschsprachige, erweiterte Bearbeitung der amerikanischen Originalversion der SCID von RL. Spitzer, JB.Williams, M. Gibbon und MB. First. Belz, Weinheim
- 25.Wobrock T, Kamer T, Roy A, Vogeley K, Schneider-Axmann T, Wagner M, Maier W, Rietschel M, Schulze TG, Scherk H, Schild HH, Block W, Träber F, Tepest R, Honer WG, Falkai P. Reduction of the internal capsule in families affected with schizophrenia. Biol Psychiatry. 2008;63(1):65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Wölwer W, Buchkremer G, Häfner H, Klosterkötter J, Maier W, Möller HJ, Gaebel W. German Research Network on Schizophrenia-Bridging the gap between research and care. Eur Arch Psychiat Clin Neurosci. 2003;253:321–329. doi: 10.1007/s00406-003-0468-8. [DOI] [PubMed] [Google Scholar]
- 27.Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 28.Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, Yamashita I, Seto H, Kurachi M. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol Psychiatry. 2003;54:427–436. doi: 10.1016/S0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]


