Abstract
The 14-3-3 protein test has been shown to support the clinical diagnosis of sporadic Creutzfeldt-Jakob disease (CJD) when associated with an adequate clinical context, and a high differential potential for the diagnosis of sporadic CJD has been attributed to other cerebrospinal fluid (CSF) proteins such as tau protein, S100b and neuron specific enolase (NSE). So far there has been only limited information available about biochemical markers in genetic transmissible spongiform encephalopathies (gTSE), although they represent 10–15% of human TSEs. In this study, we analyzed CSF of 174 patients with gTSEs for 14-3-3 (n = 166), tau protein (n = 78), S100b (n = 46) and NSE (n = 50). Levels of brain-derived proteins in CSF varied in different forms of gTSE. Biomarkers were found positive in the majority of gCJD (81%) and insert gTSE (69%), while they were negative in most cases of fatal familial insomnia (13%) and Gerstmann-Sträussler-Scheinker syndrome (10%). Disease duration and codon 129 genotype influence the findings in a different way than in sporadic CJD.
Keywords: Creutzfeldt-Jakob disease, CSF proteins, 14-3-3 protein, Tau
Introduction
The analysis of cerebrospinal fluid (CSF) in patients with suspected Creutzfeldt-Jakob disease (CJD) is an important investigation for the differential diagnosis of CJD from among other forms of rapid progressive dementia. The 14-3-3 protein levels are often increased in the CSF of sporadic CJD patients, and this test has therefore been included in the diagnostic criteria for sporadic CJD when associated with an appropriate clinical profile [6, 31]. Increased levels of other brain-derived proteins, such as tau protein, S100b or neuron-specific enolase (NSE) have also been reported, but information on these markers is limited [20, 27]. However, in most gTSE, studies of CSF proteins report 14-3-3 test results only in gCJD with E200K and V210I point mutations. Levels of brain-derived proteins are low in Gerstmann-Sträussler-Scheinker syndrome (GSS) and almost all fatal familial insomnia (FFI) patients reported to date are negative for 14-3-3 [11, 15, 20, 30].
A very few data are available on the levels of the other marker proteins in the CSF of patients with gTSEs, and no analyses on factors influencing the percentage of elevated levels have been done. In this paper, we perform such an analysis on brain-derived proteins in patients having gTSE with various mutations.
Methods
Patients and database
The study was performed in the framework of the EC-supported multinational study on biomarkers in prion diseases (CJD markers) [20].
A database was set up which included detailed data on 180 patients with genetic TSE, 61 (34%) gTSE patients from Germany, 58 (32%) from Italy, 36 (20%) from Spain, 13 (7%) from the UK, 10 (5%) from Slovakia and 2 (1%) from Switzerland.
The diagnoses of gTSE were carried out according to recent surveillance criteria after PRNP analysis [11, 12]. For the estimation of the disease stage when the LP was performed we divided the individual disease duration in thirds and calculated the time of lumbar puncture according to the first third of the total duration of the disease (early stage), the second (middle) or the third (advanced stage) of the disease.
CSF tests
14-3-3 and other tests were performed according to previously described methods [20].
Statistical analyses
Differences in CSF levels of tau, S100b and NSE proteins across the four genetic groups of gTSE were separately assessed by the Kruskal–Wallis test, followed, when statistically significant, by the Mann–Whitney test. Since different techniques were employed for the determination of S100b and NSE, we included in the quantitative assessment levels only the results of the most frequently used kits (Byk Sangtec for S100b and -Hofman LaRoche for NSE).
We then calculated the percentage of positive results out of the total for each CSF marker in the four genetic groups. All samples were included in the analysis, since a positive result was calculated according to the cut-off of the different techniques. The χ2 test was used to assess differences between categorical variables.
A multiple logistic regression model was used to assess the effect of a set of clinically relevant variables on the percentage of elevated levels of the different biomarkers in gTSE patients. These were: type of disease causing mutation, disease duration (in months), age at onset (in years), disease stage when the lumbar puncture was performed (during the first, second or third period of disease duration), and PRNP codon 129 genotype. Country of patient and gender were entered as covariates. Age at onset was categorized in four clinically meaningful groups, with younger than 40 years as the reference group. Disease duration was categorized in two groups (short and long duration), according to the median survival time. Due to the small numbers of patients, some of the statistical analyses were restricted to gCJD groups or to the 14-3-3 test.
Results
General description
Demographic and clinical information on gTSE cases is given in Table 1. A definite neuropathological diagnosis was available in 52% of the cases, ranging from more than 87% of FFI to 22% of GSS patients, while in 47% of gCJD autopsy was not performed. Data on the polymorphic codon 129 of PRNP were available in 86% of all cases. For some patients the only available information was about PRNP with no information on codon 129 genotype. The majority of gTSE patients were either homozygous for methionine (MM, 58%) or heterozygous (MV, 36%), only a few were homozygous for valine (6%). In a few cases, where the PrPSc typing was available, it was invariable PrPSc type I pattern.
Table 1.
Summary of clinical features of patients in the study
| Forms of gTSE | Number of cases total (female gender) | Percentage of neuropatho-logically confirmed cases (n) | Age at onset, years, median (range) | Clinical duration, months, median (range) | Codon 129 polymorphism | PrP type I | ||
|---|---|---|---|---|---|---|---|---|
| MM % (n) | MV % (n) | VV % (n) | ||||||
| gCJD | 124 (68) | |||||||
| E200K | 64 (37) | 54.7 (35) | 59 (29–86) | 6 (0.43–34) | 63.5 (33) | 36.5 (19) | 0.0 (0) | 4/4 |
| V210I | 40 (19) | 47.5 (19) | 56.5 (43–87) | 4 (1–31) | 67.5 (27) | 32.5 (13) | 0.0 (0) | 5/5 |
| Other | 20 (12) | 28.6 (6) | 65 (32–77) | 6.5 (2–12) | 37.5 (6) | 31.3 (5) | 31.3 (5) | 3/3 |
| FFI | 23 (5) | 87 (20) | 56 (24–83) | 12.5 (1–97) | 60.9 (14) | 39.1 (9) | 0.0 (0) | |
| GSS | 10 (4) | 22 (2) | 50 (25–70) | 80.5 (54–87) | 30.0 (3) | 60.0 (6) | 10.0 (1) | |
| A117V | 1 | |||||||
| P102L | 8 | |||||||
| P105L | 1 | |||||||
| Insert gTSE | 17 (11) | 52.9 (9) | 63 (36–77) | 11 (3–19) | 46.7 (7) | 33.3 (5) | 20.0 (3) | 1/1 |
Quantitative levels of brain-derived proteins
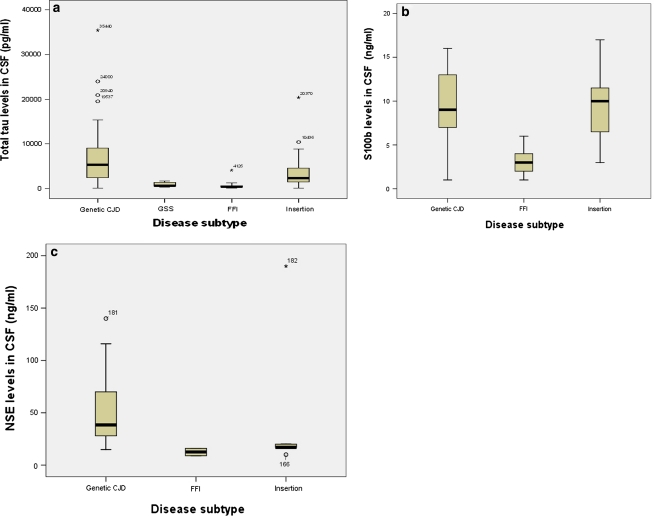
The CSF concentrations of tau protein ranged from 70.0 to 35,440.0 pg/ml (median 6,255.5 pg/ml) in the gCJD group, from 75.0 to 20,370.0 pg/ml (median 2,354.0 pg/ml) in the insert gTSE group, from 296.0 to 1,698.0 pg/ml (median 675.0 pg/ml) in the GSS group, and from 97.0 to 4,126.0 pg/ml (median 464.5 pg/ml) in the FFI group (Fig. 1a). CSF levels of tau protein significantly differ in the four groups of gTSEs (p < 0.001, Kruskal–Wallis test), but did not vary within the gCJD group of patients (p = 0.13).
Fig. 1.
a Box plots of CSF markers: total CSF tau protein levels in gCJD (n = 52), GSS (n = 6), FFI (n = 18), and insert gTSE subjects (n = 17). b Box plots of CSF S100b protein levels in gCJD (n = 15), FFI (n = 13), and insert gTSE subjects (n = 9). c Box plots of CSF levels of NSE in gCJD, FF1 (n = 13), and insert gTSE subjects (n = 9). The line in the middle of the boxes represents the median. The box extends from the 25th to the 75th percentile, bars indicate the range of data distribution. The lines emerging from the box represent the upper and lower adjacent values. Asterisks represent values more extreme than the adjacent values referred to as outliers
The median CSF concentrations of S100b protein were above the cut-off level in all groups but one (FFI), ranging from 1.0 to 16.0 ng/ml in gCJD (median 9.0 ng/ml), from 3.0 to 17.0 ng/ml in insert gTSE (median 11.0 ng/ml), and from 1.0 to 6.0 ng/ml in FFI (median 3.0 ng/ml), Fig. 1b. The only GSS patient tested for S100b protein had a CSF concentration of 23.0 ng/ml. Statistical analysis showed a significant difference among the three groups (p = 0.002, Kruskal–Wallis test).
The CSF concentrations of NSE protein ranged from 15.0 to 140.0 ng/ml (median 38.5 ng/ml) in only 10 gCJD patients where it was tested, from 10.0 to 190.0 ng/ml (median 17.0 ng/ml) in insert gTSE (n = 5), and from 9.0 to 16.0 ng/ml (median 12.5 ng/ml) in FFI (n = 2). The GSS patient tested for NSE protein had a CSF concentration of 13.0 ng/ml. Difference in CSF levels of NSE protein within the four groups of gTSE patients did not reach significance (p = 0.08, Kruskal–Wallis test) (Fig. 1c).
Abnormal cerebrospinal fluid findings in gTSEs
14-3-3
There was a statistically significant difference in the rate of elevated 14-3-3 levels among the four groups of gTSEs, (p < 0.001), see Table 2. A 14-3-3 positive test was present in the majority of gCJD patients, irrespective of the mutation they carried (R208H, V203I, E211Q, T188K, and R148H). Thus, no significant difference in percentage of abnormal tests for 14-3-3 was found between E200K (81%) andV210I (85%) or versus other forms of gCJD (72%). Insert gTSE patients had also a high proportion of positive tests (69%), while the 14-3-3 test was positive in only 10% of GSS and in 13% of FFI cases.
Table 2.
Sensitivity (positive/total) of CSF investigations by forms of gTSE
| 14-3-3 | Tau | S100b | NSE | |
|---|---|---|---|---|
| gCJD | 83.0 (97/117) | 86.4 (38/44) | 87.0 (20/23) | 64.3 (18/28) |
| E200K | 80.3 (49/61) | 75.0 (15/20) | 92.9 (13/14) | 45.4 (5/11) |
| V210I | 85.0 (34/40) | 100 (13/13) | 100 (4/4) | 75.0 (6/8) |
| D178N-Val | 33.3 (1/3) | 100 (1/1) | 0.0 (0/1) | 100.0 (1/1) |
| R208H | 100 (3/3) | 100 (2/2) | 100.0 (1/1) | |
| V203I | 100 (2/2) | |||
| E211Q | 100 (2/2) | 100 (2/2) | 100 (1/1) | 100 (1/1) |
| E196K | 100 (2/2) | 100 (3/3) | 100 (2/2) | 100 (3/3) |
| T188K | 100 (2/2) | 100 (1/1) | 50.0 (1/2) | |
| G114V | 0.0 (0/1) | 0.0 (0/1) | 0.0 (0/1) | 0.0 (0/1) |
| R148H | 100 (1/1) | 100 (1/1) | ||
| Insert gTSE | 68.8 (11/16) | 80.0 (12/15) | 77.8 (7/9) | 50.0 (4/8) |
| GSS | 10.0 (1/10) | 40.0 (2/5) | 50.0 (2/4) | 0.0 (0/2) |
| A117V | 0.0 (0/1) | 0.0 (0/1) | 0.0 (0/1) | |
| P102L | 12.5 (1/8) | 66.7 (2/3) | 100 (2/2) | 0.0 (0/2) |
| P105L | 0.0 (0/1) | 0.0 (0/1) | 0.0 (0/1) | |
| FFI | 13.0 (3/23) | 7.1 (1/14) | 20.0 (2/10) | 0.0 (0/12) |
| Stratification by codon 129 genotype | ||||
| E200K | ||||
| MM | 78.1 (25/32) | |||
| MV | 88.2 (15/17) | |||
| VV | 0.0 (0/0) | |||
| V210I | ||||
| MM | 77.8 (21/27) | p = 0.06 | ||
| MV | 100.0 (13/13) | |||
| VV | 0.0 (0/0) | |||
| Others | ||||
| MM | 100.0 (6/6) | |||
| MV | 80.0 (4/5) | |||
| VV | 50.0 (2/4) | |||
Tau
A high rate of tau levels was found in gCJD and insert gTSE groups, while in GSS only 40% of cases had tau levels above the cut-off level of 1,300 pg. Only a single FFI patient had abnormal tau levels in CSF (Table 2). The levels of tau protein in the CSF were statistically different in the four groups (p < 0.001).
S100b
A similar distribution was observed for S100b values. Elevated S100b levels were found in 87% of gCJD and 77.8% of insert gTSE patients. These values were lower in GSS and FFI patients. The differences in the gTSE groups were statistically significant, (p = 0.002).
NSE
Only 64.3% of gCJD patients and 50% of insert gTSE patients had NSE values above the cut-off level, while NSE values were normal in GSS and FFI patients.
Effect of octapeptide repeats
The number of octapeptide repeats was inversely correlated with the values of tau (Spearman ρ −0.51 (p = 0.036)); patients with high number of repeats (5 × 24) had low tau levels. A similar trend was found for the 14-3-3 test; patients with high number of repeats had low percentage of abnormal tests : 1 × 24 (1/1); 3 × 24 (1/1); 4 × 24 (5/5); 5 × 24 (5/10) Fisher’s p value = 0.22.
Effects of patient characteristics on abnormal findings
The effect of clinical characteristics on percentage of abnormal findings in gCJD patients was the age at disease onset (p = 0.014) and the PRNP codon 129 genotype (VV vs. MV p = 0.043). Sensitivity of 14-3-3 was higher in old patients compared to young ones (25.0% in patients younger than 40 years, 83.9 in patients aged 40–60 years, 86.5% in 60–80 years and 100% in patients older than 80 years).
Regarding the percentage of abnormal findings in the 14-3-3 test in relation to the PRNP codon 129 genotype, this was lower in gCJD patients homozygous for valine than in heterozygous patients (VV vs. MV p = 0.043). However, when we adjusted by type of mutation, this statistical significance was lost. Although the 14-3-3 test performed worse in homozygous for methionine than in heterozygous gCJD patients, this difference was not significant (χ2 test, p = 0.08) (Table 3). In E200K and V210I patients, the 14-3-3 sensitivity was higher in heterozygous (88.2 and 100%) than in methionine homozygous patients (78.1 and 77.8%), but these differences did not reach any significance (Table 2).
Table 3.
Sensitivity of tests in gCJD
| Characteristics | Number of 14-3-3 positive patients/total | Number of tau positive patients/total | Number of S100b positive patients/total | Number of NSE positive patients/total |
|---|---|---|---|---|
| Age at onset | ||||
| <40 | 1/4 | 1/1 | 1/1 | 1/1 |
| 40–60 | 47/57 | 18/19 | 9/11 | 11/15 |
| 60–80 | 46/53 | 18/21 | 9/9 | 6/11 |
| >80 | 2/2 | 1/1 | – | – |
| p-value | 0.016 | 0.76 | 0.34 | 0.47 |
| Codon 129 | ||||
| MM | 52/65 | 22/22 | 7/8 | 9/12 |
| MV | 32/35 | 11/14 | 9/10 | 6/12 |
| VV | 2/4 | 2/2 | 1/1 | 2/2 |
| p value | 0.075 | 0.06 | 0.93 | 0.25 |
| Disease duration | ||||
| > median | 22/26 | 10/11 | 5/5 | 7/9 |
| < median | 48/55 | 21/21 | 9/10 | 8/12 |
| p value | 0.74 | 0.16 | 0.46 | 0.58 |
| Time point of LP during diseasea | ||||
| Early stage | 8/8 | 1/1 | 1/1 | 0/1 |
| Middle stage | 23/26 | 7/7 | 3/3 | 3/3 |
| Advanced stage | 28/34 | 7/7 | 3/3 | 2/2 |
| p value | 0.40 | – | – | 0.17 |
aFor the estimation of the disease stage when the LP was performed we divided the individual disease duration in thirds and calculated the time of lumbar puncture according to the first third of the total duration of the disease (early stage), the second (middle) or the third (advanced stage) of the disease
Disease duration had no influence on 14-3-3 test results (84.6% abnormal findings in patients with disease duration below the median, and 87.3% in patients with disease duration above the median survival time, Table 3). In gCJD patients, there was no significant influence of the disease stage at the time of lumbar puncture in relation to 14-3-3 results (p = 0.4) (100% in te first third of disease duration, 89% in the second third and 82% in the last third, Table 3, as reported in sporadic CJD [21].
Regarding the other CSF tests, there was a lower percentage of abnormal findings for the S100b test in gCJD patients homozygous for methionine than in heterozygous patients, while the opposite was found for tau and NSE (Table 3), though the differences were not statistically significant (p = 0.06 for tau, p = 0.92 for S100b, and p = 0.24 for NSE, χ2 test).
Multivariate analysis, performed on all gTSE patients, included as variables disease duration, age at onset, gender, type of mutation and 129 genotype, and as covariates gender and country of residence. This analysis revealed that the type of mutation is the only variable that significantly influenced 14-3-3 test (p < 0.004). Particularly, in patients carrying the D178N-Met mutation (FFI), the percentage of abnormal findings of 14-3-3 decreased significantly in comparison to gCJD (p < 0.007) and insert gTSE (p < 0.001). Interestingly, regarding the 129 codon polymorphism, 14-3-3 had lower percentage of abnormal tests in patients homozygous for valine than in patients homozygous for methionine or heterozygous, though no significant differences were found (crude p value = 0.5; adjusted p value = 0.76). Table 4.
Table 4.
CSF biomarker in genetic TSE (reports in the literature)
| Author | Journal | Mutation | n | 14-3-3 | tau | nse | S100b |
|---|---|---|---|---|---|---|---|
| E200K | |||||||
| Rosenmann [18] | Neurology 1999 | E200K | 16 | 94% | |||
| Cataldi [4] | Neurol Sci 2000 | E200K | 1 | + | |||
| Kovacs [11] | Hum Gen 2005 | E200K | 62 | 89% | |||
| Sanchez-Valle [23] | Eur J Neurol 2004 | E200K | 5 | 100% | |||
| Ladogana [15] | Neurology 2005 | E200K V210I | 95 | 81% in MM, 95% in MV + in VV | |||
| V210I | |||||||
| Kovacs [11] | Hum Gen 2005 | V210I | 35 | 100% | |||
| Huang [7] | Arq neuropsiquiatr 2001 | V210I | 1 | – | |||
| D178N | |||||||
| Zerr [30] | Neurology 1998 | D178N-129M | 8 | – | |||
| Sanchez-Valle [23] | Eur J Neurol 2004 | D178N-129M | 2 | 0% | |||
| Kovacs [11] | Hum Gen 2005 | D178N 129V | 22 | 10% | |||
| Rosenmann [18] | Neurology 1999 | D178N 129V | 1 | + | |||
| Zarranz [29] | JNNP 2005 | D178N | 4 | 0% 50% |
|||
| 129MM | 2 | ||||||
| D178N +MV | |||||||
| Rosenmann [19] | Acta Neurol Scand 1998 | D178N-129V | 2 | + | |||
| P102L | |||||||
| Imaiso [8] | Rinsho Shinkeigaku 1998 | P102L | 1 | + | Elevated | ||
| Kovacs [11] | Hum Gen 2005 | P102L | 7 | 57% | |||
| Others | |||||||
| Krebs [13] | Neurogenetics 2005 | R148H | 1 | + | |||
| Iwaski [9] | Rinsho Shinkeigaku. 1999 | V180I | 1 | Not done | 30 | ||
| Collins [5] | Arch Neurol 2000 | T188A | 1 | + | |||
| Kotta [10] | BMC Infect dis 2006 | T193I | 1 | + | |||
| Tumani [25] | DMW 2002 | E196K | 1 | + | Elevated | Elevated | |
| Capellari [3] | Neurology 2005 | R208H | 1 | + | |||
| Roeber [17] | Acta Neuropathol 2005 | R208H | 1 | + | |||
| Ladogana [14] | Am J Med Gen 2001 | E211Q | 1 | + | |||
| Sanchez-Vallez [22] | JNNP 2008 | 9OPRI | 1 | − | |||
Multivariate analysis did not revealed any variables that influenced tau, NSE or S100b levels in gTSE patients.
In all gTSE there was a statistically significant negative correlation between tau levels and disease duration (Spearman ρ2 between duration and tau levels = −0.52 (p < 0.001). However, when the data were stratified by diagnostic groups, numbers become too low and were not significant (only borderline for gCJD: r2 = −0.32, p = 0.057).
Discussion
Data on brain-derived proteins in the CSF of patients with genetic TSE are limited and conflicting results have been reported, mostly because they are frequently based on single case observations (see Table 4). In these reports, sensitivity of biochemical markers in CSF is reported to be lower than in sporadic CJD and this was explained in terms of prolonged disease duration and relatively slow disease progression. Because of the limited numbers of patients, no detailed analysis on this topic is available.
In this study we provide data on four brain-derived proteins in a cohort of patients with various forms of genetic TSE. We found firm evidence for elevated concentrations of 14-3-3, tau, S100b and NSE in the CSF of patients with genetic CJD, but not in FFI or GSS patients. Of interest, the median concentrations for tau, S100b and NSE were similar to those detected in sporadic CJD in other studies [1, 16, 20]. In our previous study on sporadic CJD, we reported median tau levels in the range of 6,000 pg/ml, which is not significantly different from what we found in gCJD patients [21]. These results are concordant with the observation that some gCJD might present clinical similarities with sporadic CJD [11, 15]. Indeed, these cases are often misclassified as sporadic CJD if family history and genetic testing are not done.
The rate of elevated levels of 14-3-3, tau, NSE and S100b in genetic CJD was comparable to that observed in sporadic CJD [3, 20, 26, 27]. In other forms of gTSE, such as FFI and GSS, these tests were consistently negative. Although levels of tau in FFI and GSS patients were lower than the cut-off levels given for CJD, they were still elevated if compared to non-demented controls [24].
The crude analyses of disease modifying factors of the 14-3-3 test in gCJD revealed that age at onset and PRNP codon 129 genotype influenced sensitivity. 14-3-3 test sensitivity was lower in patients with disease onset before 40 years. These data parallel the results performed on sporadic CJD [20]. However, while in the multivariate analysis age remained as an independent variable in sporadic CJD, in gCJD it did not. Interestingly, in gCJD the PRNP codon 129 genotype influences 14-3-3 sensitivity in a different way with respect to what has been observed in sporadic CJD. Valine homozygous gCJD patients had a lower sensitivity in the 14-3-3 test than heterozygous patients. Though there are too few patients to draw any definite conclusions, a possible explanation might be that the PRNP mutations coupled with the valine alleles (R208H, D178N, E196K) confer low sensitivity to 14-3-3.
The biological significance of brain-derived proteins in the CSF of patients with TSEs remains to be determined. It is generally assumed that the release of 14-3-3, tau and NSE proteins in the CSF is a consequence of leakage into the CSF following rapid neuronal damage. Recently, a systematic analysis of brain-derived proteins in CSF and neuropathological lesions has shown that the levels of these proteins are the consequence of both the degree of neuronal damage and the localization of the most affected areas [2]. For example NSE levels correlated with damage of subcortical areas (such as the thalamus) and tau protein levels correlated with the degree of spongiform changes in the frontal cortex. Our findings on inverse correlation of tau levels and the number of octapeptide repeats are of interest, since the number of repeats has been correlated to the type of cerebellar PrPSc deposits [28].
In conclusion, the validity of the biomarkers varied among the different forms of gTSEs. Sensitivity of biomarkers was high in those forms, which are clinically more similar to sporadic CJD, such as genetic CJD and insert gTSEs.
Acknowledgments
We thank all physicians in the participating countries for sending us the cerebrospinal fluid and blood samples and for providing pertinent clinical and neuropathological data on those patients. We thank Dr. Gabor Kovacs for his help and advice. The collaborative study was funded by grants from the European Commission (EC) (QLG3-CT-2002-81606). The national studies were supported in Greece by the Greek Ministry of Health, through KEEL (Center for Control of Infectious Diseases), in Italy by the Ministry of Health and the Istituto Superiore di Sanità, in the Netherlands, in Slovakia by Ministry of Health and European Commission (SEEC-CJD project), in Spain a grant from the Ministerio de Ciencia y Tecnología (MCyT EET 2001/2216) and Spanish Ministerio de Sanidad y Consumo, grant number DGVI 1312/04-1, in Switzerland by Bundesamt für Gesundheit, Bern (NRPE-BAG contracts n° 03.001297and n° 04.002363), in United Kingdom by the Department of Health and the Scottish Home Office Department of Health and in Germany by the Bundesministerium für Gesundheit und Soziale Sicherung (BMGS) (GZ: 325-4471-02/15) and by the Bundesministerium für Bildung und Forschung (BMBF) (KZ: 0312720 to I.Z).
Conflict of interest statement The authors report no conflicts of interest.
Contributor Information
Anna Ladogana, Email: Ladogana@iss.it.
Pascual Sanchez-Juan, Email: ifimav.uapoyo@fmdv.org.
Eva Mitrová, Email: eva.mitrova@szu.sk.
Alison Green, Email: Alison.Green@ed.ac.uk.
Natividad Cuadrado-Corrales, Email: ncuadrado@isciii.es.
Raquel Sánchez-Valle, Email: 31799rsd@comb.es.
Silvia Koscova, Email: silvia.koscova@szu.sk.
Adriano Aguzzi, Email: adriano.aguzzi@usz.ch.
Theodoros Sklaviadis, Email: sklaviad@pharm.auth.gr.
Jerzy Kulczycki, Email: jkulczycki@data.pl.
Joanna Gawinecka, Email: epicjd@med.uni-goettingen.de.
Albert Saiz, Email: asaiz@clinic.ub.es.
Miguel Calero, Email: mcalero@isciii.es.
Cornelia M. van Duijn, Email: c.vanduijn@erasmusmc.nl
Maurizio Pocchiari, Email: Maurizio.pocchiari@iss.it.
Inga Zerr, Phone: +49-551-396636, FAX: +49-551-397020, Email: epicjd@med.uni-goettingen.de.
References
- 1.Bahl JM, Heegaard NH, Falkenhorst G, Laursen H, Hogenhaven H, Molbak K, Jespersgaard C, Hougs L, Waldemar G, Johannsen P, Christiansen M (2008) The diagnostic efficiency of biomarkers in sporadic Creutzfeldt-Jakob disease compared to Alzheimer’s disease. Neurobiol Aging. doi:101016/j.neurobiolaging.2008.01.013 [DOI] [PubMed]
- 2.Boesenberg-Grosse C, Schulz-Schaeffer WJ, Bodemer M, Ciesielczyk B, Kretzschmar HA, Green AJ, Zerr I. Brain-derived proteins in the CSF, do they correlate with brain pathology in CJD? BMC Neurol. 2006;6:35. doi: 10.1186/1471-2377-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capellari S, Cardone F, Notari S, Schininà ME, Maras B, Sità D, Baruzzi A, Pocchiari M, Parchi P. Creutzfeldt-Jakob disease associated with the R208H mutation in the prion protein gene. Neurology. 2005;64:905–907. doi: 10.1212/01.WNL.0000152837.82388.DE. [DOI] [PubMed] [Google Scholar]
- 4.Cataldi ML, Restivo O, Reggio E, Restivo DA, Reggio A. Deafness: an unusual onset of genetic Creutzfeldt-Jakob disease. Neurol Sci. 2000;21:53–55. doi: 10.1007/s100720070119. [DOI] [PubMed] [Google Scholar]
- 5.Collins S, Boyd A, Fletcher A, Byron K, Harper C, McLean CA, Masters CL. Novel prion protein gene mutation in an octogenarian with Creutzfeldt-Jakob disease. Arch Neurol. 2000;57:1058–1063. doi: 10.1001/archneur.57.7.1058. [DOI] [PubMed] [Google Scholar]
- 6.Cuadrado-Corrales N, Jiménez-Huete A, Albo C, Hortiguela R, Vega L, Cerrato L, Sierra-Moros M, Rábano A, Pedro-Cuesta J, Calero M. Impact of the clinical context on the 14-3-3 test for the diagnosis of sporadic CJD. BMC Neurol. 2006;6:25. doi: 10.1186/1471-2377-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang N, Marie SK, Kok F, Nitrini R. Familial Creutzfeldt-Jakob disease associated with a point mutation at codon 210 of the prion protein gene. Arq Neuropsiquiatr. 2001;59:932–935. doi: 10.1590/s0004-282x2001000600017. [DOI] [PubMed] [Google Scholar]
- 8.Imaiso Y, Mitsuo K. Gerstmann-Sträussler-Scheinker syndrome with a Pro102Leu mutation in the prion protein gene and atypical MRI findings, hyperthermia, tachycardia, and hyperhidrosis. Rinsho Shinkeigaku. 1998;38:920–925. [PubMed] [Google Scholar]
- 9.Iwaski Y, Sone M, Kato T, Yoshida E, Indo T, Yoshida M, Hashizume Y, Yamada M. Clinicopathological characteristics of Creutzfeldt-Jakob disease with a PrP V180I mutation and M129V polymorphism on different alleles. Rinsho Shinkeigaku. 1999;39:800–806. [PubMed] [Google Scholar]
- 10.Kotta K, Paspaltsis I, Bostantjopoulou S, Latsoudis H, Plaitakis A, Kazis D, Collinge J, Sklaviadis T. Novel mutation of the PRNP gene of a clinical CJD case. BMC Infect Dis. 2006;6:169. doi: 10.1186/1471-2334-6-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs GG, Puopolo M, Ladogana A, Pocchiari M, Budka H, Duijn C, Collins S, Boyd A, Guilivi A, Coulthart M, Delasnerie-Laupretre N, Brandel JP, Zerr I, Kretzschmar H, Pedro-Cuesta J, Calero-Lara M, Glatzel M, Aguzzi A, Bishop M, Knight R, Belay G, Will R, Mitrova E. Genetic prion disease: the EUROCJD experience. Hum Genet. 2005;118:166–174. doi: 10.1007/s00439-005-0020-1. [DOI] [PubMed] [Google Scholar]
- 12.Kovács GG, Trabattoni G, Hainfellner JA, Ironside JW, Knight RS, Budka H. Mutations of the prion protein gene. Phenotypic spectrum. J Neurol. 2002;249:1567–1582. doi: 10.1007/s00415-002-0896-9. [DOI] [PubMed] [Google Scholar]
- 13.Krebs B, Lederer R-M, Windl O, Grasbon-Frodl E-M, Zerr I, Kretzschmar HA. Creutzfeldt-Jakob disease associated with an R148H mutation of the prion protein gene. Neurogenetics. 2005;6:97–100. doi: 10.1007/s10048-004-0208-x. [DOI] [PubMed] [Google Scholar]
- 14.Ladogana A, Almonti S, Petraroli R, Giaccaglini E, Ciarmatori C, Liu QG, Bevivino S, Squitieri F, Pocchiari M. Mutation of the PRNP gene at codon 211 in familial Creutzfeldt-Jakob disease. Am J Med Genet. 2001;103:133–137. doi: 10.1002/ajmg.1511. [DOI] [PubMed] [Google Scholar]
- 15.Ladogana A, Puopolo M, Poleggi A, Almonti S, Mellina V, Equestre M, Pocchiari M. High incidence of genetic human transmissible spongiform encephalopathies in Italy. Neurology. 2005;64:1592–1597. doi: 10.1212/01.WNL.0000160118.26865.11. [DOI] [PubMed] [Google Scholar]
- 16.Otto M, Wiltfang J, Cepek L, Neumann M, Mollenhauer B, Steinacker P, Ciesielczyk B, Schulz-Schaeffer W, Kretzschmar HA, Poser S. Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2002;58:192–197. doi: 10.1212/wnl.58.2.192. [DOI] [PubMed] [Google Scholar]
- 17.Roeber S, Krebs B, Neumann M, Windl O, Zerr I, Grasbon-Frodl EM, Kretzschmar HA. Creutzfeldt-Jakob disease in a patient with an R208H mutation of the prion protein gene (PRNP) and a 17-kDa prion protein fragment. Acta Neuropathol. 2005;109:443–448. doi: 10.1007/s00401-004-0978-0. [DOI] [PubMed] [Google Scholar]
- 18.Rosenmann H, Kahana E, Korczyn AD, Kahana I, Chapman J, Gabizon R. Preliminary evidence for anticipation in genetic E200K Creutzfeldt-Jakob disease. Neurology. 1999;53:1328–1329. doi: 10.1212/wnl.53.6.1328. [DOI] [PubMed] [Google Scholar]
- 19.Rosenmann H, Vardi J, Finkelstein Y, Chapman J, Gabizon R. Identification in Israel of 2 Jewish Creutzfeldt-Jakob disease patients with a 178 mutation at their PrP gene. Acta Neurol Scand. 1998;97:184–187. doi: 10.1111/j.1600-0404.1998.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Sanchez-Valle R, Mitrova E, Stoeck K, Sklaviadis T, Kulczycki J, Hess K, Bodemer M, Slivarichova D, Saiz A, Calero M, Ingrosso L, Knight R, Janssens C, Duijn C, Zerr I. CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology. 2006;67:637–643. doi: 10.1212/01.wnl.0000230159.67128.00. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Juan P, Sanchez-Valle R, Green A, Ladogana A, Cuadrado-Corrales N, Mitrova E, Stoeck K, Sklaviadis T, Kulczycki J, Hess K, Krasnianski A, Equestre M, Slivarichova D, Saiz A, Calero M, Pocchiari M, Knight R, Dujin CM, Zerr I. Influence of timing on CSF tests value for Creutzfeldt-Jakob disease diagnosis. J Neurol. 2007;254:901–906. doi: 10.1007/s00415-006-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Valle R, Aróstegui JI, Jague J, Rami R, Lladó A, Molinuevo JL. First demonstrated de novo insertion in the prion protein gene in a young patient with dementia. J Neurol Neurosurg Psychiatry. 2008;79(7):845–846. doi: 10.1136/jnnp.2007.137463. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Valle R, Nos C, Yague J, Graus F, Dominguez A, Saiz A. Clinical and genetic features of human prion diseases in Catalonia: 1993–2002. Eur J Neurol. 2004;11:649–655. doi: 10.1111/j.1468-1331.2004.00967.x. [DOI] [PubMed] [Google Scholar]
- 24.Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM. Decreased ß-amyloid 1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 25.Tumani H, Windl O, Kretzschmar HA, Ludolph AC. Clinically atypical CJD: diagnostic relevance of cerebrospinal fluid markers and molecular genetic analysis? Dtsch Med Wochenschr. 2002;127:318–320. doi: 10.1055/s-2002-20148. [DOI] [PubMed] [Google Scholar]
- 26.Everbroeck B, Boons J, Cras P. Cerebrospinal fluid biomarkers in Creutzfeldt-Jakob disease. Clin Neurol Neurosurg. 2005;107:355–360. doi: 10.1016/j.clineuro.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Everbroeck B, Quoilin S, Boons J, Martin JJ, Cras P. A prospective study of CSF markers in 250 patients with possible Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2003;74:1210–1214. doi: 10.1136/jnnp.74.9.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vital C, Gray F, Vital A, Parchi P, Capellari S, Petersen RB, Ferrer X, Jarnier D, Julien J, Gambetti P. Prion encephalopathy with insertion of octapeptide repeats: the number of repeats determines the type of cerebellar deposits. Neuropathol Appl Neurobiol. 1998;24:125–130. doi: 10.1046/j.1365-2990.1998.00098.x. [DOI] [PubMed] [Google Scholar]
- 29.Zarranz JJ, Digon A, Atares B, Rodriguez-Martinez AB, Arce A, Carrera N, Fernandez-Manchola I, Fernandez-Martinez M, Fernandez-Maiztegui C, Forcadas I, Galdos L, Gomez-Esteban JC, Ibanez A, Lezcano E, Lopez de Munain A, Marti-Masso JF, Mendibe MM, Urtasun M, Uterga JM, Saracibar N, Velasco F, Pancorbo MM. Phenotypic variability in familial prion diseases due to the D178N mutation. J Neurol Neurosurg Psychiatry. 2005;76:1491–1496. doi: 10.1136/jnnp.2004.056606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zerr I, Giese A, Windl O, Kropp S, Schulz-Schaeffer W, Riedemann C, Skworc K, Bodemer M, Kretzschmar HA, Poser S. Phenotypic variability in fatal familial insomnia (D178N–129M) genotype. Neurology. 1998;51:1398–1405. doi: 10.1212/wnl.51.5.1398. [DOI] [PubMed] [Google Scholar]
- 31.Zerr I, Pocchiari M, Collins S, Brandel JP, Pedro Cuesta J, Knight RSG, Bernheimer H, Cardone F, Delasnerie-Lauprêtre N, Cuadrado Corrales N, Ladogana A, Fletcher A, Bodemer M, Awan T, Ruiz Bremón A, Budka H, Laplanche JL, Will RG, Poser S. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology. 2000;55:811–815. doi: 10.1212/wnl.55.6.811. [DOI] [PubMed] [Google Scholar]