Abstract
Bone marrow–derived mesodermal stem cells may differentiate toward several lines and are easily cultured in vitro. Some putative progenitors of these cells have been described in both humans and mice. Here, we describe a new mesodermal progenitor population [mesodermal progenitors cells (MPCs)] able to differentiate into mesenchymal cells upon appropriate culture conditions. When cultured in presence of autologous serum, these cells are strongly adherent to plastic, resistant to trypsin detachment, and resting. Mesodermal progenitor cells may be pulsed to proliferate and differentiate by substituting autologous serum for human cord blood serum or fetal calf serum. By these methods cells proliferate and differentiate toward mesenchymal cells and thus may further differentiate into osteoblats, chondrocytes, or adipocytes. Moreover MPCs are capable to differentiate in endothelial cells (ECs) showing characteristics similar to microvessel endothelium cells. Mesodermal progenitors cells have a defined phenotype and carry embryonic markers not present in mesenchymal cells. Moreover MPCs strongly express aldehyde dehydrogenase activity, usually present in hematopoietic precursors but absent in mesenchymal cells. When these progenitors are pulsed to differentiate, they lose these markers and acquire the mesenchymal ones. Interestingly, mesenchymal cells may not be induced to back differentiate into MPCs. Our results demonstrate the adult serum role in maintaining pluripotent mesodermal precursors and allow isolation of these cells. After purification, MPCs may be pulsed to proliferate in a very large scale and then induced to differentiate, thus possibly allowing their use in regenerative medicine.
Introduction
Stem cells are ideal candidates for regenerative medicine, tissue engineering, and cell replacement therapies because of their ability to commit to multiple cell lineages. Stem cells can be retrieved from adult tissues, and the bone marrow represents one of the richest sources for these cells. The stem cells derived from the bone marrow, as well as from immature tissues such as umbilical cord blood or the placenta, or from fetal tissues such as the liver and pancreas, are in general indicated as “adult stem cells.” It is well established that the bone marrow contains two main types of stem cells: hematopoietic cells and the less-differentiated stromal mesenchymal cells (MSCs). Mesenchymal cells are able to differentiate in vivo and in vitro into a variety of adult mesenchymal tissues, such as bone, cartilage, adipose tissue or muscle [1–4]. Therefore, MSCs represent a highly promising cellular population and regenerative applications.
Mesenchymal cells are poorly characterized due to the absence of manifest physical, phenotypic, and functional properties in cultured, heterogeneous cell populations [5,6]. Parameters such as origin, extraction procedures, and culturing conditions make it difficult to establish whether a particular cell type results from the selection imposed by these conditions or is induced by the experimental setting [7].
Recent evidence suggests that MSCs are more heterogeneous than previously described, due to the presence of multipotent mesenchymal stromal cells capable of differentiating into various mesodermal cell lineages, along with pluripotent cells with embryonic-like characteristics. This latter cell type may further differentiate into cells of the mesoderm lineage as well as into endoderm and neuroectoderm lineages (such as the MIAMI, VSEL, MAPCs) [8–10]. Little information is available on the hierarchical relationship between MSC subpopulations and their specific physiological roles, but the presence of embryonic features in adult mesenchymal cells offers potential for regenerative medicine, particularly in view of their easy access along with a reduced risk of tumorigenicity compared to embryonic stem cells (ESCs).
As with all stem cells, isolation, expansion, and subsequent differentiation of adult multipotent mesenchymal cells require strictly defined culture conditions for selection. We have used autologous human serum to grow and expand bone marrow–derived cells under conditions that minimize the risk of transmitting infectious diseases or inducing immunological reactions. Under these culture conditions, we have been able to observe two different mesenchymal cell types: the usual small, spindle-shaped cells and other, unusually round and large cells.
In this report we describe these peculiar large cells, that show embryonic markers, high aldehyde dehydrogenase activity (ALDH) activity, and the potential to differentiate into several mesodermal lineages including mesenchymal and endothelial cells. We identify these cells as mesodermal progenitor cells (MPCs).
Materials and Methods
Isolation
Bone marrow aspirates were obtained under local anesthesia from orthopedic patients, undergoing routine total hip replacement surgery, after written consent. A total of 20 mL of marrow was drawn into two 20-mL syringes containing 2500U of heparin (Roche, Basel, Switzerland) to prevent clotting and promptly shipped to the cell culture facility. After dilution 1:4 with Dulbecco's phosphate-buffered saline (D-PBS; Invitrogen, Eugene, OR) bone marrow was carefully layered over Lymphoprep (Axis-Shield, Oslo, Norway) and centrifuged at 400g for 20 min. Bone marrow mononuclear cells were collected at the media–Lymphoprep interface, washed twice in D-PBS, and plated in T-150 or T-75 culture flasks (Corning, New York, NY) containing low-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM l-glutamine, 100 μg/mL gentamicin, and 5 or 10% fetal bovine serum (FBS), human cord serum (hCS), or human autologous serum (hAS) at a cell density of 2 × 105/cm2.
Medium was changed after 48 h to remove nonadherent cells and then two times/week. At day 15, cells from FBS and hAS culture were detached using 0.25% trypsin/EDTA acid (Invitrogen) at 37°C from 1 to 3 min. Flasks were washed extensively (four to five times) in D-PBS and checked for undetached cells under inverted microscope.
We estimated the presence of MPC cells in the cultures derived from the different samples scoring them as: negative (no MPCs present), positive (1–10 MPCs/microscopical field) and highly positive (>10 cells MPCs/microscopical field).
Mesenchymal cells culture from undetached MPCs
Flasks from hAS cultures that presented undetached large cells were refilled with an appropriate amount of DMEM culture medium supplemented with 10% FBS or hCS and further cultured for 15 days. After that MSCs were detached by trypsin/EDTA digestion, representing the first generation (T1 culture). The flasks were then refilled with DMEM/FBS 10% and the procedure was repeated until the cells were able to reach a new confluence to obtain T2 culture, T3 culture, T4 culture, and so on. Further flasks from hAS were cultured for 2 months in autologous serum.
Cells from FBS cultures and from Tx (T1, T2, T3, etc.) cultures were characterized by immunophenotyping incubating with fluorescein isothiocyanate FITC-CD105, PE-CD166, PE-CD34, PE-CD133, PE-Cy5-CD90, PerCP-CD45 (all antibodies from Becton Dickinson) and their proliferative, clonogenic and differentiative potential were evaluated by population doubling assay, colony-forming units fibroblastoids (CFU-F) assay and osteogenic, adipogenic, and chondrogenic differentiation assays.
Morphological and immunological characterization of MPCs
Bone marrow mononuclear cells were also cultured as described above in Permanox double-chamber slides (Nunc, Rochester, NY) to perform immunofluorescence assays and scanning electron microscopy.
Scanning electron microscopy
Slides from FBS and hAS cultures were fixed in 4% neutral buffered formalin diluted in PBS 0.1 M pH 7.2 overnight at 48°C, dehydrated with a series of graded ethanol washes and critical-point dried with liquid CO2. Fixed slides were sputter-coated with gold, and examined with a scanning electron microscope (SEM) JSM-5200 (JEOL Ltd, Tokyo, Japan).
Tri-color immunofluorescence
Slides from FBS and hAS were fixed for 15 min in periodate-lysine-paraformaldheyde (PLP) and permeabilized by Triton-X100 0.05% 30 min then processed for either direct or indirect immunofluorescence staining by Goat antimouse SFX kit (Invitrogen) according to manufacturer's instructions using Alexa Fluor 488 anti-mouse IgG and Alexa Fluor 555 antimouse IgM as secondary antibodies. Primary monoclonal antibodies against following specificity were used: Oct-3/4 from Santa Cruz Biotechnology (Santa Cruz, CA); Nanog, Ki-67 Alexa Fluor 488-conjugated, CD34 FITC-conjugated, CD45 FITC-conjugated, CD271 (LNGFR), CD146 from Becton Dickinson (San Jose, CA); SSEA-4, SSEA-1 Alexa Fluor 488-conjugated, and TRA-1–81 from Millipore (Billerica, MA); vascular endothelial growth factor receptors 2 (VEGFR-2) from R&D Systems (Minneapolis, MN) and CD133 from Miltenyi (Bergisch Gadbach, Germany). Slides were counterstained with anti-CD90 PE-conjugated (Becton Dickinson) for 1 h at 4°C or with Phalloidin Alexa Fluor 555-conjugated (Invitrogen) for 30 min, and mounted in Prolong Gold antifade reagent with DAPI (Invitrogen). Slides were inspected using standard fluorescence DMR Leica microscope (Leica, Wetzlar, Germany) and by confocal microscopy using inverted Eclipse TE2000-U microscope equipped with D-Eclipse C1-si spectral optical system (Nikon, Tokyo, Japan).
Detaching by TrypLE Select
Attempting to detach MPCs, digestion by trypsin-substitute reagent was performed using TrypLE Select (Invitrogen) according to manufacturer's instructions. Cells detached from FBS and hAS cultures were analyzed cytofluorimetrically for the same specificity used for MSCs described above. Suspended cells were also cytocentrifuged on glass slides and stained with May Grunwald-Giemsa to evaluate morphology.
RT-PCR for OCT3/4 and NANOG
Primary cultures were first detached by trypsin digestion to harvest MSCs but not MPCs that were detached subsequently by TrypLE Select. Total RNA was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse transcripted using iScript cDNA synthesis kit (Biorad, Hercules, CA). Polymerase chain reaction (PCR) was performed by Taq DNA Polymerase kit from Invitrogen using primer pairs from Human Pluripotent Stem Cell Assessment kit (R&D Systems, Minneapolis, MN) according to manufacturer's instructions.
Cytofluorimetric evaluations
Mesodermal progenitor cells were partially detached by incubating them in TrypLE Select proteases (Invitrogen). Approximately 2.5 × 105 cells were washed with D-PBS supplemented with 0.1% NaN3 (Sigma) and 0.05% of bovine serum albumin (Sigma, St. Louis, MO) and incubated with direct fluorochrome-conjugated monoclonal antibodies for 30 min at 4°C, according to manufacturer's instructions. After incubation cells were washed in D-PBS/NaN3/BSA and 30,000 events were acquired by FACScan cytometer (BectonDickinson, San Jose, CA) and analyzed by CellQuest analysis software. Cells were incubated with CD105 FITC-conjugated, CD166 PE-conjugated, CD34 PE-conjugated, CD133 PE-conjugated, CD90 PE-Cy5-conjugated, CD45 PerCP-conjugated (all antibodies from BectonDickinson). Aldehyde dehydrogenase activity was measured by the conversion of bodipy–aminoaceteldehyde diethyl acetal into the fluorescent product bodipy–aminoacetate using Aldefluor reagents as suggested by manufacturer (Stem Cell, Vancouver, Canada).
Mesodermal progenitor cell differentiation to endothelial cell and angiogenesis response
Differentiation of MPCs to endothelial cells (ECs) was performed with minor modification of Levenberg et al. [11]. Mesodermal progenitor cells were grown in culture media (EndoCult liquid medium, Stem Cell Technologies, Vancouver, Canada): 2.5 × 104 cells were plated in six-well fibromedium-coated plates in triplicate and incubated for 2 days at 37°C, 5% CO2. Media were changed into EGM-2 media (Clonetic, San Diego, CA) and 50 ng/mL of VEGF (R&D system) for 5 days culturing. The cells were maintained on EGM-2 medium for one passage. Antibody staining, Dil-AC-LDL uptake assay, matrigel assay, and reverse transcriptase (RT)-PCR were used to confirm EC phenotypes, CD146, VE-Cadherin, eNOS and HO-1.
In vitro capillary tube–like formation
Assessment of in vitro capillary tube–like formation utilized growth factor–reduced basement membrane Matrigel matrix (BD Biosciences-Discovery Labware). The Matrigel was thawed overnight at 4°C and mixed to homogeneity using cooled pipette tips. Aliquots of Matrigel (250 μL) were distributed as a thin layer on the bottom of 12-well cell culture plates and left for polymerization at 37°C for 30 min. Quiescent MPCs were resuspended in DMEM containing 0.5% FBS to give a final cell concentration of 5 × 105 cells/mL, plated onto the Matrigel-coated surface, and incubated for 1 h in a 37°C humidified incubator. The medium was aspirated to remove non attached cells and substituted by fresh medium containing either vehicle or the test compounds. Tube-like structure formation was examined 8, 16, 24, and 48 h after treatment. Cultures were photographed, and the length of the tube-like structures was quantified using Image Pro-Express Software (Cyber Media).
Statistical analysis
The data are presented as mean ± SE. Statistical significance (P < 0.05) between the experimental groups was determined by the Fisher method of analysis for multiple comparisons. For comparison between treatment groups, the null hypothesis was tested by a single factor analysis of variance (ANOVA) for multiple groups or unpaired t-test for two groups.
Results
Morphological characterization
Human bone marrow–derived mononuclear cells were plated in culture medium used for mesenchymal stromal cells expansion supplemented with different sera: hAS, FBS, or hCS. Under all these conditions, adherence time, growth rates, and cell vitality remained unchanged.
The use of different sera led to the appearance of mono-layers containing morphologically similar cells. These cells are MSCs and may differentiate, as expected, into osteoblats, chondrocytes, or adipocytes (data not shown). However, only in the presence of hAS, large round cells, with characteristic thin membranous lamellipodia are seen.
After removing adherent cells by trypsin, the round cells remained attached on the bottom and, it was not possible to detach them by any conventional method (screeching, washing by cold saline or calcium- and magnesium-free PBS, long time incubation in trypsin). Conversely, by adding TrypLE Select cells were partially detached.
Under phase–contrast microscopy, these cells appear as round cells of ∼30–50 μm in diameter, with a highly refractive central core region rounded by fringed periphery (Fig. 1A). The cells could be detected after culturing mesenchymal cells in the presence of either 5% or 10% hAS, but were not detected after culturing with comparable amounts of either FBS or hCS. Cells remaining attached to the bottom after trypsin treatment did not proliferate nor die when cultured for extended periods in hAS-containing medium.
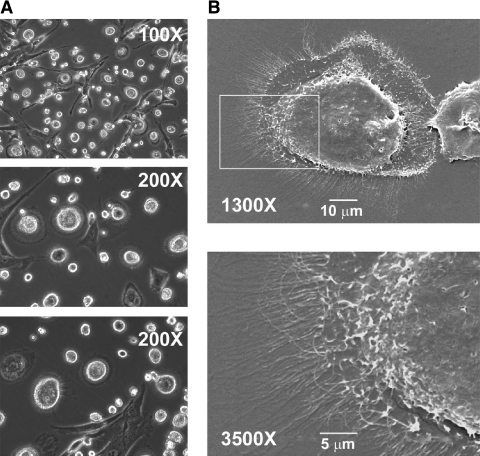
FIG. 1.
Morphological characterization of cells from autologous serum cultures. Culturing bone marrow mononuclear cells in autologous serum for ∼15 days allows the identification of two major adherent cell populations. The use of contrast–phase microscopy (A) reveals the usual MSCs fibroblastoid population and, in addition, rounded cells, strongly rifrangent in the core region, with a fried egg appearance. Scanning electron microscopy (B) reveals that these cells are thick in the core region and surrounded by a complex of microvillus structures.
Scanning electron microscope morphology
On SEM, MPCs appear as convex cells with a centrally located thick region framed by a thin and flat area (Fig. 1B). The cell periphery is uniformly characterized by the presence of numerous slender projections. Altogether, they give the impression of forming a flat and continuous lamellipodium with no preferential expansion. It is likely that these projections may be internally sustained by such elements of the cytoskeleton as microtubules (Fig. 1B). Whether they are restricted to the cell contour for substrate adhesion or also protruding upward to sustain other functions remains to be clarified.
Frequency of MPC isolation
Cultures derived from bone marrow of 39 donors, 16 females and 23 males, were scored to estimate the frequency by which MPCs could be isolated from bone marrow–derived mononuclear cells. The median age of the donor patients was 75 years (range 32–86). Three samples did not give rise to any cell type when cultured in vitro. Of the 36 samples from which cells were successfully isolated, 25 were positive for MPCs while 11 produced MSCs only. Among the 25 positive samples, 5 were highly positive (>10 MPCs/microscopical field), while 20 showed an intermediate number of MPCs (1–10 MPCs/microscopical field) (Table 1).On these basis we may suggest that ratio of MPCs to mononuclear bone marrow–derived cells approximately range between 1:103 and 1:104.
Table 1.
Frequency of MPC Isolation
| MPCs | Freq. | %Freq. | Sex | Age | Median age |
|---|---|---|---|---|---|
| (−) | 11 | 30.5 | 5 F/6 M | 32–90 | 59 |
| (+) | 20 | 55.5 | 7 F/13 M | 42–86 | 65.5 |
| (++) | 5 | 13.9 | 1 F/4 M | 38–80 | 57 |
| Total | 36 | 100.0 | 13 F/23 M | 32–90 | 64 |
Bone marrow samples were classified for the amount of mesodermal progenitors cells (MPCs) in the culture. Three groups were identified and characterized for the frequency of MPC isolation: no MPCs (−), 1–10 MPCs/microscopical field (+), and >10 MPCs/microscopical field (++). Gender, range of age, and median age of donors are reported. No significant differences were identified for gender of donors or median age of all subgroups.
Mesenchymal cell generation from MPCs
After trypsinization, residual recultured MPCs gave rise to different cell types depending on the serum supplementation. In the presence of either FBS or hCS, a new generation of typical MSCs along with residual MPCs were obtained. In contrast, MPCs maintained in a medium supplemented with hAS did not proliferate, but persisted in a quiescent and fully vital status at least for up to 2 months of in vitro culturing. Mesodermal progenitor cells remained viable and were able to differentiate into mesenchymal cells when cultured in FBS or hCS.
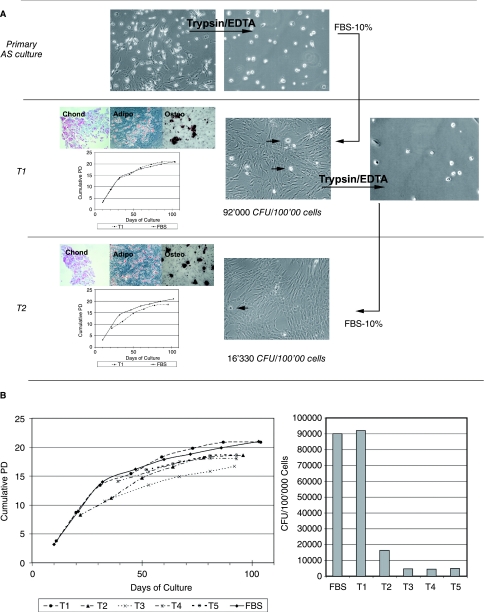
In the presence of FBS, cell generations derived from undetached MPCs (T1, T2, T3, etc.) showed typical morphological and phenotypical features of MSCs (spindle shape, CD90, CD105 and CD166 positive, CD45 and CD133 negative). The first MSCs generation (T1) derived from MPCs demonstrates the same proliferative potential as MSCs generated by bone marrow cells when cultured in FBS-supplemented medium. After removing MSCs, a reduction in density of MPCs was detected and these cells were again able to differentiate to MSCs. The remaining MPCs, after a new trypsinization cycle, were able to differentiate to MSCs until MPCs remained adherent to the flask. Interestingly, the derived MSCs maintained their proliferative capability unmodified compared to the first detached cells but their clonogenic potential decrease (Fig. 2B). In addition, they proved to be capable of differentiating into mesodermal lineages (adipocytes, condrocytes, and osteoblasts) (Fig. 2A). However, MPCs are not able to directly differentiate into the mesenchymal lineage (osteogenic, chondrogenic, adipogenic) even in the presence of appropriate stimuli. Interestingly replated MSCs recovered after trypsinzation did not generated MPCs even if recultured in hAS. Thus, while MPCs may generate MSCs, MSCs are not able to complete the reverse process.
FIG. 2.
MSCs MPCs-derived: proliferative and clonogenic potential. (A) Digestion of hAS culture by trypsin/EDTA allows the isolation of MPCs (trypsin resistant) from primary MSCs. If subsequently cultured in FBS, cells are able to sustain a confluence of fibroblastoid cells (T1) that show tri-lineage mesodermic differentiation potential. Once MSCs are detached from T1 culture, some MPCs remain undetached and are capable of producing a second confluence of MSCs (T2). This procedure could be repeated; however, some MPCs remain undetached. (B) Shows one of five experiments where the life-span of T1, T2, T3, T4, and T5 cultures is similar to primary MSCs cultured on FBS; however, the clonogenic potential decreases. For all experiments we were able to obtain at least a T5 with similar results.
Immunological and molecular characterization
Mesodermal progenitor cells that survived long term in the presence of hAS without proliferating or modifying their morphology were constantly negative for Ki-67 expression revealing a state of quiescence. In addition, MPCs were positive for CD105, but compared to classical MSCs, negative for CD90 (Fig. 3).
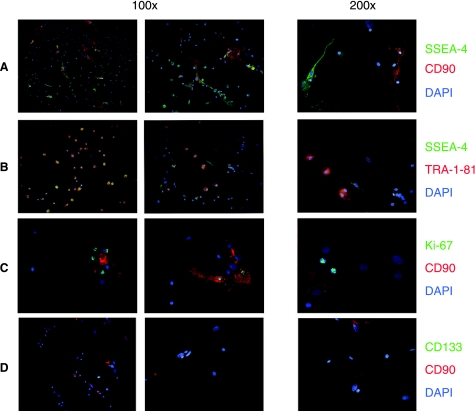
FIG. 3.
Tri-color immunofluorescence characterization of MPCs. Staining of autologous serum cultures shows two main cell populations, easily distinguishable by shape and positive stain for CD90. Mesodermal progenitor cells are defined as rounded and negative for CD90, differing from fibroblastoids, CD90 positive MSCs. The figure shows that SSEA-4 is strongly expressed by MPCs (A) and is rarely on MSCs (yellow on merged picture indicates double positive, green and red, stain), while TRA-1–81 is exclusively expressed by MPCs (B). Furthermore, most of the growing MSCs show intense nuclear fluorescence when stained for Ki-67 (C) but none of MPCs. Panel (D) shows negative stain for CD133 on both MPCs cells and MSCs.
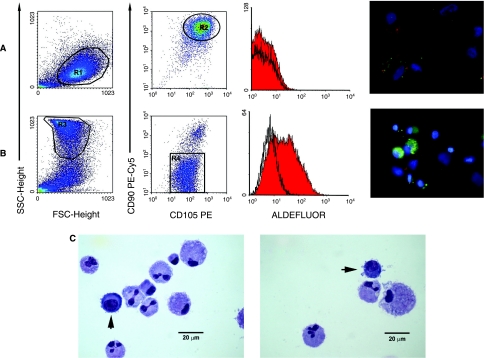
Cytofluorimetric analysis on MPCs recovered from culture after partial detachment with TrypLE Select confirmed that these cells are CD90 negative and CD105 positive whereas MSCs are both CD90 and CD105 positive. Interestingly, SSC analysis clearly reveals the two cell populations, that were extremely different for ALDH (Fig. 4): MPCs expressed high levels of ALDH, while the ALDH activity is lost when MSC differentiation is induced.
FIG. 4.
Characterization of TrypLE Select–detached cells from FBS or hAS cultures. Cells from FBS cultures (A) detached by trypsin alternative proteases show typical MSCs scattergram with low levels of SSC signals. Events on region R1 express bright levels of CD105 and CD90. In detached hAS cultures (B) we define the R3 population as characterized by high SSC signals. R3 gated events express a dim positive stain to CD105 and are negative to CD90. ALDH activity is present in MPCs while almost completely absent in MSCs as shown both by citofluorimetric analysis (Aldefluor) and by cytochemistry (green in the picture, where CD105 red is present in ALDH-negative cells only). Pictures of cytospins from hAS culture (C) stained by May Grünwald-Giemsa, show two population morphologies. MSCs appear with a low nucleus/cytoplasm (N/C) ratio and, often, with polylobular nuclei. It is also possible to identify cells with higher N/C ratio (arrows), intense basophilic cytoplasm, rounded nuclei, and with lots of membrane extrusions.
To clarify the origin of MPCs we excluded the hemangioblastic origin (CD133, CD45, KDR/Flk-1 or anti-VEGFR2, CD34 negative), the immature mesenchymal stage (low affinity nerve growth receptor or CD271 negative) and the pericytes' contamination (CD146 negative).
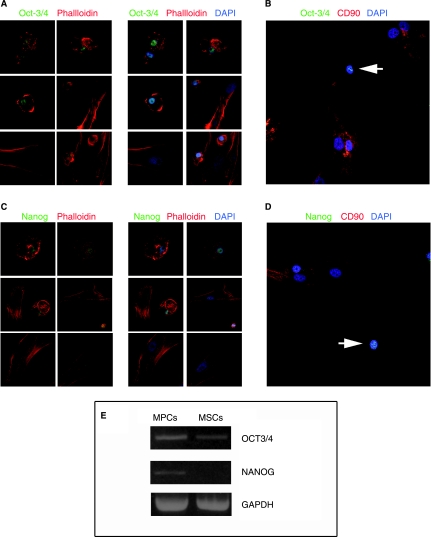
We found that MPCs strongly express the embryonic marker SSEA-4, whereas this marker is only mildly expressed in some MSCs (Fig. 3A and B). Bright stain for TRA-1–81 was reported only on MPCs (Fig. 3B) while SSEA-1 was negative in both populations. In addition, MPCs were positive for nuclear embryonic markers Oct-3/4 and Nanog (Fig. 5) and confirmed by RT-PCR to exclude pseudogenes. Counterstaining with phalloidin highlighted the considerable differences in cytoskeletal organization: a mainly not organized actin disposition on MPCs versus a mostly filamentous complex organization in classical MSCs.
FIG. 5.
Detection of embryonic markers Oct-3/4 and Nanog. Mesodermal progenitors cells (MPCs) appear rounded and with not organized globular actin (red in A and C). Furthermore, cells show a bright positive stain for Oct-3/4 (A) and Nanog (C) into the nucleus. The nuclear localization of green fluorescence is confirmed by confocal microscopy in spectral mode (B and D) where nuclei of MPCs are identified by negative CD90 stain (arrows). In contrast, co-cultured MSCs show typical spindle-shaped morphology and well-organized F-actin cytoskeleton (A and C). MSCs' nuclei are negative for Oct-3/4 and Nanog. DAPI: 4′,6-diamidino-2-phenylindole. RT-PCR analysis (E) reveals, in all three assays performed, high expression of OCT3/4 on MPCs and low level on trypsin-detached cells (MSCs), while NANOG is exclusively expressed by MPCs.
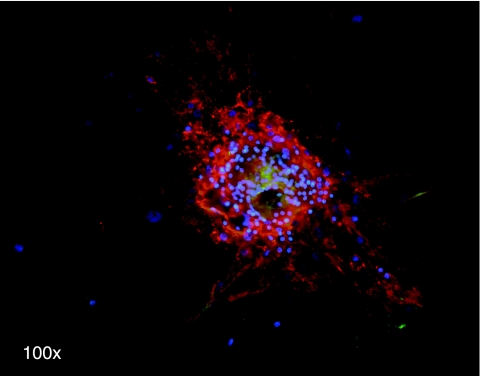
The relative expression of all these markers changed in our cultured cells, moreover CFU-F derived from a single-seeded MPC showed an interesting immunological pattern with the central area reacting with embryonic/immature mesenchymal marker (SSEA-4) and the peripheral area positive for peculiar MSCs markers (Fig. 6).
FIG. 6.
Single MPC-derived fibroblastoid colony. Microscopic fluorescence picture shows bright stain for SSEA-4 (green) in the central area, while cells from periphery express high levels of CD90 (red) but not SSEA-4. Nuclei were stained with DAPI (blue).
Mesodermal progenitor cell-mediated angiogenesis requires VEGF
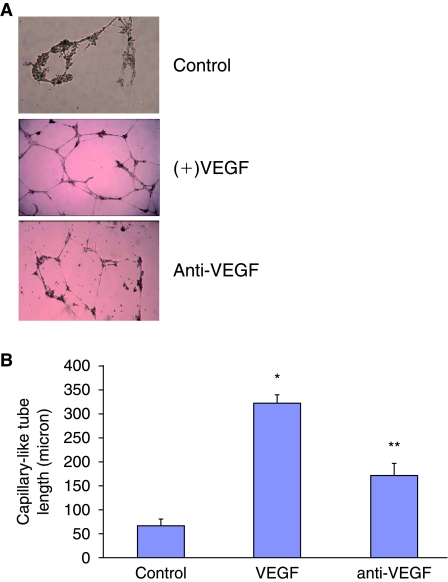
Mesodermal progenitor cells differentiated into typical cobblestone-shaped cells after 2–3 days. After 5 days of culture these cell express eNOS, AMPK, HO-1, and VE-Cadherin. As seen in Fig. 7A, VEGF (50 ng/mL) stimulated capillary tube–like formation within 24 h. This effect was concentration-dependent, with maximal stimulation achieved at 50 ng/mL (data not shown). Neither MSCs nor MPCs express CD146 antigen; however, when cultured in the appropriate medium including VEGF, cells were able to perform angiogenesis in the matrigel test and newly MPCs-derived endothelial cell expressed VE-Cadherin, eNOS, AMPK, and HO-1. Addition of VEGF antibodies to the incubation medium greatly attenuated MPCs-mediated capillary tube–like formation (Fig. 7B), indicating that the MPCs may produce other growth factors needed for capillary tube–like formation mediated, at least in part, by VEGF.
FIG. 7.
VEGF stimulation of capillary-like network formation by mesodermal progenitor cells (MPCs). Mesodermal progenitor cells plated in six-well culture plates (4 × 104 cells/well) pre-coated with Matrigel were incubated with various concentrations of VEGF (50 ng/mL yield ideal angiogenesis) for 16, 24, and 48 h. Photographs were taken at 10× magnification. Maximal response was obtained at 24 h after the addition of VEGF (A). Capillary length was measured by image analysis using Image Pro software as described. Results of capillary length after 24 h as a function of VEGF or anti-VEGF are the mean ± SE; n = 6; *P < 0.001 from control VEGF-treated cells and **P < 0.05 from VEGF versus anti-VEGF-treated MPCs (B).
Discussion
The main finding of this article is the identification and isolation in culture of a previously uncharacterized multipotent cellular population in human bone marrow cells. These cells are selected in the presence of human autologous serum and are characterized as large quiescent cells able, under the appropriate conditions, to differentiate into mesenchymal stromal cells that are able to proliferate for several generations. Mesodermal progenitor cells share with MSCs the expression of CD105 but not CD90. Interestingly, these cells do not express Ki-67 thus confirming they are resting cells. Mesodermal progenitor cells express several embryonic markers indicating a very early level of differentiation. SSEA-4, a stage-specific embryonic antigen, also identifies an adult mesenchymal stem cell population [12]. Oct-3/4, an embryonic transcription factor expressed in the inner mass of a blastocyst, is downregulated during development [13]. It has been detected in pluripotent stem cells in adult bone marrow [14]. Nanog, a gene expressed in ESCs, is thought to be a key factor in maintaining pluripotency. Over expression of Nanog in mouse embryonic stem cells causes these cells to self-renew. In the absence of Nanog, mouse embryonic stem cells differentiate into visceral/parietal endoderm [15]. TRA-1–81 antigen is a keratan sulfate proteoglycan that is expressed in undifferentiated cells and dramatically downregulated in differentiated cells [16]. The expression of these markers suggests that MPCs share some aspects with undifferentiated embryonic cells. It should be noted, however, the MPCs did not express SSEA-1, a putative embryonic marker whose functions have been associated with cell adhesion, migration, and differentiation and is often differentially expressed during development [17]. Several expressing embryonic markers multipotent progenitors present in bone marrow–derived cells have been previously described. These included in vivo detected VSEL [10] cells that are small sized and contain a large nucleus surrounded by narrow cytoplasm and have been reported in mice thus appearing as a population different from MPCs. MAPCs [9] express SSEA-1 and have a 8–10-μm diameter with a large nucleus and scant cytoplasm. These MAPCs as well as MIAMI cells [8] require regulated oxygen tension and do not appear to be comparable to MPCs. Among multipotent mesenchymal stromal cells (MUSC) two populations have been reported [18], but only later Colter et al. identified a rapidly self-renewing cells that are small and spindle-shaped (RS 1) and slowly replicating large flat cells (RS 2) [19] both of which have aspects different from the human cell population reported here despite the fact that the so called RS 2 are slow growing, large, and CD90-negative cells as MPCs. After serum deprivation SD-MSCs may be detected [20] and while these cells may express embryonic markers they appear morphologically different from MPCs. Thus, MPCs appear to be a newly described cell population capable of expressing embryonic markers in humans. We, obviously, cannot rule out that these cells, as well as previously reported populations, may result in different aspects of the same precursor cultured in different conditions [14]. The number and the proliferating capability of MPCs when cultured in FBS or hCS is not influenced by the donor age thus confirming their presence in humans as embryonic-like cells through out life. The importance of our findings is the identification and isolation in culture of a multipotent cellular population in human bone marrow cells, selected only in the presence of adult autologous serum and derived by unsorted bone marrow mononuclear cells. These cells are isolated simply by plastic adherence and cultured in medium conventionally used for the expansion of mesenchymal cells, without exogenous growth factors or critical condition of culture (e.g., oxygen tension regulation).
Thus MPCs that strictly adhere to the flask and are not removed by trypsin may be purified. The role of different sera on MSC proliferation/differentiation has already been reported [21] and specific lots of sera have been commercialized to enhance proliferation of MSCs. However, to the best of our knowledge, this is the first demonstration of the ability of FBS or hCS to induce proliferation and differentiation of human resting cells carrying embryonic markers toward typical mesenchymal cells. Enormous differences exist between these different sera: for instance, the levels of sex steroids or of other anabolic hormones may determine striking differences in cell adhesion (e.g., for their effects on cytoskeleton remodeling) [22] or on proliferation and differentiation signaling. However, it is intriguing to note that sera from immature individuals are able to induce proliferation of multipotent progenitor cells carrying embryonic features.
Mesodermal progenitors cells express high levels of ALDH similar to what previously shown in mixed progenitor cells or in hemopoietic stem cells with the highest proliferative and engraftment potential [23]. Interestingly ALDH is lost when MSC differentiation is induced.
Mesenchymal cells differ in several aspects from MPCs including morphology, adherence properties, marker expression, and cytoskeletal organization. However, MPCs easily differentiate toward mesenchymal lineage capable to follow several proliferation cycles without loosing their proliferative potential. It is important to note that CFU-F colonies derived from a single MPC show, at the centre, embryonic markers detected in MPCs and that these markers are lost on progression to the peripheral area where typical mesenchymal cells are detected. Thus, MPCs could be considered MSC precursors. Our study demonstrates that MPC supports the generation of ECs as evidenced by the increase in capillary area and angiogenesis. It is important to stress that MPCs require VEGF for maximum angiogenesis but also secrete a growth factor that generates a basal level of angiogenesis and the expression of eNOS, AMPK, VE-Cadherin, and HO-1 protein. Moreover, while these cells may generate angiogenesis the lack of CD146 expression rules out the possibility that they are endothelial cells or pericytes, the latter being another intriguing cell population that may represent embryonic “mesoangioblasts” present after birth [24].
Our data suggest that few modifications in mesenchymal culture conditions allow isolating the MPCs present in adult bone marrow. Further investigations will be required to determine the optimal conditions to enrich cultures in MPCs. Better understanding on MPCs could lead to identify a new valuable source for reparative medicine especially considering that these cells are easily isolated in autologous conditions.
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Bruder SP. Jaiswal N. Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Woodbury D. Schwarz EJ. Prockop DJ. Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Barry FP. Boynton RE. Haynesworth S. Murphy JM. Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 6.Reyes M. Lund T. Lenvik T. Aguiar D. Koodie L. Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 7.Wagner W. Ho AD. Mesenchymal stem cell preparations–comparing apples and oranges. Stem Cell Rev. 2007;3:239–248. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 8.D'Ippolito G. Diabira S. Howard GA. Menei P. Roos BA. Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y. Jahagirdar BN. Reinhardt RL. Schwartz RE. Keene CD. Ortiz-Gonzalez XR. Reyes M. Lenvik T. Lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low WC. Largaespada DA. Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 10.Kucia M. Reca R. Campbell FR. Zuba-Surma E. Majka M. Ratajczak J. Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 11.Levenberg S. Golub JS. Amit M. Itskovitz-Eldor J. Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gang EJ. Bosnakovski D. Figueiredo CA. Visser JW. Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 13.Boiani M. Scholer HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- 14.Ratajczak MZ. Machalinski B. Wojakowski W. Ratajczak J. Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 15.Chambers I. Colby D. Robertson M. Nichols J. Lee S. Tweedie S. Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 16.Gerecht-Nir S. Itskovitz-Eldor J. Human embryonic stem cells: a potential source for cellular therapy. Am J Transplant. 2004;4(Suppl. 6):51–57. doi: 10.1111/j.1600-6135.2004.0345.x. [DOI] [PubMed] [Google Scholar]
- 17.Fox N. Damjanov I. Martinez-Hernandez A. Knowles BB. Solter D. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev Biol. 1981;83:391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- 18.Friedenstein AJ. Deriglasova UF. Kulagina NN. Panasuk AF. Rudakowa SF. Luria EA. Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 19.Colter DC. Sekiya I. Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pochampally RR. Smith JR. Ylostalo J. Prockop DJ. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103:1647–1652. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- 21.Nimura A. Muneta T. Koga H. Mochizuki T. Suzuki K. Makino H. Umezawa A. Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 22.Giretti MS. Simoncini T. Rapid regulatory actions of sex steroids on cell movement through the actin cytoskeleton. Steroids. 2008;73:895–900. doi: 10.1016/j.steroids.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Hess DA. Craft TP. Wirthlin L. Hohm S. Zhou P. Eades WC. Creer MH. Sands MS. Nolta JA. Widespread nonhematopoietic tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells. 2008;26:611–620. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellavalle A. Sampaolesi M. Tonlorenzi R. Tagliafico E. Sacchetti B. Perani L. Innocenzi A. Galvez BG. Messina G. Morosetti R. Li S. Belicchi M. Peretti G. Chamberlain JS. Wright WE. Torrente Y. Ferrari S. Bianco P. Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]