Introduction
The copper catalyzed Huisgen 1,3-dipolar cycloaddition ”click” reaction,1 has become an invaluable coupling reaction due to its uncommon combination of extremely high coupling yields and broad functional group tolerance. This versatility has been demonstrated in numerous applications2 including the labeling of biological molecules (DNA, proteins, cell surfaces, etc.) both in vitro and in vivo,3,4 synthesis of biomedical materials,5 the cross-linking self assembled nanostructures,6 and the functionalization of two-dimensional surfaces.7
In the field of macromolecular synthesis, the broad utility of click coupling has been proven through the assembly of an exceptional range of complex macromolecular architectures,8 including diblock copolymers,9 triblock copolymers,10 star polymers,11 miktoarm star polymers, 12 graft polymers,13 hyperbranched polymers,14 dendrimers,15 dendronized linear polymers,16 cyclic polymers,17 cyclic block copolymers,18 polyrotaxanes,19 and even dendrimers with polymeric repeat units.20 As a consequence, characterization of well-defined azide-functionalized macromolecules has become an increasingly important aspect of polymer chemistry.
One of the most valuable tools for the characterization of polymers is matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS), which provides a means to determine the Mn and PDI of a polymer sample, as well as to accurately calculate the repeat unit and end group masses. In most traditional end group identification techniques, such as NMR or IR, the end group signal becomes vanishingly small relative to that of the repeat units as molecular weight increases. However, in a polymer’s mass spectrum, each signal contains information about that n-mer’s end groups, and therefore the mass of the end groups can be easily calculated as long as the mass of the individual signals can be accurately determined.21
Despite the importance of mono-azide functionalized polymers, their reported MALDI-TOF reflectron mass spectra have frequently been misinterpretated; a result of the unusual susceptibility of the azide functionality to fragment during matrix-assisted laser desorption ionization. Mass spectra obtained in our labs and elsewhere suggest that fragmentation of the azide functionality via expulsion of N2 gives rise to ions 28 mass units less than the mass of the n-mer.22 However, a distribution is observed in addition to the [M-28] ions in reflector mass spectra, which has a mass loss less than 28 mass units, thus exhibiting the characteristics of metastable ions. This later distribution can be misinterpreted if one is not familiar with the metastable ion formation process in mass spectrometry. In order to confirm the proposed metastable ion formation in reflector mass spectra of polymer azides, a variety of different mono-azide functionalized polymers were examined, including polycaprolactone (PCL), poly(ethylene glycol) (PEG), and polystyrene (PS) (scheme 1).
Scheme 1.
Chemical structures of the PCL, cyclic PCL, PEG, and PS monoazide polymers used in this study (PMDETA = N,N,N,′,N″,N″-pentamethyldiethylene triamine).
Results and Discussion
Metastable ions are formed in MALDI-TOF mass spectrometry when a parent ion generated during laser desorption fragments at some point during the flight path in the field free region between the ion source and detector.23 For the azide functional group, facile fragmentation has precedence in small molecule analogs, including the thermal fragmentation of phenyl azides by expulsion of N2 to generate reactive nitrene intermediates,24 as well as analogous fragmentation pathways in the mass spectra of low molecular weight aryl25 and aliphatic azides.26
In order to verify post-source metastable ion formation occurs for polymeric azides, highly precise end group mass determinations must be carried out. In this study, the accurate determination of end group masses within 0.01 Daltons (in the case of PEG) was made possible by a combination of high resolving power instrumentation (Bruker Autoflex III) and a rigorous eight point calibration across the molecular weight range (using monodisperse dendrimer standards). 27
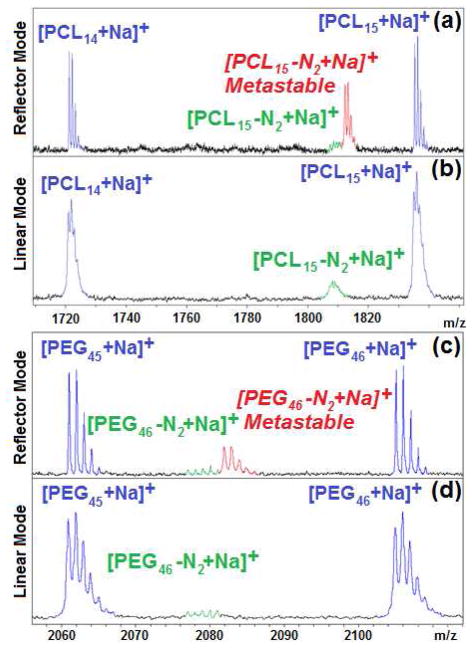
As seen in figure 1, mono-azide functionalized PCL and PEG exhibit three distinct distributions in their mass spectra when analyzed using reflector mode: the parent ion ([M+Na]+ in blue), the in-source expulsion of N2 ([M-28+Na]+ in green) and the proposed post-source expulsion of N2 (metastable ions in red). The first observation that provides strong evidence for the metastable nature of this third distribution involves its disappearance from the spectra when linear mode is used instead of reflector mode. In linear mode, post-source metastable ion formation in the field free region will produce smaller neutral and ionic fragments with the same velocity, and hence time-of-flight, as the parent ion. As a result, such fragmentation will not be apparent in mass spectra acquired in linear mode. In reflector mode, however, ions are decelerated partway through their flight path and then reaccelerated in a different direction by a “mirror” towards the second detector, in order to provide enhanced resolving power. Any post-source metastable ions formed before arriving at the mirror will undergo a different deceleration, reacceleration than their parent ion. As a result, the time-of-flight, and thus the mass for those metastable ions (the red series) will be some intermediate value between that of the parent mass (the blue series) and the in-source fragment mass (the green series). It should be noted that the intensity of the metastable signal increases with laser power (figure S1, supporting information). As a result, polymers that require higher laser power for ionization will exhibit more intense metastable signals (for example, polystyrene in figure 2c).
Figure 1.
Comparison of MALDI-TOF mass spectra obtained for polycaprolactone (Mn =2040 PDI = 1.04) in a) reflector mode, and b) in linear mode, and for poly(ethylene glycol) (Mn =2193 PDI =1.01) in c) reflector mode, and d) in linear mode
Figure 2.
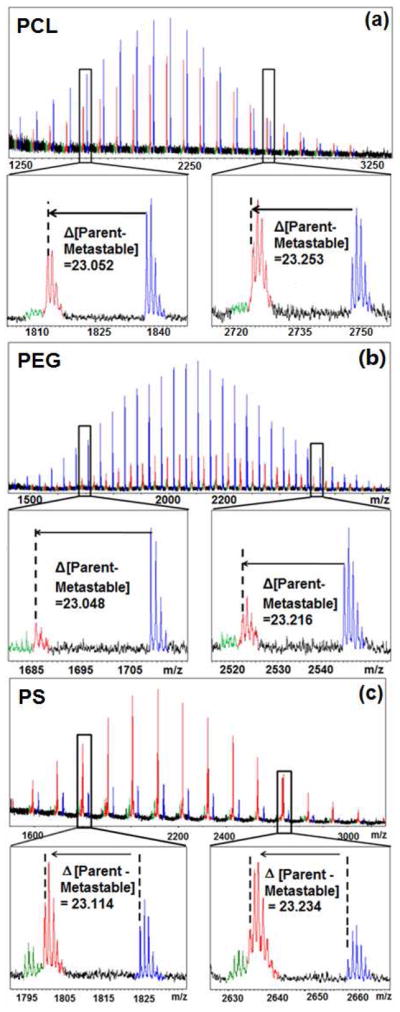
Comparison of reflector mode MALDI-TOF mass spectra for (a) poly(ε-caprolactone) (Mn =2040 PDI = 1.04) (b) polyethylene glycol (Mn =2190 PDI =1.01) and (c) polystyrene (Mn =2160 PDI = 1.03), highlighting the difference between the parent ion and the metastable ion at both the low and high extremes of the mass distributions.
A further indication of metastable ion formation in polymer azides is the non-uniform, non-integer mass offset of the third (red) distribution relative to parent azide distribution. If this distribution were the result of a degradation product or a different mode of ionization, the offset should be uniform across the entire molecular weight range. In the case of PEG-N3, the parent (blue distribution) and in-source fragment (green distribution) ions correspond closely with the theoretical masses (each observed parent ion within 0.01 Daltons of the theoretical value). However, the metastable (red distribution) ions exhibit a non-uniform mass offset that increases at higher molecular weights (tables S1–S3 in supporting information). For example, for the PEG 13-mer near 652 Daltons, the mass offset relative to its parent ion is approximately 22.67 Daltons, however, near 2633, the analogous mass offset is greater than 23.25 Daltons (figure 2). This discrepancy of nearly 0.60 Daltons is almost two orders of magnitude greater than the observed error for the calibrated parent ion data over the same mass range. A similar trend is observed for the other two polymers investigated, (though the signal of the other backbones was more obscured by background noise due to inefficient ionization) where increasing molecular weight of the parent ion results in an increased offset in the metastable ion (figure 3). This non-integer mass results from post-source metastable ion formation, since the ion mass changes after its original acceleration from the source and before its deceleration/reacceleration by the mirror. Because the lighter metastable ions lose a greater percentage of their mass during the azide fragmentation, they are affected more strongly by the reflector voltage, and exhibit an observed mass closer to the parent ion, while heavier ions exhibit an observed mass closer to the in-source fragment ion. This non-uniform metastable mass shift has been modeled elsewhere,28 and the previously reported trends agree with the data observed in this study.
Figure 3.
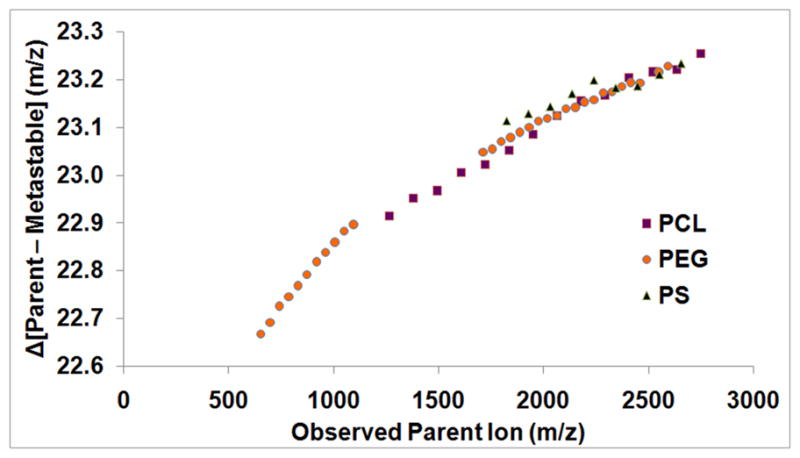
Graph comparing the mass discrepancies between the metastable signals the parent signals for three mono-azide functionalized polymers: PCL (purple), PEG (orange), and PS (black).
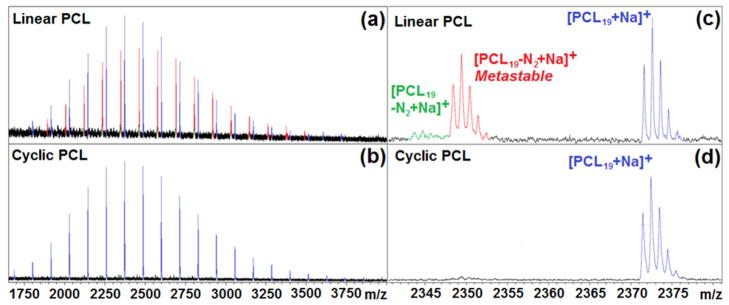
Additional confirmation of the metastable nature of the azide end groups is the disappearance of both the in-source and post-source fragmentation ions in the polymer mass spectra after their “click” coupling, because the product triazole is aromatic and therefore is no longer susceptible to fragmentation. A particularly clear example of this is after the intramolecular cyclization of polycaprolactone with complimentary azide and alkyne end groups on opposite ends of the polymer.29 Because the Huisgen 1,3-dipolar cycloaddition is an intramolecular pericyclic reaction, there is no loss or gain in the molecular mass; however, the fragmentation of the parent ion is no longer plausible in the triazole product and as a result the signal corresponding to the parent ions were retained while those corresponding to the azide fragments are lost (figure 4).30
Figure 4.
Comparison of MALDI-TOF mass spectra obtained for (a) linear poly(ε-caprolactone) (Mn = 2440 PDI = 1.02) and (b) cyclic poly(ε-caprolactone) (Mn = 2400 PDI = 1.02). Insets (c) and (d) correspond to linear and cyclic poly (ε-caprolactone), respectively, where the expanded region between 2340 and 2380 m/z, highlights the disappearance of the (red) post-source fragment ions and the (green) in-source fragment ions as a result of the aromatic stabilization of the triazole product.
Conclusion
In conclusion, the mass spectra of mono-azide functionalized polymers have been investigated in detail to verify the prevalence of fragment ions resulting from a loss of N2 via both in-source and post-source metastable ion formation. The post-source metastable ions are identified by a non-uniform, non-integer mass offset relative to the parent ion, and can be most easily confirmed by their disappearance using linear mode detection. With the increasing importance of macromolecular azides in polymer research, the authors hope this study will assist others examining their mass spectra by facilitating the identification and interpretation of the metastable signals.
Supplementary Material
Acknowledgments
The authors thank Michael A. Grayson and Paul Kowalski for helpful discussions, the NSF for providing MALDI-TOF MS instrumentation (MRI 0619770), as well as Tulane University, the National Institutes of Health (5R01EB6493), the Louisiana Board of Regents (LEQSF(2006-09)-RD-A-29), the American Chemical Society Petroleum Research Fund (47108-G7), and the National Science Foundation (NSF-DMR 0844662 (ARRA)) for financial support.
Footnotes
Supporting Information: Experimental synthetic and analytical procedures as well as tables of MALDI-TOF data. This material is available free of charge and via the internet at http://pubs.acs.org.
References
- 1.Huisgen R. In: 1,3-Dipolar Cycloaddition Chemistry. Padwa A, editor. Wiley; New York: 1984. [Google Scholar]; Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 2.Meldal M, Tornøe CW. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 3.Amblard F, Cho JH, Schinazi RF. Chem Rev. 2009;109:4207–4220. doi: 10.1021/cr9001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best MD. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 5.van Dijk M, Rijkers DTS, Liskamp RMJ, van Nostrum CF, Hennink WE. Bioconjugate Chem. 2009;20:2001–2016. doi: 10.1021/bc900087a. [DOI] [PubMed] [Google Scholar]; Lutz J, Zarafshani Z. Adv Drug Delivery Rev. 2008;60:958–970. doi: 10.1016/j.addr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Joralemon MJ, O’Reilly RK, Hawker CJ, Wooley KL. J Am Chem Soc. 2005;127:16892–16899. doi: 10.1021/ja053919x. [DOI] [PubMed] [Google Scholar]
- 7.Bryan MC, Fazio F, Lee HK, Huang CY, Chang A, Best MD, Calarese DA, Blixt O, Paulson JC, Burton D, Wilson IA, Wong CH. J Am Chem Soc. 2004;126:8640–8641. doi: 10.1021/ja048433f. [DOI] [PubMed] [Google Scholar]; Collman JP, Devaraj NK, Chidsey CED. Langmuir. 2004;20:1051–1053. doi: 10.1021/la0362977. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meng JC, Siuzdak G, Finn MG. Chem Commun. 2004;10:2108–2109. doi: 10.1039/b408200a. [DOI] [PubMed] [Google Scholar]; Sun XL, Stabler CL, Cazalis CS, Chaikof EL. Bioconjugate Chem. 2006;17:52–57. doi: 10.1021/bc0502311. [DOI] [PubMed] [Google Scholar]; Santoyo-Gonzalez F, Hernandez-Mateo F. Chem Soc Rev. 2009;38:3449–3462. doi: 10.1039/b909363j. [DOI] [PubMed] [Google Scholar]
- 8.Fournier D, Hoogenboom R, Schubert US. Chem Soc Rev. 2007;36:1369–1380. doi: 10.1039/b700809k. [DOI] [PubMed] [Google Scholar]; Lutz JF. Angew Chem Int Ed. 2007;46:1018–1025. doi: 10.1002/anie.200604050. [DOI] [PubMed] [Google Scholar]
- 9.Opsteen JA, van Hest JCM. Chem Commun. 2005:57–59. doi: 10.1039/b412930j. [DOI] [PubMed] [Google Scholar]; Quémener D, Davis TP, Barner-Kowollik C, Stenzel MH. Chem Commun. 2006:5051–5053. doi: 10.1039/b611224b. [DOI] [PubMed] [Google Scholar]
- 10.Durmaz H, Dag A, Altintas O, Erdogan T, Hizal G, Tunca U. Macromolecules. 2007;40:191–198. [Google Scholar]
- 11.Gao H, Matyjaszewski K. Macromolecules. 2006;39:4960–4965. [Google Scholar]
- 12.Whittaker MR, Urbani CN, Monteiro MJ. J Am Chem Soc. 2006;128:11360–11361. doi: 10.1021/ja0645990. [DOI] [PubMed] [Google Scholar]
- 13.Gao H, Matyjaszewski K. J Am Chem Soc. 2007;129:6633–6639. doi: 10.1021/ja0711617. [DOI] [PubMed] [Google Scholar]
- 14.Scheel AJ, Komber H, Voits BI. Macromol Rapid Commun. 2004;25:1175–1180. [Google Scholar]
- 15.Wu P, Feldman AK, Nugent AK, Hawker CJ, Scheel A, Voit B, Pyun J, Frechet JMJ, Sharpless KB, Fokin VV. Angew Chem Int Ed. 2004;43:3928–3982. doi: 10.1002/anie.200454078. [DOI] [PubMed] [Google Scholar]; Joralemon MJ, O’Reilly RK, Matson JB, Nugent AK, Hawker CJ, Wooley KL. Macromolecules. 2005;38:3663–3678. [Google Scholar]
- 16.Helms B, Mynar JL, Hawker CJ, Fréchet JMJ. J Am Chem Soc. 2004;126:15020–15021. doi: 10.1021/ja044744e. [DOI] [PubMed] [Google Scholar]
- 17.Laurent BA, Grayson SM. J Am Chem Soc. 2006;128:4238–4239. doi: 10.1021/ja0585836. [DOI] [PubMed] [Google Scholar]
- 18.Eugene DM, Grayson SM. Macromolecules. 2008;41:5082–5084. [Google Scholar]
- 19.Loethen S, Ooya T, Choi HS, Yui N, Thompson DH. Biomacromolecules. 2006;7:2501–2506. doi: 10.1021/bm0602076. [DOI] [PubMed] [Google Scholar]
- 20.Urbani CN, Bell CA, Whittaker MR, Monteiro MJ. Macromolecules. 2008;41:1057–1060. [Google Scholar]
- 21.The use of MALDI-TOF mass spectrometry for polymer end group analysis has evolved alongside instrument development, a brief review and leading references can be found in: Li Y, Hoskins JN, Sreerama SG, Grayson MA, Grayson SM. J Mass Spectrom. 2010;45:587–611. doi: 10.1002/jms.1743.in addition, a number of the foundational papers include: Danis PO, Karr DE, Mayer F, Holle A, Watson CH. Org Mass Spectrom. 1992;27:843–846.Maloney DR, Hunt KH, Lloyd PM, Muir AVG, Richards SN, Derrick PJ, Haddleton DM. Chem Commun. 1995:561–562.Montaudo G, Montaudo MS, Puglisis C, Samperi F. Macromolecules. 1995;28:4562–4569.Danis PO, Karr DE, Simonsick WJ, Jr, Wu DT. Macromolecules. 1995;28:1229–1232.Zammit MD, Davis TP, Haddleton DM, Suddaby KG. Macromolecules. 1997;30:1915–1920.Williams JB, Gusev AI, Hercules DM. Macromolecules. 1997;30:3781–3787.
- 22.For polystyrene: Matyjaszewski K, Nakagawa Y, Gaynor SG. Macromol Rapid Commun. 1997;18:1057–1066.Coessens V, Matyjaszewski KJ. Macromol Sci, Part A: Pure Appl Chem. 1999;A36:667–679.Lutz JF, Borner HG, Weichenhan K. Macromol Rapid Commun. 2005;26:514–518.Guillaneuf Y, Dufils PE, Autissier L, Rollet M, Gigmes D, Bertin D. Macromolecules. 2010;43:91–100.For poly(ethylene glycol): Raynaud J, Absalon C, Gnanou Y. J Am Chem Soc. 2009;131:3201–3209. doi: 10.1021/ja809246f.
- 23.Spengler B, Kirsch D, Kaufmann R, Cotter RJ. Rapid Commun Mass Spectrom. 1991;5:198–202. [Google Scholar]; Kaufmann R, Chaurand P, Kirsch D, Spengler B. Rapid Commun Mass Spectrom. 1996;10:1199–1208. doi: 10.1002/(SICI)1097-0231(19960731)10:10<1199::AID-RCM643>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]; Spengler B. J Mass Spectrom. 1997;32:1019–1036. [Google Scholar]
- 24.Bertho A. Chem Berichte. 1924;57:1138–1142. [Google Scholar]
- 25.Crow WD, Wentrup C. Tetrahedron Lett. 1967;44:4379–4384. [Google Scholar]; Abramovitch RA, Kyba EP, Scriven EFV. J Org Chem. 1971;36:3796–3803. [Google Scholar]
- 26.Olivera AM, Barros MT, Martins AM, Cabral MAR, Dias AA, Costa ML, Cabral MH, Moutinho AMC, Jennings KR. Rapid Commun Mass Spectrom. 1999;13:559–561. [Google Scholar]
- 27.Grayson SM. 2010/023087. US Patent. pending.
- 28.Harvey DJ, Hunter AP, Bateman RH, Brown J, Critchley G. Int J Mass Spectrom. 1999;188:131–146. [Google Scholar]; Stairs JR, Dermota TE, Wisniewski ES, Castleman AW., Jr Int J Mass Spectrom. 2002;213:81–89. [Google Scholar]; Shard AG, Gilmore IS. Int J Mass Spectrom. 2008;269:85–94. [Google Scholar]
- 29.Hoskins JN, Grayson SM. Macromolecules. 2009;43:6406–6413. [Google Scholar]
- 30.The very low remaining signal at 2349 Daltons is likely caused by a trace amount of unreacted linear precursor.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.