Summary
Moral judgments, whether delivered in ordinary experience or in the courtroom, depend on our ability to infer intentions. We forgive unintentional or accidental harms and condemn failed attempts to harm. Prior work demonstrates that patients with damage to the ventromedial prefrontal cortex (VMPC) deliver abnormal judgments in response to moral dilemmas, and that these patients are especially impaired in triggering emotional responses to inferred or abstract events (e.g., intentions), as opposed to real or actual outcomes. We therefore predicted that VMPC patients would deliver abnormal moral judgments of harmful intentions in the absence of harmful outcomes, as in failed attempts to harm. This prediction was confirmed in the current study: VMPC patients judged attempted harms including attempted murder as more morally permissible relative to controls. These results highlight the critical role of the VMPC in processing harmful intent for moral judgment.
Introduction
When we attempt to understand and evaluate other people's actions, we often draw inferences about their beliefs and intentions (Cushman, 2008; Knobe, 2005; Mikhail, 2007; Young et al., in press). For example, did they believe they would cause harm? Did they intend to cause harm? Typically, these beliefs and intentions match the action's outcomes: when someone thinks she is sweetening her friend's coffee by putting sugar in it, she is usually not mistaken (Young and Saxe, 2009a). Mismatches occur, however, in the case of accidents (e.g., when the “sugar” is in fact poison) and failed attempts to harm (e.g., when the “poison” is in fact sugar). The aim of the current study is to understand the causal role of the ventromedial prefrontal cortex (VMPC) for such moral judgments that rely on assessments of intent (Casebeer and Churchland, 2003; Gazzaniga, 2005; Haidt, 2007; Mikhail, 2007). Using a neuropsychological approach, we show that bilateral damage to the VMPC leads to moral judgments that largely neglect harmful intent, focusing instead on the outcome of the action (e.g., the moral judgment of a failed murder attempt as permissible). Consequently, we suggest that the VMPC plays an integral role in processing negatively valenced intentions for moral judgment.
Prior neuroimaging and neuropsychological evidence has suggested a role for the VMPC in evaluating harmful actions (Borg et al., 2006; Ciaramelli et al., 2007; Glenn et al., 2009; Greene et al., 2004; Greene et al., 2001; Harenski and Hamaan, 2006; Heekeren et al., 2003; Koenigs et al., 2007; Luo et al., 2006; Mendez et al., 2005; Moll et al., 2002; Young and Saxe, 2009b). Specifically, the VMPC was robustly recruited when subjects evaluated emotionally salient harms to an individual that were intended as a means to maximize aggregate welfare, for example, pushing a person into the path of a trolley in order that his body stop the trolley from hitting five other people (Greene et al., 2004; Greene et al., 2001). Furthermore, patients with bilateral damage to the VMPC were more likely to deliver utilitarian moral judgments, that is, to endorse such harmful actions as appropriate, compared to brain damaged and healthy comparison participants (Ciaramelli et al., 2007; Koenigs et al., 2007; Mendez et al., 2005). These results were taken to indicate a causal role for emotional processing, as subserved by the VMPC, in evaluating harmful actions in this context (Young and Koenigs, 2007). This body of work, however, leaves open an important question that we seek to address in the current study: do VMPC patients endorse harmful actions because a failure to process harmful outcomes or harmful intentions?
Here we probe moral judgment in patients with adult-onset bilateral damage to the VMPC using scenarios that critically disentangle the contributions of intentions and outcomes to moral judgment. By studying patients with damage to this region, we therefore directly investigate the causally necessary role of the VMPC in the processing of intentions and outcomes for moral judgment. We note that the current study also differs from the prior work in several methodological respects: (1) the presentation of more ordinary and perhaps familiar scenario settings (e.g., eating at a restaurant, driving home from work), rather than the somewhat contrived contexts previously tested (e.g., halting runaway trolleys, facing terrorists in a jungle); (2) a focus on third-person moral judgments, as opposed to hypothetical first-person action predictions (e.g., what would you do in this situation?); (3) a departure from moral dilemmas (i.e. competing norms and no clear socially or legally mandated answers) of stereotypical form (e.g., would you kill one to save many?). These methodological changes allow us to determine whether the role of the VMPC in moral judgment extends to (1) more ordinary contexts, (2) judgments as opposed to predictions of behavior, and (3) moral scenarios that feature pure transgressions (e.g., murder attempts) as opposed to moral dilemmas that force a choice between violations of competing moral norms (e.g., “the lesser of two evils”).
We tested a sample of nine patients with adult-onset, focal bilateral VMPC lesions (Figure 1) and comparison groups of neurologically normal (NC) and brain-damaged (BDC) participants (Table 1; see Experimental Procedures). Based on prior neuropsychological testing, all of the VMPC patients in the current study exhibited characteristic deficits in social emotional processing (Table 2), while presenting generally intact intellect and cognitive function (Table S1). In general, despite preserved general intelligence, logical reasoning, and declarative knowledge of social and moral norms (Burgess et al., 2006; Saver and Damasio, 1991), patients with VMPC lesions commonly fail to apply such knowledge in daily living and exhibit impairments in processing social emotions such as empathy and embarrassment (Anderson et al., 2006; Barrash et al., 2000; Beer et al., 2003; Camille et al., 2004), as well as counterfactual emotional responses such as guilt and regret (Camille et al., 2004; Krajbich et al., 2009). Other work has demonstrated that VMPC patients are specifically impaired in triggering emotional responses when they must infer an emotional event (Bechara et al., 1997; Camille et al., 2004), as opposed to when they are presented with an actual emotional outcome (e.g., losing money), in which case their emotional responses are relatively spared or even exaggerated (Bechara et al., 2000; Koenigs and Tranel, 2007). This neuropsychological profile is best understood in the context of the functional connectivity of the VMPC. The VMPC projects to the basal forebrain and brainstem regions, which regulate and execute bodily components of emotional responses (Ongur and Price, 2000), while neurons within the VMPC encode the emotional value of stimuli (Rolls, 2000).
Figure 1.
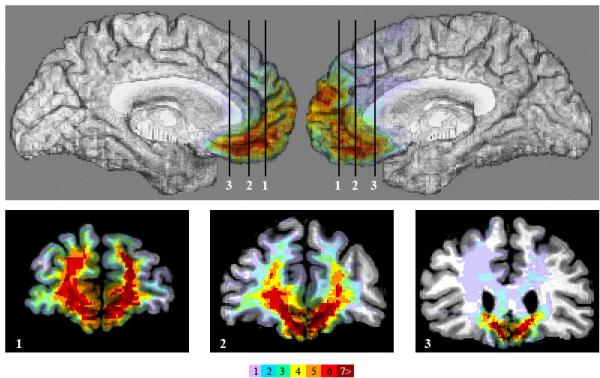
Lesion overlap of the 9 VMPC subjects using the MAP-3 technique. Top panel shows the left and right mesial views of the template brain. Panels 1,2,3 show three coronal sections through VMPC at the levels indicated in the top panel. The numbers of overlaps at each voxel is shown in the color bar.
Table 1.
Demographic and clinical data. Age, in years. Educ., years of formal schooling. Hand., degree of right- or left-handedness on a scale ranging from full right-handedness (+100) to full left-handedness (-100). Chronicity, years between lesion onset and current experiment. Etiology, cause of brain damage (SAH = subarachnoid hemorrhage; ACoA = anterior communicating artery). The 7 brain-damaged comparison patients had brain damage caused by cerebrovascular disease. For Age and Education, there were no significant differences between the 3 groups, per one-way ANOVA. For Chronicity, the VMPC and BDC groups did not differ, per t-test.
| Participant | Age | Educ. | Hand. | Sex | Chronicity | Etiology |
|---|---|---|---|---|---|---|
| 0318 | 69 | 14 | +100 | M | 34 | Meningioma resection |
| 0770 | 67 | 16 | +100 | F | 24 | Meningioma resection |
| 1424 | 73 | 13 | +100 | M | 24 | Head trauma |
| 1815 | 57 | 20 | +100 | M | 11 | Meningioma resection |
| 1983 | 46 | 13 | +100 | F | 13 | SAH; ACoA aneurysm |
| 2352 | 60 | 14 | +100 | F | 10 | SAH; ACoA aneurysm |
| 2391 | 63 | 13 | +100 | F | 9 | Meningioma resection |
| 2577 | 69 | 11 | +100 | M | 10 | SAH; ACoA aneurysm |
| 3383 | 59 | 12 | -100 | F | 3 | SAH; ACoA aneurysm |
| VMPC | ||||||
| Mean | 62.6 | 14.0 | 8 RH | 4 M | 15.3 | |
| SD | (8.2) | (2.6) | 1 LH | 5 F | (9.8) | |
| BDC (n = 7) | ||||||
| Mean | 62.4 | 16.6 | 7 RH | 4 M | 8.9 | |
| SD | (9.5) | (3.0) | 0 LH | 3 F | (6.8) | |
| NC (n = 8) | ||||||
| Mean | 64.1 | 14.1 | 7 RH | 5 M | ||
| SD | (9.7) | (1.7) | 1 LH | 3 F |
Table 2.
Emotional and social functioning data for VMPC patients. Skin conductance responses (SCRs) to emotionally charged social stimuli (e.g., pictures of social disasters, mutilations, and nudes, using methods described previously (Damasio et al., 1990)). None of the 7 brain-damaged comparison patients had SCR impairments to emotionally charged stimuli. Social Emotions, the patient's demonstrated capacity for empathy, embarrassment, and guilt, as determined from reports from a collateral source (spouse or family member) provided on the Iowa Scales of Personality Change (Barrash et al., 2000) and from data from clinical interviews. Acquired Personality Changes, post-lesion changes in personality (e.g., irritability, emotional dysregulation, and impulsivity), as determined from data from the Iowa Scales of Personality Change. For Social Emotions and Acquired Personality Changes, the degree of severity is designated in parentheses (1 = mild, 2 = moderate, 3 = severe). None of the 7 brain-damaged comparison patients had defective social emotions or post-morbid personality changes.
| Patient | SCRs | Social Emotions | Acquired Personality Changes |
|---|---|---|---|
| 0318 | Lower SCR | Diminished (3) | Yes (3) |
| 0770 | Lower SCR | Diminished (3) | Yes (3) |
| 1424 | Lower SCR | Diminished (2) | Yes (2) |
| 1815 | Lower SCR | Diminished (2) | Yes (2) |
| 1983 | Lower SCR | Diminished (3) | Yes (3) |
| 2352 | Lower SCR | Diminished (2) | Yes (3) |
| 2391 | Lower SCR | Diminished (3) | Yes (2) |
| 2577 | Lower SCR | Diminished (3) | Yes (3) |
| 3383 | Lower SCR | Diminished (3) | Yes (3) |
Scenarios presented to participants followed a 2 × 2 design (see Figure 2 and Supplemental Information for full text): (1) the protagonist either intended to cause harm to another person (negative intent) or intended to cause no harm (neutral intent), and (2) the protagonist either caused harm to another person (negative outcome) or caused no harm (neutral outcome) (Young et al., 2007). More precisely, the stimuli explicitly specified the agent's belief about whether he or she would cause harm, and, on this basis, participants could infer the agent's intention to cause harm or not. This design contained two conditions where intentions and outcomes matched, and two where they mismatched (i.e. attempted harms and accidental harms). Participants made moral judgments of the protagonist's action on a scale of 1 (morally forbidden) to 7 (morally permissible).
Figure 2.

Experimental design and stimuli. (top) The combination of intent (neutral vs. negative) and outcome (neutral vs. negative) factors yielded a 2×2 design with four conditions. (bottom) Full text of an example “failed attempt to harm” scenario. Bold sections indicate words that differed across conditions.
Given the critical role of the VMPC in triggering emotional responses to inferred or abstract events (Bechara et al., 1997; Damasio et al., 1990), we predicted that patients with VMPC damage would fail to perceive the emotional significance of harmful intentions (e.g., unobservable mental states), and therefore deliver abnormal moral judgments in the case that judgments depend on emotional responses to such abstract representational content. We predicted that, as a direct result, VMPC patients would instead judge actions primarily on the basis of the actions’ outcomes, which are represented concretely in the world. In particular, we predicted patients with VMPC damage would judge attempted harms as more morally permissible than control participants, and, consequently, use the neutral outcome as the relevant moral metric. Notably, moral judgment of accidental harms (neutral intent, negative outcome) also requires the processing of an unobservable mental state; however, in this case, the mental state is a neutral intent, which does not necessarily elicit an emotional response that is critical for moral judgment. We therefore predicted that VMPC patients would show a selective deficit only when moral judgment requires an emotional response to mental state content. In other words, we predicted a deficit for attempted harms, and not accidental harms. This pattern of results would indicate that in the absence of a normally functioning VMPC, and normal emotional responses subserved by the VMPC that are typically associated with perceiving harmful intentions, individuals will deliver abnormal moral judgments.
Results
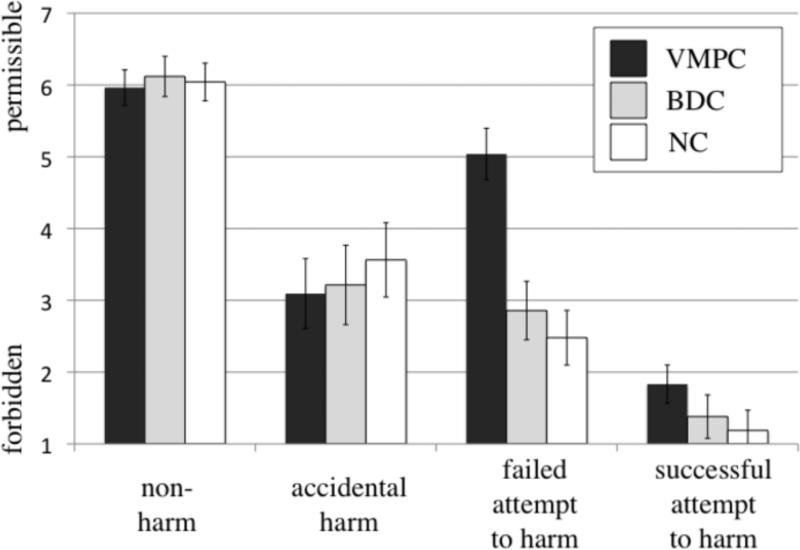
A 2 (intent: neutral vs. negative) × 2 (outcome: neutral vs. negative) × 3 (group: VMPC vs. BDC vs. NC) mixed effects ANOVA of participants’ moral judgments yielded main effects of intent (F(1,21)=136.0 p=1.2×10-10), outcome (F(1,21)=94.4 p=3.2×10-9), and an interaction between intent and outcome (F(1,21)=7.0 p=0.015) (Figure 3). Importantly, these effects were observed in the context of interaction effects involving the participant group variable, specifically, a two-way interaction between intent and participant group (F(1,21)=9.7 p=0.001) and a three-way interaction between intent, outcome, and participant group (F(1,21)=3.9 p=0.036). There were no statistically significant interaction effects involving the participant group variable for reaction time (intent × participant group, F(1,21)=1.4 p=0.27; belief × outcome by participant group, F(1,21)=0.50 p=0.61; see also Supplemental Analyses).
Figure 3.
Moral judgments for all four conditions. Judgments are shown for each participant group, on 7-point scale. Error bars represent standard error of the mean. VMPC participants judged failed attempts to harm as significantly more permissible than the brain-damaged comparison (BDC) participants and the normal comparison (NC) participants (P values < 0.001).
To interpret these interaction effects, planned comparisons were conducted, yielding significant differences between participant groups only for attempted harms. VMPC participants judged attempted harms as more permissible than BDC participants (t(14)=4.0, p=0.001) and NC participants (t(15)=4.6, p=3.3×10-4). There was no difference between BDC and NC participants in their moral judgments of attempted harms (t(13)=0.73, p=0.48) or any other condition. Moreover, there were no other significant differences for any pair of participant groups (VMPC, BDC, NC) on any of the other conditions: non-harm, accidental harm, or successful attempt to harm. Importantly, there were no differences between VMPC participants and either comparison group on non-harms (BDC: t(14)=-0.40, p=0.70; NC: t(15)=-0.21, p=0.84), accidental harms (BDC: t(14)=-0.16, p=0.89; NC: t(15)=-0.71, p=0.49), or successful attempts to harm (BDC: t(14)=0.94, p=0.37; NC: t(15)=1.6, p=0.13).
VMPC participants’ judgments did reflect a difference between attempted harms and non-harms (t(8)=2.97, p=0.018), and a difference between accidental harms and successful attempts to harm (t(8)=6.2, p=2.5×10-4). Thus, VMPC participants were able to distinguish between these conditions by representing the content of negative beliefs and intentions. The difference between attempted harms and non-harms also emerged in the NC group (t(6)=7.3, p=3.5×10-4) and the BDC group (t(7)=12.7, p=4.5×10-6), as did the difference between accidental harms and successful attempts to harm (NC: t(6)=2.7, p=0.038, BDC: (t(7)=4.9, p=0.002).
Notably, VMPC participants also judged attempted harms as significantly more permissible than accidental harms (t(8)=3.7, p=0.006), a pattern that was significantly different from the pattern observed in the BDC participant group (F(1,14)=5.3 p=0.037) and the NC participant group (F(5,10)=12.0 p=0.003). Moral judgments of accidental and attempted harms in the BDC and NC groups reflected a difference in the opposite direction, though this difference did not reach significance (combined analysis for BDC and NC groups: t(14)=1.3, p=0.2). Strikingly, all nine VMPC participants showed the same reversal of judgments of attempted and accidental harms; this pattern was significantly different from the pattern of judgments in the BDC and NC participant groups (Kruskal-Wallis test, H=8.3, 2 d.f., p=0.016). Furthermore, this difference was significant for the comparison between both VMPC and BDC participants (Mann-Whitney U test, U=13.5, p=0.01), and between VMPC and NC participants (U=13.5, p=0.006).
Discussion
The primary aim of this study was to examine the causal role of the VMPC in specific aspects of moral judgment: processing intentions versus outcomes. Because the emotional valence of an intention (e.g., negative versus neutral) greatly influences normal moral judgments (e.g., negative intentions are judged as immoral), and because neuropsychological studies of these VMPC patients reveal deficits in emotional processing, we predicted that VMPC patients would show a selective neglect of negative intentions in moral judgment. The current results are consistent with this prediction: VMPC participants judged attempted harms as more morally permissible than both comparison groups. VMPC participants even judged attempted harms (e.g., attempting, but failing to poison someone) as more permissible than accidental harms (e.g., accidentally poisoning someone).
Notably, the pattern of moral judgments delivered by the VMPC patients represents not just a departure from but also a reversal of the normal pattern of moral judgments. Among healthy adults and even young children, attempted harms are generally judged quite harshly and usually more harshly than accidental harms (Cushman, 2008; Piaget, 1965/1932). In contrast, when VMPC patients confront the same cases, they neglect the protagonist's negative intention, focusing instead on the action's neutral outcome. This results in unusually lenient moral judgments of failed attempts to harm. Importantly, VMPC participants did not exhibit a global deficit in moral judgment in judging all actions as either more permissible or more forbidden. Instead, their deficit was highly selective, restricted to the context of attempted harms.
In conjunction with prior evidence (Bechara et al., 1997; Beer et al., 2003; Damasio et al., 1990), we suggest that the current pattern of results may be due to impaired emotional processing, subserved by the VMPC. That is, the results are consistent with the possibility that when VMPC participants encounter a failed attempt to harm, they may not experience the aversive emotions that normally arise from perceiving that one person intends to harm another. More specifically, due to a deficit in triggering emotions in response to inferred, abstract, imagined, or recalled events, previously termed as secondary emotion induction (Bechara and Damasio, 2005), VMPC patients may fail to respond appropriately to an agent's intention to cause harm. We note that in the current study, this information is both abstract insofar as mental state representations are abstract representations and inferred insofar as the agent's intention is inferred from the agent's belief that he or she would cause harm. Indeed, future research ought to characterize in further cognitive detail the dimensions of representational content that fails to elicit appropriate emotional responding in VMPC patients, in both moral and non-moral contexts. Engaging an emotional response to harmful intent may normally lead to judging attempted harms as morally forbidden (Valdesolo and DeSteno, 2006; Wheatley and Haidt, 2005). We suggest that VMPC patients may lack this guiding emotional response (Koenigs et al., 2007; Saver and Damasio, 1991). VMPC patients may therefore rely instead on explicit outcome information to formulate their moral judgments. Because failed attempts to harm result in neutral outcomes (e.g., no harm), VMPC patients judge failed attempts as more permissible. By the same logic, VMPC patients judge successful attempts to harm as forbidden, on the basis of negative outcomes. This pattern is therefore consistent with intact processing of outcome information in VMPC patients, but impaired processing of emotional aspects of intention for moral judgment.
In the current study, we did not measure emotional responding during the moral judgment task itself. However, the characteristics of the emotional deficit exhibited in these patients, i.e., impaired emotional responding to inferred events (‘secondary induction’), but not actual outcomes (‘primary induction’), have been studied and documented over almost two decades of research (for a review, see (Bechara and Damasio, 2005). Although a specific impairment in triggering emotions from inferred or abstract events is the most parsimonious explanation for the observed results given the available evidence, here we consider two alternative hypotheses for the pattern of judgments provided by the VMPC participants.
First, VMPC participants may have produced an abnormal pattern of moral judgments because of deficits in domain-general cognitive abilities, rather than social-emotional deficits. This alternative hypothesis appears unlikely for a number of reasons. VMPC patients, including the ones we tested, showed preserved general intelligence, logical reasoning, and declarative knowledge of social and moral norms (Burgess et al., 2006; Saver and Damasio, 1991). Consistent with this neuropsychological profile, these patients also provided normal moral judgments on all but one condition (i.e., attempted harms) in the current study, and showed no reaction time differences as compared to either control group on any condition. Furthermore, the attempted harm condition was not more difficult for any group, as indicated by reaction time. Given the VMPC participants’ cognitive profile, as well as their performance on the current task, it is unlikely that their selective deficit on attempted harms is due to generic cognitive deficits.
A second alternative hypothesis is that VMPC participants’ performance on the moral judgment task may be attributed to a deficit in basic theory of mind or false belief understanding. In other words, damage to the VMPC in the current participants could have resulted in a deficit in attributing intentions across all conditions. This alternative hypothesis also appears unlikely because VMPC participants did not make abnormal moral judgments across all conditions. Instead, VMPC participants showed a selective deficit for attempted harms. Importantly, VMPC participants exhibited normal performance on accidental harms, and distinguished accidental harms from successful attempts to harm. Moral judgments of successful attempts to harm could be made on the basis of outcome information alone. However, moral judgments of accidental harms require attributing beliefs and intentions. VMPC participants’ moral judgments of accidental harms may therefore reflect intact processing of neutral intentions as well as negative outcomes. Indeed, VMPC participants were even able to discriminate between attempted harms and non-harms, suggesting an intact capacity to represent the specific content of negative beliefs and intentions. VMPC participants’ selective failure on attempted harms cannot therefore be due to a deficit in representing the content of either a negative or a neural mental state, a belief or an intention. In light of the current pattern of results, as well as prior work on the role of the VMPC in emotional processing, we suggest instead that VMPC participants’ abnormal responding to attempted harms may be mediated by a specific deficit in triggering a sufficiently robust emotional response to these representations, in this case, an aversive response to harmful intent (Bechara et al., 1997; Beer et al., 2003; Damasio et al., 1990). While we did not measure VMPC participants’ theory of mind or false belief understanding outside the moral judgment task, nor did we measure explicit intention understanding during the task, the full pattern of results suggests that VMPC patients are not impaired in basic theory of mind.
Prior evidence has suggested a specific role for the VMPC in processing affective aspects of another person's mental states (Jenkins and Mitchell, 2009; Mitchell et al., 2006; Shamay-Tsoory and Aharon-Peretz, 2007; Vollm et al., 2006). The current finding that the VMPC is associated with processing intentions with high emotional content, i.e. negative intentions, for moral judgment, is consistent with the role of the VMPC in “affective” or “hot” theory of mind (Jenkins and Mitchell, 2009; Mitchell et al., 2006; Shamay-Tsoory and Aharon-Peretz, 2007; Vollm et al., 2006). The current results are also consistent with a recent fMRI finding of a selective positive correlation between the average response in the VMPC and moral judgments of attempted harms (Young and Saxe, 2009b). Healthy adult participants with a high VMPC response assigned more moral blame to agents for harmful intentions, in the absence of any actual harmful outcome. Together, these results support the significance of the VMPC in moral judgments of harmful intentions and therefore attempted harms. We note, though, that the VMPC targeted in neuroimaging and neuropsychological work spans a large cortical region; future work is therefore needed in order to further elucidate the functional organization of the VMPC, and its contribution to different aspects of the decision-making process.
A fundamental component of normal moral judgment is the ability to blame those who intend harm, even when they fail to cause harm. We recognize failed attempts to harm as deserving of moral blame; failed attempts represent instances in which we might even be motivated to punish at a cost to ourselves (Cushman et al., 2009; de Quervain et al., 2004; Moll et al., 2006). In fact, the ability to blame for failed attempts not only features prominently in mature moral judgments but emerges quite early in development: typically developing children use mental state information (i.e. harmful intent) to assign blame for attempted harms, well before they are able to use mental state information (i.e. neutral intent) to mitigate blame for accidental harms (Baird and Astington, 2004; Piaget, 1965/1932). Thus, while the standard challenge for healthy children and adults lies in forming exculpatory moral judgments or forgiveness (e.g., judging accidental harms as morally permissible on the basis of agents’ neutral intentions), the opposite seems to hold true in the case of VMPC damage. Attributing moral blame even for failed murder attempts therefore poses a unique challenge for VMPC patients.
The current results reveal an important aspect of VMPC function for moral judgment, specifically, its role in evaluating harmful intent. In conjunction with prior work on the role of the VMPC in emotional processing, these results further suggest that an emotional response to harmful intent is crucial for condemning failed attempts. Given the critical role of intent in moral judgment, and social cognition more generally, understanding the neural basis of how intent is processed will be essential in helping us understand human moral judgment.
Experimental Procedures
Subjects
Nine patients with bilateral, adult-onset damage to the VMPC and seven brain damaged comparison patients who had lesions that excluded structures thought to be important for emotions (VMPC, amygdala, insula, right somatosensory cortices) were recruited from the Patient Registry of the Division of Cognitive Neuroscience at the University of Iowa. Five of these nine VMPC participants were previously tested on a moral dilemmas task, described above (Koenigs et al., 2007); see Supplemental Analyses. Eight healthy comparison subjects with no brain damage were recruited from the Iowa community. Groups were age-, gender-, and ethnicity-matched. All participants gave written informed consent.
Neuroanatomical analysis
All subjects had MR scans (3) or CT scans (6) obtained in the chronic epoch of their lesions, and all scans were reconstructed in 3 dimensions using Brainvox (Damasio and Frank, 1992; Frank et al., 1997). The lesions were analyzed on each individual scan. Subsequently, the contours of the nine target lesions were mapped onto a non-lesioned standard brain, using the MAP-3 technique (Damasio, 2005), to visualize the region of maximal overlap (Figure 1).
Stimuli and task
We presented participants with 24 scenarios, selected from a previously published set (Young et al., 2007; Young and Saxe, 2008). There were four variations (conditions) of each scenario, following a 2 × 2 design: (1) protagonists either harmed another person (negative outcome) or did no harm (neutral outcome); (2) protagonists either believed they would cause harm (negative intent) or believed they would cause no harm (neutral intent). Each possible belief was true for one outcome and false for the other outcome; the agent held true beliefs in the all-neutral and all-negative conditions and false beliefs in the accidental harm and attempted harm conditions. Subjects saw one version of each scenario. Stimuli were presented in a pseudorandom order; conditions were counterbalanced across subjects. Subjects read six stimuli per each of the four conditions. Across subjects, every scenario occurred in each of the four conditions.
Word count was matched across conditions (mean ± s.d. for the all-neutral condition: 103 ± 10; accidental harm: 101 ± 9; attempted harm: 103 ± 10; intentional harm: 103 ± 9). On average, scenarios featuring negative beliefs contained the same number of words as scenarios featuring neutral beliefs (F(1, 23)=0.15 p=0.70, partial h2 =0.006); scenarios featuring negative outcomes contained the same number of words as scenarios featuring neutral outcomes (F(1, 23)=0.17 p=0.68, partial h2=0.007).
We presented each story in four cumulative segments (previous segments remained on the screen when later segments were added): (1) background information to set the scene, (2) facts foreshadowing the eventual outcome, (3) the protagonist's belief (from which intent could be inferred), (4) the protagonist's action and its outcome. The question and response scale were then added to the screen. Participants made moral judgments of the protagonist's action on a scale of 1 (forbidden) to 7 (permissible), using a computer keyboard. Participants read and responded at their own pace, pressing the spacebar to add the next segment of the story and finally the question. There was no time limit for reading or responding.
Supplementary Material
Acknowledgements
We thank Rebecca Saxe and Josh Greene for their helpful comments on an earlier draft of this manuscript. This research was supported in part by a grant from the NSF-HSD (A.B., A.D., M.H.), NINDS (P01 NS19632) (A.D., H.D., D.T.), gift funds from J. Epstein and S. Shuman (M.H.), by NIDA (DA022549) (D.T.), and the NSF (L.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. J Int Neuropsychol Soc. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Baird JA, Astington JW. The role of mental state understanding in the development of moral cognition and moral action. New Directions for Child and Adolescent Development. 2004;103:37–49. doi: 10.1002/cd.96. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson S. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio A. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. J Pers Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Borg JS, Hynes C, Van Horn J, Grafton S, Sinnott-Armstrong W. Consequences, action, and intention as factors in moral judgments: an FMRI investigation. Journal of Cognitive Neuroscience. 2006;18:803–817. doi: 10.1162/jocn.2006.18.5.803. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Forbes C, Costello A, Coates LM, Dawson DR, Anderson ND, Gilbert SJ, Dumontheil I, Channon S. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. J Int Neuropsychol Soc. 2006;12:194–209. doi: 10.1017/S1355617706060310. [DOI] [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Casebeer W, Churchland P. The Neural Mechanisms of Moral Cognition: A Multiple-Aspect Approach to Moral Judgment and Decision-Making. Biology and Philosophy. 2003;18:169–194. [Google Scholar]
- Ciaramelli E, Muccioli M, Ladavas E, di Pellegrino G. Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Social Cognitive and Affective Neuroscience. 2007;2:84–92. doi: 10.1093/scan/nsm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman F. Crime and Punishment: Distinguishing the roles of causal and intentional analysis in moral judgment. Cognition. 2008;108:353–380. doi: 10.1016/j.cognition.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Cushman F, Dreber A, Wang Y, Costa J. Accidental outcomes guide punishment in a “trembling hand” game. PLoS One. 2009;4:e6699. doi: 10.1371/journal.pone.0006699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio H. Human Brain Anatomy in Computerized Images. 2nd edn Oxford University Press; New York: 2005. [Google Scholar]
- Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans. Arch Neurol. 1992;49:137–143. doi: 10.1001/archneur.1992.00530260037016. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. NeuroImage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. The ethical brain. Dana Press; New York: 2005. [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Mol Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Haidt J. The new synthesis in moral psychology. Science. 2007;316:998–1002. doi: 10.1126/science.1137651. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Hamaan S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A. An fMRI study of simple ethical decision-making. Neuroreport. 2003;14:1215–1219. doi: 10.1097/00001756-200307010-00005. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Mentalizing under Uncertainty: Dissociated Neural Responses to Ambiguous and Unambiguous Mental State Inferences. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobe J. Theory of Mind and Moral Cognition: Exploring the Connections. Trends in Cognitive Sciences. 2005;9:357–359. doi: 10.1016/j.tics.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29:2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Nakic M, Wheatley T, Richell R, Martin A, Blair RJ. The neural basis of implicit moral attitude-An IAT study using event-related fMRI. Neuroimage. 2006;30:1449–1457. doi: 10.1016/j.neuroimage.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Mendez M, Anderson E, Shapira J. An Investigation of Moral Judgment in Frontotemporal Dementia. Cognitive and Behavioral Neurology. 2005;18:193–197. doi: 10.1097/01.wnn.0000191292.17964.bb. [DOI] [PubMed] [Google Scholar]
- Mikhail JM. Universal moral grammar: theory, evidence and the future. Trends in Cognitive Sciences. 2007;11:143–152. doi: 10.1016/j.tics.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiulo PA, Pessoa L. The neural correlates of moral sensitivity: A functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002;22:2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci U S A. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price J. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Piaget J. The Moral Judgment of the Child. Free Press; New York: 1965/1932. [Google Scholar]
- Rolls E. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;3:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–1249. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45:3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Valdesolo P, DeSteno D. Manipulations of Emotional Context Shape Moral Judgment. Psychological Science. 2006;17:476. doi: 10.1111/j.1467-9280.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Haidt J. Hypnotic disgust makes moral judgments more severe. Psychological Science. 2005;16:780–784. doi: 10.1111/j.1467-9280.2005.01614.x. [DOI] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proc Natl Acad Sci U S A. 2007;104:8235–8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Koenigs M. Investigating emotion in moral cognition: A review of evidence from functional neuroimaging and neuropsychology. British Medical Bulletin. 2007;84:69–79. doi: 10.1093/bmb/ldm031. [DOI] [PubMed] [Google Scholar]
- Young L, Nichols S, Saxe R. Investigating the neural and cognitive basis of moral luck: It's not what you do but what you know. Review of Philosophy and Psychology. doi: 10.1007/s13164-010-0027-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Saxe R. The neural basis of belief encoding and integration in moral judgment. NeuroImage. 2008;40:1912–1920. doi: 10.1016/j.neuroimage.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R. An FMRI investigation of spontaneous mental state inference for moral judgment. J Cogn Neurosci. 2009a;21:1396–1405. doi: 10.1162/jocn.2009.21137. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R. Innocent intentions: a correlation between forgiveness for accidental harm and neural activity. Neuropsychologia. 2009b;47:2065–2072. doi: 10.1016/j.neuropsychologia.2009.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.