Abstract
Diminished thiamine (vitamin B1) dependent processes and oxidative stress accompany Alzheimer’s disease (AD). Thiamine deficiency in animals leads to oxidative stress. These observations suggest that thiamin may act as an antioxidant. The current experiments first tested directly whether thiamin could act as an antioxidant, and then examined the physiological relevance of the antioxidant properties on oxidant sensitive, calcium dependent processes that are altered in AD. The first group of experiments examined whether thiamin could diminish reactive oxygen species (ROS) or reactive nitrogen species (RNS) produced by two very divergent paradigms. Dose response curves determined the concentrations of t-butyl-hydroperoxide (t-BHP) (ROS production) or 3-morpholinosydnonimine ((SIN-1) (RNS production) to induce oxidative stress within cells. Concentrations of thiamine that reduced the RNS in cells did not diminish the ROS. The second group of experiments tested whether thiamine alters oxidant-sensitive aspects of calcium regulation including endoplasmic reticulum (ER) calcium stores and capacitative calcium entry (CCE). Thiamin diminished ER calcium considerably, but did not alter CCE. Thiamine did not alter the actions of ROS on ER calcium or CCE. On the other hand, thiamine diminished the effect of RNS on CCE. These data are consistent with thiamine diminishing the actions of the RNS, but not ROS, on physiological targets. Thus, both experimental approaches suggest that thiamine selectively alters RNS. Additional experiments are required to determine whether diminished thiamine availability promotes oxidative stress in AD or whether the oxidative stress in AD brain diminishes thiamine availability to thiamine dependent processes.
Keywords: Thiamine, oxidative stress, neurodegenerative disease, calcium
INTRODUCTION
Thiamine (vitamin B1) may serve several roles in brain in addition to its role as a cofactor of vital enzymes. For example, recent studies report the first adenine nucleotide containing vitamin B1, adenosine thiamine triphosphate (AThTP) or thiaminylated ATP [1]. Many thiamine-dependent processes are diminished in brains from patients with Alzheimer’s disease (AD) [2] so that an understanding of thiamine’s other roles may be clinically important. Several lines of evidence suggest that thiamine may serve as an antioxidant. In mice, thiamine deficiency (TD) reduces thiamine-dependent enzymes [3,4] and increases many markers of oxidative stress that are also elevated in brains from AD patients [5,6]. Furthermore, homogenates of thalamus and cortex from TD rats produce excess reactive oxygen species (ROS) [7]. The antioxidant vitamin E provides significant neuroprotection to TD neurons in vitro [8]. The elevation in lipid peroxidation and reduction in glutathione reductase (Grx) observed during cardiac hypertrophy are normalized by thiamine [9]. Together these observations suggest that thiamine may act as an antioxidant. Brains from AD patients reveal considerable damage from oxidative stress [10]. Thiamine diphosphate (TDP) is a cofactor for enzymes of major pathways of energy metabolism and these thiamine-dependent enzymes are reduced in AD brain as well as in several other neurodegenerative disorders [3,6,11]. In AD, levels of TDP are significantly reduced in the three cortical brain areas that were examined [12]. Clinical data suggest that high-dose thiamine may have a mild beneficial effect in some patients with AD [2]. The experiments first tested whether thiamine could act as an antioxidant for reactive oxygen species [13]or reactive nitrogen species (RNS). The subsequent experiments also determined whether thiamine would alter the actions of ROS and RNS on physiological targets (i.e., intracellular calcium pools that are altered in AD).
The selections of sources and concentrations of ROS and RNS were based on the ability of the oxidants to selectively interact with fluorescent probes and to alter cellular calcium pools. The addition of tert-butyl-hydroxyperoxide (t-BHP) to cells increases ROS as detected by 6-carboxy-2',7'- dichlorodihydrofluorescein diacetate, di(acetoxymethyl ester) (DCF). On the other hand, the addition of 3-morpholinosydnonimine (SIN-1) increases ROS as measured with DCF or RNS as detected by 4-amino-5-methylamino-2', 7'- difluorofluorescein (DAF). The two approaches also vary in the way they induce ROS. t-BHP acts directly whereas SIN-1 involves tissue generation of RNS. Previous studies demonstrated selective effects of t-BHP and SIN-1 on endoplasmic reticulum (ER) bombesin releasable calcium stores (BRCS) [14,15]. t-BHP increases BRCS without altering cytosolic calcium, whereas SIN-1 does not affect BRCS. The depletion of ER Ca2+ content triggers Ca2+ influx through plasma membrane Ca2+ channels, a process known as capacitative calcium entry (CCE) [16]. The effects of t-BHP and SIN-1 on CCE are unknown. The t-BHP induced increase in DCF and BRCS can be selectively blocked by α-keto-β-methylvalerate (KMV). The increase in BRCS in fibroblasts from AD patients can also be blocked by KMV, which suggests the same radicals may be involved in producing the AD related changes [15]. The current studies confirmed the effects of t-BHP and SIN-1 on BRCS, tested their effect on CCE, and then determined whether thiamine altered their action on cellular calcium regulation.
EXPERIMENTAL PROCEDURE
The supplies were from the indicated companies: Cell culture reagents (GIBCO; Grand Island, NY); 6-carboxy-2',7'- dichlorodihydrofluorescein diacetate, di(acetoxymethyl ester) DCF, fura-2 acetoxymethyl ester (Fura-2), 4-amino-5-methylamino-2',7'- difluorofluorescein diacetate (DAF), 3-morpholinosydnonimine (SIN-1) (Molecular Probes; Eugene, Oregon), bombesin, tert-butyl-hydroxyperoxide (t-BHP) and thiamine (Sigma Chemical, St Louis, MO).
A human skin fibroblast cell line from a young male control (8399) was purchased from Coriell Cell Repository (Camden, NJ). Cells were maintained exactly as described in our published protocol [17].
ROS measurement
Fibroblasts were seeded at 2.8×103 cells/ well in 96 well plates seven days before experiments [14,15]. On the day of experiment, cells were washed with balanced salt solution (BSS) buffer [(mM): NaCl (140), KCl (5), CaCl2 (2.5), MgCl2 (1), glucose (5), HEPES (10), pH 7.4] and loaded with DCF (10 µM) or DAF (10 µM) with BSS for 1 hr at 37°C. After loading, cells were rinsed once and incubated with oxidants in Ca2+-free BSS. Fluorescent signals were read in a plate reader (Molecular Devices, Sunnyvale, CA) at 25°C at excitation /emission wavelengths of 485/538 nm for DCF-ROS and of 490 /515 nm for DAF.
Bombesin releasable calcium stores (BRCS)
Internal calcium stores were monitored as described previously [14,15]. Fibroblasts were loaded with 2 µM Fura-2 in BSS for one hr at room temperature and rinsed twice with Ca2+-free BSS. [Ca2+]i was monitored on the stage of an inverted Olympus IX70 microscope at room temperature with a Delta Scan System from PTI (Photon Technology International, Lawrenceville, NJ). Excitation wavelengths were alternated between 350 and 378 nm (band pass 4 nm) and emission was monitored at 510 nm with a Hamamatsu C2400 SIT camera at 5 sec intervals. Basal [Ca2+]i was measured for 1 min. Bombesin (200 nM) and [Ca2+]i was added to release ER calcium and the signal was measured for another 5 min. Each value was the average of 32 images taken within 5 sec. Standard images of Fura-2 solutions with minimum and maximum [Ca2+]i were taken at the end of each day's experiment to calculate the intracellular calcium concentrations.
Measurement of capacitative calcium entry (CCE)
After cells were preincubated in Ca2+-free media with or without bombesin or the ER Ca2+-ATPase inhibitor cyclopiazonic acid (CPA), CaCl2 (2.5 mM) was added. In Ca2+-free media, bombesin will release ER Ca2+ from InsP3 sensitive stores, and CPA without bombesin will release InsP3 insensitive Ca2+ stores. The resulting increase in [Ca2+]i is an estimate of CCE.
Statistical analysis
All data are expressed as mean ± SEM. A Student’s t-test was used to compare two variables. For multiple variable comparisons, data were analyzed by a one-way analysis of variance (ANOVA) followed by a Student Newman-Keul’s test.
RESULTS
Thiamine diminished DCF-detectable ROS production induced by t-BHP in fibroblasts
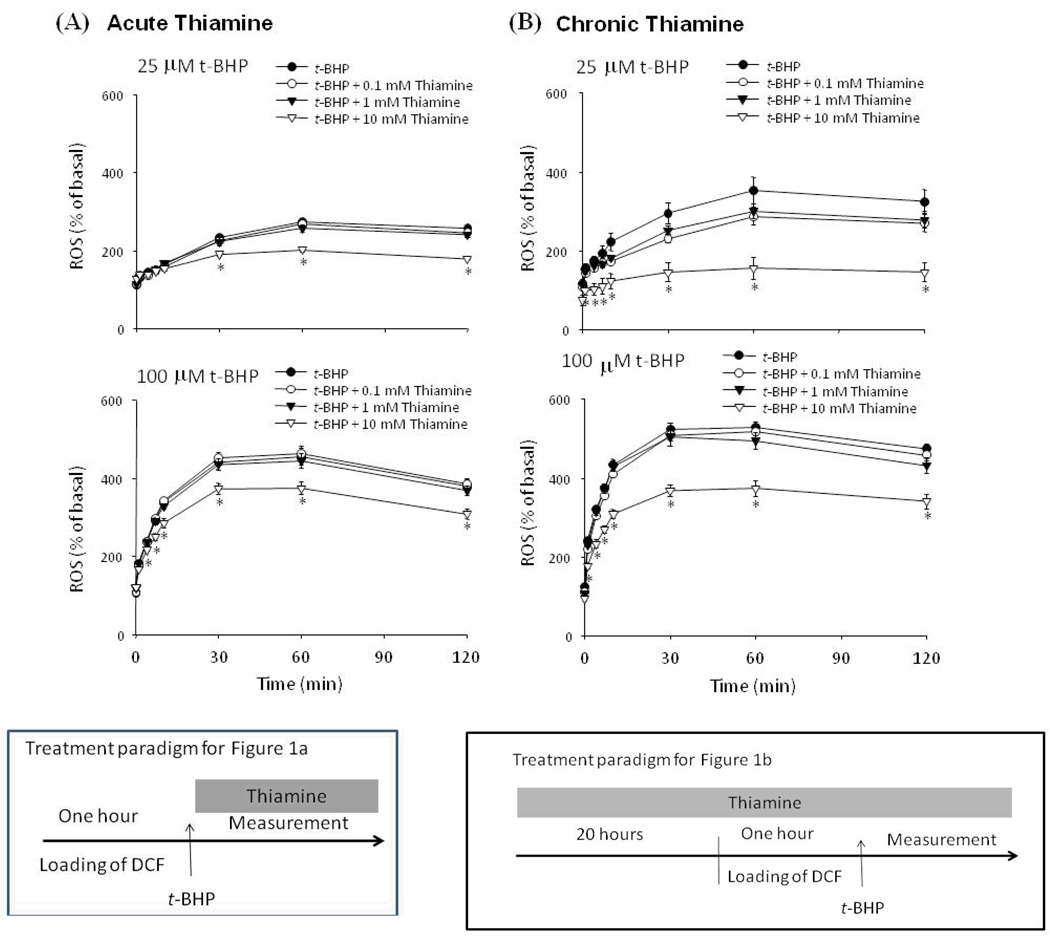
Treatment of fibroblasts with t-BHP increased DCF-detectable ROS in a dose dependent manner (Fig. 1). Acute treatment with a high concentration of thiamine (10 mM) diminished DCF-detectable ROS production induced by either 25 (−32%) or 100 (−20%) µM t-BHP (Fig. 1a). Lower concentrations of thiamine (0.1 and 1 mM) were ineffective (Fig. 1a). Chronic thiamine treatment (20 hr) was more effective at reducing t-BHP-induced DCF-detectable ROS production than acute treatment. Chronic treatment with 10 mM thiamine reduced DCF-detectable ROS induced by 25 (−76%) or 100 µM (−37%) t-BHP, respectively (Fig. 1b). Lower concentrations of thiamine (0.1 and 1 mM) were still not effective.
Figure 1.
Only high concentrations of thiamine diminished DCF-detectable ROS production by t-BHP. (a) t-BHP (25, 100 µM) and/or thiamine (0.1, 1 and 10 mM) were incubated with cells after they were loaded with DCF.
(b) Fibroblasts were treated with thiamine (0.1, 1 and 10 mM) for 20 hr prior to loading with DCF. The cells were then rinsed once and were incubated with Ca2+-free BSS containing t-BHP (25, 100 µM) and thiamine (0.1, 1 and 10 mM), the fluorescent signals were monitored for 0, 1, 4, 7, 10, 30, 60 and 120 min.
Values are expressed as a percentage increase of basal fluorescence. Data are mean ± SEM (n=10). * Denotes value varies significantly (p<0.05) from the group without thiamine by ANOVA followed by Student Newman Keul’s test.
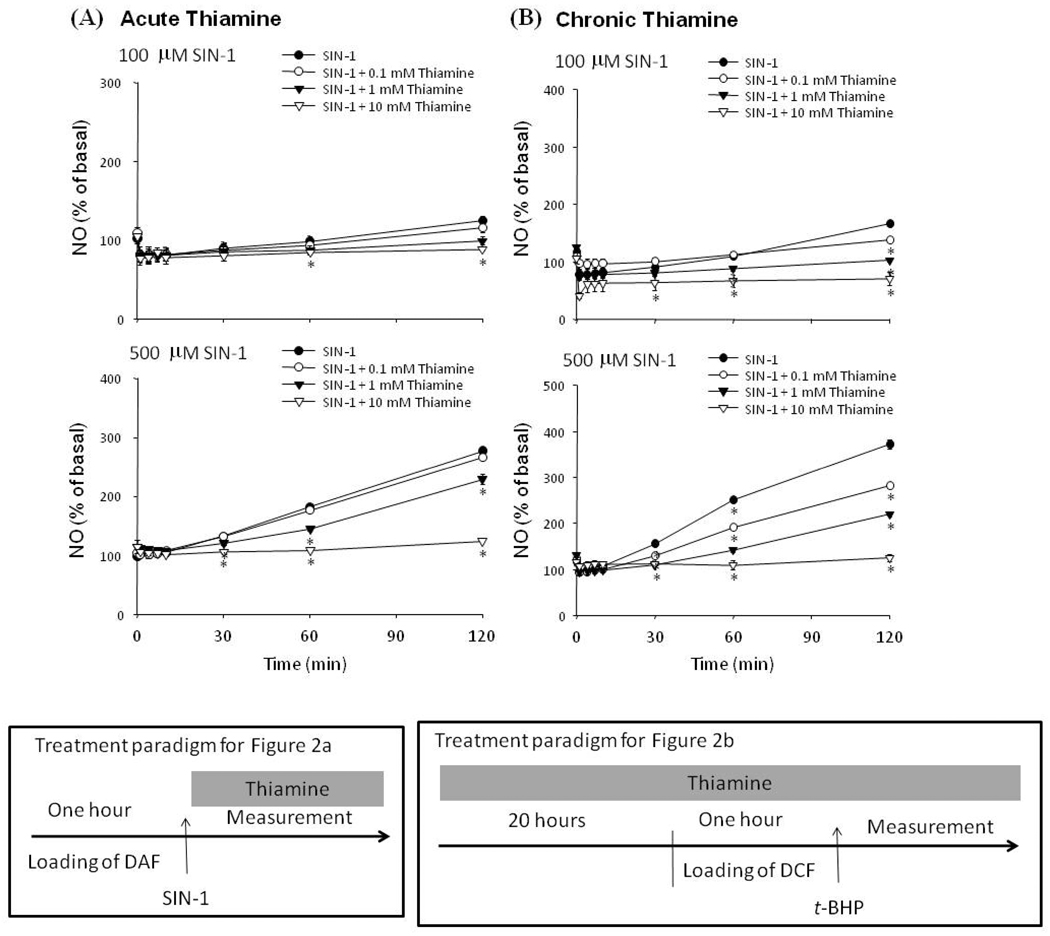
Thiamine diminished SIN-1 induced DAF-detectable NO˙ production in a dose- and time-dependent manner in fibroblasts
SIN-1 spontaneously decomposes to yield NO˙ and superoxide (O2˙−) radicals, which subsequently form peroxynitrite (ONOO−) (see discussion). SIN-1 produced a dose-dependent increase in DAF-detectable RNS (Fig. 2). Low concentrations of SIN-1 (100 µM) induced small but detectable DAF signals, whereas 500 µM SIN-1 increased DAF-FM-detectable NO˙ production to 132 % of control within 30 min and to 278 % of control within 120 min (Fig. 2a). Both acute and chronic thiamine treatments diminished SIN-1 induced DAF-detectable RNS in a dose-dependent manner. With acute treatment, the lowest concentration of thiamine (0.1 mM) treatment did not affect SIN-1 induced DAF-detectable RNS˙ production. One mM thiamine diminished SIN-1 induced DAF- NO˙ by 46% by 60 min, and 10 mM thiamine reduced SIN-1 induced DAF-detectable NO˙ production by >90% (Fig. 2a). Chronic thiamine treatment diminished DAF-detectable NO˙ production by SIN-1 in a time and dose dependent manners and the effect was stronger than with acute administration (Fig. 2b). Chronic thiamine at a low concentration (0.1 mM) effectively diminished SIN-1 induced DAF-detectable NO˙ by 30 min. At 60 min, thiamine (0.1, 1 and 10 mM) reduced SIN-1 induced DAF- detectable NO˙ production (−40, −72 and −94%, respectively).
Figure 2.
Thiamine treatment diminished DAF-detectable NO˙ production by SIN-1 (a) SIN-1 (100, 500 µM) and/or thiamine (0.1, 1 and 10 mM) were incubated with cells after they were loaded with DAF.
(b) Cells were pretreated with thiamine (0, 0.1, 1 and 10 mM) for 20 hr prior to loading with DAF for 60 min, and then incubated with SIN-1 (100, 500 µM) in the presence of thiamine. Values are expressed as a percentage increase of basal fluorescence. Data are mean ± SEM (n=10). * Denotes value varies significantly (p<0.05) from the group without thiamine by ANOVA followed by Student Newman Keul’s test.
Oxidants selectively modify the BRCS and CCE
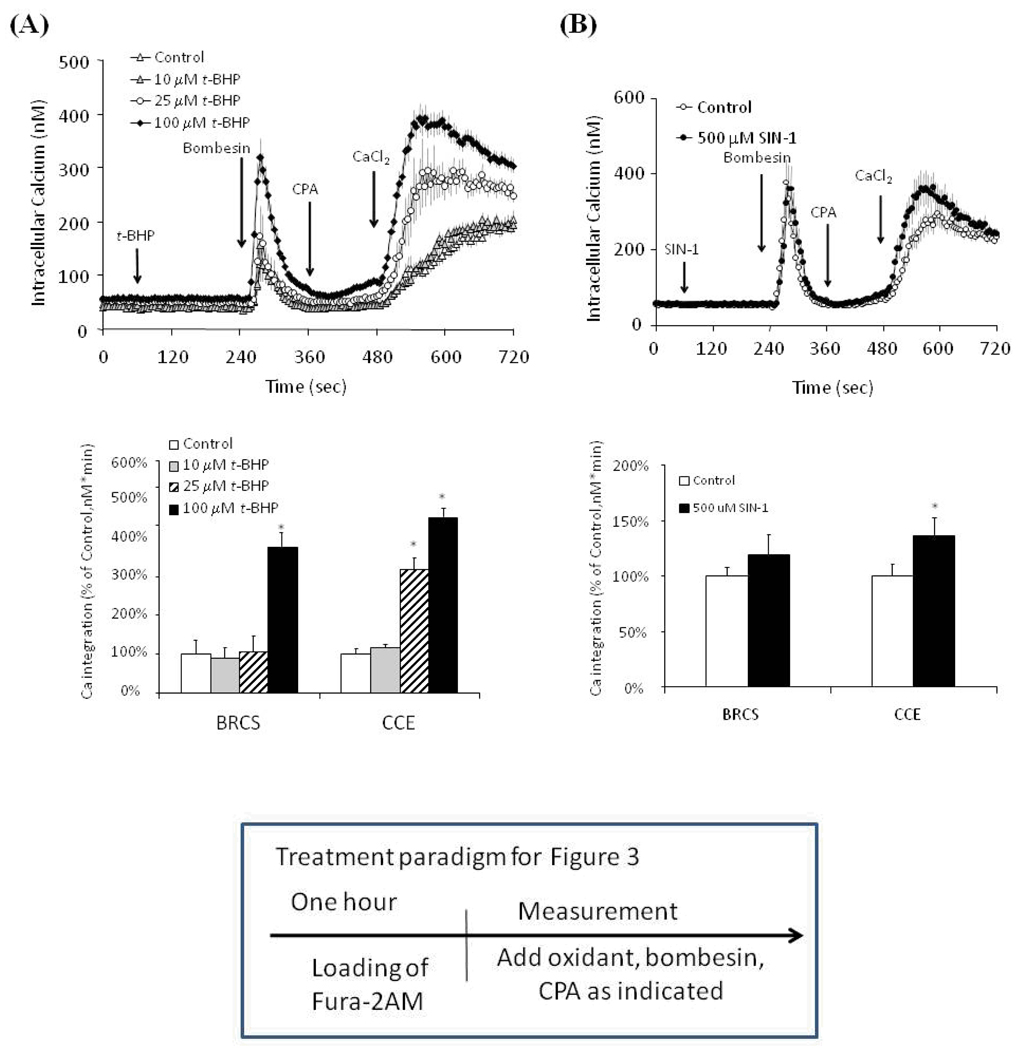
Subsequent experiments tested whether physiological targets (i.e., intracellular calcium pools) of the oxidants were altered by thiamine. Previous studies demonstrated selective effects of t-BHP and SIN-1 on ER bombesin releasable calcium stores (BRCS). They were increased by t-BHP, but SIN-1 did not affect them. The bombesin-induced release of ER calcium stimulates capacitative calcium entry (CCE). The current studies first tested the effects of oxidants on BRCS [(a confirmation of previous studies [14,15] and CCE and then determined whether thiamine showed a selective action of the oxidant induced changes in the cells. t-BHP (100 µM) exaggerated BRCS (as in our previous studies) [14,15] and elevated CCE (Fig. 3a). t-BHP at 25 and 100 µM increased BRCS from control by 5% and 377%, respectively. Subsequent, CCE was elevated significantly from control by 318% and 454%, respectively.
Figure 3.
Effects of t-BHP (a) and SIN-1 (b) on BRCS and CCE.
Fibroblasts were loaded with Fura 2 for 60 min. After loading, the media were changed to calcium free BSS and 3 min later the calcium measurements were initiated.
(a) t-BHP (0, 10, 25 and 100 µM) or (b) SIN-1 (100 and 500 µM) were added after 1 min, and bombesin (1 µM) was added 3 min later. After an additional 3 min, CPA (2 µM) was added and 3 min later CaCl2 (2.5 mM) was added. The top panel shows the tracings taken from 94–129 cells for t-BHP and from 160–183 cells for SIN-1 groups. The bottom panel shows the integration of the [Ca2+]i peak after bombesin addition or calcium addition. The values for integration [Ca2+]i peak over 1 min interval started from 255 sec and 495 sec, respectively. Data are means ± SEM for t-BHP (n=94–129 cells) and SIN-1 (n=160–183 cells). Values for BRCS or CCE with different letters vary significantly (p<0.05) from each other by ANOVA followed by Student Newman Keul’s test.
SIN-1-induced-DAF-detectable NO˙ affected BRCS and CCE very differently from t-BHP. In contrast to t-BHP, SIN-1 did not alter BRCS (Fig. 3b; in agreement with our previous studies [14] but elevated CCE by 37% (Fig. 3b). Thus, the two oxidants selectively alter BRCS and CCE.
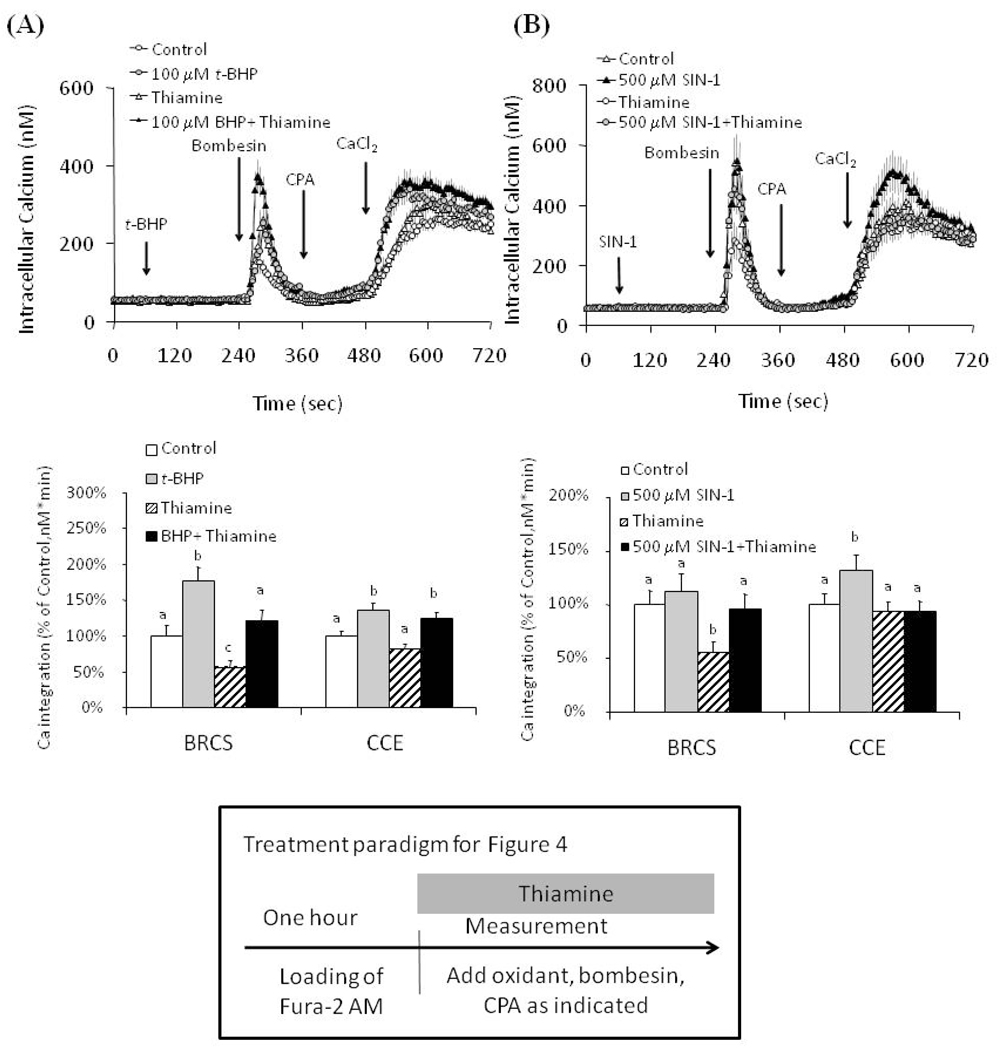
Thiamine selectively altered the BRCS and CCE in the presence and absence of t-BHP or SIN-1
To test whether thiamine altered BRCS and CCE with oxidant, cells were treated with thiamine (1 mM) in the presence or absence of oxidants prior to measurement of BRCS and CCE. The concentration of thiamine was selected because this was the minimum concentration that was required to block RNS under both acute and chronic conditions. Thiamine diminished BRCS by 44%. The depression was similar with (−31%) or without (−44%) t-BHP suggesting there was not an interaction t-BHP and thiamine. This is in agreement with Fig. 1 where thiamine (1 mM) did not reduce t-BHP induced changes in BRCS. Thiamine did not affect CCE in the presence or absence of t-BHP (Fig. 4a).
Figure 4.
Thiamine acts on the BRCS and CCE in the presence or absence of oxidants.
a. Thiamine diminished BRCS in the presence or absence of t-BHP, but did not affect CCE in the presence or absence of t-BHP. Fibroblasts were loaded with Fura 2 (2 µM) for 60 min. After loading, the media were changed to calcium free BSS with or without 1 mM-thiamine, and 3 min later the calcium measurements were initiated in the presence or absence of thiamine. t-BHP (100 µM) was added after 1 min basal [Ca2+]i measurement, and bombesin (1 µM) was added 3 min after t-BHP treatment. After an additional 3 min, CPA (2 µM) was added, and 3 min later CaCl2 (2.5 mM) was added. The top panel shows the tracings taken from 94–113 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 1 min interval after calcium addition. Data are means ± SEM (n = 94–113 cells). Values for BRCS or CCE with different letters vary significantly (p<0.05) from each other by ANOVA followed by Student Newman Keul’s test.
b. Thiamine diminished the exaggeration of CCE by SIN-1. Fibroblasts were loaded with Fura 2 (2 µM) for 60 min. After loading, the media were changed to calcium free BSS with or without thiamine, and 3 min later the calcium measurements were initiated in the presence or absence of thiamine. SIN-1 (500 µM) was added after 1 min basal [Ca2+]i measurement, and bombesin (1 µM) was added 3 min after SIN-1 treatment. After an additional 3 min, CPA was added, and 3 min later CaCl2 (2.5 mM) was added. The top panel shows the tracings taken from 79–128 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 1 min interval after bombesin or calcium addition. Data are means ± SEM (n= 79–128 cells). Values for BRCS or CCE with different letters vary significantly (p<0.05) from each other by ANOVA followed by Student Newman Keul’s test.
SIN-1 had no effect on BRCS but elevated CCE. Although thiamine had no effect on CCE, it completely blocked the SIN-1-induced exaggeration in CCE. This supports the suggestion that thiamine could diminish a physiological effect of SIN-1 induced RNS (Fig. 4b).
DISCUSSION
Two diverse oxidants were selected to test the role of thiamine as an antioxidant. The concentrations of SIN-1 and t-BHP were selected based on their ability to induce ROS or RNS in cells, respectively. The concentrations of the two oxidants that were used were very different, but at the utilized concentrations they produced similar magnitude of increases in cellular ROS or RNS. It is not surprising that a higher concentration of SIN-1 would be required because t-BHP acts directly whereas SIN-1 involves tissue generation of RNS. The selective oxygen species produced by SIN-1 and t-BHP showed different actions on BRCS and CCE.
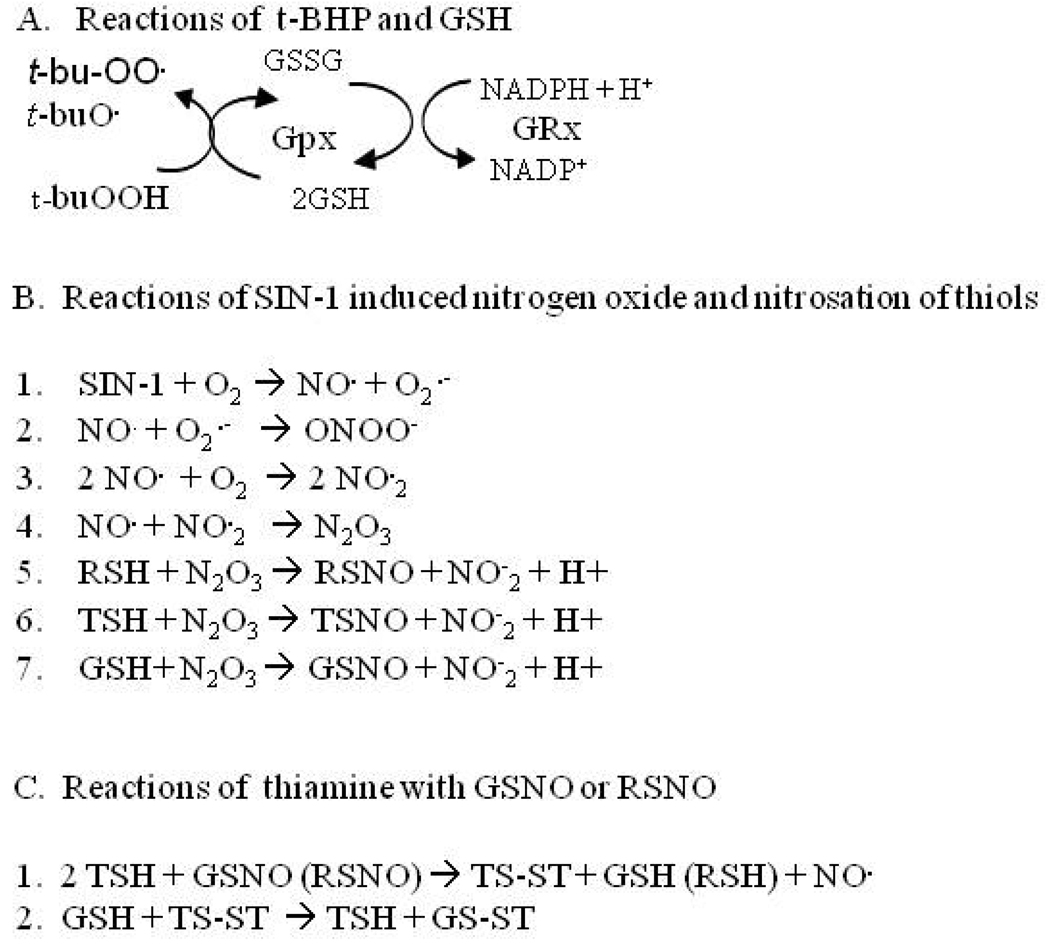
t-BHP increased BRCS and CCE. t-BHP produces the radicals tert-butyloxyl (t-bu-O˙) and t- butylperoxyl (t-bu-OO˙) [14,18], induces lipid peroxidation, activates glutathione peroxidase (Gpx) to oxidize glutathione (GSH) to form glutathione disulfide (GSSG) [19,20], decreases glutathione reductase (GRx) [9]and depletes endogenous GSH (Fig. 5A). When GSH is oxidized to form GSSG, the levels of t-bu-O˙ radicals will be increased (Fig. 5A). The t-BHP induced exaggeration of BRCS could be mediated by Ca2+ channels proteins, InsP3 or ryanodine receptors [21], endoplasmic reticulum Ca2+-ATPase, proteins of the Bcl-2/Bax family, the mitochondria Na+/Ca2+ exchanger or the Ca2+ uniporter that regulates interactions between mitochondria and ER Ca2+ [22]. Previous studies suggest that t-BHP increases Ca2+ release through modification of the SH groups of the InsP3 receptor [23]or ryanodine receptor rather than by inhibition of the ER Ca2+-ATPase activity or activation of passive Ca2+ leak pathway in ER [24]. Since our only measure in these studies was [Ca2+]i, the detailed mechanisms cannot be assessed. However, these data support the suggestion of a selective action of oxidants on different aspects of the interaction of BRCS and CCE, and that these interactions can be revealed in cell systems by the use of multiple oxidants and multiple detection systems [14,15].
Figure 5.
Reactions of t-BHP and SIN-1 with antioxidants and with thiamine (references are in the text. See also [29].
SIN-1 altered CCE but not BRCS. In the presence of O2, SIN-1 releases both NO˙ and O2˙− (Fig. 5B Reaction 1), which generates OONO− (Fig. 5B Reaction 2) [25]. O2 reacts with NO˙ to form NO˙2 (Fig. 5B Reaction 3), which can react with NO˙ to produce dinitrogen trioxide (N2O3) (Fig. 5B Reaction 4) [26,27,28,29]. N2O3 is known to be highly effective in nitrosating sulfhydryl groups [28], which can react with the thiol groups of proteins (RSH), thiamine (TSH) or glutathione (GSH) to form S-nitrosothiol (RSNO) (Fig. 5B Reaction 5), nitroso-thiamine (TSNO) (Fig. 5B Reaction 6) or S-nitrosoglutathione (GSNO) (Fig. 5B; Reaction 7). Depending on the cell type, NO˙ has been reported to either potentiate CCE in pancreatic acinar cells and colonic epithelial cells [30] or to inhibit CCE in platelets [31] and smooth muscle cells [32]. NO• also has been reported to deplete ER calcium stores by inhibiting Ca2+-ATPase activity [33,34] or by activating ryanodine receptors [35]. Although the data suggests that SIN-1 altered CCE but not BRCS, the experimental approach did not rule out the possibility that SIN-1 facilitated the Ca2+ release from the ER, and this Ca2+ was taken up by mitochondria. In some cells, the actions of NO˙ on CCE may be through effects on mitochondrial Ca2+ handling [13,22]. Nevertheless, the results clearly show that regulation of CCE is sensitive to different oxidants than the steps regulating BRCS and is cell type specific.
The current results show that thiamine is only modestly effective in reducing t-BHP-induced DCF-ROS but that it is much more effective with SIN-1-induced-DAF-ROS. This contrasts with α-keto-β-methyl valerate, which did not effectively diminish DAF-detectable ROS but did reduce DCF-detectable ROS [15]. Thiamine may serve as an effective scavenger to neutralize nitrogen species produced by SIN-1 by forming S- nitrosothiols. The concentrations of thiamine that were used are far higher than physiological. In rat brain the concentrations (µM) are 11.4. 1.5 and <0.3 for thiamine diphosphate, thiamine monophosphate and thiamine, respectively. Others suggest that TDP may be an even more effective antioxidant [36]. Furthermore, the concentrations may be far higher in subcellular compartments [37]. Evidence suggests that the interactions of thiamine with NO•, glutathione, thiol containing proteins and oxidants may be important in cellular regulation of NO˙ and may change protein functions [29]. The reduced form of thiamine thiol (TSH) can be oxidized by GSNO to form GSH and thiamine disulfide (TS-ST) and release NO˙ [Fig. 5C Reaction 1]. GSH reacts with TS-ST through a thiol-disulfide exchange reaction to form thiol thiamine (TSH) and a combination of glutathione with thiamine disulfide (GS-ST) [Fig. 5C Reaction 2] [29]. Reactions of thiamine with GSNO and RSNO always release NO• to be neutralized again (Fig. 5).
These results are consistent with the results of others. Thiamine and thiamine diphosphate suppress superoxide generation by hypoxanthine and xanthine oxidase system. Their 50% inhibition (IC50) values were estimated to be 158 and 56 µM, respectively. They also depressed hydroperoxide generation derived from oxidized linoleic and their IC50 values were 260 and 46 µM. They further prevented the oxygen radical generation in opsonized zymosan-stimulated human blood neutrophils, and their IC50 values were 169 and 38 µM. In contrast, they caused weak suppression of hydroxyl radical generation by Fenton reaction (i.e., the IC50 values were 8.45 and 1.46 mM respectively. Thus, in all of these experiments thiamine diphosphate (TDP) was a better antioxidant than thiamine [36].
Thiamine blocked the downstream effects of SIN-1induced DAF-ROS on CCE. Thiamine had no effect on CCE, but abolished the exaggeration of CCE by SIN-1 [Fig. 4b]. This occurred at concentrations at which thiamine was effective as an antioxidant [Fig. 2]. In the presence of O2, GSH reacts with nitrogen oxygen species to form GSNO (Figure 5B, Reaction 7), which depletes ER calcium stores and down regulates Ca2+ ATPase [38]. Thus, thiamine competes with GSH to bind peroxynitrite or N2O3 and forms S-nitrosothiol (Figure 5B Reactions 6 vs. 7), which transnitrosates protein thiols in the ER and reduces Ca2+ release. Studies of thiamine deficiency provide indirect support for an interaction of nitric oxide or peroxynitrite with thiamine, and for an interaction of thiamine with the ER Ca2+. In brain, TD induces endothelial nitric oxide synthase isoform (eNOS) [39,40] increases lipid peroxidation [41] and tyrosine nitration in neurons within susceptible areas [5]. Furthermore, genetic deletion of eNOS protects against TD [5]. Thus, these results indirectly suggest that thiamine interacts with NO˙ or its oxidation product N2O3 that may modify the proteins that regulate BRCS and CCE.
Thiamine interacted with BRCS and CCE. Acute thiamine diminished BRCS approximately the same in the presence or absence of t-BHP. This suggests the two are acting by independent mechanisms. This is supported by the observation that thiamine effectively altered calcium stores at thiamine concentrations that were not effective as antioxidants. Thiamine may diminish BRCS by its capacity to abolish lipid peroxidation and reduce glutathione reductase as has been shown to occur in cardiac tissue. Thiamine increased the GSH level and stabilized SH groups of the InsP3 receptors [9]. The property of thiamine to normalize the elevated BRCS or CCE effectively may be because of thiamine’s ability to preserve the GSH and prevent S-nitrosothiolation of the thiol proteins in the ER or in the plasma membrane channels.
SUMMARY
Thiamine diminished ROS production by t-BHP and NO• production by SIN-1, but it was much more effective toward RNS. Several indirect lines of evidence suggest that thiamine acts as an NO• buffer. The results in this paper demonstrate directly that thiamine interacts with RNS. The results revealed a selective action of oxidants on calcium regulation. t-BHP exaggerated BRCS, whereas SIN-1 exaggerated only CCE. Thiamine diminished BRCS and CCE, but the effects appeared independent of the presence of t-BHP. Thiamine selectively diminished oxidant-induced RNS production and blocked their actions on CCE. Plausible mechanisms of oxidants interacting with the thiols group of thiamine may underlie these changes, and the greater effectiveness of thiamine on SIN-1 induced ROS. The results suggest that thiamine may selectively modify BRCS and CCE.
Acknowledgements
The work was supported by AG14930, AG14600, AG19589 and Burke Medical research Institute. The authors thank Dr. Arthur Cooper for his constructively critical comments.
Abel Lajtha has been an inspiration to me for my whole career as a neurochemist. I had the honor of interacting with him in his roles in the American Society for Neurochemistry, Neurochemical Research and the Handbook for Neurochemistry. His activities have had a large impact on my career and the whole neurochemistry community. His vision for each of these has been fulfilled. He has always made time for me and has always encouraged me. His faith in me enabled me to accomplish goals that I would not have attempted. I am very grateful to Abel for being such a positive influence for so many years.
Abbreviations
- BRCS
Bombesin-releasable calcium store
- BSS
balanced salt solution
- CCE
capacitative calcium entry
- CPA
cyclopiazonic acid
- [Ca2+]i
cytosolic free calcium concentration
- DCF
6-carboxy-2’,7’-dichlorodihydro-fluorescein diacetate (acetoxymethyl ester)
- DAF
diacetate (4-amino-5-methylamino- 2’,7’-difluorofluorescein diacetate)
- N2O3
dinitrogen trioxide
- DMEM
Dulbecco's modified Eagle's medium
- ER
endoplasmic reticulum
- Fura-2
fura-2-acetoxymethyl ester
- GSNO
S-nitrosoglutathione
- GSH
glutathione
- GSSG
glutathione disulfide
- Gpx
glutathione peroxidase
- GRx
glutathione reductase
- NO
nitric oxide
- OONO−
peroxinitrite
- PBS
phosphate-buffered saline
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SIN-1
3-morpholinosyndnonimine
- t-BHP
tert-butyl-hydroxyperoxide
- tert-butyloxyl (t-bu-O˙)
t- butylperoxyl (t-bu-OO˙)
- TD
thiamine deficiency
REFERENCES
- 1.Bettendorff L, Wirtzfeld B, Makarchikov AF, Mazzucchelli G, Frederich M, et al. Discovery of a natural thiamine adenine nucleotide. Nat Chem Biol. 2007;3:211–212. doi: 10.1038/nchembio867. [DOI] [PubMed] [Google Scholar]
- 2.Meador K, Loring D, Nichols M, Zamrini E, Rivner M, et al. Preliminary findings of high-dose thiamine in dementia of Alzheimer's type. J Geriatr Psychiatry Neurol. 1993;6:222–229. doi: 10.1177/089198879300600408. [DOI] [PubMed] [Google Scholar]
- 3.Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch Neurol. 1988;45:836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- 4.Sheu KF, Calingasan NY, Lindsay JG, Gibson GE. Immunochemical characterization of the deficiency of the alpha-ketoglutarate dehydrogenase complex in thiamine-deficient rat brain. J Neurochem. 1998;70:1143–1150. doi: 10.1046/j.1471-4159.1998.70031143.x. [DOI] [PubMed] [Google Scholar]
- 5.Calingasan NY, Gibson GE. Vascular endothelium is a site of free radical production and inflammation in areas of neuronal loss in thiamine-deficient brain. Ann N Y Acad Sci. 2000;903:353–356. doi: 10.1111/j.1749-6632.2000.tb06386.x. [DOI] [PubMed] [Google Scholar]
- 6.Gibson GE, Zhang H. Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem Int. 2002;40:493–504. doi: 10.1016/s0197-0186(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 7.Langlais PJ, Anderson G, Guo SX, Bondy SC. Increased cerebral free radical production during thiamine deficiency. Metab Brain Dis. 1997;12:137–143. [PubMed] [Google Scholar]
- 8.Pannunzio P, Hazell AS, Pannunzio M, Rao KV, Butterworth RF. Thiamine deficiency results in metabolic acidosis and energy failure in cerebellar granule cells: an in vitro model for the study of cell death mechanisms in Wernicke's encephalopathy. J Neurosci Res. 2000;62:286–292. doi: 10.1002/1097-4547(20001015)62:2<286::AID-JNR13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Tolstykh OI, Khmelevskii Iu V. The role of alpha-tocopherol and thiamine in the correction of lipid peroxidation in compensatory myocardial hypertrophy. Vopr Pitan. 1991:38–42. [PubMed] [Google Scholar]
- 10.Ding Q, Dimayuga E, Keller JN. Oxidative damage, protein synthesis, and protein degradation in Alzheimer's disease. Curr Alzheimer Res. 2007;4:73–79. doi: 10.2174/156720507779939788. [DOI] [PubMed] [Google Scholar]
- 11.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 12.Mastrogiacoma F, Bettendorff L, Grisar T, Kish SJ. Brain thiamine, its phosphate esters, and its metabolizing enzymes in Alzheimer's disease. Ann Neurol. 1996;39:585–591. doi: 10.1002/ana.410390507. [DOI] [PubMed] [Google Scholar]
- 13.Thyagarajan B, Malli R, Schmidt K, Graier WF, Groschner K. Nitric oxide inhibits capacitative Ca2+ entry by suppression of mitochondrial Ca2+ handling. Br J Pharmacol. 2002;137:821–830. doi: 10.1038/sj.bjp.0704949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang HM, Chen HL, Xu H, Gibson GE. Modification of endoplasmic reticulum Ca2+ stores by select oxidants produces changes reminiscent of those in cells from patients with Alzheimer disease. Free Radic Biol Med. 2005;39:979–989. doi: 10.1016/j.freeradbiomed.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Huang HM, Zhang H, Ou HC, Chen HL, Gibson GE. alpha-keto-beta-methyl-n-valeric acid diminishes reactive oxygen species and alters endoplasmic reticulum Ca(2+) stores. Free Radic Biol Med. 2004;37:1779–1789. doi: 10.1016/j.freeradbiomed.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Putney JW, Jr, Ribeiro CM. Signaling pathways between the plasma membrane and endoplasmic reticulum calcium stores. Cell Mol Life Sci. 2000;57:1272–1286. doi: 10.1007/PL00000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson G, Tofel-Grehl B, Scheffold K, Cristofalo V, Blass J. A reproducible procedure for primary culture and subsequent maintenance of multiple lines of human skin fibroblasts. Age. 1998;21 doi: 10.1007/s11357-998-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton D, Le Gloahec V, Patin H, Launay F. Radical chemistry of tert-butyl hydroperoxide (tBHP). Part 1. Studies of the Fe3+-tBHP mechanism. New J Chem. 1998:559–563. [Google Scholar]
- 19.Lin WL, Wang CJ, Tsai YY, Liu CL, Hwang JM, et al. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch Toxicol. 2000;74:467–472. doi: 10.1007/s002040000148. [DOI] [PubMed] [Google Scholar]
- 20.Ochi T, Miyaura S. Cytotoxicity of an organic hydroperoxide and cellular antioxidant defense system against hydroperoxides in cultured mammalian cells. Toxicology. 1989;55:69–82. doi: 10.1016/0300-483x(89)90175-3. [DOI] [PubMed] [Google Scholar]
- 21.Bird GS, Burgess GM, Putney JW., Jr Sulfhydryl reagents and cAMP-dependent kinase increase the sensitivity of the inositol 1,4,5-trisphosphate receptor in hepatocytes. J Biol Chem. 1993;268:17917–17923. [PubMed] [Google Scholar]
- 22.Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- 23.Poirier SN, Poitras M, Laflamme K, Guillemette G. Thiol-reactive agents biphasically regulate inositol 1,4,5-trisphosphate binding and Ca(2+) release activities in bovine adrenal cortex microsomes. Endocrinology. 2001;142:2614–2621. doi: 10.1210/endo.142.6.8195. [DOI] [PubMed] [Google Scholar]
- 24.Anzai K, Ogawa K, Kuniyasu A, Ozawa T, Yamamoto H, et al. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Commun. 1998;249:938–942. doi: 10.1006/bbrc.1998.9244. [DOI] [PubMed] [Google Scholar]
- 25.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 26.Jourd'heuil D, Jourd'heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 27.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J Biol Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 28.Kirsch M, Fuchs A, de Groot H. Regiospecific nitrosation of N-terminal-blocked tryptophan derivatives by N2O3 at physiological pH. J Biol Chem. 2003;278:11931–11936. doi: 10.1074/jbc.M300237200. [DOI] [PubMed] [Google Scholar]
- 29.Stepuro AI, Piletskaya TP, Stepuro II. Role of thiamine thiol form in nitric oxide metabolism. Biochemistry (Mosc) 2005;70:339–349. doi: 10.1007/s10541-005-0120-5. [DOI] [PubMed] [Google Scholar]
- 30.Bischof G, Brenman J, Bredt DS, Machen TE. Possible regulation of capacitative Ca2+ entry into colonic epithelial cells by NO and cGMP. Cell Calcium. 1995;17:250–262. doi: 10.1016/0143-4160(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 31.Trepakova ES, Cohen RA, Bolotina VM. Nitric oxide inhibits capacitative cation influx in human platelets by promoting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase-dependent refilling of Ca2+ stores. Circ Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- 32.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, et al. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- 33.Doutheil J, Althausen S, Treiman M, Paschen W. Effect of nitric oxide on endoplasmic reticulum calcium homeostasis, protein synthesis and energy metabolism. Cell Calcium. 2000;27:107–115. doi: 10.1054/ceca.1999.0099. [DOI] [PubMed] [Google Scholar]
- 34.Viner RI, Williams TD, Schoneich C. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38:12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 36.Okai Y, Higashi-Okai K, E FS, Konaka R, Inoue M. Potent Radical-Scavenging Activities of Thiamin and Thiamin Diphosphate. J Clin Biochem Nutr. 2007;40:42–48. doi: 10.3164/jcbn.40.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batifoulier F, Verny MA, Besson C, Demigné C, Rémésy C. Determination of thiamine and its phosphate esters in rat tissues analyzed as thiochromes on a RP-amide C16 column. Journal of Chromatography B. 2005;816:67–72. doi: 10.1016/j.jchromb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 38.He J, Kang H, Yan F, Chen C. The endoplasmic reticulum-related events in S-nitrosoglutathione-induced neurotoxicity in cerebellar granule cells. Brain Res. 2004;1015:25–33. doi: 10.1016/j.brainres.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Calingasan NY, Huang PL, Chun HS, Fabian A, Gibson GE. Vascular factors are critical in selective neuronal loss in an animal model of impaired oxidative metabolism. J Neuropathol Exp Neurol. 2000;59:207–217. doi: 10.1093/jnen/59.3.207. [DOI] [PubMed] [Google Scholar]
- 40.Galdhar NR, Pawar SS. Hepatic drug metabolism and lipid peroxidation in thiamine deficient rats. Int J Vitam Nutr Res. 1976;46:14–23. [PubMed] [Google Scholar]
- 41.Kruse M, Navarro D, Desjardins P, Butterworth RF. Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochem Int. 2004;45:49–56. doi: 10.1016/j.neuint.2003.12.007. [DOI] [PubMed] [Google Scholar]