Abstract
Phosphodiesterase 4 (PDE4) is a member of the PDE enzyme superfamily that inactivates cyclic adenosine monophosphate and cyclic guanosine monophosphate, and is the main PDE isoenzyme occurring in cells involved in inflammatory airway disease such as chronic obstructive pulmonary disease (COPD). COPD is a preventable and treatable disease and is characterized by airflow obstruction that is not fully reversible. Chronic progressive symptoms, particularly dyspnoea, chronic bronchitis and impaired overall health are worse in those who have frequent, acute episodes of symptom exacerbation. Although several experimental PDE4 inhibitors are in clinical development, roflumilast, a highly selective PDE4 inhibitor, is the first in its class to be licensed, and has recently been approved in several countries for oral, once-daily treatment of severe COPD. Clinical trials have demonstrated that roflumilast improves lung function and reduces exacerbation frequency in COPD. Furthermore, its unique mode of action may offer the potential to target the inflammatory processes underlying COPD. Roflumilast is effective when used concomitantly with all forms of bronchodilator and even in patients treated with inhaled corticosteroids. Roflumilast thus represents an important addition to current therapeutic options for COPD patients with chronic bronchitis, including those who remain symptomatic despite treatment. This article reviews the current status of PDE4 inhibitors, focusing on the pharmacokinetics, efficacy and safety of roflumilast. In particular, it provides an overview of the effects of roflumilast on lung function and exacerbations, glucose homoeostasis and weight loss, and the concomitant use of long-acting beta2-adrenergic receptor agonists and short-acting muscarinic receptor antagonists.
LINKED ARTICLES
This article is part of a themed issue on Respiratory Pharmacology. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-1
Keywords: Anti-inflammatory, chronic obstructive pulmonary disease, phosphodiesterase 4 inhibitor, roflumilast
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex syndrome that involves airway inflammation and airway limitation, oedema, mucociliary dysfunction and hypoxic vasoconstriction of pulmonary arterioles, which reduces perfusion, and consequent airway structural changes, in addition to significant systemic effects that lead to comorbid conditions (Global Initiative for Chronic Obstructive Lung Disease, 2009). The observation that the functions of inflammatory cells could be inhibited by raising their intracellular levels of 3′5′-cyclic adenosine monophosphate (cAMP), and the wide distribution of phosphodiesterase 4 (PDE4) in inflammatory cells and the lung, prompted the exploration of isoenzyme-selective PDE4 inhibitors as a way of reducing inflammation in patients with COPD (Boswell-Smith et al., 2006b; Halpin, 2008).
Phosphodiesterases (PDEs) are a superfamily of enzymes that hydrolyse cAMP and 3′5′-cyclic guanosine monophosphate (cGMP) to their inactive 5′ monophosphates, and thereby regulate the intracellular levels of secondary messengers (Halpin, 2008). One isoenzyme of the PDE family, PDE4, is the major regulator of cAMP levels in leukocytes and other inflammatory cells. As cAMP-specific PDE4 is expressed in all of the inflammatory cells and several other airway cells involved in the pathogenesis of COPD, inhibition of PDE4 should interfere with their function (Torphy and Undem, 1991; Giembycz, 1992; Souness et al., 2000; Burnouf and Pruniaux, 2002; Sanz et al., 2005).
This article will review the current status of PDE4 inhibitors, focusing on the pharmacokinetics, efficacy and safety of roflumilast, the first specific PDE4 inhibitor to be licensed for the treatment of COPD. In particular, it will provide an overview of the effects of roflumilast on lung function and exacerbations, glucose homoeostasis and weight loss and the concomitant use of long-acting beta2-adrenergic receptor agonists (LABAs) and short-acting muscarinic receptor antagonists (SAMAs).
Overview of the field
The first generation of PDE4 inhibitors (e.g. rolipram) were shown to be effective in inhibiting inflammatory cell functions, but their use was limited by side effects, particularly those affecting the gastrointestinal tract (Barnette and Underwood, 2000). There remained a need for a molecule with more specific anti-inflammatory activity and an improved safety profile. Development of such a molecule was potentially achievable as the PDE4 family is highly complex, with four genes (A, B, C and D) coding for the enzyme and each gene having several splice variants (Muller et al., 1996). Indeed, more than 20 isoforms of PDE4 have been identified and there are significant differences in the tissue distribution of the mRNA for each of these isoforms.
Several experimental PDE4 inhibitors are in clinical development, but only cilomilast and roflumilast have reached Phase III clinical trials. Data from Phase I and II studies showed that cilomilast significantly improved lung function and reduced exacerbation rates in COPD (Giembycz, 2006). However, because of its greater selectivity for the PDE4D subtype, cilomilast is associated with gastrointestinal disturbances, such as emesis and nausea (Boswell-Smith and Spina, 2007), and drug development for COPD was terminated (Field, 2008; Rennard et al., 2008). Current investigational drugs include oglemilast (GRC 3886), an oral PDE4 inhibitor under investigation in inflammatory airway diseases. In animal models in vitro and in vivo, oglemilast inhibited pulmonary cell infiltration, including eosinophilia and neutrophilia (Vakkalanka et al., 2004; Giembycz, 2008). Tetomilast is a once-daily oral PDE4 inhibitor that is currently in development for COPD and ulcerative colitis (O'Mahony, 2005; Schreiber et al., 2007); two recent multicentre Phase III studies in ulcerative colitis reported that efficacy was generally numerically better with tetomilast than placebo, but statistically significant improvement was not demonstrated (Keshavarzian et al., 2007). ONO-6126 has been tested in healthy subjects (Furuie et al., 2003; Giembycz, 2008) and is believed to be in Phase II development, while ELB353 has exhibited a good efficacy profile in animal models of pulmonary neutrophilia (Pages et al., 2009), and a further Phase I trial is underway to study its safety and pharmacokinetics in healthy subjects. Several inhaled PDE4 inhibitors are in the early stages of development: GSK256066, SCH900182 and ibudilast (Knowles et al., 2009; Chapman et al., 2010; Etsuko et al., 2010), while several more have been discontinued due to a lack of efficacy: AWD 12–281, UK-500,001 and tofimilast (CP 325366) (Giembycz, 2008).
Pharmacodynamics, pharmacokinetics and metabolism
Roflumilast (3-cyclopropylmethoxy-4-difluoromethoxy-N-[3,5-dichloropyrid-4-yl]-benzamide) is an oral PDE4 inhibitor for the treatment of COPD (Figure 1) (Boswell-Smith et al., 2006b). Roflumilast was identified in 1993 from a series of benzamides in a comprehensive screening programme (Amschler, 1995). The high potency and selectivity of roflumilast for competitive inhibition of PDE4, without affecting PDE1, 2, 3 or 5 isoenzymes, within various cells and tissues indicated its potential as a therapeutic agent (Hatzelmann et al., 2010).
Figure 1.
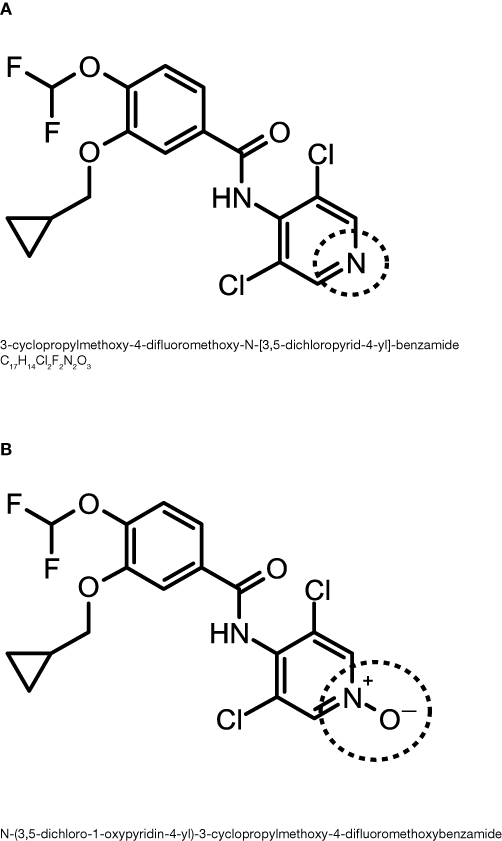
The chemical structure of (A) roflumilast and (B) roflumilast N-oxide.
The potency and selectivity of roflumilast and its active metabolite have been studied for PDE1–11 (Table 1) (Hatzelmann et al., 2010). Roflumilast does not affect PDE enzymes apart from PDE4, and is a subnanomolar inhibitor of most PDE4 splicing variants tested. It showed no PDE4 subtype selectivity apart from PDE4C, which is inhibited with a slightly lower potency. Roflumilast N-oxide is only twofold to threefold less potent than roflumilast, with respect to PDE4 inhibition, maintains high selectivity to other PDE isoenzymes and shows no selectivity for PDE4 subtypes. In contrast, cilomilast shows some subtype selectivity for PDE4D (Table 1).
Table 1.
Phosphodiesterase (PDE) selectivities of roflumilast, roflumilast N-oxide and cilomilast. (Adapted with permission from Hatzelmann et al., 2010)
| 4A1 | 4A4 | 4B1 | 4B2 | 4C1 | 4C2 | 4Cshort | 4D2 | 4D3 | 4D4 | 4D5 | 1A3 | 1B1 | 1C1 | 2A3 | 3A1 | 5A1 | 6 (bovine) | 7A1 | 8B | 9A3 | 10A | 11A4 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roflumilast | nM | 0.7 | 0.9 | 0.7 | 0.2 | 3 | 4.3 | 3.5 | 0.3 | 0.4 | 0.2 | 0.4 | >10 000 | >10 000 | >10 000 | >10 000 | >10 000 | 4500 | 4200 | >10 000 | >10 000 | >10 000 | >10 000 | >10 000 |
| Roflumilast N-oxide | nM | 1.4 | 2.3 | 0.95 | 1.1 | 5.9 | 7.8 | 3.9 | 0.4 | 0.8 | 0.5 | 0.8 | >10 000 | >10 000 | >10 000 | >10 000 | >10 000 | >10 000 | 6300 | >10 000 | >10 000 | >10 000 | >10 000 | >10 000 |
| Cilomilast | µM | 0.11 | 0.16 | 0.14 | 0.09 | 0.47 | 0.63 | 0.65 | 0.018 | 0.02 | 0.02 | 0.025 | 48 | >100 | 74 | 84 | >100 | >100 | 24 | >100 | 21 | >100 | >100 | 92 |
Selectivity was assessed using human recombinant enzymes except PDE6, which is from a bovine source.
Values given are IC50 for inhibition of PDE catalysis; these are nM values for roflumilast and its N-oxide and µM-values for cilomilast.
Inhibitors of PDE4, such as roflumilast, interfere with the breakdown of cAMP, leading to its intracellular accumulation. In turn, an elevated concentration of intracellular cAMP activates protein kinase A, which enhances phosphorylation of proteins (Figure 2) (Torphy and Undem, 1991; Giembycz, 1992; Souness et al., 2000; Burnouf and Pruniaux, 2002; Conti et al., 2003; Sanz et al., 2005). In vitro, inhibition of PDE4 results in a wide range of effects, including decreased apoptosis (which may result in the clearance of sputum) and release of inflammatory mediators in neutrophils (a reduction in influx resulting in a reduction of neutrophils in the airways), decreased expression of cell surface markers in many cell types (e.g. adhesion molecules in T-cells), and decreased release of cytokines in many cell types (such as tumour necrosis factor alpha, interleukin-1β and interleukin-10 in macrophages) (Hatzelmann et al., 2010). In vivo, inhibition of PDE4 leads to a broad spectrum of effects, such as the inhibition of cell trafficking, and cytokine and chemokine release from inflammatory cells such as neutrophils, eosinophils, macrophages and T-cells (Sanz et al., 2005).
Figure 2.
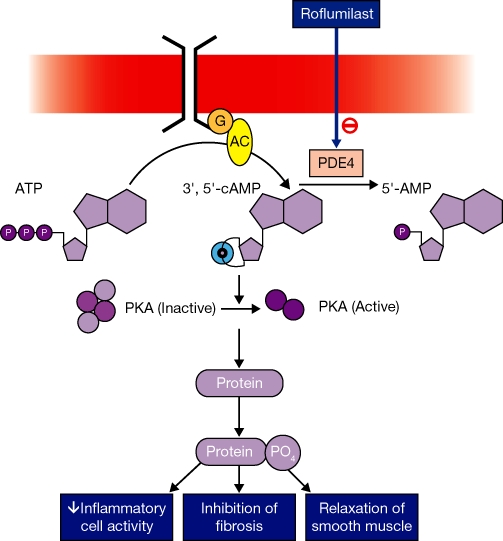
PDE4 inhibitors increase levels of cyclic adenosine monophosphate (cAMP) through inhibition of its metabolism. The resulting increase in protein kinase A (PKA) activation stimulates increased protein phosphorylation, with subsequent inhibition of pro-inflammatory cells and mediators and inhibition of fibrosis. Although increased cAMP levels generally have smooth muscle relaxant effects, roflumilast does not have acute bronchodilator effects (Grootendorst et al., 2003), which are not a property of selective PDE4 inhibitors. It may be speculated that smooth muscle has several PDE isoenzymes, and that selective inhibition of PDE4 is insufficient to afford an acute dilator effect.
Animal studies with roflumilast demonstrated that it reduced the accumulation of neutrophils in bronchoalveolar lavage fluid following short-term exposure of guinea pigs, mice or rats to tobacco smoke, and following exposure of rats to a combination of tobacco smoke and bacterial lipopolysaccharide, and abolished the lung parenchymal influx of inflammatory cells seen in rats exposed to tobacco smoke for 7 months (Fitzgerald, 2001; Martorana et al., 2005; 2008; Fitzgerald et al., 2006; Le Quement et al., 2008; Weidenbach et al., 2008; Carnini et al., 2009; Hardaker et al., 2009; 2010; Stevenson et al., 2009). Similarly, roflumilast was able to prevent bleomycin-induced lung infiltration of neutrophils and macrophages in mice (Cortijo et al., 2009). Furthermore, roflumilast was not only able to reduce smoke-induced emphysema in mice exposed to tobacco smoke for 7 months but also to prevent progression of emphysema when given to mice after exposure for 4 months (Martorana et al., 2005).
Following oral dosing, roflumilast is rapidly converted by cytochrome P450 (CYP) 3A4 and 1A2 to its active metabolite roflumilast N-oxide (Bethke et al., 2007). This metabolite has similar potency and specificity to the parent compound, and has been estimated to contribute ∼90% of the total PDE4 inhibitory (tPDE4i) activity of roflumilast (Hatzelmann and Schudt, 2001; Hauns et al., 2006; Hermann et al., 2006; Bethke et al., 2007; Lahu et al., 2009). Roflumilast N-oxide is primarily cleared by CYP3A4, with some contribution from CYP2C19 and extrahepatic CYP1A1. A number of covariates, including age, gender and smoking, can affect the activity of CYP3A4 and CYP1A2 (Bebia et al., 2004; Mangoni and Jackson, 2004; Cotreau et al., 2005; Funck-Brentano et al., 2006). However, a recent modelling study that analysed the effects of a range of covariates on tPDE4i activity of roflumilast found them to have a limited impact on this parameter (Lahu et al., 2010c).
Roflumilast is rapidly and almost completely absorbed after oral administration, with maximum plasma concentrations (Cmax) reached within ∼1 h in healthy volunteers (Bethke et al., 2007). Absolute bioavailability is ∼80% when administered as an immediate-release tablet (David et al., 2004). The pharmacokinetic profile of roflumilast is linear and predictable over the dose range of 250–1000 µg (Bethke et al., 2007). The therapeutic dose is 500 µg, taken once daily (which can be either in the morning or the evening). On repeated oral dosing with roflumilast 500 µg once daily in healthy subjects, the free drug concentration of roflumilast N-oxide in plasma over 24 h was estimated to be about 1–2 nM, following the measurement of plasma protein binding of roflumilast N-oxide of approximately 97% (Bethke et al., 2007). The apparent effective plasma half-life (t½) of roflumilast ranges from 8 to 31 h (median 17 h) and steady-state plasma concentrations are achieved after 3–4 days of oral, once-daily dosing (Lahu et al., 2010c). The Cmax for roflumilast N-oxide is reached ∼8 h after drug intake (Bethke et al., 2007; Hermann et al., 2007a,b; Nassr et al., 2007a; von Richter et al., 2007), and the t½ is ∼30 h (Bethke et al., 2007; Hermann et al., 2007a,b; von Richter et al., 2007). Steady-state plasma concentrations of roflumilast N-oxide are achieved within 6 days of oral, once-daily dosing (Bethke et al., 2007). There is no effect of food on the pharmacokinetics of roflumilast or roflumilast N-oxide (Hauns et al., 2006).
The pharmacokinetics of roflumilast 500 µg once daily in patients with severe renal impairment and roflumilast 250 µg once daily in patients with mild/moderate hepatic impairment were assessed (Drollmann et al., 2002). In patients with severe renal impairment, the area under the concentration–time curve (AUC) was 21% and 7% lower for roflumilast and roflumilast N-oxide, respectively, compared with healthy subjects. A decrease of 16% and 12% in the Cmax of roflumilast and roflumilast N-oxide was also observed in the renally impaired group. In patients with severe renal impairment, tPDE4i was decreased by 9% compared with healthy controls (Bethke et al., unpublished data). In patients with mild hepatic impairment, the AUC of roflumilast increased by 51% (roflumilast N-oxide increased by 24%), and the Cmax increased by 3% (roflumilast N-oxide increased by 26%), compared with healthy controls. For those patients with moderate hepatic impairment, the AUC of roflumilast increased by 92% (roflumilast N-oxide increased by 41%), while the Cmax of roflumilast increased by 26% (roflumilast N-oxide increased by 40%), compared with healthy controls. Relative to healthy subjects, mean increases in tPDE4i were 26% and 46% in patients with mild or moderate hepatic impairment respectively (Hermann et al., 2007a).
Drug interaction studies have shown that no dose adjustment of roflumilast 500 µg was required when co-administered with erythromycin (Lahu et al., 2009), ketoconazole (Lahu et al., 2008), midazolam (Nassr et al., 2007b), digoxin (Lahu et al., 2010a) and Maalox®, an antacid containing magnesium hydroxide and aluminium hydroxide (Nassr et al., 2007a). Furthermore, no interaction was noted between roflumilast and cigarette smoke (Nycomed GmbH, 2010b). However, co-administration of rifampicin 600 mg, a potent CYP3A4 inducer, and roflumilast led to a reduction of 58% in tPDE4i activity of roflumilast, suggesting that co-administration may have reduced the therapeutic effect of roflumilast (Nassr et al., 2009). In addition, co-administration with the antidepressant fluvoxamine 50 mg, a strong multiple pathway inhibitor blocking more than one relevant CYP isoenzyme, led to an increase of 59% in tPDE4i activity of roflumilast. This increase would not be expected when co-administering roflumilast with a more selective CYP1A2 inhibitor (von Richter et al., 2007).
Furthermore, although roflumilast and theophylline should not be used concomitantly, a drug interaction study in healthy volunteers has reported that the pharmacokinetics of theophylline were not altered by repeated oral administration of roflumilast. The AUC of roflumilast increased by 28% following repeated theophylline co-administration, but the AUC of roflumilast N-oxide was unaffected and there was no change in the Cmax of roflumilast or roflumilast N-oxide. In addition, no safety concerns were raised when theophylline and roflumilast were administered concomitantly for a short period of up to 5 days. However, as there are no clinical data available to support the co-administration of both drugs, concomitant treatment is not recommended for maintenance therapy (Böhmer et al., unpublished data).
The effects of co-medications frequently used in COPD on the pharmacokinetics of roflumilast and roflumilast N-oxide have also been studied in healthy subjects. Co-administration of roflumilast with salbutamol, budesonide or formoterol had no significant effect on the mean tPDE4i activity of roflumilast (Bethke et al., 2006; Hermann et al., 2007b; Lahu et al., 2010a). No dose adjustment of roflumilast is required with co-administration of montelukast (Böhmer et al., 2009). The effects of concomitant therapy with warfarin, enoxacin and cimetidine were also evaluated. Co-administration of warfarin and roflumilast had no significant effect on the mean tPDE4i activity of roflumilast, and no clinically relevant adverse events were reported during the study (Lahu et al., 2010a). Co-administration of enoxacin and roflumilast resulted in a mean increase in tPDE4i activity of 25%, although this is unlikely to be clinically relevant (Lahu et al., 2010b). Co-administration of cimetidine and roflumilast resulted in a mean increase of 47% in tPDE4i activity, but dose adjustment of roflumilast is not required (Böhmer et al., 2010).
Efficacy
Early studies
Early studies with roflumilast used lung function [primary endpoint being post-bronchodilator forced expiratory volume in 1 s (FEV1)] and quality of life as efficacy endpoints (Table 2). The beneficial effect of roflumilast on lung function was initially demonstrated in a 6-month study in subjects with moderate-to-severe COPD (M2-107) (Rabe et al., 2005), followed by a 12-month study in severe COPD (M2-112) (Calverley et al., 2007).
Table 2.
Phase III studies with roflumilast
| Study | Patients | Study design | Therapy | Key findings |
|---|---|---|---|---|
| M2-107 (Rabe et al., 2005) | N = 1411Post-bronchodilator FEV1 30–80% predicted; post-bronchodilator FEV1:FVC ratio ≤ 70% | Multicentre, double-blind, parallel-group study | Roflumilast 250 µg (n = 576) or 500 µg (n = 555) or placebo (n = 280) once daily for 24 weeks | Roflumilast 500 µg significantly improved post-bronchodilator FEV1 compared with placebo (difference 97 mL, P < 0.0001) and health-related quality of life |
| M2-111(Data on file Nycomed GmbH) (Nycomed GmbH, 2010a) | N = 1176Post-bronchodilator FEV1:FVC ratio ≤ 70%Post-bronchodilator ≤ 50% predicted | Multicentre, double-blind, parallel-group study | Roflumilast 500 µg (n = 568) or placebo (n = 608) once daily for 52 weeks | Roflumilast 500 µg significantly improved pre-bronchodilator FEV1 compared with placebo (difference 36 mL, P < 0.0001)Roflumilast 500 µg significantly improved post-bronchodilator FEV1 compared with placebo (difference 38 mL, P < 0.0001) |
| M2-112 (Calverley et al., 2007) | N = 1513Post-bronchodilator FEV1≤ 50% predicted, post-bronchodilator FEV1:FVC ratio ≤ 0.70 | Multicentre, randomized, placebo-controlled, double-blind, parallel-group trial (identical to study M2-111) | Roflumilast, 500 µg once daily or placebo for 1 yearNote: unlike M2-124/125, patients in this study were not required to have symptoms of chronic bronchitis or a history of exacerbations | Post-bronchodilator FEV1 increased by 39 mL with roflumilast compared with placebo by 52 weeks (P = 0.001)The mean exacerbation rate per patient per year was low and comparable (0.86 vs 0.92 for roflumilast and placebo respectively). In a retrospective analysis, the exacerbation rate per patient per year in patients in GOLD stage IV disease was 36% lower in patients treated with roflumilast than in those treated with placebo (1.01 vs. 1.59, respectively; P = 0.024) |
| M2-124 (Calverley et al., 2009) | N = 1523Post-bronchodilator FEV1≤ 50% predicted, bronchitic symptoms, and a history of exacerbations | Multicentre, double-blind, randomized, parallel-group study | Roflumilast 500 µg (n = 765) once daily or placebo (n = 758) for 1 year | Pre-bronchodilator FEV1 increased in the roflumilast group by 46 mL, but was almost unchanged (8 mL increase) in the placebo group (P = 0.0003)There was also 14.9% reduction in the exacerbation rate in the roflumilast group vs placebo (P = 0.0278) |
| M2-125 (Calverley et al., 2009) | N = 1568Post-bronchodilator FEV1≤ 50% predicted, bronchitic symptoms, and a history of exacerbations | Multicentre, double-blind, randomized, parallel-group study | Roflumilast 500 µg once daily (n = 772) or placebo (n = 796) for 1 year | Roflumilast increased pre-bronchodilator FEV1 by 33 mL compared with a 25 mL decrease in the placebo group (P < 0.0001)Roflumilast also reduced the rate of moderate or severe COPD exacerbations compared with placebo by 18.5%; P = 0.0035 |
| M2-127 (Fabbri et al., 2009) | N = 933Post-bronchodilator FEV1 40–70% predicted | Double-blind, randomized, parallel-group, multicentre study | Roflumilast 500 µg (n = 466) or placebo (n = 467), once daily for 24 weeks, in addition to salmeterol 50 µg twice daily | Compared with placebo, roflumilast consistently improved mean pre-bronchodilator FEV1 by 49 mL (P < 0.0001) in patients treated with salmeterol. Similar improvement in post-bronchodilator FEV1 was noted. Furthermore, roflumilast had beneficial effects on other lung function measurements and on selected patient-reported outcomes |
| M2-128 (Fabbri et al., 2009) | N = 743Post-bronchodilator FEV1 40–70% predicted | Double-blind, randomized, parallel-group, multicentre study | Roflumilast 500 µg (n = 371) or placebo (n = 372) once daily for 24 weeks, in addition to tiotropium 18 µg once daily | Compared with placebo, roflumilast consistently improved mean pre-bronchodilator FEV1 by 80 mL (P < 0.0001) in those treated with tiotropium. Similar improvement in post-bronchodilator FEV1 was noted. Furthermore, roflumilast had beneficial effects on other lung function measurements and on selected patient-reported outcomes |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Study M2-107 was a double-blind, multicentre, placebo-controlled study that involved 1411 patients with moderate-to-severe COPD (FEV1 30–80% predicted), treated with roflumilast, 250 µg or 500 µg, or placebo once daily for 24 weeks (Rabe et al., 2005). There were significant improvements in post-bronchodilator FEV1 in both roflumilast groups compared with placebo (74 mL for roflumilast 250 µg and 97 mL for roflumilast 500 µg; P < 0.0001). Improvements were also seen in quality of life, as assessed by St. George's Respiratory Questionnaire (SGRQ) total score (−1.6 for roflumilast 250 µg and −1.7 for roflumilast 500 µg compared with placebo; P = 0.077 and P = 0.053 respectively).
M2-112 was a randomized, double-blind, placebo-controlled, parallel-group study of 1513 patients with severe COPD (FEV1≤ 50% predicted) treated with roflumilast 500 µg or placebo once daily for 1 year (Calverley et al., 2007). The primary efficacy variables were the change from baseline to endpoint in post-bronchodilator FEV1, and the number of moderate or severe exacerbations per patient per year.
Concomitant inhaled corticosteroids (ICS) were permitted and were used by about 60% of patients. As in study M2-107, post-bronchodilator lung function improved significantly with roflumilast treatment (39 mL compared with placebo, P = 0.001). This improvement was achieved in a patient population with poor baseline reversibility. In addition, fewer moderate-to-severe exacerbations were observed among patients receiving roflumilast than in the placebo group, but this reduction failed to reach significance.
The heterogeneous nature of COPD needs to be considered in the development of novel therapies as different drugs are likely to benefit different patient subpopulations. Consequently, it was suggested that certain subgroups of patients with COPD would be more likely to benefit from the anti-inflammatory action of roflumilast than others. In order to improve understanding of the effects of roflumilast on COPD exacerbations and to examine which patient populations might gain the most benefit from roflumilast, a post-hoc, pooled analysis of two replicate 12-month studies (M2-111 and M2-112) was performed (Rennard et al., 2011). This analysis showed a 14.3% reduction in the rate of moderate-to-severe exacerbations with roflumilast vs. placebo (0.52 vs. 0.61 exacerbations per year; P = 0.026). It was also able to identify responsive patient subgroups that showed greatest benefit with roflumilast: patients with chronic bronchitis or with high cough or sputum scores in the week prior to randomization, and patients receiving concomitant ICS or SAMAs (Figure 3) (Rennard et al., 2011). The preferential effect of roflumilast in certain patient subgroups suggested that a tailored approach was required to optimize treatment for COPD. This analysis facilitated the design of subsequent clinical trials, which consistently demonstrated the efficacy of roflumilast, focussing on the identified patient groups (Calverley et al., 2009; Fabbri et al., 2009).
Figure 3.
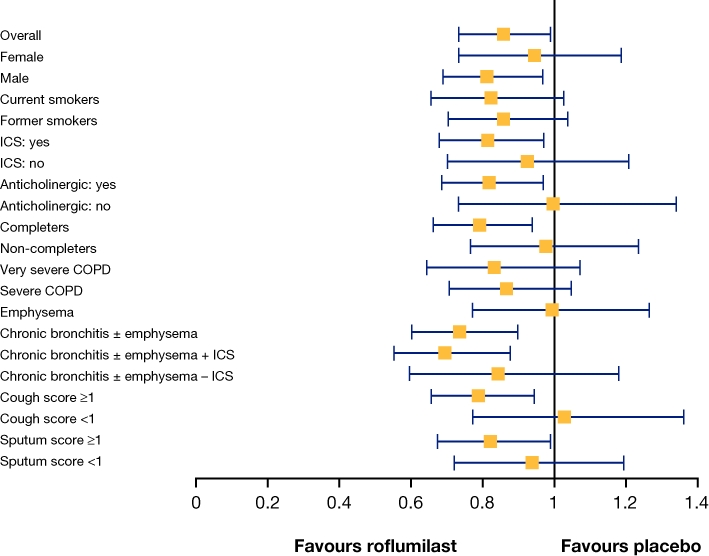
Effect of roflumilast treatment by disease severity according to rate ratios for reduction in moderate-to-severe COPD exacerbations (Rennard et al., 2011). Error bars represent 95% confidence intervals. (Reproduced by courtesy of BioMed Central).
Recent studies
The two pivotal, 12-month studies that followed (M2-124 and M2-125) used the information gained from the pooled analysis to investigate the effects of roflumilast in a patient population with a background of chronic bronchitis who are at higher risk of exacerbations (Calverley et al., 2009). These two trials were randomized, double-blind studies comparing roflumilast 500 µg once daily (n = 1537) with placebo (n = 1554), and were designed to examine the effects of roflumilast on lung function and exacerbation rate. Patients had severe-to-very-severe COPD (FEV1≤ 50% predicted) and were required to have bronchitic symptoms and a history of exacerbations. ICS were not allowed during the study but LABAs or SAMAs could be used. As well as significantly improving pre-bronchodilator FEV1, treatment with roflumilast was associated with a significant reduction in the annual rate of exacerbations. In a pooled analysis, the mean rate of moderate or severe exacerbations per patient per year was 1.14 with roflumilast and 1.37 with placebo [17% reduction, rate ratio 0.83 (95% CI 0.75–0.92); P = 0.0003].
The most effective pharmacological treatment for patients with moderate-to-severe COPD is the regular use of inhaled long-acting bronchodilators, combined with ICS in patients with severe disease who are at risk of exacerbations (Global Initiative for Chronic Obstructive Lung Disease, 2009). As roflumilast is an anti-inflammatory agent rather than a bronchodilator, it should be used concomitantly with other treatments, most notably long-acting bronchodilators. Consequently, two recent 6-month studies examined the effects of roflumilast on pre-bronchodilator FEV1 when used concomitantly with salmeterol (study M2-127) or tiotropium (study M2-128) (Fabbri et al., 2009). These trials were multicentre, double-blind, randomized, parallel-group studies evaluating patients with moderate-to-severe COPD (FEV1 40–70% predicted). M2-128 recruited patients who were more symptomatic as they were required to have a history of chronic cough and sputum production and frequent use of short-acting beta2 agonists. Patients were randomly assigned to receive roflumilast 500 µg or placebo once daily for 24 weeks, in addition to concomitant treatment with salmeterol 50 µg twice daily (n = 933 treated) or tiotropium 18 µg once daily (n = 743 treated).
In the salmeterol study, mean pre-bronchodilator FEV1 improved by 49 mL in the roflumilast 500 µg group compared with placebo (P < 0.0001), while in the tiotropium study, the improvement was 80 mL (P < 0.0001). Furthermore, similar improvements were seen in post-bronchodilator FEV1, in the roflumilast 500 µg group compared with placebo, with improvements of 60 mL and 81 mL (both P < 0.0001) in the salmeterol and tiotropium studies respectively. In view of the poor baseline reversibility of these patients (partial reversibility to albuterol ≤ 12%), these improvements in FEV1 show that roflumilast can provide additional benefit to patients already receiving long-acting bronchodilator therapy. Although these studies were powered to detect an improvement in lung function, M2-127 noted a reduction in the mean annual exacerbation rate (moderate or severe) by 36.8% (P = 0.0315; post-hoc). This reduction was not significant in M2-128: concomitant treatment reduced the mean annual exacerbation rate (moderate or severe) by 23.2% (rate ratio = 0.768; P = 0.196; Figure 4) (Chapman and Rabe, 2010). The low levels of exacerbations in the 6-month versus 12-month trials are illustrated in Figure 4. However, these 6-month studies in moderate-to-severe COPD may have been of an insufficient duration to permit reliable detection of an effect on exacerbations. Furthermore, patients were included in these studies who were not at risk from exacerbations or who had no prior history of exacerbations.
Figure 4.
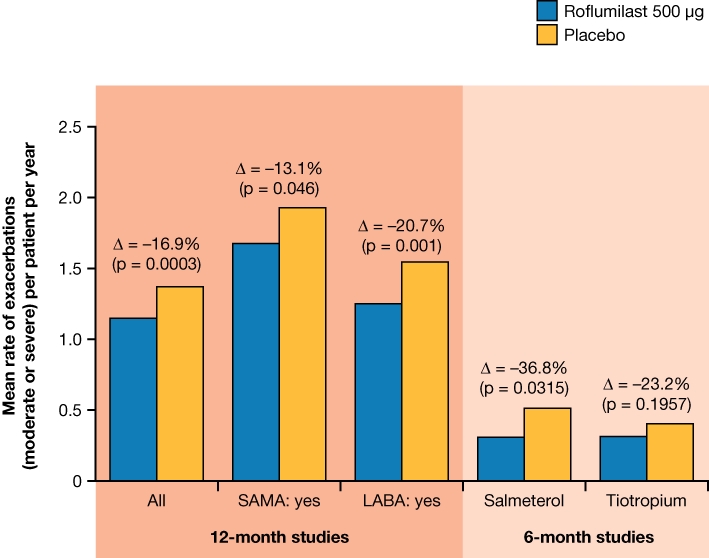
Effects on moderate or severe exacerbations after treatment with roflumilast 500 µg or placebo in patients receiving concomitant short-acting muscarinic antagonists (SAMAs) or long-acting beta2-adrenergic receptor agonists (LABAs) in studies M2-124 and M2-125 (12-month studies) and M2-127 (concomitant administration with salmeterol) and M2-128 (concomitant administration with tiotropium) (Calverley et al., 2009; Fabbri et al., 2009; Bateman et al., unpublished data).
In order to further examine the effects of roflumilast in patients receiving concomitant treatments, a pooled analysis of studies M2-124 and M2-125 was performed. This analysis focused on the impact of roflumilast on exacerbations, not only when used concomitantly with long-acting bronchodilators but also in patients previously treated with an ICS (Bateman et al., unpublished data). The mean rate of moderate and severe exacerbations per patient per year in patients receiving roflumilast was significantly lower than in those receiving placebo, regardless of concomitant use of LABA (20.7%; P = 0.0110), SAMA (13.1%; P = 0.0458) or previous ICS treatment (19.3%; P = 0.0038 all pre-specified analyses). Both pre-bronchodilator (improvement of 48 mL) and post-bronchodilator FEV1 (improvement of 55 mL) were also significantly improved in patients treated with roflumilast compared with placebo, irrespective of concomitant LABA or SAMA use or previous ICS treatment (P < 0.0001). Furthermore, patients receiving concomitant ICS responded well to roflumilast, with an 18.8% reduction in exacerbations compared with placebo (P = 0.014) in a pooled analysis of two other 12-month studies (M2-111 and M2-112) (Rennard et al., 2011). These findings, together with the results of M2-127 and M2-128 (Figures 4 and 5) (Calverley et al., 2009; Fabbri et al., 2009; Bateman et al., unpublished data) support the addition of roflumilast in the treatment of patients whose symptoms are not adequately controlled with other therapies.
Figure 5.
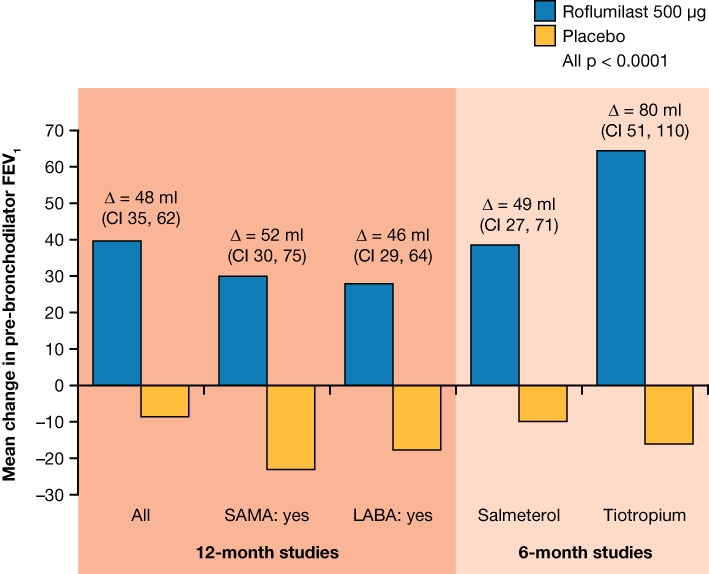
Effects on pre-bronchodilator FEV1 after treatment with roflumilast 500 µg or placebo in patients receiving concomitant short-acting muscarinic antagonists (SAMAs) or long-acting beta2-adrenergic receptor agonists (LABAs) in studies M2-124 and M2-125 (12-month studies) and in patients randomized to concomitant administration of roflumilast or placebo with salmeterol (M2-127) or concomitant administration of roflumilast or placebo with tiotropium (M2-128) (Calverley et al., 2009; Fabbri et al., 2009; Bateman et al., unpublished data).
Safety
Roflumilast has been generally well tolerated in clinical studies to date. The most common adverse events observed are those that would be expected with PDE4 inhibitors, namely gastrointestinal effects and weight loss. In a pooled analysis of over 6000 patients receiving roflumilast in clinical trials, the overall adverse event rate was similar to that among patients receiving placebo (Calverley et al., 2010a). Higher rates of diarrhoea, weight decrease, nausea, headache, back pain, insomnia, decreased appetite and dizziness were reported with roflumilast 500 µg than with placebo. By contrast, the incidence of COPD exacerbations, dyspnoea, upper respiratory tract infections, bronchitis, pneumonia and hypertension were lower with roflumilast 500 µg than with placebo (Calverley et al., 2010b) (Table 3). In a pooled analysis of M2-124 and M2-125 (pivotal COPD studies pool), a range of adverse events occurred with roflumilast that were centrally mediated, namely insomnia, nausea, headache and gastrointestinal (predominantly diarrhoea) disturbance. Vomiting was not observed, in contrast to studies of cilomilast (Calverley et al., 2009). These side effects were most evident in the first 4–12 weeks of therapy and were mostly mild or moderate in intensity. In this analysis, there were no cases of mesenteric vasculitis, as demonstrated by the absence of its most common clinical manifestation, ischaemic colitis (Nycomed GmbH, 2009), and no neurological or cardiac toxicity was noted. The incidence of adverse events was 67% with roflumilast and 62% with placebo; serious adverse events were reported in 19 and 22% of patients respectively. Discontinuations due to adverse events, however, were more common with roflumilast (14%) than with placebo (11%) in the 12-month M2-124 and M2-125 studies.
Table 3.
Adverse event (AE) frequency with roflumilast (COPD safety pool) (Calverley et al., 2010b)
| Roflumilast 500 µg·day−1 | Placebo | |
|---|---|---|
| (n = 5766) | (n = 5491) | |
| n (%) | n (%) | |
| Patients with AEs | 3873 (67.2) | 3447 (62.8) |
| Patients with SAEs | 781 (13.5) | 782 (14.2) |
| Deaths | 84 (1.5) | 86 (1.6) |
| AEs with suggested causality (investigator) | 1003 (17.4) | 294 (5.4) |
| Study discontinued due to AEs | 824 (14.3) | 503 (9.2) |
| Most common adverse events (frequency ≥2% of patients in any treatment group) | ||
| Infections and infestations | 1492 (25.9) | 1508 (27.5) |
| Nasopharyngitis | 364 (6.3) | 346 (6.3) |
| Upper respiratory tract infection | 219 (3.8) | 234 (4.3) |
| Bronchitis | 177 (3.1) | 192 (3.5) |
| Influenza | 145 (2.5) | 132 (2.4) |
| Pneumonia | 104 (1.8) | 110 (2.0) |
| Respiratory, thoracic and mediastinal disorders | 1476 (25.6) | 1607 (29.3) |
| COPD (exacerbation) | 1142 (19.8) | 1271 (23.1) |
| Dyspnoea | 84 (1.5) | 120 (2.2) |
| Gastrointestinal disorders | 1271 (22) | 587 (10.7) |
| Diarrhoea | 585 (10.1) | 143 (2.6) |
| Nausea | 297 (5.2) | 79 (1.4) |
| Investigations | 811 (14.1) | 584 (10.6) |
| Weight decreased | 394 (6.8) | 101 (1.8) |
| Nervous system disorders | 615 (10.7) | 304 (5.5) |
| Headache | 266 (4.6) | 110 (2.0) |
| Dizziness | 139 (2.4) | 65 (1.2) |
| Musculoskeletal and connective tissue disorders | 590 (10.2) | 445 (8.1) |
| Back pain | 176 (3.1) | 117 (2.1) |
| General disorders | 369 (6.4) | 321 (5.8) |
| Psychiatric disorders | 344 (6.0) | 164 (3.0) |
| Insomnia | 148 (2.6) | 50 (0.9) |
| Cardiac disorders | 326 (5.7) | 326 (5.9) |
| Metabolism and nutrition disorders | 311 (5.4) | 186 (3.4) |
| Decreased appetite | 125 (2.2) | 22 (0.4) |
| Injury, poisoning and procedural complications | 209 (3.6) | 220 (4.0) |
| Skin and subcutaneous tissue disorders | 206 (3.6) | 154 (2.8) |
| Vascular disorders | 196 (3.4) | 229 (4.2) |
| Hypertension | 95 (1.6) | 136 (2.5) |
| Neoplasms (benign, malignant and unspecified) | 118 (2.0) | 92 (1.7) |
MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Class-specific adverse events
Gastrointestinal disturbances are class-related adverse events for PDE4 inhibitors (Spina, 2003). Investigations into the possible causes of these effects have focused on the tissue distribution of the PDE4 isoforms. For example, PDE4B is the predominant PDE4 subtype in monocytes and neutrophils and is thought to have a role in the inflammatory processes (Wang et al., 1999), whereas PDE4D is highly expressed in the lung, cortex, cerebellum and in T-cells (Erdogan and Houslay, 1997; Jin et al., 1998), and plays an important role in airway smooth muscle contraction (Mehats et al., 2003). Studies in knockout mice have suggested that PDE4D is the main isoform associated with emesis (Robichaud et al., 2002), while PDE4B appears to be the main isoform responsible for mediating release of tumour necrosis factor alpha (Jin and Conti, 2002). Further, binding of inhibitors to PDE4 is influenced by the N-terminal domain structure. There appears to be two binding sites: a high-affinity site with a Ki approximately 50–1000 times greater than binding to the low-affinity site. High-affinity binding predominates in the central nervous system, while low-affinity binding predominates in inflammatory cells, leading to important clinical differences in the pharmacological properties of inhibitors (Halpin, 2008). Strategies to improve the therapeutic ratio of PDE4 inhibitors have been suggested, such as targeting isoforms that appear to be expressed only as part of the inflammatory process in COPD, such as PDE4A4, or to develop dual-specificity inhibitors that inhibit PDE4 and either PDE1, PDE3 or PDE7 (Giembycz, 2005). However, as research progresses, the picture continues to become more complex. The complex arrangement of transcriptional units and multiple promoters have led to the identification of over 20 PDE4 isoforms (splice variants), each with unique N-terminal properties, enabling complex regulation mechanisms and intracellular compartmentalization (Peter et al., 2007). Although roflumilast shows a similar specificity for PDE4D4 as for other subtypes, the gastrointestinal adverse events are less severe than with other PDE4 inhibitors. For example, cilomilast is 10 times more selective for PDE4D than other isozymes, and this selectivity for PDE4D-type nausea-inducing neurons could explain the lower tolerability seen with this compound (Lipworth, 2005).
Weight loss and glucose metabolism
Weight loss has been reported in subjects treated with roflumilast (Stanescu et al., 1996; Calverley et al., 2009), and is also seen with the non-selective PDE inhibitor theophylline (Boswell-Smith et al., 2006a). Investigation of the mechanisms of lipolysis in human adipocytes found that PDE3B and PDE4 regulate cAMP pools that affect the activation/phosphorylation state of AMP-activated protein kinase, thereby influencing lipolysis (Omar et al., 2009). Rolipram, a selective PDE4 inhibitor has been shown to increase plasma glucagon-like peptide-1 (GLP-1) concentrations in rats, suggesting PDE4D may play an important role in regulating intracellular cAMP, linked to the regulation of GLP-1 release (Ong et al., 2009). Furthermore, GLP-1 analogues used for the treatment of diabetes mellitus type 2 (DM2) have demonstrated weight loss in obese patients without DM2 (Astrup et al., 2009).
The pivotal COPD studies pool (M2-124 and M2-125) showed that the incidence of patients with measured weight loss was higher with roflumilast than with placebo (Calverley et al., 2009). However, the weight loss observed with roflumilast was generally small (<3% of baseline weight). Body weight in the placebo groups remained almost unchanged. Most weight loss occurred in the first 6 months of treatment and was partially reversible within 12 weeks of stopping treatment (Martinez et al., 2010). When stratified by body mass index (BMI) category, all patient subsets showed a greater weight loss in the roflumilast arm than with placebo. The most pronounced weight change was observed in obese patients; this subgroup also had the highest proportion of patients with weight loss and with weight loss classified as clinically relevant (16.5% of patients vs. 9.1% in the overweight subgroup, 12.3% in the normal weight subgroup and 12.6% in the underweight subgroup). Underweight patients did not show a more notable weight loss than patients in the other BMI categories (Calverley et al., 2010b). As might be expected from the involvement of PDE in lipolysis, the weight loss with roflumilast is primarily due to loss of fat mass. Bioimpedance measurements in study M2-128, which included an investigation into the effects of roflumilast on weight change as part of the safety assessment, showed that with roflumilast, the decline in fat-free mass index was most pronounced during the first 4 weeks and then reached a plateau until the end of treatment (Wouters et al., 2010b). In contrast, the corresponding BMI values declined progressively during the 6-month duration of the study; these decreases levelled out after 3–4 months, and thereafter reached a plateau, the differential between fat-free mass and BMI constituting a reduction in fat mass. At the end of 6 months, almost two-thirds of the total weight decrease of −2.1 kg could be attributed to a loss of fat mass. These changes were reversible after withdrawal of study drug (Wouters et al., 2010b). These findings are in accordance with those of Losco et al. (2004), who also found an association between weight loss and loss of fat mass with the PDE4 inhibitor, SCH351591, in monkeys (Losco et al., 2004). In this study, weight loss was dose-dependent and reached a plateau after 2–3 weeks, with some animals (males) gradually regaining the weight lost.
As COPD is associated with insulin resistance and an increased risk of DM2 (Bolton et al., 2007), which may be the result of elevated levels of systemically active pro-inflammatory molecules contributing to an altered metabolic state and insulin resistance, it is of interest to examine the effect of roflumilast on glucose metabolism, body composition and weight loss in patients with diabetes. In pooled analyses of roflumilast studies, roflumilast-treated patients with concomitant diabetes showed either no change in fasting or non-fasting blood glucose levels [pivotal COPD studies pool (M2-124 and M2-125)] or even decreased glucose levels (COPD safety pool; pooled data from the safety populations of 14 COPD studies). Placebo-treated patients with diabetes showed an increase in glucose levels from baseline to last visit (Wouters et al., 2010a). In patients without concomitant diabetes, there were slight increases in glucose levels from baseline to last visit in both treatment groups. The difference in blood glucose level between the roflumilast and placebo groups was statistically significant only for patients with concomitant diabetes in the COPD safety pool (P = 0.0135) (Wouters et al., 2010a).
In a 12-week, placebo-controlled study of roflumilast 500 µg once daily in 205 newly diagnosed, treatment-naïve patients with DM2 (study M2-401), plasma glucose levels decreased significantly more in the roflumilast group than in the placebo group [least square (LS) mean (standard error, SE) difference −1.04 (0.30), P = 0.0006] (Wouters et al., 2010a). Marked attenuation of the glucagon response after the fixed meal test was observed in the roflumilast treatment group, and the rise in glucose levels following a fixed meal was also reduced in this group (EFM Wouters, unpublished data). Patients in both groups lost weight over the course of the study; LS means (SE) −1.9 kg (0.3) versus −1.2 kg (0.3) for roflumilast versus placebo, respectively [LS means (SE) difference −0.7 kg (0.4), P = 0.0584] (EFM Wouters, unpublished data). Type II diabetes is characterized by a severely reduced or absent incretin effect (Nauck et al., 1986), and these effects of roflumilast on glucose and glucagon levels, and effects on weight, may involve incretins – gastrointestinal hormones released from endocrine cells in the distal ileum and the colon in response to food intake with subsequent amplification of insulin secretion (Holst, 2007).
Other adverse events
Cardiac adverse events have been seen with some PDE4 inhibitors, namely rolipram (Larson et al., 1996) and SCH351591 (Losco et al., 2004). In the pooled safety results for roflumilast, however, the incidence of cardiac adverse events was similar in the roflumilast 500 µg and placebo groups (5.7% vs. 5.9%) (Calverley et al., 2010a).
Of the other adverse events that have been suggested as potential problems with PDE4 inhibitors, the safety analyses from the roflumilast trials have not been able to show any reason for concern with respect to proconvulsant effects, infections or tumours (Calverley et al., 2010a). Moreover, no proconvulsant effect would be expected with roflumilast due to PDE4 selectivity. In clinical COPD studies, there was no difference between roflumilast and placebo in the frequency of adverse events associated with potential proconvulsant effects of roflumilast (i.e. convulsions, epilepsy, partial seizure, petit mal seizure) (Calverley et al., 2010a). The incidence of infections was similar across all groups: 27.5% in the placebo group, 23.6% with roflumilast 250 µg and 25.9% with roflumilast 500 µg. As COPD patients are known to be prone to pneumonia, these events were analysed separately and showed no appreciable differences between roflumilast and placebo (1.8% vs. 2.0%) (Calverley et al., 2010a). The rate of tumours in the COPD safety pool was slightly higher with roflumilast than placebo (1.5% vs. 1.3%). None of these tumour events was assessed as related to the study medication (Calverley et al., 2010b). Comparison of these results with tumour incidence in the COPD general population using data from an epidemiological study based on 35 772 COPD patients revealed a higher incidence in the time-adjusted incidence of tumours in this COPD population than in the COPD safety pool (27.8 vs. 27.3 respectively) (Schneider et al., 2010). The majority of cancers observed in the COPD safety pool were solid tumours (175/185; (Calverley et al., 2010b); these are known to require several years to develop before diagnosis. The risk of a tumour adverse event did not increase over time but remained constant over the treatment period of up to 1 year, suggesting that the tumours were not causally related to roflumilast treatment. Furthermore, the events were detected during the first 6 months of treatment with no difference in incidence between placebo and roflumilast detected after 6 months of treatment, suggesting against a causal relationship to treatment.
In the COPD safety pool, psychiatric disorders occurred in 6.0% of patients receiving roflumilast 500 µg compared with 3.0% of placebo patients. Although there were more cases of depression (1.21% vs. 0.82%) and suicidal ideation/attempt (0.03% vs. 0.02%) with roflumilast 500 µg compared with placebo, overall this affected very few patients (Food and Drug Administration, 2010). The incidence of completed suicide in patients receiving roflumilast in the COPD safety pool (two in the 500-µg group and one in the 250-µg group vs. none receiving placebo) has recently been identified as a subject of significant concern by the US Food and Drug Administration (Food and Drug Administration, 2010); however, none was identified as being related to study medication.
Conclusions
Current management of COPD requires an incremental approach in which patients are first treated with bronchodilators (beta-adrenergic receptor agonists or anticholinergic agents), which can be followed by anti-inflammatory treatment (inhaled or oral corticosteroids) if needed. However, this approach has limited overall efficacy in this disease that presents with a wide variety of clinical phenotypes.
PDE4 inhibitors are the first novel class of drug to emerge for the treatment of COPD in the last decade. Roflumilast is the first member of this class to be licensed, and is indicated in the European Union for the maintenance treatment of severe COPD associated with chronic bronchitis and a history of frequent exacerbations as an add-on to bronchodilator treatment. Clinical trials have demonstrated that roflumilast improves lung function and, more importantly, reduces exacerbation frequency in COPD. Furthermore, its mode of action may provide a unique approach to targeting the inflammatory process underlying COPD compared with other currently available medication. Roflumilast is effective when used concomitantly with all forms of bronchodilator and even in patients treated with ICS. Roflumilast thus represents an important addition to current therapeutic options for COPD patients with chronic bronchitis, including those who remain symptomatic despite treatment.
Acknowledgments
Editorial assistance with the preparation of the paper was provided by Caroline Howell, Sarah Brown and Paul Wilmott, for Caudex Medical Ltd, supported by Nycomed, Konstanz, Germany.
Glossary
Abbreviations
- AUC
area under the concentration–time curve
- BMI
body mass index
- cAMP
3′5′-cyclic adenosine monophosphate
- cGMP
3′5′-cyclic guanosine monophosphate
- Cmax
maximum plasma concentrations
- COPD
chronic obstructive pulmonary disease
- CYP
cytochrome P450
- DM2
diabetes mellitus type 2
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GLP-1
glucagon-like peptide-1
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- ICS
inhaled corticosteroids
- LABAs
long-acting beta2-adrenergic receptor agonists
- LS
mean, least squares mean
- PDE4
phosphodiesterase 4
- SAMAs
short-acting muscarinic receptor antagonists
- SGRQ
St George's Respiratory Questionnaire
- t½
half-life
- tPDE4i
total PDE4 inhibitory
Conflict of interest
KFR has served as a consultant, participated in advisory board meetings, has received lecture fees from AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Pfizer, Novartis, Nycomed, Merck Sharp and Dohme, and GlaxoSmithKline, and has received research funding from Altana Pharma, Novartis, AstraZeneca, Boehringer Ingelheim, Roche and GlaxoSmithKline.
Supporting Information
Teaching Materials; Figs 1–5 as PowerPoint slide.
References
- Amschler H. Fluoroalkoxy-substituted benzamides and their use as cyclic nucleotide phosphodiesterase inhibitors. PCT Patent. 1995 WO95/01338. [Google Scholar]
- Astrup A, Rossner S, Van GL, Rissanen A, Niskanen L, Al HM, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- Barnette MS, Underwood DC. New phosphodiesterase inhibitors as therapeutics for the treatment of chronic lung disease. Curr Opin Pulm Med. 2000;6:164–169. doi: 10.1097/00063198-200003000-00014. [DOI] [PubMed] [Google Scholar]
- Bebia Z, Buch SC, Wilson JW, Frye RF, Romkes M, Cecchetti A, et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther. 2004;76:618–627. doi: 10.1016/j.clpt.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Bethke TD, Giessmann T, Westphal K, Weinbrenner A, Hauns B, Hauschke D, et al. Roflumilast, a once-daily oral phosphodiesterase 4 inhibitor, lacks relevant pharmacokinetic interactions with inhaled salbutamol when co-administered in healthy subjects. Int J Clin Pharmacol Ther. 2006;44:572–579. doi: 10.5414/cpp44572. [DOI] [PubMed] [Google Scholar]
- Bethke TD, Böhmer GM, Hermann R, Hauns B, Fux R, Morike K, et al. Dose-proportional intraindividual single- and repeated-dose pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor. J Clin Pharmacol. 2007;47:26–36. doi: 10.1177/0091270006294529. [DOI] [PubMed] [Google Scholar]
- Böhmer GM, Nassr N, Wenger M, Hunnemeyer A, Lahu G, Templin S, et al. The targeted oral, once-daily phosphodiesterase 4 inhibitor roflumilast and the leukotriene receptor antagonist montelukast do not exhibit significant pharmacokinetic interactions. J Clin Pharmacol. 2009;49:389–397. doi: 10.1177/0091270008330980. [DOI] [PubMed] [Google Scholar]
- Böhmer GM, Gleiter CH, Morike K, Nassr N, Walz A, et al. No dose adjustment on coadministration of the PDE4 inhibitor roflumilast with a weak CYP3A, CYP1A2, and CYP2C19 inhibitor: an investigation using Cimetidine. J Clin Pharmacol. 2010 doi: 10.1177/0091270010368282. DOI: 10.1177/0091270010768282 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bolton CE, Evans M, Ionescu AA, Edwards SM, Morris RH, Dunseath G, et al. Insulin resistance and inflammation – A further systemic complication of COPD. COPD. 2007;4:121–126. doi: 10.1080/15412550701341053. [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D. PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int J Chron Obstruct Pulmon Dis. 2007;2:121–129. [PMC free article] [PubMed] [Google Scholar]
- Boswell-Smith V, Cazzola M, Page CP. Are phosphodiesterase 4 inhibitors just more theophylline? J Allergy Clin Immunol. 2006a;117:1237–1243. doi: 10.1016/j.jaci.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006b;147(Suppl 1):S252–S257. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf C, Pruniaux MP. Recent advances in PDE4 inhibitors as immunoregulators and anti-inflammatory drugs. Curr Pharm Des. 2002;8:1255–1296. doi: 10.2174/1381612023394665. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Fabbri LM, Rabe KF, Mosberg H. Roflumilast in the treatment of COPD: a pooled safety analysis. Eur Respir J Suppl. 2010a;36(Suppl 54):Abstract P4001. [Google Scholar]
- Calverley PMA, Fabbri LM, Rabe KF, Mosberg H. Roflumilast in the treatment of COPD: a pooled safety analysis. 2010b. Presented at the European Respiratory Society Annual Congress, Barcelona, Spain 18-22 September, 2010: Poster 4732.
- Carnini C, Caruso P, Bassani F, Pisano AR, Riccardi B, Gallo PM, et al. Cigarette smoke-induced inflammatory and oxidative response in mice and pharmacological intervention studies involving compounds with different mechanism of action. Am J Respir Crit Care Med. 2009;179:A2025. [Google Scholar]
- Chapman KR, Rabe KF. Efficacy and safety of roflumilast in patients with chronic obstructive pulmonary disease (COPD) concomitantly treated with tiotropium or salmeterol. Prim Care Respir J. 2010;19:A12. [Google Scholar]
- Chapman RW, House A, Richard J, Fernandez X, Jones H, Prelusky D, et al. Pharmacology Of SCH900182, A Potent, Selective Inhibitor Of PDE4 For Inhaled Administration. Am J Respir Crit Care Med. 2010;181:A5671. [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Cortijo J, Iranzo A, Milara X, Mata M, Cerda-Nicolas M, Ruiz-Sauri A, et al. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol. 2009;156:534–544. doi: 10.1111/j.1476-5381.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- David M, Zech K, Seiberling M, Weimar C, Bethke TD. Roflumilast, a novel, oral, selective PDE4 inhibitor, shows high oral bioavailability. J Allergy Clin Immunol. 2004;113:S220–S221. [Google Scholar]
- Drollmann A, Hartmann M, Zech K, David M, Weimar C, Bethke T. Patients with severe renal impairment do not require dose adjustments of roflumilast. Eur Respir J. 2002;20(Suppl 38):108s. Abstract P743. [Google Scholar]
- Erdogan S, Houslay MD. Challenge of human Jurkat T-cells with the adenylate cyclase activator forskolin elicits major changes in cAMP phosphodiesterase (PDE) expression by up-regulating PDE3 and inducing PDE4D1 and PDE4D2 splice variants as well as down-regulating a novel PDE4A splice variant. Biochem J. 1997;321(Pt 1):165–175. doi: 10.1042/bj3210165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etsuko T, Tamaoki J, Arai N, Kondo M, Nagai A. Treatment of airway mucus hypersecretion and postnasal drip syndrome by the PDF4 inhibitor ibudilast in patients with chronic airway inflamation. Am J Respir Crit Care Med. 2010;181:A5620. [Google Scholar]
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- Field SK. Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma. Expert Opin Investig Drugs. 2008;17:811–818. doi: 10.1517/13543784.17.5.811. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MF. Efficacy of the PDE4 inhibitor, Bay 19-8004, in a smoke-induced model of pulmonary inflammation in the guinea pig. Am J Respir Crit Care Med. 2001;163:A905. [Google Scholar]
- Fitzgerald MF, Spicer D, McAulay AE, Wollin L, Beume R. Roflumilast but not methylprednisolone inhibited cigarette smoke-induced pulmonary inflammation in guinea pigs. Eur Respir J. 2006;28(Suppl 50):663s. [Google Scholar]
- Food and Drug Administration. Daxas (roflumilast) tablets NDA 22-522. 2010. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM208711.pdf. (last accessed 19 October 2010)
- Funck-Brentano C, Raphael M, Lafontaine M, Arnould JP, Verstuyft C, Lebot M, et al. Effects of type of smoking (pipe, cigars or cigarettes) on biological indices of tobacco exposure and toxicity. Lung Cancer. 2006;54:11–18. doi: 10.1016/j.lungcan.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Furuie H, Nakagawa S, Kawashima M, Murakami M, Irie S. Suppressive effect of novel phosphodiesterase4 (PDE4) inhibitor ONO-6126 on TNF-α release was increased after repeated oral administration in healthy Japanese subjects. 2003. Presented at the 13th Annual European Respiratory Society's Annual Congress, Vienna, Austria. September 27: Abstract 2557.
- Giembycz MA. Could isoenzyme-selective phosphodiesterase inhibitors render bronchodilator therapy redundant in the treatment of bronchial asthma? Biochem Pharmacol. 1992;43:2041–2051. doi: 10.1016/0006-2952(92)90160-k. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. Phosphodiesterase-4: selective and dual-specificity inhibitors for the therapy of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:326–333. doi: 10.1513/pats.200504-041SR. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. An update and appraisal of the cilomilast Phase III clinical development programme for chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2006;62:138–152. doi: 10.1111/j.1365-2125.2006.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz MA. Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking? Br J Pharmacol. 2008;155:288–290. doi: 10.1038/bjp.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2009) Bethesda, MD: National Heart, Lung and Blood Institute; 2009. [Google Scholar]
- Grootendorst DC, Gauw SA, Benschop N, Sterk PJ, Hiemstra PS, Rabe KF. Efficacy of the novel phosphodiesterase-4 inhibitor BAY 19-8004 on lung function and airway inflammation in asthma and chronic obstructive pulmonary disease (COPD) Pulm Pharmacol Ther. 2003;16:341–347. doi: 10.1016/S1094-5539(03)00090-7. [DOI] [PubMed] [Google Scholar]
- Halpin DM. ABCD of the phosphodiesterase family: interaction and differential activity in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:543–561. doi: 10.2147/copd.s1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaker EL, Freeman MS, Dale N, Raza F, Mok J, Banner KH, et al. Characterisation of a model that mimicks aspects of the hyper-inflammatory response observed during an acute exacerbation of COPD. Am J Respir Crit Care Med. 2009;179:A5351. [Google Scholar]
- Hardaker EL, Freeman MS, Dale N, Bahra P, Raza F, Banner KH, et al. Exposing rodents to a combination of tobacco smoke and lipopolysaccharide results in an exaggerated inflammatory response in the lung. Br J Pharmacol. 2010;160:1985–1996. doi: 10.1111/j.1476-5381.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–279. [PubMed] [Google Scholar]
- Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, et al. The preclinical pharmacology of roflumilast – a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23:235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Hauns B, Hermann R, Hunnemeyer A, Herzog R, Hauschke D, Zech K, et al. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46:1146–1153. doi: 10.1177/0091270006291621. [DOI] [PubMed] [Google Scholar]
- Hermann R, Lahu G, Hauns B, Bethke T, Zech K. ‘Total PDE4 inhibitory activity’: a concept for evaluating pharmacokinetic alterations of roflumilast and roflumilast N-oxide in special populations and drug-drug interactions. Eur Respir J. 2006;28:436s. [Google Scholar]
- Hermann R, Nassr N, Lahu G, Peterfai E, Knoerzer D, Herzog R, et al. Steady-state pharmacokinetics of roflumilast and roflumilast N-oxide in patients with mild and moderate liver cirrhosis. Clin Pharmacokinet. 2007a;46:403–416. doi: 10.2165/00003088-200746050-00003. [DOI] [PubMed] [Google Scholar]
- Hermann R, Siegmund W, Giessmann T, Westphal K, Weinbrenner A, Hauns B, et al. The oral, once-daily phosphodiesterase 4 inhibitor roflumilast lacks relevant pharmacokinetic interactions with inhaled budesonide. J Clin Pharmacol. 2007b;47:1005–1013. doi: 10.1177/0091270007300950. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Jin SL, Bushnik T, Lan L, Conti M. Subcellular localization of rolipram-sensitive, cAMP-specific phosphodiesterases. Differential targeting and activation of the splicing variants derived from the PDE4D gene. J Biol Chem. 1998;273:19672–19678. doi: 10.1074/jbc.273.31.19672. [DOI] [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci USA. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Mutlu E, Guzman JP, Forsyth C, Banan A. Phosphodiesterase 4 inhibitors and inflammatory bowel disease: emerging therapies in inflammatory bowel disease. Expert Opin Investig Drugs. 2007;16:1489–1506. doi: 10.1517/13543784.16.9.1489. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Ball DI, Gascoigne MH. In vivo characterisation of GSK256066, an exceptionally high affinity and selective inhibitor of PDE4 suitable for topical administration. Am J Respir Crit Care Med. 2009;179:A4582. [Google Scholar]
- Lahu G, Hünnemeyer A, von Richter O, Hermann R, Herzog R, McCracken N, et al. Effect of single and repeated doses of ketoconazole on the pharmacokinetics of roflumilast and roflumilast N-oxide. J Clin Pharmacol. 2008;48:1339–1349. doi: 10.1177/0091270008321941. [DOI] [PubMed] [Google Scholar]
- Lahu G, Hünnemeyer A, Herzog R, McCracken N, Hermann R, Elmlinger M, et al. Effect of repeated dose of erythromycin on the pharmacokinetics of roflumilast and roflumilast N-oxide. Int J Clin Pharmacol Ther. 2009;47:236–245. doi: 10.5414/cpp47236. [DOI] [PubMed] [Google Scholar]
- Lahu G, Goehring UM, Hunnemeyer A, Nassr N. Roflumilast in coadministration with medications commonly prescribed for COPD: an overview of existing studies. Eur Respir J. 2010a;36(Suppl 54):Abstract P4593. [Google Scholar]
- Lahu G, Nassr N, Herzog R, Elmlinger M, Ruth P, Hinder M, et al. Effect of steady-state enoxacin on single-dose pharmacokinetics of roflumilast and roflumilast N-Oxide. J Clin Pharmacol. 2010b doi: 10.1177/0091270010370590. DOI: 10.1177/0091270010370590 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lahu G, Hünnemeyer A, Diletti E, Elmlinger M, Ruth P, Zech K, et al. Population pharmacokinetic modelling of roflumilast and roflumilast N-Oxide by total phosphodiesterase 4 inhibitory activity and development of a population pharmacodynamic-adverse event model. Clin Pharmacokinet. 2010c;49:589–606. doi: 10.2165/11536600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Larson JL, Pino MV, Geiger LE, Simeone CR. The toxicity of repeated exposures to rolipram, a type IV phosphodiesterase inhibitor, in rats. Pharmacol Toxicol. 1996;78:44–49. doi: 10.1111/j.1600-0773.1996.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Le Quement C, Guenon I, Gillon JY, Valenca S, Cayron-Elizondo V, Lagente V, et al. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br J Pharmacol. 2008;154:1206–1215. doi: 10.1038/bjp.2008.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–175. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- Losco PE, Evans EW, Barat SA, Blackshear PE, Reyderman L, Fine JS, et al. The toxicity of SCH 351591, a novel phosphodiesterase-4 inhibitor, in Cynomolgus monkeys. Toxicol Pathol. 2004;32:295–308. doi: 10.1080/01926230490431493. [DOI] [PubMed] [Google Scholar]
- Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FJ, Rabe KF, Wouters EFM, Brose M, Goehring UM, Fabbri LM, et al. Time course and reversibility of weight decrease with roflumilast, a phosphodiesterase 4 inhibitor. Am J Respir Crit Care Med. 2010;181:A4441. [Google Scholar]
- Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;172:848–853. doi: 10.1164/rccm.200411-1549OC. [DOI] [PubMed] [Google Scholar]
- Martorana PA, Lunghi B, Lucattelli M, De CG, Beume R, Lungarella G. Effect of roflumilast on inflammatory cells in the lungs of cigarette smoke-exposed mice. BMC Pulm Med. 2008;8:17. doi: 10.1186/1471-2466-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehats C, Jin SL, Wahlstrom J, Law E, Umetsu DT, Conti M. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003;17:1831–1841. doi: 10.1096/fj.03-0274com. [DOI] [PubMed] [Google Scholar]
- Muller T, Engels P, Fozard JR. Subtypes of the type 4 cAMP phosphodiesterases: structure, regulation and selective inhibition. Trends Pharmacol Sci. 1996;17:294–298. doi: 10.1016/0165-6147(96)10035-3. [DOI] [PubMed] [Google Scholar]
- Nassr N, Lahu G, Hünnemeyer A, von Richter O, Knoerzer D, Reutter F, et al. Magnesium hydroxide/aluminium hydroxide-containing antacid does not affect the pharmacokinetics of the targeted phosphodiesterase 4 inhibitor roflumilast. J Clin Pharmacol. 2007a;47:660–666. doi: 10.1177/0091270006297920. [DOI] [PubMed] [Google Scholar]
- Nassr N, Lahu G, von Richter O, Reutter F, Knoerzer D, Zech K, et al. Lack of a pharmacokinetic interaction between steady-state roflumilast and single-dose midazolam in healthy subjects. Br J Clin Pharmacol. 2007b;63:365–370. doi: 10.1111/j.1365-2125.2006.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassr N, Huennemeyer A, Herzog R, von RO, Hermann R, Koch M, et al. Effects of rifampicin on the pharmacokinetics of roflumilast and roflumilast N-oxide in healthy subjects. Br J Clin Pharmacol. 2009;68:580–587. doi: 10.1111/j.1365-2125.2009.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- Nycomed GmbH. Data on file. Safety evaluation of roflumilast – mesenteric vasculitis. 2009. Nycomed GmbH (previously Altana Pharma AG) 358/2008.
- Nycomed GmbH. Data on file, Nycomed GmbH (previously Altana Pharma AG) 134/2006. 2010a.
- Nycomed GmbH. Data on file, Nycomed GmbH (previously Byk Gulden Lomberg Chemische Fabrik GmbH) 213/2000. 2010b.
- O'Mahony S. Tetomilast. Idrugs. 2005;8:502–507. [PubMed] [Google Scholar]
- Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21:760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WK, Gribble FM, Reimann F, Lynch MJ, Houslay MD, Baillie GS, et al. The role of the PDE4D cAMP phosphodiesterase in the regulation of glucagon-like peptide-1 release. Br J Pharmacol. 2009;157:633–644. doi: 10.1111/j.1476-5381.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages L, Gavalda A, Lehner MD. PDE4 inhibitors: a review of current developments (2005-2009) Expert Opin Ther Pat. 2009;19:1501–1519. doi: 10.1517/13543770903313753. [DOI] [PubMed] [Google Scholar]
- Peter D, Jin SL, Conti M, Hatzelmann A, Zitt C. Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: predominant role of PDE4D. J Immunol. 2007;178:4820–4831. doi: 10.4049/jimmunol.178.8.4820. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast – an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Knobil K, Rabe KF, Morris A, Schachter N, Locantore N, et al. The efficacy and safety of cilomilast in COPD. Drugs. 2008;68(Suppl 2):3–57. doi: 10.2165/0003495-200868002-00002. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPD. Respir Res 12: 18. 2011 doi: 10.1186/1465-9921-12-18. DOI: 10.1186/1465-9921-12-18 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Richter O, Lahu G, Hünnemeyer A, Herzog R, Zech K, Hermann R. Effect of fluvoxamine on the pharmacokinetics of roflumilast and roflumilast N-oxide. Clin Pharmacokinet. 2007;46:613–622. doi: 10.2165/00003088-200746070-00006. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther. 2005;106:269–297. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Schneider C, Jick SS, Bothner U, Meier CR. Cancer risk in patients with chronic obstructive pulmonary disease. Pragmatic and Observational Research. 2010;1:15–23. doi: 10.2147/POR.S13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Keshavarzian A, Isaacs KL, Schollenberger J, Guzman JP, Orlandi C, et al. A randomized, placebo-controlled, phase II study of tetomilast in active ulcerative colitis. Gastroenterology. 2007;132:76–86. doi: 10.1053/j.gastro.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Souness JE, Aldous D, Sargent C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology. 2000;47:127–162. doi: 10.1016/s0162-3109(00)00185-5. [DOI] [PubMed] [Google Scholar]
- Spina D. Phosphodiesterase-4 inhibitors in the treatment of inflammatory lung disease. Drugs. 2003;63:2575–2594. doi: 10.2165/00003495-200363230-00002. [DOI] [PubMed] [Google Scholar]
- Stanescu D, Sanna A, Veriter C, Kostianev S, Calcagni PG, Fabbri LM, et al. Airways obstruction, chronic expectoration, and rapid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax. 1996;51:267–271. doi: 10.1136/thx.51.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CS, Wan WYH, Mok J, Pearce W, Morris A, Kinnear G. Does the impact of anti-inflammatory compounds in acute smoking mouse models predict therapeutic efficacy in more chronic models. Am J Respir Crit Care Med. 2009;179:A2224. [Google Scholar]
- Torphy TJ, Undem BJ. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991;46:512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkalanka SKVS, Balasubramaniam G, Gharat LA, Joshi VD, Hussain N, Rao J. The pharmacological and safety profile of a novel selective phosphodiesterase-4(PDE4) inhibitor:- GRC- 3886. Eur Respir J. 2004;24:220s. [Google Scholar]
- Wang P, Wu P, Ohleth KM, Egan RW, Billah MM. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol. 1999;56:170–174. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- Weidenbach A, Braun C, Schwoebel F, Beume R, Marx D. Therapeutic effects of various PDE4 inhibitors on cigarette smoke-induced pulmonary neutrophilia in mice. Am J Respir Crit Care Med. 2008;178:A651. [Google Scholar]
- Wouters EF, Teichmann P, Brose M, Fabbri L, Rabe K. Effect of roflumilast on glucose levels in patients with COPD and diabetes mellitus type 2. 2010a. Presented at the European Respiratory Society Annual Congress, Barcelona, Spain 18-22 September, 2010: Poster 4002. [DOI] [PubMed]
- Wouters EFM, Teichmann P, Brose M, Rabe KF, Fabbri LM. Effects of roflumilast, a phosphodiesterase 4 inhibitor, on body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010b;181:A4473. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


