Abstract
This study evaluated the clinicopathological and prognostic implications of genetic alterations characterizing oral squamous cell carcinoma (OSCC). Comparative genomic hybridization was used to identify chromosomal alterations present in primary OSCCs obtained from 97 patients. In this population, tobacco use was a significant risk factor for OSCC. By contrast, the 97 samples were negative for human papillomavirus (HPV) DNA integration, another known risk factor for OSCC in certain populations. Results of the Fisher’s exact test, followed by the Benjamini-Hochberg correction for multiple testing, showed a correlation of 7p gain and 8p loss with node-positive OSCC (p≤0.04 for both genetic alterations) and an association of 11q13 gain with high-grade OSCC (p≤0.05). Univariate Cox-proportional hazard models, also corrected for multiple testing, showed a significant association of 11q13 gain and 18q loss with decreased survival (p≤0.05). The findings were supported by multivariate analysis, which revealed that 11q13 gain and 18q loss together serve as a strong bivariate predictor of poor prognosis. In conclusion, our study identified genetic alterations that correlate significantly with nodal status, grade and the poor survival status of OSCC. These potential biomarkers may aid the current tumor node metastasis system for better prediction of clinical outcome.
Keywords: oral cancer, comparative genomic hybridization, prognosis, survival analysis, gain of 11q13, loss of 18q
Introduction
Oral cancer is the sixth most common malignancy worldwide (1). India has a higher incidence compared to developed countries due to the habits of tobacco chewing and bidi smoking (2,3). Despite improved treatment modalities, the 5-year survival rates have not changed during the past few decades. The tumor node metastasis (TNM) staging system used for treatment decisions should be complemented with genomic biomarkers, which may aid in overcoming its limitations (4). Genomic biomarkers may predict the biological variability in tumors that alters their clinical behavior.
To obtain biomarkers, a better understanding of the molecular basis of oral carcinogenesis is required, which can be achieved via a genomic method of analysis, such as comparative genomic hybridization (CGH). CGH has revealed a recurrent pattern of genetic alterations characterizing oral squamous cell carcinomas (OSCC) (5–14). The clinical and prognostic evaluation of these alterations is crucial in order to determine their clinical utility as biomarkers. Studies investigating chromosomal CGH of OSCC are descriptive in nature and their patient cohort is limited, rendering it difficult to find biomarkers. A comprehensive clinicopathological evaluation of genomic alterations associated with OSCC is lacking.
The existence of divergent pathways of oral cancer progression has recently been demonstrated using oncogenetic tree models (15). The distinct sets of genetic alterations underlying the progression of node-negative (pN0) and node-positive (pN+) OSCC indicated that specific alterations characterize subtypes of oral cancers. To elaborate on these findings, clinicopathological associations are required to define genetic biomarkers that may aid in predicting the aggressive behavior of primary oral cancers.
In this study, genetic alterations were correlated with clinicopathological parameters, such as site, size, stage, lymph node involvement and tumor grade. The prognostic significance of these alterations was also determined for those patients for whom complete follow-up information was available (n=40). To obtain candidate genetic biomarkers, stringent statistical methods for data analysis were applied.
Results of previous studies showed that human papillomavirus (HPV) infection is associated with a subset of head and neck squamous cell carcinoma (HNSCC). HPV-positive HNSCC constitutes a distinct molecular and clinicopathological disease entity and has better prognosis than HPV-negative HNSCC (16–18). Thus, the presence of HPV is considered to be an additional factor in the sub-grouping of tumors. This study therefore aimed to evaluate the HPV status in all tumors profiled by CGH.
Materials and methods
Study population and sample collection of the primary data set
Fresh frozen tumor samples were collected from 97 patients with primary oral carcinomas treated at the Tata Memorial Hospital, India, between 2001 and 2005. Written informed consent was obtained from all 97 participants prior to the collection of tissues. The tumor tissues used for CGH analysis were microdissected and only those with >70% of tumor cells were included for analysis. The clinicopathological characteristics of the cases were as previously described (15). Staging and differentiation were determined according to the joint TNM and American Joint Committee on Cancer classification of 2002, and the World Health Organization international histological classification of tumors, respectively. Follow-up information was available for 40 (41%) patients. The overall survival time was defined as the time from the date of surgery to the date of the last follow-up visit. Of the 41 patients, 27 were alive and remained disease-free throughout the time of follow-up, with a median follow-up of 24.4 months (range 4.9–75).
Detection of HPV by general primer PCR
The integrity and quality of the DNA extracted was checked by 0.8% agarose gel electrophoresis and DNA quantification was performed on a Nanodrop-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). To screen oral cancer samples for HPV integration into the human genome, a general HPV PCR was carried out using 250–300 ng of sample DNA and the primer pair GP5+/GP6+ for L1 consensus sequence (19). The CaSki cell line was used as a positive control. Samples with bands obtained at 150 bp were considered to be positive. To determine the quality of amplification of the extracted tumor DNA, β-globin PCR was performed (20).
CGH and digital image analysis
CGH was performed as previously described (15). The digital image analysis was carried out using the ISIS software (Metasystems, Altlussheim, Germany). Altered chromosomal regions were determined as the ratio of green to red (G/R) fluorescence intensities. G/R thresholds were set to 1.25 and 0.75 to determine gains and losses, respectively (21,22).
Interphase FISH
To validate the CGH predicted gains of 11q13, interphase fluorescence in situ hybridization (FISH) was performed on archival tumor samples with known CGH results. Dual-colored FISH was performed on 4-μm sections of archival OSCC samples (n=32) and the corresponding normal oral mucosal tissues (n=3) as previously described (15).
Statistical analysis
Statistical analysis was performed using the R software package (www.r-project.org). The frequency of copy number alterations (CNAs) was determined and only those alterations occurring in at least 30% of the 97 cases were considered for further analysis. The threshold of 30% was selected to limit multiple testing. The Fisher’s exact test was used to determine the association of CNAs with various clinicopathological parameters. To correct for multiple testing, p-values were adjusted using the Benjamini-Hochberg method (23). A corrected p-value of ≤0.05 was considered to be statistically significant for all analyses. Survival analysis was performed using the Cox proportional hazards model (24). The risk of death is shown by the hazard function, h(t) = h0(t)exp(∑iβiχi), where h0(t) is the baseline hazard. The co-efficient βi determines the relative contribution of the clinical factors and CNAs χi determines the contribution to the overall risk. The prognostic value of each CNA and each clinicopathological parameter was initially analyzed in a univariate model. P-values were obtained from a likelihood ratio test. Stepwise model selection was performed for combinations of prognostic factors in a multivariate Cox model based on the Bayesian information criterion (BIC) (25). In each step, the factor reducing the BIC score most was added to (or removed from) the Cox model. This procedure was repeated until no further BIC improvement was possible. Additionally, parameter selection was performed using the least absolute shrinkage and selection operator (LASSO) method (26,27). In this method, the size of the risk co-efficients is constrained by a shrinkage parameter s, such that ∑i|βi|≤s. Since a number of co-efficients vanish for small values of s, parameter selection is achieved by selecting an appropriate value of s, via generalized cross validation.
Results
CNAs detected by CGH in 97 primary OSCC
The frequencies of chromosomal aberrations detected by CGH are shown in Table I. The average number of total CNAs was 9.9 (range 1–30), including 6.73 gains and 3.05 losses on average. For further analysis, we considered alterations occurring in at least 30% of samples. The bandless CGH data are available at ftp://ftp.ncbi.nlm.nih.gov/pub/oral_cancer (chromosomal gains are denoted as ‘+’ and chromosomal losses as ‘−’).
Table I.
Frequencies of CNAs among 97 oral cancer patients.
| Genetic alteration | Frequency (%) |
|---|---|
| +8q | 74.2 |
| −3p | 49.5 |
| −8p | 47.4 |
| +9q | 41.2 |
| +11q13 | 41.2 |
| +7p | 37.1 |
| +3q | 35.1 |
| −18q | 34.0 |
| +20q | 33.0 |
| +20p | 27.8 |
| +14q | 25.8 |
| +5p | 24.7 |
| −11q14-qter | 20.6 |
CNAs, copy number alterations.
HPV integration
The 97 tumor samples screened for HPV were found to be HPV-negative, indicating that HPV is not causally associated with the tumors profiled in this study.
Confirmation of CGH results with interphase FISH
To further assess some of the CNAs detected by CGH on chromosome arm 11q, FISH was performed on 32 samples and sub-band 11q13, which is known to be frequently gained, was evaluated (11,12,14). The results obtained from tumor samples were compared to three normal oral mucosal samples. For all samples for which FISH signals were obtained, the concordance rate between CGH and FISH was high (83.33%, Table II). For 11q13, the five discrepancies comprised two non-informative tissues and three cases where FISH detected a gain that was not found by CGH.
Table II.
Concordance of CGH and FISH results for region 11q13.
| 11q13 | |||
|---|---|---|---|
|
|
|||
| Gains | No gains | Total concordant (n) | |
| CGH | 13 | 19 | 32 |
| FISH | 10 | 15 | 25 |
For 11q13, two samples showed no signals in fluorescene in situ hybridization (FISH), so the concordance rate was 25/30 (83.33%). CGH, comparative genomic hybridization.
Association of CNAs with clinicopathological parameters
The recurrent CNAs in OSCC were found to be associated with high grade and positive lymph node status (Table III). In particular, +7p and −8p significantly correlated with pN+ as opposed to pN0 (Table III). Furthermore, 11q13 gain was more prominent in high-grade (moderately and poorly differentiated) than in low-grade (well-differentiated) OSCC (Table IV). The CNAs did not show any correlation with clinicopathological parameters, such as size, site and stage of OSCC (data not shown).
Table III.
Correlation of CNAs with nodal status.
| Genetic alteration | Nodal status | Fisher | BH | |
|---|---|---|---|---|
|
|
||||
| N0 (n=43) | N+ (n=54) | p-valuea | p-valueb | |
| +3q | 13 (30.2) | 21 (38.9) | 0.250 | 0.28 |
| +11q13 | 16 (37.2) | 24 (44.4) | 0.310 | 0.31 |
| +20q | 12 (27.9) | 20 (37.0) | 0.230 | 0.28 |
| +7p | 10 (23.0) | 26 (48.1) | 0.010 | 0.04 |
| −8p | 14 (32.5) | 32 (59.3) | 0.008 | 0.04 |
| +9q | 13 (30.2) | 27(50.0) | 0.040 | 0.09 |
| −18q | 10 (23.0) | 23 (42.6) | 0.040 | 0.09 |
| +8q | 28 (65.1) | 44 (81.5) | 0.060 | 0.10 |
| −3p | 19 (44.1) | 29 (53.7) | 0.230 | 0.28 |
Number and percentage (in parentheses) of cases are shown.
One-sided Fisher’s exact test, p<0.05 is significant.
BH, Benjamini-Hochberg (1995) method of adjusting for multiple tests.
CNAs, copy number alterations.
Table IV.
Correlation of CNAs with tumor grade.
| Genetic alteration | Grade | Fisher | BH | ||
|---|---|---|---|---|---|
|
|
|||||
| Well (n=14) | Moderate (n=53) | Poor (n=30) | p-valuea | p-valueb | |
| +3q | 1 (7.10) | 24 (45.3) | 9 (30.0) | 0.020 | 0.091 |
| +11q13 | 1 (7.10) | 22 (41.5) | 17 (56.7) | 0.005 | 0.050 |
| +20q | 2 (14.3) | 16 (30.2) | 14 (46.7) | 0.088 | 0.260 |
| +7p | 3 (21.3) | 21 (40.0) | 12 (40.0) | 0.500 | 0.740 |
| −8p | 7 (50.0) | 25 (47.2) | 14 (46.7) | 1.000 | 1.000 |
| +9q | 6 (42.3) | 21 (40.0) | 13 (43.3) | 0.960 | 1.000 |
| −18q | 4 (28.6) | 20 (37.8) | 9 (30.0) | 0.760 | 0.970 |
| +8q | 8 (57.1) | 40 (75.4) | 24 (80.0) | 0.270 | 0.500 |
| −3p | 4 (28.6) | 28 (52.8) | 18 (60.0) | 0.280 | 0.500 |
Number and percentage (in parentheses) of cases are shown.
One-sided Fisher’s exact test, p<0.05 is significant.
BH, Benjamini-Hochberg (1995) method of adjusting for multiple tests.
CNAs, copy number alterations.
Association of CNAs and clinicopathological parameters with prognosis
Follow-up data were available for 40 patients, including 12 patients with reduced survival (median survival time 6.1 months). Survival was tested against clinical parameters and genetic alterations. Univariate Cox proportional hazards models were used to determine the effect of clinicopathological parameters and genetic alterations on survival (Table V). The analysis revealed that clinical parameters, including TNM stage, did not show any significant association with survival. Among the genetic alterations, univariate analysis revealed correlations of +11q13 and −18q with poor survival (Table V; Fig. 1A and B).
Table V.
Univariate Cox proportional hazards regression analysis of single predictors for overall survival for 40 patients.
| Factor | p-valuea | BHb |
|---|---|---|
| +11q13 | 0.005 | 0.05 |
| −18q | 0.007 | 0.05 |
| +3q | 0.220 | 0.41 |
| −3p | 0.100 | 0.30 |
| +7p | 0.630 | 0.86 |
| +8q | 0.500 | 0.75 |
| −8p | 0.170 | 0.36 |
| +9q | 0.170 | 0.36 |
| +20q | 0.920 | 0.92 |
| Age | 0.080 | 0.30 |
| Nodal status | 0.090 | 0.30 |
| Grade | 0.300 | 0.50 |
| TNM stage | 0.880 | 0.92 |
| Size | 0.840 | 0.92 |
| Site | 0.760 | 0.92 |
Log-ratio test.
Benjamini-Hochberg (1995) method of adjusting for multiple tests.
Figure 1.
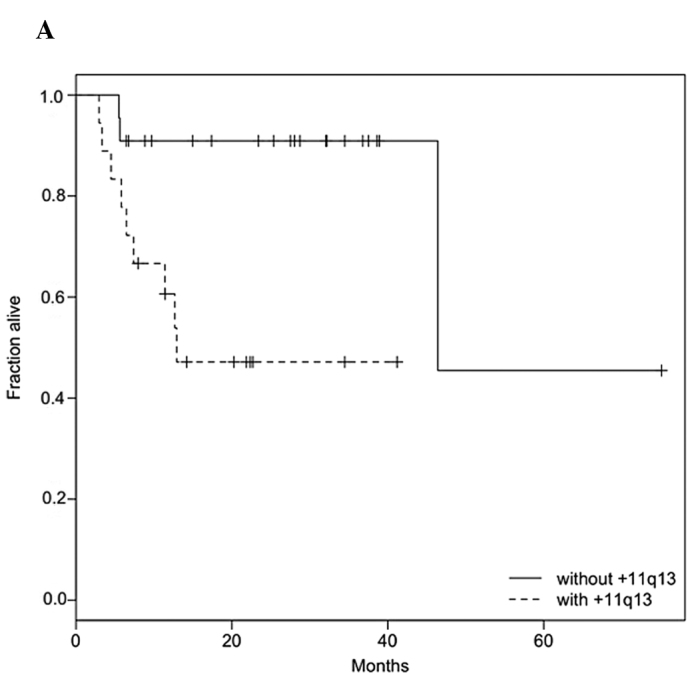
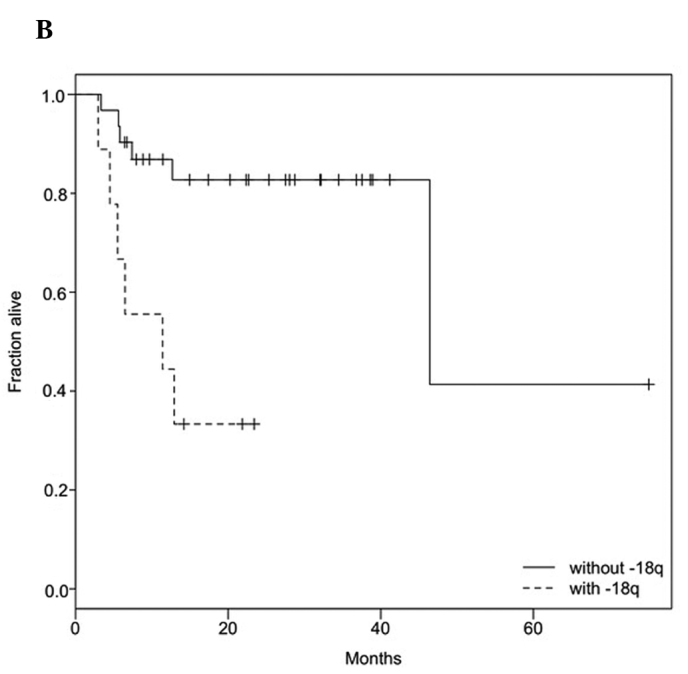
Kaplan-Meier estimates for (A) 11q13 gain and (B) 18q loss. In both cases, the survival rate was significantly higher in the group lacking alterations. (A) For patients with 11q13 gain, the median survival time was 12.9 months, while (B) for patients with 18q loss, it was 11.4 months. The survival time compares to a half-life of 46.4 months in the absence of either alteration.
To determine the best prognostic combination of CNAs, we used a multivariate Cox proportional hazards model. To avoid overparameterization, model selection based on the LASSO method and stepwise model selection using BIC was performed. The LASSO method revealed the bivariate combination of +11q13 and −18q as the best model (Fig. 2). For this combination, the risk is distributed relatively evenly among 11q13 (hazard: expβi=4.3; p=0.08) and 18q (expβi=3.1; p=0.08). A Kaplan-Meier plot with patient subgroups having none, one or both of the two CNAs is shown in Fig. 3. The results of the LASSO method were confirmed by stepwise BIC, and it was shown that a combination of +11q13, −18q, +9q and +7p is the best multivariate predictor (BIC=73.70). This model has a slightly better (i.e., lower) BIC value than the bivariate combination of +11q13 and −18q (BIC=74.58). In the best BIC model, the main effect on survival stems again from the gain of 11q13 (expβi=15.2; p=0.004). The remaining risk was distributed among +9q (expβi=5.3; p=0.03), −18q (expβi=4.7; p=0.03), and +7p (expβi=0.13; p=0.02). A Kaplan-Meier plot with four patient groups based on the calculated risk is shown in Fig. 4.
Figure 2.
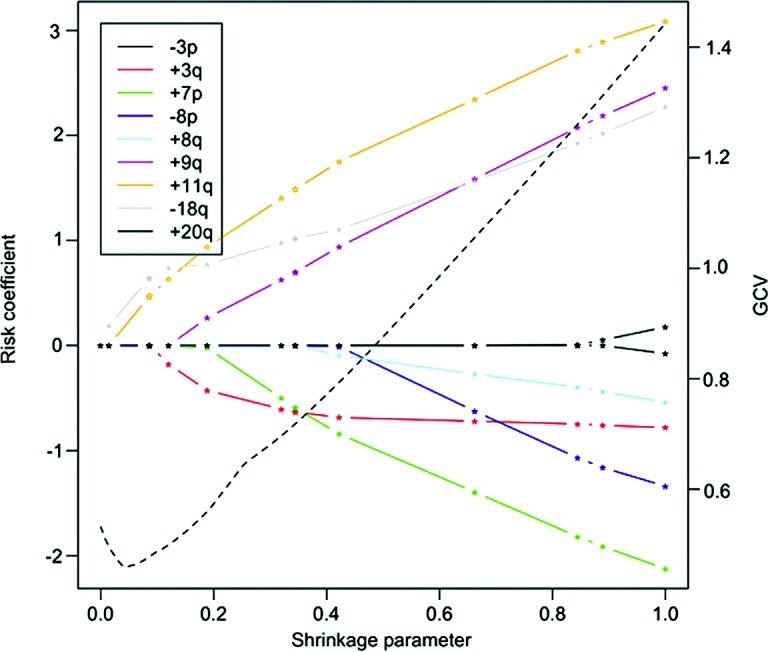
LASSO method estimates of the risk co-efficients in the Cox proportional hazards model. Co-efficients of high-level CNAs are shown as a function of the shrinkage parameter (straight lines). A shrinkage parameter of 1 denotes the unconstrained model. The first two non-vanishing co-efficients are those of +11q and −18q, followed by +3q and +9q. An evaluation of the generalized cross validation statistic (dashed line) reveals that it is minimal for a shrinkage parameter of 0.05. Only +11q and −18q contribute to the overall risk, making this combination an ideal multivariate marker.
Figure 3.
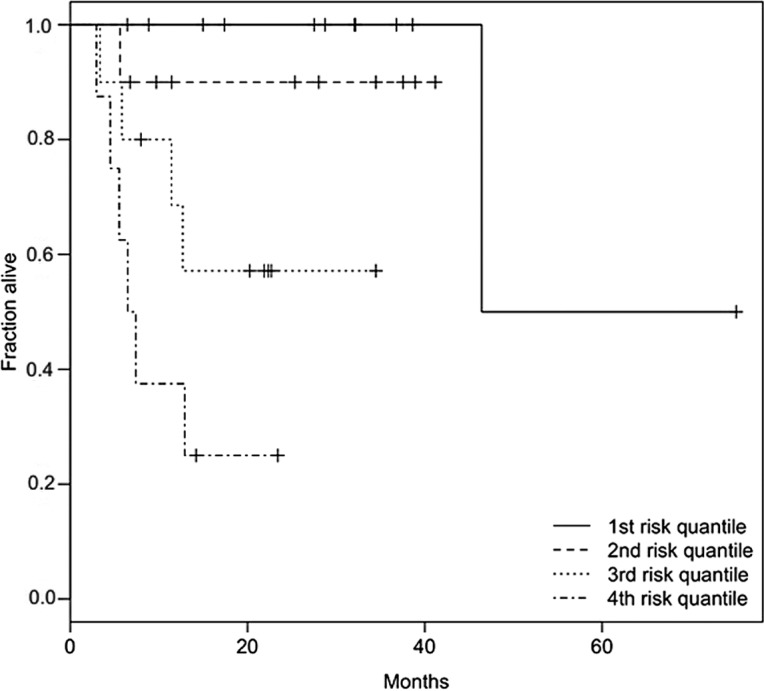
Kaplan-Meier plot of the multivariate predictor with +11q13, −18q, +9q and +7p as determined by BIC. Four cohorts defined by the 25, 50, 75 and 100% quantiles of the risk function of the Cox proportional hazards model are shown. As expected, survival was shortest in the high-risk group and longest in the low-risk group. With the exception of one outlier, the lowest risk quantile had complete survival, whereas in the highest risk cohort the half-life was <10 months.
Figure 4.
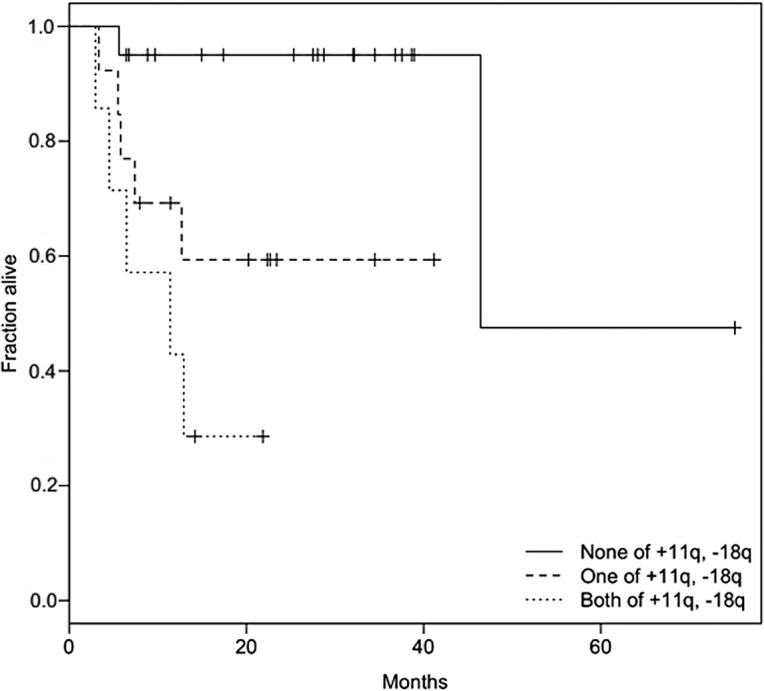
Bivariate predictor based on +11q13 and −18q as yielded by the LASSO method. The fraction lacking the two CNAs exhibited the lowest risk, whereas the cohort with the two alterations had the highest risk. The median survival time in the high-risk group was only 11.4 months, with 29% of the patients surviving after 24 months. By contrast, 95% of patients survived in the lowest risk group after 40 months. The presence of either of the two alterations formed an intermediate risk group with approximately 60% patient survival after 40 months.
Discussion
The chromosome-based CGH has identified recurrent chromosomal alterations in oral cancers (5–14). The recurrent chromosomal imbalances may play a significant role in the complex process of oral carcinogenesis. However, none of the 10 studies cited above has yielded genetic biomarkers for predicting the clinical outcome of oral carcinomas. The majority of these studies have not investigated the association of recurrent alterations with clinicopathological parameters and clinical outcome. In studies where the presence of prognostic CNAs has been suggested, a rigorous statistical assessment of the multivariate data is lacking. In the present study, oral cancers with CGH were analyzed and clinical associations of the recurrent alterations were evaluated by applying stringent statistical procedures, including correction for multiple testing and BIC- and LASSO-based model selection.
A recurrent CGH pattern for OSCC was obtained in this study, which is in accordance with previous CGH studies (5–14). CGH results were validated with interphase FISH for gains of region 11q13. Our statistical analysis revealed associations of specific alterations with lymph node involvement, high grade and poor clinical outcome. This result suggests that these alterations are clinically viable.
We analyzed oral cancers that are associated with tobacco use. Notably, none of the 97 samples exhibited HPV integration. This result is in agreement with former studies, in which there was little or no prevalence of HPV infection in oral cancers as compared to other HNSCC anatomic sites (28,29). Additionally HPV-related OSCCs are characterized by loss of 16q, whereas HPV-unrelated tumors, such as those of the present study, exhibited gains/amplifications of 11q13 and more losses at 3p, 5q, 9p, 15q and 18q (30). Thus, our data support the hypothesis that HPV is an etiological factor for only a subset of HNSCC.
A significant correlation of +7p and −8p with pN+ was noted in this study. This finding is consistent with the results of a previous analysis based on oncogenetic trees (15). The occurrence of +7p and −8p in pN+ oral cancers has been reported (31–34). Genes at chromosomal regions 7p12 are known to affect the biological characteristics of OSCC; for example, activation of the oncogene EGFR on 7p12 has been shown to play a role in the nodal metastasis of OSCC. Loss of tumor suppressor genes on 8p, which contributes to the lymph node involvement of primary OSCC, remains to be determined. Our findings indicate that the two alterations confer aggressive behavior of OSCC. Thus, they may serve as biomarkers for predicting the propensity of nodal metastases in OSCC.
The correlation between genetic alterations and histological grade of OSCC is seldom studied. However, this study found a significant correlation of +11q13 with high-grade OSCC. One possible explanation for 11q13 gains affecting these characteristics may be that +11q13 is associated with an increased copy number of CCND1 and the overexpression of its protein cyclin D1 (35). Previous studies have noted the role of cyclin D1 as a potential marker of proliferation, since the overexpression of D1 has been shown to positively correlate with other proliferation markers, such as Ki-67 and PCNA, and to inversely correlate with the expression of the anti-apoptotic gene BCL2 (36).
In their study, Angadi et al showed an association between the overexpression of cyclin D1 and a high grade (poor differentiation) of oral cancers (37). A similar finding was reported by Mate et al in lung carcinomas. The authors speculated that differentiation, in addition to proliferation, is a major target of cyclin D1 impinging upon mechanisms of development of neoplasia (38). This raises the possibility that cyclin D1 overexpression plays a role in the inhibition of tumor cell differentiation in certain cell types (39).
Clinical follow-up data were available for a subset of patients, and univariate and multivariate Cox proportional hazards analyses were performed. Since the full multivariate model was likely to be overparameterized, key parameters were identified by means of model selection. When the prognostic impact of the clinical variables was evaluated on a univariate basis, the clinicopathological parameters, including TNM stage, did not show any prognostic association. Among the CGH-detected alterations, +11q13 and −18q were most significantly associated with poor clinical outcome. The two model selection criteria (LASSO and BIC) have shown +11q13 together with −18q to be a strong combined predictor of poor prognosis in the multivariate analysis. Moreover, the stepwise BIC selection indicates that this bivariate marker may be further improved by including genetic lesions on 9q and 7p. Our survival analysis shows that genetic aberrations define subsets of poor survival among oral cancers beyond clinical criteria.
Survival analysis of CGH data of head and neck cancers has consistently found +11q13 to be a predictor of poor prognosis (40,41). Although the chromosome was detected by interphase FISH analysis in earlier studies (42), such an association has not been found by CGH studies of oral cancers. To the best of our knowledge, the present study is the first to report an association of +11q13 with poor clinical outcome in the more homogeneous group of oral cancers. Understanding the role of different oncogenes, such as CCND1, EMS1 and FGF3/4 (located at 11q13), may aid in the development of better therapeutic strategies.
Multivariate statistical analysis identified −18q in combination with +11q13 as a predictor of poor prognosis. Loss of 18q has been associated with increased angiogenesis, recurrence and short survival of HNSCC patients (43,44). Thus, 18q loss may play a role in the aggressive behavior of oral cancers. Loss of maspin, a TSG on 18q, was shown to correlate with increased angiogenesis and reduced survival in OSCC (45,46). Notably, the multivariate analysis revealed the involvement of +9q and +7p as prognostic markers. The latter has been associated, not only with nodal metastasis, but also with poor prognosis. However, evidence shows 9q gain to correlate with an unfavorable outcome. Our study suggests that these alterations, if used in combination, may be useful in prognosticating OSCC patients.
The present study is the first to analyze oral cancers using rigorous statistical analysis and the first to obtain genetic alterations that are clinically relevant biomarkers. We have identified genetic alterations that correlate with clinicopathological parameters and the survival status of oral cancers. The bivariate predictor based on +11q13 and −18q may serve as a valuable biomarker for identifying patients with poor clinical outcome. Following confirmation in a larger set of samples, this candidate predictor serves as a biomarker that enhances the current TNM staging system for better prediction of the clinical behavior of oral cancers.
Acknowledgements
The authors are thankful to the Indian Council of Medical Research (ICMR; Grant no.5/13/2/TF/2001-NCD-III) for funding the project. This study was also supported by the Intramural Research Program of the National Institutes of Health, NLM. We thank CSIR for providing fellowship to Ms. Swapnali Pathare during her tenure as a graduate (PhD) student.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Notani P. Epidemiology and prevention of head and neck cancer: a global view. In: Saranath D, editor. Contemporary Issues in Oral Cancer. Oxford University Press; New Delhi: 2000. pp. 1–29. [Google Scholar]
- 3.Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19:251–262. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 4.Akervall J. Genomic screening of head and neck cancer and its implications for therapy planning. Eur Arch Otorhinolaryngol. 2006;263:297–304. doi: 10.1007/s00405-006-1039-1. [DOI] [PubMed] [Google Scholar]
- 5.Hermsen M, Joenje H, Arwert F, et al. Assessment of chromosomal gains and losses in oral squamous cell carcinoma by comparative genomic hybridisation. Oral Oncol. 1997;33:414–418. doi: 10.1016/s0964-1955(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 6.Wolff E, Girod S, Liehr T, et al. Oral squamous cell carcinomas are characterized by a rather uniform pattern of genomic imbalances detected by comparative genomic hybridisation. Oral Oncol. 1998;34:186–190. doi: 10.1016/s1368-8375(97)00079-1. [DOI] [PubMed] [Google Scholar]
- 7.Weber R, Scheer M, Born I, et al. Recurrent chromosomal imbalances detected in biopsy material from oral premalignant and malignant lesions by combined tissue microdissection, universal DNA amplification, and comparative genomic hybridization. Am J Pathol. 1998;153:295–303. doi: 10.1016/S0002-9440(10)65571-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okafuji M, Ita M, Hayatsu Y, Shinozaki F, Oga A, Sasaki K. Identification of genetic aberrations in cell lines from oral squamous cell carcinomas by comparative genomic hybridization. J Oral Pathol Med. 1999;28:241–250. doi: 10.1111/j.1600-0714.1999.tb02032.x. [DOI] [PubMed] [Google Scholar]
- 9.Okafuji M, Ita M, Oga A, et al. The relationship of genetic aberrations detected by comparative genomic hybridization to DNA ploidy and tumor size in human oral squamous cell carcinomas. J Oral Pathol Med. 2000;29:226–231. doi: 10.1034/j.1600-0714.2000.290506.x. [DOI] [PubMed] [Google Scholar]
- 10.Oga A, Kong G, Tae K, Lee Y, Sasaki K. Comparative genomic hybridization analysis reveals 3q gain resulting in genetic alteration in 3q in advanced oral squamous cell carcinoma. Cancer Genet and Cytogenet. 2001;127:24–29. doi: 10.1016/s0165-4608(00)00430-1. [DOI] [PubMed] [Google Scholar]
- 11.Lin S, Chen Y, Kao S, et al. Chromosomal changes in betel-associated oral squamous cell carcinomas and their relationship to clinical parameters. Oral Oncol. 2002;38:266–273. doi: 10.1016/s1368-8375(01)00054-9. [DOI] [PubMed] [Google Scholar]
- 12.Hannen E, Macville M, Wienk S, et al. Different chromosomal imbalances in metastasized and nonmetastasized tongue carcinomas identified by comparative genomic hybridization. Oral Oncol. 2004;40:364–371. doi: 10.1016/j.oraloncology.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Noutomi Y, Oga A, Uchida K, et al. Comparative genomic hybridization reveals genetic progression of oral squamous cell carcinoma from dysplasia via two different tumourigenic pathways. J Pathol. 2006;210:67–74. doi: 10.1002/path.2015. [DOI] [PubMed] [Google Scholar]
- 14.Martin C, Reshmi S, Ried T, et al. Chromosomal imbalances in oral squamous cell carcinoma: examination of 31 cell lines and review of the literature. Oral Oncol. 2008;44:369–382. doi: 10.1016/j.oraloncology.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathare S, Schäffer A, Beerenwinkel N, Mahimkar M. Construction of oncogenetic tree models reveals multiple pathways of oral cancer progression. Int J Cancer. 2009;124:2864–2871. doi: 10.1002/ijc.24267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 17.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 19.De Roda Husman AM, Walboomers JM, Meijer CJ, et al. Analysis of cytomorphologically abnormal cervical scrapes for the presence of 27 mucosotropic human papillomavirus genotypes, using polymerase chain reaction. Int J Cancer. 1994;56:802–806. doi: 10.1002/ijc.2910560607. [DOI] [PubMed] [Google Scholar]
- 20.Khoja S, Ojwang P, Khan S, Okinda N, Harania R, Ali S. Genetic analysis of HIV-1 subtypes in Nairobi, Kenya. PLoS One. 2008;3:e3191. doi: 10.1371/journal.pone.0003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoir SD, Schröck E, Bentz M, et al. Quantitative analysis of comparative genomic hybridization. Cytometry. 1995;19:27–41. doi: 10.1002/cyto.990190105. [DOI] [PubMed] [Google Scholar]
- 22.Jeuken J, Sprenger S, Wesseling P. Comparative genomic hybridization: practical guidelines. Diagn Mol Pathol. 2002;11:193–203. doi: 10.1097/00019606-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 24.Cox DR. Regression models and life-tables. J Roy Statist Soc Ser B. 1972;34:187–220. [Google Scholar]
- 25.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 26.Tibshirani R. The Lasso method for variable selection in the Cox model. Statistics in Medicine. 1997;16:385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Park MY, Hastie T. L1-regularization path algorithm for generalized linear models. J Roy Statist Soc Ser B. 2007;69:659–677. [Google Scholar]
- 28.Ha PK, Pai SI, Westra WH, et al. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8:1203–1209. [PubMed] [Google Scholar]
- 29.Klussmann JP, Weissenborn SJ, Wieland U, et al. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92:2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::aid-cncr10130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Klussmann JP, Mooren JJ, Lehnen M, et al. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res. 2009;15:1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 31.Gebhart E, Ries J, Wiltfang J, Liehr T, Efferth T. Genomic gain of the epidermal growth factor receptor harboring band 7p12 is part of a complex pattern of genomic imbalances in oral squamous cell carcinomas. Arch Med Res. 2004;35:385–394. doi: 10.1016/j.arcmed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Kalyankrishna S, Grandis J. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 33.Ishwad C, Shuster M, Bockmuhl U, et al. Frequent allelic loss and homozygous deletion in chromosome band 8p23 in oral cancer. Int J Cancer. 1999;80:25–31. doi: 10.1002/(sici)1097-0215(19990105)80:1<25::aid-ijc6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Temam S, Oh M, et al. Global expression-based classification of lymph node metastasis and extracapsular spread of oral tongue squamous cell carcinoma. Neoplasia. 2006;8:925–932. doi: 10.1593/neo.06430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnellen R, Chetty R. Cyclin D1 and human neoplasia. J Clin Pathol. 1998;51:1–7. doi: 10.1136/mp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staibano S, Migonogua M, Muzio L, Alberti L, Natale E, Lucariello A. Overexpression of cyclin D1, bcl-2, and bax proteins, proliferating cell nuclear antigen, and DNA-ploidy in squamous cell carcinoma of the oral cavity. Hum Pathol. 1998;29:1189–1194. doi: 10.1016/s0046-8177(98)90244-1. [DOI] [PubMed] [Google Scholar]
- 37.Angadi P, Krishnapillai R. Cyclin D1 expression in oral squamous cell carcinoma and verrucous carcinoma: correlation with histological differentiation. Oral Surg Oral Med Oral Pathol Oral Radiol and Endod. 2007;103:30–35. doi: 10.1016/j.tripleo.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Mate J, Ariza A, Aracil C, Lopez D, Isama T, Piteira J. Cyclin D1 overexpression in non-small cell lung carcinoma: correlation with ki-67 labelling index and poor cytoplasmic differentiation. J Pathol. 1996;80:395–399. doi: 10.1002/(SICI)1096-9896(199612)180:4<395::AID-PATH688>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Shapex S, Rhee J, Spicer D, Lassar A. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 40.Bockmuhl U, Schluns K, Kuchler I, Petersen S, Petersen I. Genetic imbalances with impact on survival in head and neck cancer patients. Am J Pathol. 2000;157:369–375. doi: 10.1016/S0002-9440(10)64549-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wreesmann VB, Shi W, Thaler HT, et al. Identification of novel prognosticators of outcome in squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22:3965–3972. doi: 10.1200/JCO.2004.01.094. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto R, Uzawa N, Nagaoka S, Nakakuki K, Hirata Y, Amagasa T. Potential marker of oral squamous cell carcinoma aggressiveness detected by fluorescence in situ hybridization in fine-needle aspiration biopsies. Cancer. 2002;95:2152–2159. doi: 10.1002/cncr.10929. [DOI] [PubMed] [Google Scholar]
- 43.Pearlstein R, Benninger M, Carey T, et al. Loss of 18q predicts poor survival of patients with squamous cell carcinoma of the head and neck. Genes Chromosomes Cancer. 1998;21:333–339. doi: 10.1002/(sici)1098-2264(199804)21:4<333::aid-gcc7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 44.Takebayashi S, Hickson A, Ogawa T, et al. Loss of chromosome arm 18q with tumor progression in head and neck squamous cancer. Genes Chromosomes Cancer. 2004;41:145–154. doi: 10.1002/gcc.20066. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Volpert O, Shi Y, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 46.Xia W, Lau Y, Hu M. High tumoral maspin expression is associated with improved survival of patients with oral squamous cell carcinoma. Oncogene. 2000;19:2398–2403. doi: 10.1038/sj.onc.1203535. [DOI] [PubMed] [Google Scholar]


