Abstract
Amygdala reactivity to threat-related distractor stimuli can be abolished in perceptually-demanding contexts. Premised on the biological imperative to respond swiftly to threat, we demonstrate, however, that when participants are threatened by shock, greater amygdala responses to fearful compared to neutral distractor faces is preserved under conditions of high attentional demand. Lateral prefrontal cortices also showed selective responding to fearful distractor faces under these conditions, suggesting that threat-related distractor stimuli engaged attentional control mechanisms. We conclude that anxiety elicited by looming threat promotes neurocognitive processes that broaden attention and enhance sensitivity to potential danger cues, even when perceptual systems are taxed.
Keywords: amygdala, anxiety, attention, fearful faces, perceptual load, prefrontal cortices
1.0 Introduction
The human amygdala is activated by a variety of emotional and social stimuli (Adolphs, 2003; Fitzgerald et al., 2006). Especially potent are threat-related stimuli such as fearful facial expressions. Studies manipulating perceptual-attentional resources suggest, however, that amygdala responses to threat-related stimuli are dependent on resource allocation (Bishop et al., 2007; Pessoa et al., 2002; Pessoa et al., 2005; van Dillen et al., 2009). In general, threat-related faces lose their ability to elicit greater amygdala responses, relative to non-threatening neutral faces, when participants are engaged in demanding perceptual tasks in which attending to these faces is irrelevant to performance. Even aversively-conditioned fearful faces fail to differentially activate the amygdala when perceptual systems are overburdened (Lim et al., 2008). This body of work has challenged the ‘automaticity’ of amygdala reactivity (Dolan & Vuilleumier, 2003) and the notion that emotional stimuli constitute a privileged stimulus class with dedicated neurocognitive mechanisms for their rapid identification.
These findings also indicate that participants are quite adept at maintaining attention on a difficult perceptual task while ignoring emotionally-salient distractors. This may not always be advantageous, and—evolutionarily speaking—may be costly when real danger presents itself. There must be mechanisms in place to continuously monitor the environment for threat. Anxiety may be a powerful moderator in this regard. Anxiety entails heightened vigilance, a state of being ‘on guard’ that may require a broad distribution of attention to the environment (Cornwell et al., 2008). Anxious vigilance promotes adaptation to unpredictable perceptual input (Herry et al., 2007), and sensitizes sensory-perceptual systems to potential threat cues (Cornwell et al., 2007). To be reliably adaptive, a resetting of threat detection mechanisms to a more sensitive mode (Öhman, 1993) must occur even when perceptual systems are heavily taxed. One way to study these adaptive processes is to focus on natural between-subject variations in state or trait anxiety. Using this approach, Bishop et al. (2007) found a positive correlation between state anxiety (at experiment onset) and amygdala reactivity to threat-related distractors under low but not high attentional demand. However, such an approach does not involve manipulating anxiety, and results could be influenced by personality characteristics in addition to state anxiety. A complementary approach is to directly induce anxiety in participants and perform within-subjects comparisons (Cornwell et al., 2007; Grillon, 2002; Shackman et al., 2006).
Here we build on previous research by testing whether threat-induced anxiety overrides the blocking effects of high perceptual load on amygdala reactivity to threat-related distractors. Using fMRI, participants performed a letter detection task, which was adapted from Bishop et al. (2007), under two conditions: (1) threat of shock in which a moderately-uncomfortable shock could be delivered without warning and (2) safety in which there was no risk of shocks. In this task, letter strings were superimposed on task-irrelevant faces, with some faces displaying fearful expressions and others displaying emotionally-neutral expressions. From previous work, we predicted that amygdala responses would be greater to fearful distractor faces compared to neutral distractor faces when the task imposed low demand on attentional resources. Based on the hypothesis that anxiety enhances sensitivity to potential threat cues, we predicted that this differential amygdala response (fearful-vs.-neutral) would be preserved under conditions of high attentional demand during threat of shock. Because of the greater sensitivity to fearful distractors, we also anticipated that prefrontal attentional control mechanisms would be active under these same conditions. Finally, it is also possible that inducing anxiety overshadows the influence of individual differences in state anxiety (at experiment onset) and trait anxiety; thus while state/trait measures of anxiety may correlate with amygdala or prefrontal reactivity during safety, in line with the findings of Bishop et al. (2007), these correlations may not persist during threat.
2.0 Material and Methods
2.1 Participants
Eighteen healthy adults participated (10 men, mean age = 28 yr, range = 13 yr). One additional volunteer completed the study, but because of equipment failure, his data were excluded. Exclusion criteria included: (1) past or current psychiatric disorders as per the Structured Clinical Interview for DSM-IV (First et al., 1995), (2) current use of psychoactive medications as per self-report, and (3) current use of illicit drugs determined by urinalysis. The study was approved by the Combined Neuroscience Institutional Review Board of the NIH. All participants gave informed consent prior to participation. After consent was obtained, the Spielberger State-Trait Anxiety Inventory (STAI, Spielberger, 1983) was administered (mean ± SD; Trait: 32 ± 7; State: 28 ± 8). These STAI scores suggest that our sample was predominantly comprised of relatively low anxious participants (Spielberger, 1983).
2.2 Task procedure
Participants completed 8 task runs, alternating 4 times between threat of shock and safe conditions, with an equal number of participants beginning with a Threat or Safe run. Participants were informed that they might receive shocks during the Threat runs but not during the Safe runs. Within each run, a total of 6 blocks of 4 trials of the letter detection task were completed, 3 low and 3 high perceptual load blocks (pseudo-randomly ordered), with a 2-s interval between blocks. This mixed block/event-related design, taken from Bishop and colleagues (2007), consisted of blocks that varied by Load (Low, High) and events that varied by type of distractor Face (Fearful, Neutral). The inter-trial interval within blocks was randomly jittered using an exponential function (mean = 4.5 s, min = 3 s).
Each trial required a perceptual decision regarding the presence of an ‘X’ or ‘N’ in a letter string superimposed on a fearful or neutral face (200-ms duration). Task-irrelevant distractor faces (4 men, 4 women) were taken from a standardized set (Ekman & Friesen, 1976). Perceptual load was manipulated by the letter string composition, with letter strings for low load trials containing six instances of the target (e.g., ‘XXXXXX’) and those for high load trials containing one (e.g., ‘KHNMWZ’). Presentation software controlled stimulus delivery and recorded responses. Participants used a button box to respond. Accuracy and speed were both emphasized.
2.3 Electrode set-up and shock delivery
Two surface electrodes were prepared with conductive gel and attached to the left ankle for shock administration. Shock intensity was set individually to a moderately uncomfortable level before the subject was placed in the scanner. PsyLab software (Contact Precision Instruments, London, UK) controlled delivery of shocks. A total of 6 shocks were administered in between trials during Threat (1–2 shocks per Threat run).
2.4 Subjective anxiety and heart rate
Subjective anxiety was assessed after each run (0–10 with “0” representing “no anxiety” and “10” representing “intense anxiety”). Cardiovascular activity was monitored during each run with a GE Scanner pulsometer attached to the nondominant hand, and heart rate (beats/min) was calculated over the first 30 s of each run before a shock was administered. The pulsometer failed for two participants.
2.5 MRI data acquisition
Imaging data were collected at the Functional MRI Facility of the NIMH using a 3T General Electric Signa HDx MRI scanner (90 cm bore, whole body gradient inset 40 mT/m, slew rate 150 T/m/s, whole body RF coil) equipped with an 8-channel receive-only brain array. For each functional run, we acquired 65 T2*-weighted echoplanar images (EPI; 33 slices, slice thickness 4 mm, TR = 2000 ms, TE = 30 ms, flip angle = 90°, matrix 64 × 64, FOV 240 mm). In addition, we collected T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) structural images (124 axial slices, slice thickness 1.2 mm, TR = 7.28 ms, TE = 2.7 ms, matrix 256 × 256, FOV 220) for anatomical registration.
2.6 Behavioral data analysis
Error rates and median reaction times of correct responses only were computed for each of the 8 trial types. Three-way repeated-measures ANOVAs with Condition (Safe, Threat), Load (Low, High) and Face (Fearful, Neutral) as within subjects factors were conducted to test our hypotheses.
2.7 fMRI pre-processing and analysis
Imaging data were processed and analyzed using AFNI (Cox, 1996). For each subject, we removed the first four volumes of each EPI series to reduce T1 equilibrium effects. EPI volumes were then corrected for slice-timing and registered to the EPI volume closest in time to the MPRAGE. Following motion correction, we used a recently developed cost functional (Saad, 2009), which applies a weighted local Pearson coefficient, to coregister functional and anatomical images. The anatomical data were then registered to Talairach-Tournoux stereotaxic space (Talairach & Tournoux, 1988) using a 12-parameter affine transformation. EPI data were smoothed with a 6-mm FWHM Gaussian kernel and normalized at each time point to reflect percent signal change from the mean of the time series at each voxel. Prior to individual subject analyses, we concatenated runs.
We used multiple regression to analyze the imaging data acquired from each subject during the letter detection task. The regression model included one regressor for each of the 8 trial types. Responses to these regressors were modeled with a standard gamma-variate function. In addition, regressors of no interest included 6 head movement parameters (3 translations, 3 rotations), baseline drift, and the BOLD response to shocks. Whereas the latter was modeled with a gamma-variate function as was done for all trial types, drifting effects were modeled with a separate linear polynomial for each run.
2.8 Group fMRI analysis
Right and left amygdalae masks were anatomically defined by the Talairach Atlas Daemon (Lancaster et al., 2000) and resampled to the same grid as the EPI data. Each voxel retained for the resampled mask overlapped the original mask by at least 25% to limit inclusion of regions surrounding the amygdalae (right amygdala mask = 56 3-mm isotropic voxels, left amygdala mask = 54 3-mm isotropic voxels. Individual-subject average BOLD responses across each mask for each condition were extracted. Three-way repeated-measures ANOVAs were conducted on these data.
For prefrontal cortical regions, we used spherical masks (8-mm radius) centered at the same coordinates used by Bishop and colleagues (2007). Five regions of interest (ROI) were explored: bilateral dorsolateral regions (±34, 36, 24 in MNI space), bilateral ventrolateral regions (±38, 20, 0), and dorsal anterior cingulate cortices (4, 14, 36). Like for the amygdalae, BOLD responses for each prefrontal ROI were averaged across the mask and extracted for group-level analyses.
3. Results
3.1 Subjective anxiety and Heart rate
Self-reported anxiety and heart rate (HR) were higher during Threat runs (mean ± SD, anxiety rating: 5.31 ± 2.13 arbitrary units; HR: 65.0 ± 4.8 beats/min) than during Safe runs (anxiety rating: 1.94 ± 1.51 arbitrary units; HR: 61.0 ± 4.6 beats/min, t(17) = 7.69, p < .001 and t(15) = 2.54, p < .05, respectively).
3.2 Letter detection performance
A Condition (Safe, Threat) × Load (Low, High) × Face (Fearful, Neutral) repeated-measures ANOVA on error rates revealed a main effect of Load, F(1,17) = 49.21, p < .001, and a marginally significant effect of Condition, F(1, 17) = 3.53, p = .078. More errors were committed on high load (21.4 ± 8.4%) compared to low load trials (2.0 ± 1.7%), and there was a trend toward more errors during Threat (12.7 ± 7.6%) compared to Safe (10.8 ± 5.8%). No other main effects or interactions emerged (p’s > .19).
For reaction times (RT) of correct responses, a significant 3-way interaction was found, F(1,17) = 7.90, p = .012 (Figure 1). On low load trials, a 2-way ANOVA of RT resulted in a main effect of Condition, F(1,17) = 17.03, p = .001, but no effect of Face, F(1,17) = 1.30, p = .27, and no Condition-by-Face interaction, F < 1. Participants were faster to respond correctly during Threat (654 ± 99 ms) than during Safe (685 ± 109 ms) on low load trials. On high load trials, a significant Condition-by-Face interaction emerged, F(1,17) = 8.93, p = .008, reflecting slower responding with fearful compared to neutral distractors during Threat, F(1,17) = 9.30, p = .007, but no difference in RT as a function of Face during Safe, F(1,17) = 2.21, p = .155. Moreover, participants were, in general, faster to respond on low load compared to high load trials, F(1,17) = 77.30, p < .01, and during Threat compared to Safe, F(1,17) = 8.15, p = .011; there was no main effect of Face in the omnibus analysis, F < 1.
Figure 1.
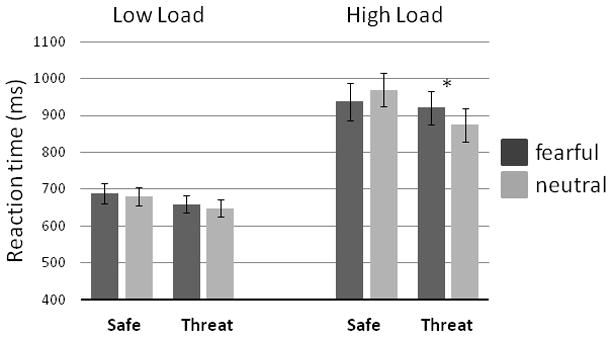
Mean reaction time (N=18) on the letter detection task (correct responses only) as a function of Load (Low, High), Condition (Safety, Threat) and distractor Face (Fearful, Neutral). Error bars represent SEMs. *p < .05
3.3 Amygdalae responses to distractors
A 3-way interaction was significant for the right amygdala, F(1,17) = 4.90, p = .041 (Figure 2), but only marginally significant for the left amygdala, F(1,17) = 3.25, p = .089. On low load trials, fearful distractors elicited greater right amygdala responses than neutral distractors, F(1,17) = 4.99, p = .039. No other effects were present, Fs < 1. On high load trials, the Condition-by-Face interaction was significant, F(1,17) = 6.84, p = .018), with fearful distractors eliciting greater right amygdala responses than neutral distractors during Threat, F(1,17) = 6.81, p = .018, but not during Safe, F(1,17) = 1.74, p = .205. Moreover, right and left amygdalae responses were greater, in general, for fearful over neutral distractors, F(1,17) = 7.84, p = .012 and F(1,17) = 4.61, p = .046, respectively. Also, high load weakened right and left amygdalae responses compared to low load, F(1,17) = 16.93, p = .001 and F(1,17) = 22.30, p < .001, respectively.
Figure 2.
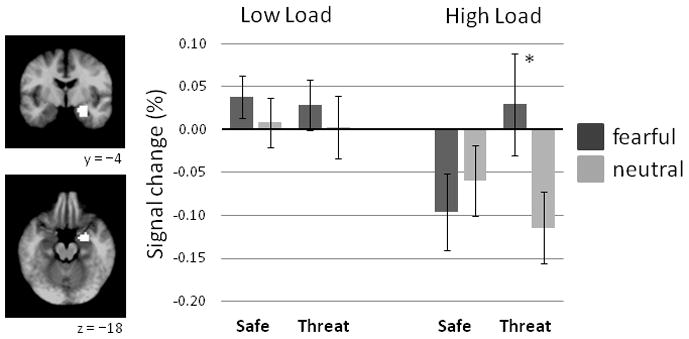
Right amygdala ROI responses (N=18) to face distractors during safety and threat of shock on low and high load trials. The anatomical mask (white) used to extract right amygdala data is overlayed on a group-averaged T1 image. Coordinates are in MNI space. Error bars represent SEMs. *p < .05
Right amygdala responses on high load trials during Threat may be related to task performance, even though error rates rose only by about 2% during Threat (Section 3.2). To test this possibility, we conducted a post-hoc analysis of right amygdala responses on high load trials in a subsample of participants (N=15) with similar error rates on high load trials during Threat and Safe (Safe, 18.2 ± 10.0% vs. Threat, 18.7 ± 11.0%, F < 1). Three participants with the greatest increases in error rates from Safe to Threat were removed. In this subsample, the Condition-by-Face interaction remained significant, F(1,14) = 5.41, p = .036, and again reflected greater responses to fearful compared to neutral distractors during Threat, F(1,14) = 5.69, p = .032, but no difference during Safe, F(1,14) = 1.17, p = .299.
3.4 Prefrontal cortical responses to distractors
Significant 3-way interactions were found for the left dorsolateral cortical (DLPFC) ROI, and left and right ventrolateral prefrontal cortical (VLPFC) ROIs, F(1,17) = 10.21, p = .005, F(1,17) = 5.03, p = .039, F(1,17) = 6.01, p = .025, and F(1,17) = 5.03, p = .039 respectively (Figure 3). No other prefrontal ROIs exhibited significant 3-way interactions (ps > .14). On low load trials, left DLPFC responses showed a significant Condition-by-Face interaction, F(1,17) = 4.51, p = .049, with greater responding to neutral compared to fearful distractors during Threat, F(1,17) = 5.36, p = .033, but no difference during Safe, F < 1. On high load trials, left DLPFC responses showed a significant Condition-by-Face interaction, F(1,17) = 8.02, p = .011, with greater responding to fearful compared to neutral distractors during Threat, F(1,17) = 18.84, p < .001, but no difference during Safe, F < 1.
Figure 3.
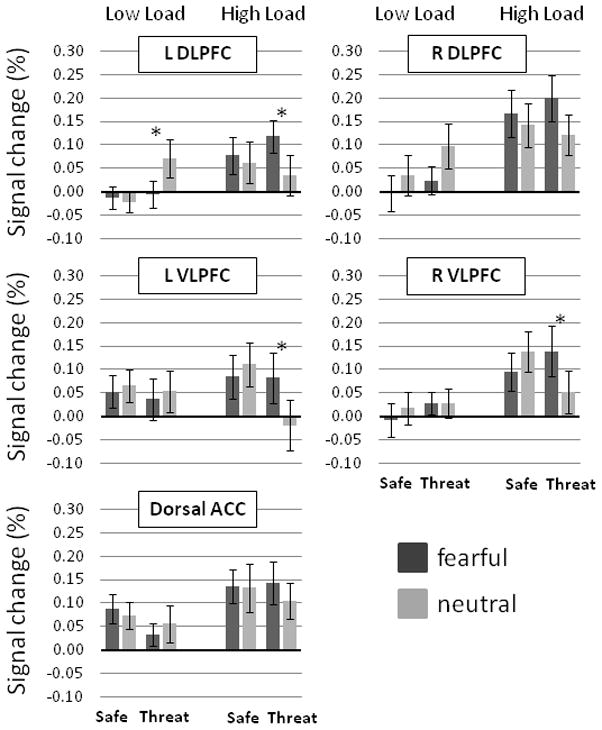
Prefrontal cortical ROI responses (N=18) to face distractors during during safety and threat of shock on low and high load trials. L = left, R = right, DLPFC = dorsolateral prefrontal cortices, VLPFC = ventrolateral prefrontal cortices, ACC = anterior cingulate cortices. Error bars represent SEMs. *p < .05
For the left and right VLPFC, no effects were found on low load trials, Fs < 1. Left VLPFC responses on high load trials showed a significant Condition-by-Face interaction, F(1,17) = 8.87, p = .008, with greater responding to fearful compared to neutral distractors during Threat, F(1,17) = 11.31, p = .004, but no difference during Safe, F < 1. Similarly, right VLPFC responses on high load trials showed a significant Condition-by-Face interaction, F(1,17) = 10.40, p = .005, with greater responding to fearful compared to neutral distractors during Threat, F(1,17) = 12.15, p = .003, but no difference during Safe, F(1,17) = 3.10, p = .096.
In addition, right DLPFC ROI showed a significant Load-by-Face interaction, F(1,17) = 14.34, p = .001, with greater responding to neutral compared to fearful distractors on low load trials, F(1,17) = 5.52, p = .031, and greater responding to fearful compared to neutral distractors on high load trials, F(1,17) = 12.88, p = .002. Finally, all prefrontal ROIs, with the exception of left VLPFC, showed greater responses on high load compared to low load trials, Fs(1,17) > 8.22, ps < .012 (Figure 3). No other effects were found in the omnibus analyses of these ROIs.
3.5 State and trait anxiety correlations
To follow-up on findings reported by Bishop et al. (2007), we performed correlation analyses to determine whether individual differences in state or trait anxiety scores (from the STAI) correlated with differential amygdala and prefrontal ROI responses during Safe and Threat conditions. We computed individual difference of differences scores (i.e., Low load [Fearful –Neutral] – High load [Fearful – Neutral]) for Safe and Threat separately. For the Safe condition, dorsal ACC ROI activity was negatively correlated with STAI-State scores, r(16) = −.57, p = .013; in addition there were statistical trends for correlations between left DLPFC ROI activity and STAI-State scores, r(16) = −.46, p = .054, and left amygdala ROI activity and STAI-Trait scores, r(16) = .42, p = .083. All other correlations for the Safe condition were not significant, ps > .10. For the Threat condition, no correlations were significant, ps > .10.
4. Discussion
Our results evidence an important distinction between threat and safe conditions in right amygdala reactivity to threat-related distractors when perceptual systems are heavily taxed. Despite a general dampening of amygdala responding under high perceptual load, participants still exhibited greater right amygdala responses to fearful faces compared to neutral faces when threatened by shock. When participants were safe, differential responding was abolished as expected, replicating previous findings under non-anxious conditions (Bishop et al., 2007; Pessoa et al., 2005). Lateral prefrontal cortical reactivity followed this same pattern, except that these regions generally showed enhanced responding under high perceptual load. Altogether, the present data suggest that under threat conditions, participants remain sensitive to threat-related stimuli even when engaged in a highly-demanding perceptual task.
These findings extend previous fMRI research using the same task that explored individual differences in state and trait anxiety, but did not manipulate state anxiety during the task (Bishop et al., 2007). State and trait anxiety – assessed with the STAI (Spielberger, 1983) – correlated with differential amygdala and prefrontal cortical reactivity under low attentional demand, but not under high attentional demand. Our results are not necessarily incompatible with these previous data. In fact, during safety, differential dorsal ACC reactivity showed a similar negative correlation with pre-existing state anxiety as Bishop et al. found for trait anxiety. Accordingly, natural variations in state and trait anxiety may influence dorsal ACC reactivity – and perhaps left DLPFC and left amygdala reactivity as well (see Section 3.5) – when attentional demand is low. Inducing anxiety with threat of shock appears necessary, however, to show that sensitivity to threat-related distractors is maintained when attentional demand is high. Moreover, threat of shock seems to promote a relatively uniform adaptive state among participants that overshadows pre-existing or dispositional anxiety attributes (Lissek et al., 2006). Individuals with a broader range of trait anxiety levels than those tested here would be needed to fully assess this possibility.
Considering task performance, evidence of increased errors in detecting target letters during threat of shock was inconclusive, but raised the possibility that amygdala responses on high load trials was driven by the poorest performers attending to the faces at the expense of the letter strings. This is not likely because the results were unchanged for participants showing comparable error rates across safe and threat conditions, suggesting instead that differential amygdala reactivity on high load trials during threat occurred despite participants’ being fully engaged in detecting letters. It is also evident from reaction times that relative to neutral distractors, fearful distractors slowed perceptual decisions on high load trials during threat, but not during safety. Based on their impact on behavioral performance, we can infer that fearful distractors captured attention during threat despite high demands on perceptual-attentional mechanisms. Still, relative to safety, participants were generally faster to respond to target letters during threat, suggesting that attending to fearful distractors on high load trials during threat imposed negligible costs on primary task performance.
Along with eliciting differential amygdala responses, fearful distractors also engaged left dorsal and bilateral ventral prefrontal cortical regions on high load trials during threat. This fits with attentional control theory that predicts enhanced recruitment of prefrontal attention mechanisms in anxiety to limit distraction by threat-related stimuli when demands on attention are high (Eysenck et al., 2007). This may be particularly true for low trait-anxious participants (Bishop et al., 2007), like those tested here. At the same time, we observed reduced DLPFC responding to fearful distractors on low load trials, which was pronounced during threat in the left DLPFC. Bishop et al. reported a similar finding in high trait-anxious participants, suggesting that a lack of left DLPFC recruitment to threat-related distractors when attentional demand is low may be both a trait anxiety marker and a characteristic of threat-induced anxiety. To cast the present findings as evidence of weakened goal-directed attentional control and greater distractibility in anxiety, however, is to miss the adaptive value of attending to multiple sources of the environment in the context of real danger. Although faces did not predict shock, they constituted a potentially relevant source worth monitoring for signs of imminent threat.
Finally, regarding the debate over the (in)dependence of amygdala reactivity on attention, we see a third alternative that does not presuppose that attentional resources are a fixed quantity. Visuo-spatial attention, that is, may not always take the form of a unified ‘spotlight,’ but may be intentionally deployed in various configurations to multiple environmental sources simultaneously based on task demands (Cave et al., 2010; Jans et al., 2010; McMains & Somers, 2005). Anxiety elicited by a looming threat may be one such condition in which attention widens and sensitivity to potential danger cues across the environment is enhanced (Cornwell et al., 2008). This could explain how, even though participants’ performance on a difficult letter detection task was relatively unchanged under threatening conditions, right amygdala, lateral prefrontal, and behavioral responses were all modulated by the content of task-irrelevant faces. In short, we contend that attentional mechanisms were optimized to process both letter strings and task-irrelevant faces during threat of shock, unlike during safety. The full extent by which threat-induced anxiety enhances perceptual-attentional processes merits further investigation.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH). We wish to thank Katye Vytal for helpful comments on an earlier draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs RA. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: Effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Cave KR, Bush WS, Taylor TGG. Split attention as part of a flexible attentional system for complex scences: Comment on Jans, Peters, and De Weerd (2010) Psychol Rev. 2010;117(2):685–695. doi: 10.1037/a0019083. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Echiverri AM, Covington MF, Grillon C. Modality-specific attention under imminent but not remote threat of shock: Evidence from differential prepulse inhibition of startle. Psychol Sci. 2008;19:615–622. doi: 10.1111/j.1467-9280.2008.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Baas JMP, Johnson L, Holroyd T, Carver FW, Lissek S, et al. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. NeuroImage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Softwared for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Ann N Y Acad Sci. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA, USA: 1976. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RI, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV (SCID) American Psychiatric Association; Washington, DC, USA: 1995. [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. NeuroImage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans B, Peters JC, De Weerd P. Visual spatial attention to multiple locations at once: The jury is still out. Psychol Rev. 2010;117(2):637–682. doi: 10.1037/a0019082. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SL, Padmala S, Pessoa L. Affective learning modulates spatial competition during low-load attentional conditions. Neuropsychologia. 2008;46:1267–1278. doi: 10.1016/j.neuropsychologia.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biol Psychol. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC. Processing efficiency of divided attention mechanisms in human visual cortex. J Neurosci. 2005;25:9444–9448. doi: 10.1523/JNEUROSCI.2647-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A. Fear and anxiety as emotional phenomena. In: Lewis M, Haviland J, editors. Handbook of emotions. Guilford Press; New York, NY, USA: 1993. pp. 511–536. [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. NeuroImage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. NeuroImage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1983. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York, NY, USA: 1988. [Google Scholar]
- van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: An fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage. 2009;45:1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]


