Abstract
Millions of people in Central and Southern America are affected by Chagas disease. Richard Reithinger and colleagues explain the difficulties of elimination and suggest a strategy
In 2007, the World Health Organization announced a renewed strategy to eliminate Chagas disease in the Americas by 2010.1 The intention was “to answer key questions about the treatment and control of Chagas disease, and to coordinate global efforts towards the prevention of transmission through a new Global Network for Chagas Elimination.”1 The announcement was welcome because it put the spotlight on Chagas disease, which has been overshadowed by other priorities, such as HIV and malaria, in recent years. However, two years later, WHO has yet to produce a clear strategy for elimination and we are unaware of any new operational activities to eliminate the disease. Too many challenges remain.
Scale of the problem
Chagas disease is both a disease of poverty and, like other neglected tropical diseases, poverty promoting.2 An estimated 10-20 million people live with the condition and it is responsible for a burden of 670 000 disability adjusted life years,3 making it the most important parasitic disease in the Americas.
The disease is caused by the protozoan parasite Trypanosoma cruzi,4 which is transmitted through the faeces of blood feeding triatomine bugs; trypomastigotes in the faeces contaminate the wound or enter through mucosal surfaces.5 It can also be transmitted by blood transfusions, organ donations, through the placenta, or by eating contaminated food. Most people do not know that they have become infected with T cruzi because the acute symptoms tend to be unspecific or benign (fever, swollen lymph glands, inflammation at the biting site or a swollen eye, and, rarely, severe myocarditis or meningoencephalitis). In mammals, T. cruzi must invade host cells in order to replicate. It ultimately destroys these cells, stimulating tissue inflammation and releasing infective extracellular trypanomastigote forms, which propagate the infection through the body.
After the acute phase, which lasts a few weeks or months, the infection is generally well controlled by the host immune response. Nonetheless, parasites continue to cycle in and out of host cells but are only transiently in the bloodstream and thus difficult to detect, with symptoms not evident for years or even decades. Eventually, however, up to 40% of infected people develop chronic Chagas disease. This chronic stage of the disease is characterised by cardiac or gastrointestinal complications, which if left untreated are severely debilitating and often fatal. Indeed, Chagas disease is the most common cause of cardiomyopathy in South and Central America and the leading cause of cardiovascular death in disease endemic areas.6 In people who have suppressed immune systems (because of HIV infection or chemotherapy, for example), Chagas disease can reactivate with abundant parasites found in the blood and tissues commonly leading to severe meningoencephalitis, which is fatal if untreated.
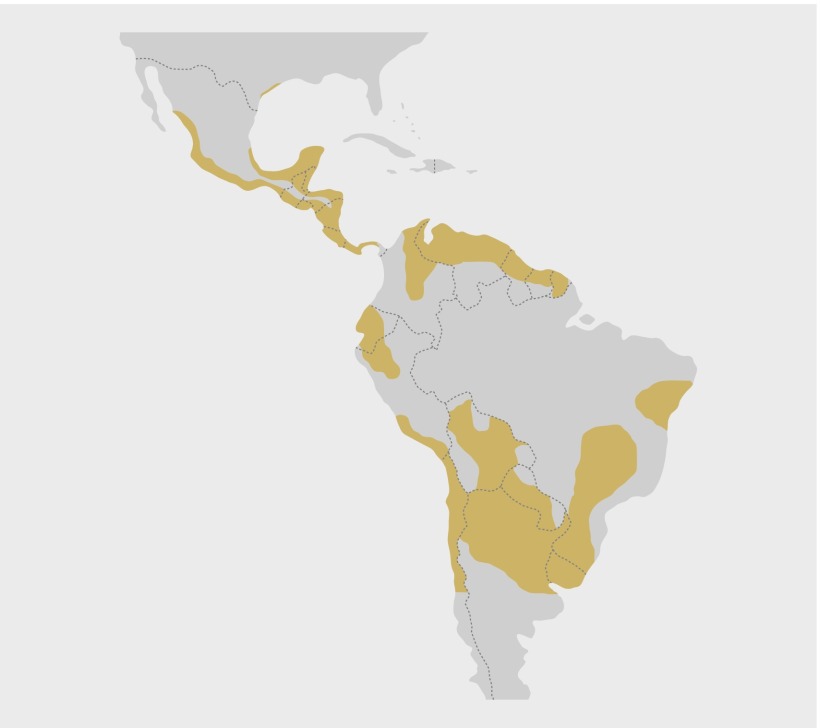
T cruzi is found in triatomine bugs, wildlife, domestic animals, and humans throughout much of rural and peri-urban areas of North, Central, and South America (figure). Although autochthonous vector borne human cases are scarce in the United States,7 8 and non-existent in Canada and Europe, T cruzi has been detected in triatomine bugs, dogs, and wildlife in the US7 9 and is increasingly detected in the US, Canada, and Europe among immigrants or tourists from Latin America.10 11 12 After the detection of a number of cases in blood donors,13 the US FDA approved a screening assay for blood donors in 2007.
Chagas disease is endemic in all Latin American countries. Brown shading shows the main areas where vector borne transmission of T cruzi to humans occurs
Large regional control programmes have reduced the incidence of Chagas disease in the southern tier of South America and some other regions in the last decade.14 Vector borne transmission has been reduced or even halted, mainly through residual spraying of domestic and peridomestic household structures with insecticide, and blood donations are routinely screened for T cruzi. Uruguay, Chile, and Brazil are currently declared free of T cruzi transmission by the main vector Triatoma infestans.
Challenges of elimination
Eradication of Chagas disease is impossible because of the zoonotic characteristics of the T cruzi transmission cycle.4 Control of the disease is also hampered by several operational and policy challenges and knowledge gaps.15 For example, increasing insecticide resistance of bugs and the recolonisation of households by bugs after insecticide treatment are reducing the impact of vector control; no consensus exists on whether benznidazole and nifurtimox is the best treatment for adult chronic infection (the most prevalent presentation) and the drugs have been in short supply 16 17; there is no agreed standard for diagnosis, with laboratories using a combination of serological tests that may give inconclusive or false negative results18; and there is no vaccine to prevent Chagas disease.
Whether the intention of WHO is to stop the development of Chagas disease in populations at risk or halt vector borne transmission of T cruzi to humans is not clear. Too many challenges persist to expect successful elimination of Chagas disease by 2010. Resurgence of vector borne transmission after apparent elimination, as witnessed in Argentina during the 1990s, shows that without proper planning or emphasis on consolidation and sustainability, successes may be short lived. T cruzi transmission still persists in many regions of Latin America (two large outbreaks of orally transmitted T cruzi were reported in Venezuela in the past 15 months) and has entered peri-urban areas in some places, such as Arequipa, Peru.19 A realistic, sustainable strategy to interrupt the human transmission of T cruzi should be developed and a strategy to treat and manage the millions of currently infected individuals needs to be established.
The first step is to get a good understanding of the extent of the disease. WHO stated “current estimates suggest that less than 8 million people remain infected,”1 but with no recent nationwide surveys of T cruzi infection (except in Brazil and Chile), the estimate is suspect. No up to date data on the burden and distribution of Chagas disease exist for Mexico, Peru, Colombia, Costa Rica, and much of the Amazonian region. Indeed, as recently as 2005 only 361 cases of the disease were reported by the Mexican Ministry of Health,20 yet focal studies in rural areas showed seroprevalence of up to 30% and up to 82.5% of patients with congestive heart failure testing seropositive for T cruzi.21
Road map
Given these challenges and gaps,15 how should we develop a comprehensive strategy for eliminating Chagas disease? Below we outline what we believe should be the main components.
Better monitoring and estimation of true burden of disease
Cost constraints mean that a disease specific regional surveillance system is probably not feasible. Instead there should be advocacy to integrate Chagas disease into a country’s health monitoring information system or other disease surveillance programmes (malaria, dengue, or tuberculosis). Additionally, representative surveys (similar to the malaria indicator surveys developed under Roll Back Malaria’s monitoring and evaluation reference group)22 should be carried out regularly to estimate domestic infestation, the incidence of Chagas disease, prevalence of coinfections, disability associated with the disease, and population knowledge of the disease’s diagnosis, treatment, prevention, and control. Both surveillance and surveys would contribute to getting more accurate data to estimate disease burden as well as to monitor and evaluate the effect of a putative elimination strategy.
We also need a better understanding of the burden of Chagas disease. Clarification should be obtained about the nature and origin of the measures used to calculate disability adjusted life years (prevalence of disease, age at death and mortality, duration of disease) and how the disability weight for Chagas disease was computed. Similarly, clarification should be obtained about how cases are detected and reported at national and regional level (active or passive case reporting; mandatory notification, etc). These efforts should be complemented by sensitivity and specificity analyses to determine more robust estimates of the disease burden as well as to show how different measures to calculate the disability adjusted life years attributed to Chagas disease affect current national and regional estimates.
Standard diagnostic procedures
The substantial number of inconclusive diagnoses18 shows we need a valid and accepted standard diagnostic procedure. The available diagnostic procedures (which include enzyme linked immunosorbent assays, immunofluorescence antibody tests, radioimmune precipitation assays, and polymerase chain reaction based assays) vary in sensitivity, particularly if not properly standardised. The absence of a standard method for detecting T cruzi not only makes it impossible to determine the true impact of Chagas disease but also makes the development of new diagnostics extremely challenging.
A WHO consultative meeting last year highlighted the need for substantial investment in the development of new and better diagnostics, using the increased understanding of the genetic makeup and diversity of T cruzi and new technologies for improved, rapid, and cost effective diagnostics.23 A uniform set of diagnostic standards obtained from the entire geographic range of T cruzi and incorporating confirmed but problematic samples (positive on some current tests but negative on others) must be established. Though for many years WHO and the Pan American Health Organisation have supported external validation of samples in selected reference laboratories, it is unclear how many local laboratories use that service. A first step in reaching this goal may be the recent decision to develop and provide a panel of reference samples to laboratories in endemic countries24 and to conduct complex multicentre trials comparing a range of diagnostic tests.
In addition, there should be a recommendation, internationally recognised and endorsed, on uniform case descriptions of Chagas disease, algorithms for diagnosis, and standardised approaches to estimate case numbers through active and passive detection in endemic settings. Although many endemic countries have standard definitions for clinical disease, definitions, algorithms, and protocols for confirmatory, laboratory diagnosis may differ within and between countries. Thus, in Argentina diagnosis of chronic Chagas disease is based on the results of two independent, standardised serological tests,25 but which tests and their protocol is not specified. El Salvador requires two confirmatory serological tests after an initial positive result,26 but again the nature of the tests is not specified.
Effective treatment strategy
The elimination of Chagas disease implies the effective treatment of millions of people already infected with T cruzi in the American continent and now in non-endemic areas as well. This is a massive undertaking that obviously requires identification of the patients as well as the availability of safe, effective, and affordable drugs. Currently available drugs (nifurtimox and benznidazole)27 are generally accepted to be effective in treating acute and early chronic infections in children younger than 15 years but their efficacy and availability is not uniform through all endemic regions. Treatment efficacy in longer term chronic infections, however, remains controversial,16 17 as are the criteria (conventional serology and direct methods to evaluate parasitaemia) currently used to assess drug efficacy. This, coupled with the common occurrence of side effects, leads many doctors to view the risk:benefit ratio of such treatments as unfavourable. Despite important advances in preclinical studies,27 no new drugs have entered clinical trials in more than a decade. In the meantime a strategy to manage these patients is urgently required and should be part of any elimination efforts.
Increased spraying coverage and monitoring of insecticide resistance
Any elimination strategy will rely on spraying of households with insecticides as its main approach for vector control. Spraying needs to be maintained in those areas where it is currently implemented and expanded to cover more endemic areas in order to significantly affect T cruzi transmission. Monitoring and reporting of insecticide resistance are required, given the recent reports of insecticide resistance in triatomine bugs. A range of insecticides may need to be used rather than just the currently used pyrethroid insecticides to prevent resistance from developing and expanding. Unfortunately, there are few non-toxic, affordable alternatives to pyrethroid insecticides that are effective against triatomine bugs. Other approaches for vector control have proved effective in preventing infection in humans, such as insecticide treated bednets28 or preventing bugs from feeding on animal reservoirs by using insecticide treated dog collars.29 However, these interventions have yet to be tested operationally on a large scale and their cost effectiveness needs to be assessed.
Coordination
Any elimination effort will have to be implemented in the context of increasingly decentralised health services,30 the loss of operational capacity and of technical knowledge about vector control. Many government disease control programmes have lost qualified staff through retirement and are overburdened by other communicable diseases. There is a shortage of public health officers and entomologists trained to design and manage vector control programmes or use the information technology and geographical information systems that are now commonly used in modern vector control. To counterbalance one of the negative impacts of decentralisation, more trained local staff are urgently needed.
Finally, for any elimination strategy to be successful, coordination between national, regional, and international policymakers, public health professionals, and academics is essential.
WHO’s efforts to highlight Chagas disease as an important public health issue and to suppress this devastating, poverty promoting disease should be fully supported. However, to successfully accomplish this bold undertaking, a realistic, sustainable strategy to interrupt the human transmission of T cruzi is prerequisite.
The opinions expressed are those of the authors and may not reflect the position of their employing organisation or of their work’s sources of funding.
Contributors and sources: RR, RT, JU, UK, and RG have all worked extensively in the diagnosis, treatment, prevention and control of Chagas disease or other vector-borne diseases, in academic as well as operational settings. The article arose following the authors’ extensive discussions on the challenges of eliminating Chagas disease. Part of this process included a comprehensive literature search of medical databases as well as non-medical search engines. All authors co-wrote and reviewed the manuscript, with RR and RT taking the lead. RR and RT are the guarantors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Cite this as: BMJ 2009;338:b1283
References
- 1. WHO. New global effort to eliminate Chagas disease. Partners set out strategy against the “kissing bug” disease. www.who.int/mediacentre/news/releases/2007/pr36/en/index.html.
- 2.Hotez P, Ottesen E, Fenwick A, Molyneux D. The neglected tropical diseases: the ancient afflictions of stigma and poverty and the prospects for their control and elimination. Adv Exp Med Biol 2006;582:23-33. [DOI] [PubMed] [Google Scholar]
- 3.WHO. The World Health Organization report 2004. Changing history. Geneva: WHO, 2004.
- 4.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis 2001;1:92-100. [DOI] [PubMed] [Google Scholar]
- 5.Communicable Disease Center. Parasites and health: trypanosomiasis , American. www.dpd.cdc.gov/dpdx/HTML/TrypanosomiasisAmerican.htm#Life%20Cycle.
- 6.Rassi A Jr, Rassi A, Little WC, Xavier SS, Rassi SG, et al. Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med 2006;355:799-808. [DOI] [PubMed] [Google Scholar]
- 7.Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Peterson AT, et al. Chagas disease in a domestic transmission cycle, southern Texas, USA. Emerg Infect Dis 2003;9:103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis 2007;13:605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabsley MJ, Noblet GP. Seroprevalence of Trypanosoma cruzi in raccoons from South Carolina and Georgia. J Wildl Dis 2002;38:75-83. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Blood donor screening for Chagas disease--United States, 2006-2007. MMWR Morb Mortal Wkly Rep 2007;56:141-3. [PubMed] [Google Scholar]
- 11.Diaz JH. Recognizing and reducing the risks of Chagas disease (American trypanosomiasis) in travellers. J Travel Med 2008;15:184-95. [DOI] [PubMed] [Google Scholar]
- 12.Lescure FX, Canestri A, Melliez H, Jauréguiberry S, Develoux M, Dorent R, et al. Chagas disease, France. Emerg Infect Dis 2008;14:644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leiby DA, Herron RM Jr, Garratty G, Herwaldt BL. Trypanosoma cruzi parasitemia in US blood donors with serologic evidence of infection. J Infect Dis 2008;198:609-13. [DOI] [PubMed] [Google Scholar]
- 14.Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz 2002;97:603-12. [DOI] [PubMed] [Google Scholar]
- 15.Tarleton RL, Reithinger R, Urbina JA, Kitron U, Gürtler RE. The challenges of Chagas disease—grim outlook or glimmer of hope. PLoS Medicine 2007;4:1852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Expert Committee. Control of Chagas disease. Brasilia, Brazil: WHO, 2002.
- 17.WHO. Strategic direction for research. Disease burden and epidemiological trends. http://www.who.int/tdr/diseases/chagas/direction.htm.
- 18.Pirard M, Iihoshi N, Boelaert M, Basanta P, Lopez F, Van der Stuyft P. The validity of serologic tests for Trypanosoma cruzi and the effectiveness of transfusional screening strategies in a hyperendemic region. Transfusion 2005;45:554-61. [DOI] [PubMed] [Google Scholar]
- 19.Bowman NM, Kawai V, Levy MZ, Cornejo del Carpio JG, Cabrera L, Delgado F, et al. Chagas disease transmission in periurban communities of Arequipa, Peru. Clin Infect Dis 2008;46:1822-8. [DOI] [PubMed] [Google Scholar]
- 20.Secretaria de Salud. Información Epidemiológica de Morbilidad 2005. Versión Ejecutiva. www.dgepi.salud.gob.mx/download/descargar/morbilidad05.pdf.
- 21.Attaran A. Chagas’ disease in Mexico. Lancet 2006;368:1768. [DOI] [PubMed] [Google Scholar]
- 22.Roll Back Malaria Monitoring and Evaluation Reference Group. Malaria indicator survey: basic documentation for survey design and implementation. Geneva: WHO, 2005. www.rbm.who.int/merg.html#MIS.
- 23.Campaign for Access to Essential Medicines, Médecins Sans Frontières. International meeting: new diagnostic tests are urgently needed to treat patients with Chagas disease. Rev Soc Bras Med Trop 2008;41:315-9. [DOI] [PubMed] [Google Scholar]
- 24.WHO consultation on international biological reference preparations for Chagas diagnostic tests, 2-3 July, 2007. www.who.int/bloodproducts/ref_materials/WHO_Report_1st_Chagas_BRP_consultation_7-2007_final.pdf.
- 25.Ministerio de Salud. Guías Para la Atención al Paciente Infectado con Trypanosoma cruzi. Argentina: Ministerio de Salud, 2006.
- 26.Ministerio de Salud Pública y de Asistencia Social. Norma Técnica de Prevención y Control de la Enfermedad de Chagas. El Salvador: Ministerio de Salud Pública y de Asistencia Social, 2007.
- 27.Duschak VG, Couto AS. An insight on targets and patented drugs for chemotherapy of Chagas disease. Recent Patents Anti-Infect Drug Disc 2007;2:19-51. [DOI] [PubMed] [Google Scholar]
- 28.Kroeger A, Villegas E, Ordoñez-González J, Pabon E, Scorza JV. Prevention of the transmission of Chagas’ disease with pyrethroid-impregnated materials. Am J Trop Med Hyg 2003;68:307-11. [PubMed] [Google Scholar]
- 29.Reithinger R, Ceballos L, Stariolo R, Davies CR, Gürtler RE. Extinction of experimental Triatoma infestans populations following continuous exposure to dogs wearing deltamethrin-treated collars. Am J Trop Med Hyg 2006;74:766-71. [PMC free article] [PubMed] [Google Scholar]
- 30.Yadón ZE, Gürtler RE, Tobar F, Medici AM, eds. Decentralización y gestión del control de las enfermedades transmisibles en América Latina. Buenos Aires: Fundación Mundo Sano y Organización Panamericana de la Salud, 2006.