Abstract
Although humans and chimpanzees share >99% identity in alignable protein sequences, they differ surprisingly in the incidence and severity of some common diseases. In general, humans infected with various viruses, such as HIV and hepatitis C virus, appear to develop stronger reactions and long-term complications. Humans also appear to suffer more from other diseases associated with over-reactivity of the adaptive immune system, such as asthma, psoriasis, and rheumatoid arthritis. In this study, we show that human T cells are more reactive than chimpanzee T cells to a wide variety of stimuli, including anti-TCR Abs of multiple isotypes, L-phytohemagglutin, Staphylococcus aureus superantigen, a superagonist anti-CD28 Ab, and in MLRs. We also extend this observation to B cells, again showing a human propensity to react more strongly to stimuli. Finally, we show a relative increase in activation markers and cytokine production in human lymphocytes in response to uridine-rich (viral-like) ssRNA. Thus, humans manifest a generalized lymphocyte over-reactivity relative to chimpanzees, a finding that is correlated with decreased levels of inhibitory sialic acid-recognizing Ig-superfamily lectins (Siglecs; particularly Siglec-5) on human T and B cells. Furthermore, Siglec-5 levels are upregulated by activation in chimpanzee but not human lymphocytes, and human T cell reactivity can be downmodulated by forced expression of Siglec-5. Thus, a key difference in the immune reactivity of chimp and human lymphocytes appears to be related to the differential expression of Siglec-5. Taken together, these data may help explain human propensities for diseases associated with excessive activation of the adaptive immune system.
The common chimpanzee (Pan troglodytes) is our closest evolutionary relative, sharing >99% identity in most protein sequences with humans (1). Thus, the chimpanzee has long been considered a good model to study human diseases. However, manifestation of diseases in humans and chimpanzees appears to differ more than previously assumed (2–4). For example, in captive chimpanzee models of HIV infection, there is a lack of progression to AIDS with the maintenance of CD4 T cell counts in the great majority of experimentally infected chimpanzees (5–8). Although less clear cut, there also appear to be differences in the late outcomes of hepatitis C virus (HCV) infection (9–12). The development of Abs to HCV envelope proteins also appears to be less frequent and less vigorous in chimpanzees, with hepatitis occurring later and being somewhat milder (13). In addition, the late complications of cirrhosis, terminal liver failure, and hepatocellular carcinoma appear to be less common in chimpanzees (10, 14, 15). Such discrepancies suggest differences in adaptive immune responses between the two species. Indeed, one current school of thought favors hyperimmune activation as playing a major role in the progression of human HIV infection to AIDS (8, 16, 17) and of progression of hepatitis virus infection toward chronic hepatitis (18–20).
Adult chimpanzees also appear to show a milder reaction to primary varicella infections (21), and require higher doses of tuberculin Ag to manifest a T cell-mediated skin response to mycobacterial infection (E. Strobert, Yerkes Primate Center, personal communication). Furthermore, some very common human T cell-mediated diseases, such as bronchial asthma, psoriasis, rheumatoid arthritis, and type 1 diabetes, have so far not been reported in chimpanzees (2, 3). Taken together, all these data suggest a differential response of humans and chimpanzees to lymphocyte activation-mediated processes, with human cells being relatively over-reactive.
Siglecs are sialic acid-recognizing Ig-superfamily lectins prominently expressed on immune cells (22). A subset of rapidly evolving CD33-related Siglecs (CD33rSiglecs, Siglec-3, and Siglecs 5–11) can downregulate immune cell activation via cytosolic ITIMs (22–27). These ITIMs recruit protein phosphatases, like Src homology region 2 domain-containing phosphatases (SHPs) SHP-1 and SHP-2, which limit activation pathways stimulated by tyrosine kinases. For example, Siglec-5 (CD170) is involved in the negative regulation of innate immune responses via ITIMs (28–30). Also, Siglec-8 on human eosinophils and mast cells and Siglec-F on murine eosinophils, have been shown to act as inhibitory receptors (23, 27, 31, 32). In addition, mouse Siglec-E is upregulated and phosphorylated after LPS stimulation to limit TLR-driven cytokine production (33). Siglec-10/G has also been recently shown to play a major role in repressing tissue damage-induced immune responses (26), and Siglec-H has been shown to affect signaling in plasmacytoid dendritic cells (34).
For T cell activation to occur, two signals are usually required: engagement of the TCR after recognition of the Ag/MHC-complex on the surface of APCs and costimulation of CD28 by its natural ligands, CD80 and CD86, which are present on mature APCs (35–38). We have previously shown that when using an anti-CD3 (TCR) Ab in conjunction with anti-CD28 costimulation, human T cells were more reactive than chimpanzee T cells (39). This difference appeared to be associated with higher levels of potentially inhibitory Siglecs (particularly Siglec-5) on chimpanzee T cells. In support of this, transfection of Siglec-5 into human T cells suppressed calcium signaling responses (39). In another study, transfection of Siglec-7 or -9 into a T cell line reduced phosphorylation of Tyr319 on the ZAP-70 kinase, which plays a key role in upregulation of gene transcription after TCR stimulation (40).
However, the situation has been complicated by the discovery of Siglec-14 as a paired receptor with Siglec-5, undergoing concerted evolution in primates, and demonstrating near-complete sequence homology of the N-terminal first 230 aa (41). In contrast to Siglec-5, Siglec-14 has activating signaling properties through an association with TYROBP (previously known as DAP12) (41–44). Because of the near identical N-terminal sequence, most commercially available “anti–Siglec-5” Abs actually recognize both Siglec-5 and Siglec-14 making it difficult to discern their respective expression patterns and functional roles (41). Recently, a Siglec-14 specific mAb was developed, which allows for distinction between Siglecs-5 and -14 (44). In humans, granulocytes express both Siglec-5 and -14, monocytes express only Siglec-14, and B cells express only Siglec-5 (44). However, these expression patterns have not been studied in chimpanzees.
As an alternative method of T cell activation, a subset of anti-CD28 mAbs are capable of activating T cells without the additional engagement of the TCR/CD3-complex, the “CD28 superagonists” (45–47). Based on their ability to preferentially induce expansion of T regulatory cells (Tregs) (48–51), it was hypothesized that CD28 superagonists could be used in the treatment of autoimmune disorders. However, when given to healthy human volunteers during a phase I clinical trial in London, U.K., in 2006, the humanized CD28 superagonist TGN1412, caused unexpected serious adverse events because of the massive release of high amounts of proinflammatory cytokines, a “cytokine storm” (52). This was in stark contrast to the preclinical testing of this approach in rats, rhesus monkeys, and cynomolgus monkeys in which doses up to 500 times that given to humans were administered and found to be safe (52–54). The exact mechanisms of these differences have not been understood, and in this study, we provide a likely explanation.
Given the potential importance to human diseases and the complexities arising because our original observations, we have greatly expanded our studies of T cell activation in humans versus chimpanzees, and in addition, determined whether a similar human hypersensitivity is also intrinsic to B cells. In this study, we show that there is indeed a clear-cut human lymphocyte over-reactivity relative to chimpanzee lymphocytes, which is independent of the method of activation used. We also show that this selective inhibitory role for Siglec-5 in chimpanzees extends to B lymphocyte populations, and we demonstrate that this finding is independent of Siglec-14.
Materials and Methods
Cells and cell culture
Chimpanzee blood samples were collected into EDTA-containing tubes at the Yerkes National Primate Center (Atlanta, GA) and shipped on ice overnight to the University of California San Diego, La Jolla, CA. Human blood was collected from healthy volunteer donors, with approval from the University of California San Diego Institutional Review Board. These samples were collected into identical EDTA-containing tubes at approximately the same time as the chimpanzee samples, and stored overnight on ice to ensure similar treatment conditions. PBMCs were isolated by centrifugation over Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden), followed by ACK buffer (0.15 M NH4Cl/10 mM KHCO3/0.1 mM EDTA) lysis of the remaining RBCs. For CFSE labeling, 1–2 × 106 PBMCs were labeled with 5 μM CFSE (Invitrogen, Carlsbad, CA), incubated for 15 min at 37°C, rested on ice for 5 min, and then washed. CFSE content was analyzed by flow cytometry after 5 d of stimulation. The 1–2 × 106 PBMCs were cultured in RPMI medium 1640 supplemented with 10% FCS, in flat bottom 24–48 well plates. For anti-CD3 stimulation, plates were coated with varying concentrations of anti-CD3 Ab in PBS. After 2–4 h, PBS was removed and cells in media with anti-CD28 Ab (0.1 μg/ml) were added. For PHA, ANC28.1/5D10, and staphylococcal enterotoxin B (SEB) stimulation, varying concentrations of each agent was added immediately after 1–2 × 106 PBMCs were added to each well.
Abs and reagents
The following Abs were obtained from BD Pharmingen (San Diego, CA): anti-human CD3 Abs (clones UCTH1, HIT3a, SK7, and SP34), anti-human CD28 (clone CD28.2), anti–CD4-FITC, anti-CD8 PE, anti-CD19 APC, anti–TNF-APC, anti–CD69-FITC. Anti-human CD28 superagonist Ab clone ANC28.1/5D10 was obtained from Ancell (Bayport, MN), and anti-human Siglec-5/Siglec-14-APC from R&D Systems (Minneapolis, MN). Anti-human Siglec-14 clone 40-1 (41) was kindly provided by Takashi Angata, Osaka University, Osaka, Japan.
In several experiments, we use Abs raised against human proteins to also study chimpanzee equivalents. Given the extremely close evolutionary relationship, chimpanzee proteins are, on average, >99% identical to human proteins (1). And with many of the conserved proteins we are studying, the identity approaches 100%. Thus, the chance of an Ab against a conserved human protein reacting differently to the chimpanzee equivalent is <1%. To be doubly certain of this, we checked all Abs used in our studies by flow cytometry, and they showed very similar binding to human and chimpanzee cells.
MLRs
MLRs were performed as previously described (55). In short, 4 × 105 CFSE-treated cells were mixed with 8 × 105 Mitomycin C-treated cells and then incubated for 8 d. The percentage of activated cells was determined by flow cytometry by following changes in cell size and granularity.
DNA nucleofection
Freshly isolated human PBMCs were labeled with CFSE as described previously and rested overnight in RPMI 1640. PBMCs were nucleofected using the Human T cell Nucleofector Kit (Amaxa, Gaithersburg, MD) according to the manufacturer’s protocol. Cells were then incubated for 72 h and analyzed for Siglec-5 expression and CFSE content.
Calcium mobilization assay
Mobilization of intracellular calcium was measured by using a real-time flow cytometric assay (39). Briefly, Fluo-4, acetoxymethyl ester (1 mM), and Fura Red (1 mM) calcium-sensing dyes (Molecular Probes, Eugene, OR) were mixed with Pluronic F-127 solution (Molecular Probes) at a volume ratio of 1:2:3. The calcium-sensing dye solution was added to PBMCs in 2 ml RPMI 1640 media and incubated at 37°C for 45 min. Cells were then washed with PBS, resuspended in 1 ml RPMI 1640 media, and allowed to rest at room temperature for 30 min before stimulation. For analysis, cells were acquired by using the time parameter on the FACSCalibur and analyzed for FL1 and FL3 fluorescence. The cell flow rate was 100–200 cells per second, ~5–10 × 104 cell events. Anti-IgM or anti-IgD Ab was added 30–45 s after beginning cell acquisition. Cells were collected for a total of 512 s. Postcollection analysis was performed using FlowJo software (TreeStar, Ashland, OR). The ratio of FL1/FL3 was derived and plotted over time. Kinetic plots are expressed as median of the FL1:FL3 ratio.
Intracellular TNF labeling and CD69 detection
One million PBMCs per well of a 48-well plate were stimulated with 10 μg/ml ssRNA (ssRNA40/LyoVec, InvivoGen, San Diego, CA), whereas unstimulated cells served as negative controls. Stimulation for all assays was conducted at 37°C and 5% CO2, in the presence of 0.5 μg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO) to inhibit cellular cytokine release. The intracellular cytokine content of live PBMCs (determined by size exclusion and forward and/side scatter gating) was determined after 20 h of stimulation. Briefly, cells were fixed, permeabilized (Perm/Wash Buffer I and Fix Buffer I, BD Biosciences, San Jose, CA) and stained for TNF. Surface expression of CD69 was detected by flow cytometry 20 h after stimulation. All samples were acquired on a FACSCalibur (BD Biosciences). The frequencies of cytokine-positive cells, as well as the frequencies of CD69-positive cells above background of unstimulated cells, were determined by subsequent analysis using FlowJo software (TreeStar).
Cytokine bead array assay and analysis
The 1 × 106 freshly isolated PBMCs were cultured in 48-well plates and incubated for 96 h at 37°C in RPMI 1640 and 10% FCS. The 50 μl culture media supernatant was removed and centrifuged to remove cellular debris. Human Th1/Th2/Th17 cytokine bead array kit (BD Biosciences) was used according to the manufacturer’s suggestions. Cytokine concentrations were analyzed on a FACSCalibur flow cytometer using Cellquest software. Cytokine concentrations were derived from the mean fluorescence intensities using Cytometric Bead Array Analysis software (BD Biosciences).
Results
Human T lymphocytes show greater reactivity than chimpanzee T cells to multiple types of lymphocyte stimulation
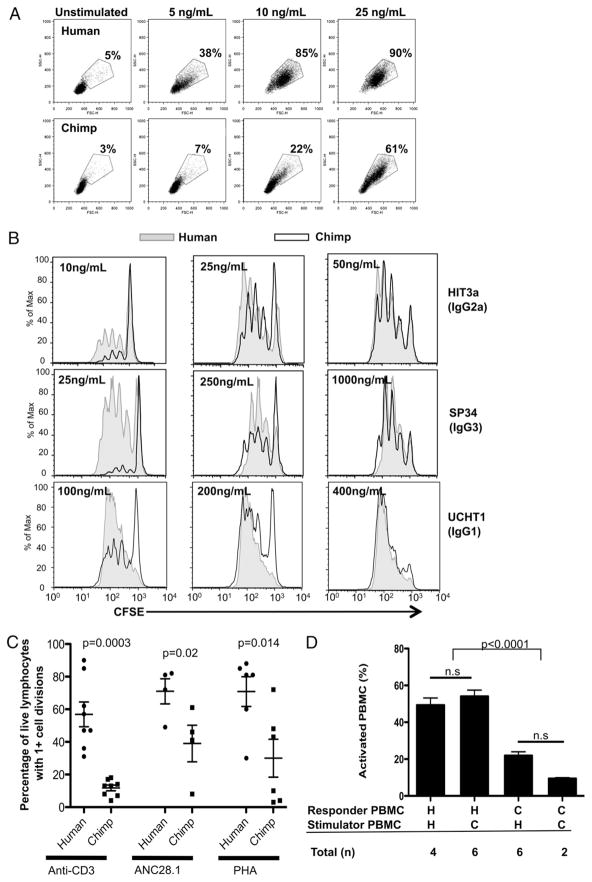
To examine potential differences in response to stimulation by anti-CD3 and anti-CD28 Abs, we compared the proliferative response of human and chimpanzee lymphocytes by using CFSE to measure cell proliferation. We performed dose titration curves using three different anti-CD3 clones of three different isotypes (Fig. 1A,1B). The profiles in Fig. 1B were generated from a constant proportion of the cultures such that the dilution of fluorescence shows the number of cell divisions, and the area under each curve is proportional to the accumulation of cells. At lower doses, we consistently found that all humans tested showed increased proliferation when compared with chimpanzees (n = 20, representing 20 different chimpanzees versus 11 different humans), regardless of the isotype of the Ab used. However, as the dose of anti-CD3 was increased, chimpanzee T cells were eventually able to respond equally. This finding is independent of the binding affinity of the anti-CD3 Abs to human and chimpanzee T cells, which was found to be comparable by flow cytometry (data not shown), and is consistent with the sequences of the CD3ε ectodomain, the subunit recognized by anti-CD3 Abs, that are known to be identical in human and chimpanzee but for a single serine-glycine substitution at 68 aa (56). In addition, this is independent of the levels of expression of CD28 on human and chimpanzee T cells, which has previously been shown to be similar (39).
FIGURE 1.
Human lymphocytes show higher levels of proliferation in response to stimulation compared with chimpanzee lymphocytes, regardless of method of stimulation. PBMCs were labeled with CFSE and stimulated for 5 d with varying amounts of three different isotypes of anti-CD3 IgG Abs, combinedwithanti-CD28 costimulation (0.01 μg/ml, clone CD28.2). A, Light scatter flow cytometry plots showing percentage of activated lymphocytes (determined by size). Top panels, human cells. Bottom panels, chimpanzee cells. B, The CFSE content in each culture was analyzed by flow cytometry. All plots shown represent analysis by gating on live lymphocytes, including both resting and blasting cells, as shown in A. One experiment, representative of at least three comparisons for each Ab isotype, is shown. C, Graph summarizing data from comparisons of PBMCs that were labeled with CFSE and stimulated with: 5–10 ng/ml anti-CD3 Ab with anti-CD28 costimulation (0.1 μg/ml), 2 μg/ml PHA, and 0.5–2 μg/ml anti-CD28 superagonist ANC28.1/5D10. The CFSE content in each culture was analyzed by flow cytometry 5 d after stimulation, and the percent age of live cells with more than one cell division was determined using FlowJo software. Errors bar represent SEM. D, MLRs. Target cells are treated with mytomycin C to prevent cell division and mixed with CFSE-labeled responder cells. The percentage of activated cells was determined by flow cytometry after 7 d. Error bars represent SD. The number of experiments shown represents comparisons between cells from different individuals.
This dose response parallels previous findings, which showed that chimpanzee T cells were able to proliferate in response to anti-CD3 Abs, but to a lesser degree than human T cells at the doses tested (56). We even found one anti-CD3 Ab clone, SK7, which results only in human but not chimpanzee T cell proliferation, despite high doses tested (Table I). Both clone SK7 and clone UCHT1 are isotype IgG1. This result indicates that these differences are not in fact related to the isotype of the anti-CD3 Ab used, as was recently suggested by others (56), based on a limited study of a few Abs tested over a limited concentration range (direct comparisons between human and chimpanzee lymphocytes were only performed with two isotypes, and concentrations used started at 300 ng/ml) (56). Rather, any differences observed are specific to individual anti-CD3 clones. Importantly, regardless of which Ab was used, human T cells exhibited a more sensitive response in every experiment.
Table I.
Summary data of T cell proliferation experiments
| Mitogen | Dosage | Hu > Cpz (No. of Comparisons) | Cpz > Hu (No. of Comparisons) | Dose Response Difference (Approximate)a |
|---|---|---|---|---|
| L-PHA | 2–10 μg/ml | 11 | 0 | 5× |
| ANC 28.1 | 0.125–2.5 μg/ml | 9 | 0 | 5× |
| SEB | 2–200 ng/ml | 2 | 0 | 100× |
| αCD3 HIT3a | 2.5–250 ng/ml | 8 | 0 | 5× |
| αCD3 UCHT1 | 2.5 ng–10 μg/ml | 3 | 0 | 5× |
| αCD3 SK7 | 2.5 ng–2.5 μg/ml | 3 | 0 | >10× |
| αCD3 SP34 | 2.5 ng–1 μg/ml | 3 | 0 | 5–10× |
| MLR | N/A | 6 | 0 | N/A |
| Total | 45 | 0 |
This table summarizes all proliferation comparisons between human and chimpanzee T lymphocytes in response to different methods of stimulation. Fig. 1 shows the p values associated with these comparisons.
Refers to the fold difference in amount of stimulating agent needed to get a response from chimpanzee cells similar to that of human cells. At the highest doses studied, the chimp and human lymphocyte responses are mostly similar, with the exception of αCD3 SK7 Ab, where the chimp lymphocytes did not proliferate even at high doses.
As we have previously shown, there is robust activation of both freshly isolated human and chimpanzee T cells when stimulated with high doses of L-PHA (39). L-PHA is a potent T cell Ag receptor-dependent, mitogenic lectin with a preference for N-acetyl-D-galactosamine (57) and for binding certain branching N-glycans (58). To better quantify any differences in response to stimulation between human and chimpanzee lymphocytes, we used CFSE labeling and tested a wide range of doses of PHA. We found that although T cells from both species do indeed respond similarly to stimulation with high doses of L-PHA (10 μg/ml), human T cells show greater levels of proliferation at lower doses (2 μg/ml). (Fig. 1C, Table I).
Next, we examined the response of human and chimpanzee T cells to varying doses of a commercially available mAb CD28 superagonist, ANC28.1/5D10 (59), which is similar to TGN1412. We found that, although human T cells proliferated in response to low doses (0.5 μg/ml), chimpanzee T cells did not proliferate at this dose even after 7 d of incubation (Fig. 1C, Table I). However, as the doses were increased, chimpanzee T cells responded to ANC28.1/5D10 but not to the same magnitude as human T cells. In fact, even at the highest dose tested (2.5 μg/ml), chimpanzee T cells did not proliferate as robustly as human T cells.
To determine whether cytokine production by cells exposed to either PHA or ANC28.1/5D10 were significantly increased in humans, we used a flow cytometry-based cytokine bead array assay. Human cells showed significantly higher levels of TNF and IL-4 secretion after stimulation by PHA or ANC28.1/5D10 (data not shown). Other cytokines tested (IL-2, IL-6, IL-10, IFN-γ, and IL-17) did not show statistically significant differences between human and chimpanzee cells, but an overall trend toward human cells producing more cytokines was observed (data not shown). These results are also consistent with previously reported findings of robust cell proliferation, transmembrane calcium flux, and cytokine production in human T cells in response to CD28 superagonist stimulation, under conditions where cells from rhesus and cynomolgus monkeys showed a lower transmembrane calcium flux (59).
We also compared human and chimpanzee lymphocyte proliferation responses after exposure with a superantigen, SEB (Table I). We note that in all species tested to date, a subpopulation of T cells responds to SEB based on the Vβ gene element expressed as part of the TCR. As such, we compared the percentage of cells proliferating within the responding population, ignoring the undivided subset of T cells. Again, we found increased human T cell proliferation after 5 d of exposure to SEB at low doses (2 ng/ml), with chimpanzee responses becoming similar at 100 times this dose (200 ng/ml). The finding that SEB reveals the least disparate proliferation differences among the mitogens tested is consistent with previously reported data comparing human and chimpanzee lymphocyte response to various superantigens (60). Further studies are needed to rule out the unlikely possibility that chimpanzees may also have a lower percentage of T cells capable of responding to SEB (61, 62)
MLRs can be used to determine foreign histocompatibility tolerance between two individuals. Traditionally, MLRs have been used in vitro as a method to predict compatibility of a transplant recipient with a potential donor (55). This in vitro test is one of the closest physiologic approximations to an in vivo test to determine T cell proliferative responses. We adapted the traditional MLR reaction to a xenogeneic comparison, and measured activation in one population (responder PBMC) in response to being exposed to a xenogeneic population that is pretreated with mitomycin C, which arrests cell division and prevents proliferation (stimulator PBMC). In multiple such studies, we found that human lymphocytes consistently showed more activation than chimpanzee lymphocytes when mixed either with human PBMCs (allogeneic) or chimpanzee (xenogeneic) PBMCs (Fig. 1D).
Human T lymphocytes show greater reactivity than chimpanzee T cells in all experimental approaches
All of our data regarding human and chimpanzee T cell proliferation comparison experiments are summarized in Table I. We see similar human and chimpanzee T cell proliferation at high doses of mitogens; however, at lower doses we did not find even a single instance wherein chimpanzee T cells were more proliferative than human cells. Lymphocyte proliferation measured with CFSE-labeling was also directly compared with alternative methods, including [3H]thymidine incorporation, EdU incorporation (Click-iT EdU, Invitrogen), and DNA quantification with fluorescence methods (CyQuant, Invitrogen). Regardless of the method used to quantify the level of lymphocyte proliferation after stimulation, all methods tested revealed findings consistent with results obtained with CFSE labeling (data not shown). Overall, human T lymphocytes show a greater reactivity than chimpanzee cells in essentially every replicate of every single experimental approach taken to activation. In fact, if we test the null hypothesis that humans and chimpanzees have an equal likelihood of responding better in any given experiment, the probability that humans will respond with higher levels of proliferation in every single experiment done (n = 45) has a p value of <10−14.
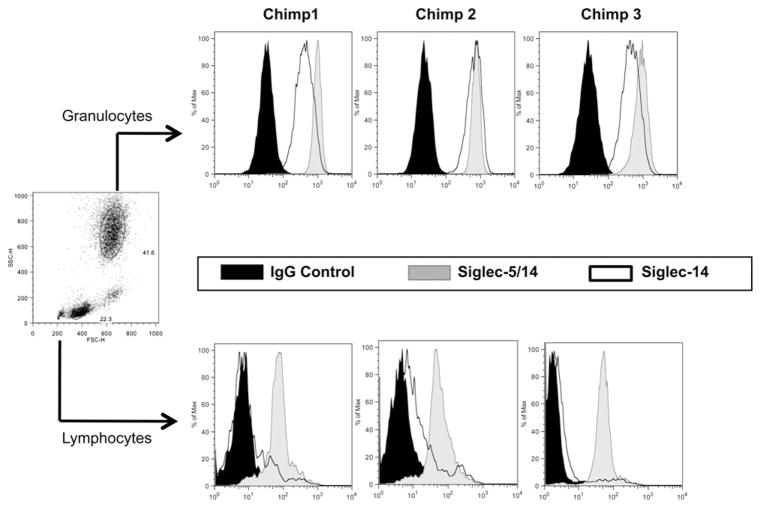
Chimpanzee lymphocytes express Siglec-5, not Siglec-14
During the recent discovery of Siglec-14, it was noted that most previously described Abs used for detection of Siglec-5 cross-react with Siglec-14, due to the high sequence homology in their two N-terminal Ig domains (41). To determine whether chimpanzee lymphocytes expressed Siglec-5, an inhibitory receptor, or Siglec-14, a potential activating receptor, we stained chimpanzee PBMCs with a Siglec-14–specific Ab (44). We found that although chimpanzee granulocytes express both Siglec-5 and -14, the great majority of chimpanzee lymphocytes express only Siglec-5 (Fig. 2). Others have shown that Siglec-14 is not found on human lymphocytes (44). These data reaffirm our previous findings of increased expression of Siglec-5 on chimpanzee lymphocytes when compared with humans (39), and supports our hypothesis that the expression of inhibitory Siglecs on chimpanzee lymphocytes dampens their response to activating stimuli.
FIGURE 2.
Chimpanzee lymphocytes express Siglec-5, not Siglec-14. Freshly isolated chimpanzee PBMCs were labeled with anti–Siglec-5/14 and anti–Siglec-14–specific Abs. Expression of Siglec-5 and/or Siglec -14 was analyzed by flow cytometry in granulocyte and lymphocyte populations, based on their forward and side scatter profiles. Results for three different individuals are shown.
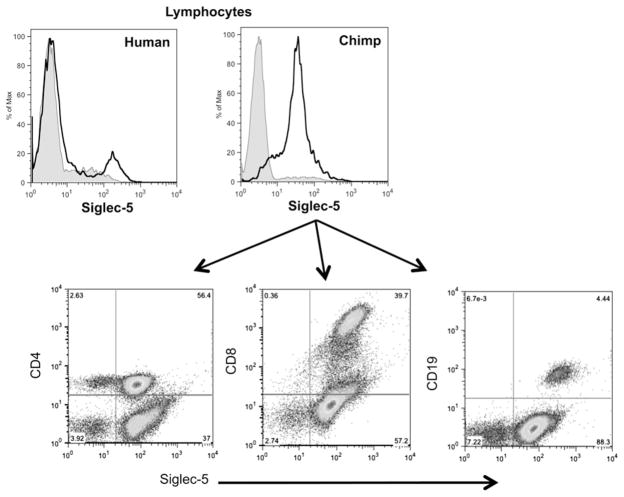
Chimpanzee lymphocyte subsets express Siglec-5 at similar levels
Our previous findings looking at the difference in Siglec expression between humans and chimpanzees show that in total lymphocyte populations, the levels of Siglec expression appear to be similar (39). To determine whether all lymphocyte subsets (CD4, CD8, and CD19 positive cells) express Siglec-5, we labeled each subset with an anti–Siglec-5 Ab. As shown in Fig. 3, all chimpanzee lymphocyte subsets express easily detectable Siglec-5, suggesting that chimpanzee B cells may also show lower levels of activation in response to stimulation when compared with human B cells (in humans this population was absent, or sometimes present at very low levels).
FIGURE 3.
Chimpanzee lymphocyte subsets express Siglec-5 at similar levels. Freshly isolated chimpanzee PBMCs were labeled with anti–Siglec-5/14 Abs and anti-CD4, anti-CD8, or anti-CD19. Expression of Siglec-5 was analyzed in each subset by flow cytometry. Results representative of three different individuals.
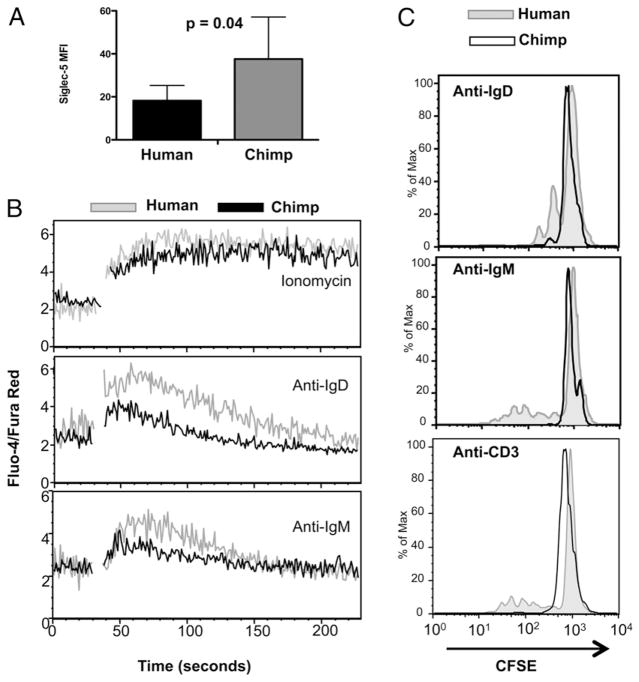
Human B lymphocytes express lower levels of Siglec-5, which correlate with increased calcium flux and higher proliferative responses
It has been previously noted that human B lymphocytes express inhibitory Siglecs, including Siglec-5 (42, 63). However, the level of cell surface expression of Siglecs on human B cells has not been directly compared with those of chimpanzees. We therefore determined the level of expression of Siglec-5 on B cells in four humans and four chimpanzees, and found higher levels on all tested chimpanzees compared with humans (Fig. 4A). We also found that a greater number of chimpanzee B cells express other inhibitory Siglecs, including Siglec-3, -6, -7, -8, -9, and -10 when compared with humans (data not shown).
FIGURE 4.
Human B lymphocytes express lower levels of Siglec-5, which correlate with increased calcium flux and higher proliferative responses. A, Siglec-5 expression on CD19+ lymphocytes was analyzed in freshly isolated PBMCs by flow cytometry. Data shown are the average of the mean fluorescence intensity from cells from four individuals from each species. Error bars represent SD. B, PBMCs were labeled with calcium sensitive dyes (Fluo-4/FuraRed) and stimulated with ionomycin (1 mM), anti-IgD (5 μg/ml), and anti-IgM (5 μg/ml) Abs. The relative change in calcium levels was recorded in CD19+ cells using a flow cytometer. One experiment representative of three comparisons is shown. C, PBMCs labeled with CFSE were stimulated with anti-IgD, anti-IgM, and anti-CD3/CD28 Abs for 5 d. The amount of proliferation in CD19+ cells was measured by flow cytometry. One experiment representative of three comparisons is shown.
We thus asked if species-specific activation and proliferation differences also occur in B cells from humans and chimpanzees. Treatment with ionomycin, a powerful ionophore produced by Streptomyces conglobatus, showed that human and chimpanzee B cells are both able to respond similarly with a calcium flux. However, when B cells are exposed to specific activators (anti-IgD and anti-IgM Abs), there was increased calcium flux noted in human B cells when compared with chimpanzee cells, indicating a propensity for increased B cell activation (Fig. 4B). We also measured human and chimpanzee peripheral blood B cell proliferation in response to the same B cell activators, and saw that human B cells show increased levels of proliferation, even under these suboptimal conditions. that is, in the absence of added cytokines (Fig. 4C). Interestingly, we also noted that human B cells present in PBMC cultures that had been exposed to anti-CD3 and anti-CD28 Abs also proliferated to some extent (presumably in response to T cell-derived cytokines), whereas chimpanzee B cells do not (Fig. 4C).
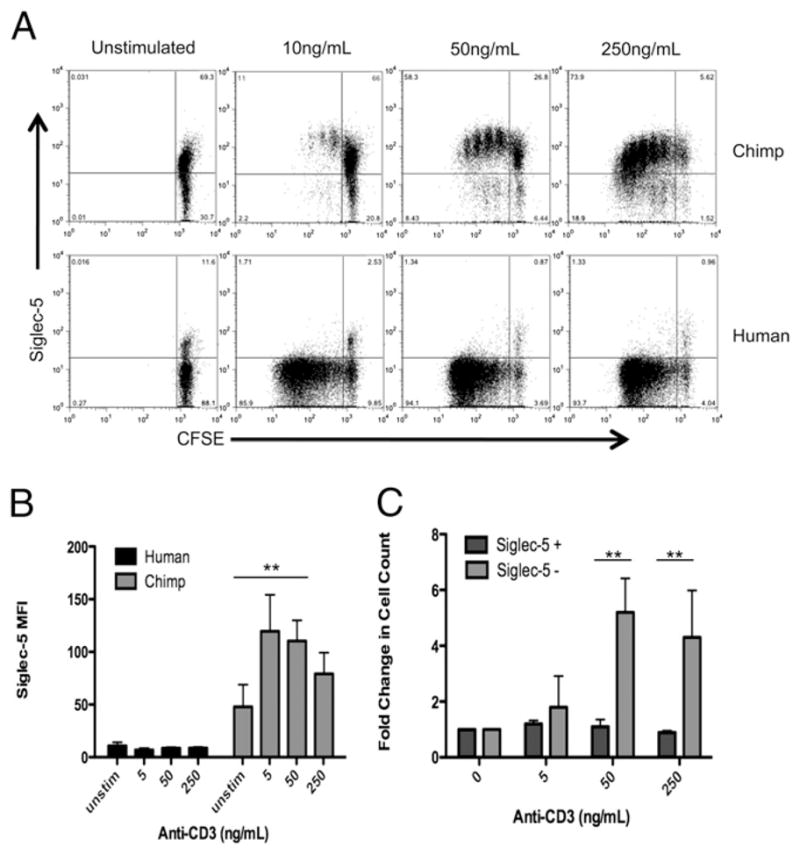
Siglec-5 expression correlates with lack of proliferation in a subset of human cells, and is upregulated by activation of chimpanzee but not human lymphocytes
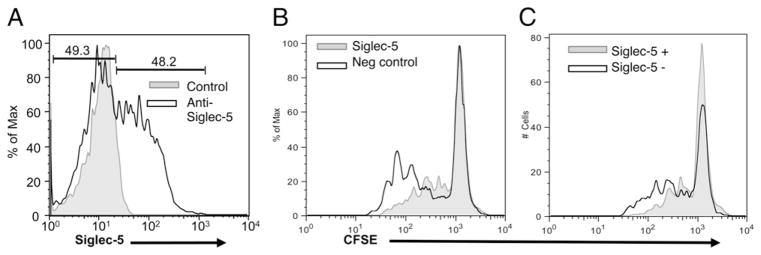
We have previously observed that some humans have a small population of Siglec-5 expressing lymphocytes, ranging from 1–10% positive (39), namely, Siglec-5 expression is not completely silenced in all human lymphocytes (see Fig. 3, for an example). Based on these observations, we sought to determine whether lymphocyte activation could result in increased expression of Siglec-5 in human lymphocytes. Likewise, we also examined the levels of Siglec-5 expression in response to activation in chimpanzee lymphocytes (Fig. 5A, 5B). We found that although Siglec-5 expression does not increase in human lymphocytes with activation, chimpanzee lymphocytes show a 2- to 3-fold increase in Siglec-5 expression after activation. Thus, although upregulation of Siglec-5 in chimpanzee lymphocytes may provide a form of negative feedback regulation, this mechanism is missing in human lymphocytes. We also examined the behavior of the small subpopulation of human lymphocytes that do express Siglec-5 at baseline, prior to activation. Importantly, although the majority of cells lacking Siglec-5 proliferated robustly, the small subpopulation of human lymphocytes that originally expressed Siglec-5 did not proliferate in response to stimulation (Fig. 5C). These data further support the notion that Siglec-5 expression, when present, dampens lymphocyte reactivity.
FIGURE 5.
Siglec-5 expression is upregulated by activation of chimpanzee but not human lymphocytes, and correlates with lack of proliferation in human cells. A, CFSE-labeled PBMCs were stimulated for 5 d with varying amounts of anti-CD3 Ab (clone HIT3a), and then stained for Siglec-5 expression. B, Siglec-5 mean fluorescence intensity of PBMCs stimulated with increasing amounts of anti-CD3. Data shown are the average of three different experiments with SD. C, Fold increase in cell number over unstimulated cells (calculated by dividing the number of live cells in stimulated cultures by the number of cells in unstimulated cultures) for human lymphocytes that are Siglec-5 positive, compared with cells that are Siglec-5 negative. Data shown are the average of three different experiments with SD. **p < 0.01.
Transfection of Siglec-5 into primary human T lymphocytes leads to decreased proliferation
In our previous study (39) we noted that when human T cells were transfected by nucleofection with a Siglec-5 expression plasmid, they showed an apparent decrease in activation, based on reduced changes in forward and side scatter on flow cytometry analysis. In this study we have re-examined the issue, using the more precise measure of changes in cellular CFSE content. We induced expression of Siglec-5 in resting human T cells by nucleofection (Nucleofector, Amaxa), and then activated them via TCR stimulation with anti-CD3 (clone HIT3a) and anti-CD28 for 72 h. Transfection efficiency was found to be ~50% (Fig. 6A). Using CFSE to quantify cell proliferation, we compared the lymphocytes that underwent sham nucleofection versus those nucleofected with a Siglec-5 containing plasmid (Fig. 6B). The mean proliferation index in these populations (the average number of cell divisions that a cell in the original population has undergone) was 2.3 in the no plasmid control population, and 1.6 in the Siglec-5 nucleofected population. Thus, lymphocytes successfully nucleofected with Siglec-5 show decreased proliferation and have a larger percentage of cells in earlier stages of division. We next compared the proliferation differences in human lymphocytes nucleofected with the Siglec-5 plasmid by comparing cells in the same population that were expressing Siglec-5 compared with those lymphocytes that were not (Fig. 6C). The cells expressing Siglec-5 underwent fewer cell divisions and were less likely to proliferate compared with those that were not expressing Siglec-5. Taken together, these data further support the proposal that low Siglec-5 expression plays a role in the relatively higher proliferative response of human T cells to activation stimuli.
FIGURE 6.
Transfection of Siglec-5 into primary human T lymphocytes leads to decreased proliferation. Freshly isolated human PBMCs were labeled with CFSE and nucleofected with Siglec-5 expression plasmid. A, Siglec-5 expression in nucleofected cells was detected by flow cytometry using an anti–siglec-5 Ab. B, Cell proliferation was analyzed by comparing CFSE content in cells nucleofected with Siglec-5 plasmid versus cells nucleofected with no plasmid (Neg control). C, CFSE profile of cells within the population of nucleofected cells that were expressing Siglec-5, compared with the CFSE profile of cells within the same population that were not expressing Siglec-5. Data shown are representative of two independent experiments.
In an attempt to further characterize the role of Siglecs in dampening the immune response, we tried to knock-down the expression of Siglec-5 in chimpanzee PBMCs using short hairpin and small interfering RNA techniques. However, despite multiple attempts with various protocols we found it difficult to effectively knockdown Siglec-5 in activated chimpanzee lymphocytes (data not shown). The likely explanation for this is that the upregulation of Siglec-5 expression in activated chimpanzee lymphocytes could not be overcome by the use of short hairpin or small interfering RNA knockdown methods. This further suggests that Siglec-5 upregulation in activated chimpanzee lymphocytes may play an important role in preventing overreactivity or apoptosis. Meanwhile, it is interesting that human lymphocytes did not show this upregulation of Siglec-5, even on activation.
Exposure of PBMCs to ssRNA leads to higher TNF production and increased cell surface expression of CD69 in human lymphocytes
HIV-1 derived ssRNA molecules are able to elicit strong immune activation of cells of both the innate and adaptive immune system. Such ssRNA can also be used in vitro to activate immune cells by mechanisms similar to infections by RNA viruses (e.g., HCV, influenza, and HIV) (64, 65). We compared the effect of ssRNA treatment on human and chimpanzee PBMCs by analyzing intracellular TNF production, and also expression of an early marker of lymphocyte activation (CD69). As shown in Fig. 7, we found that human lymphocytes showed increased levels of expression of CD69 after exposure to ssRNA (Fig. 7A), and that intracellular TNF expression was also significantly higher in human PBMC cultures when compared with chimpanzees (Fig. 7B).
FIGURE 7.
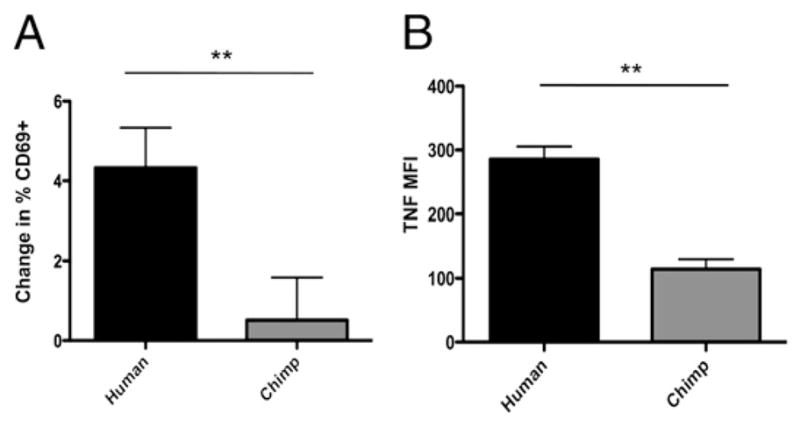
Exposure of PBMCs to ssRNA leads to more TNF production and increased cell surface expression of CD69 in human cells. Freshly isolated PBMCs were exposed to ssRNA for 20 h. A, Lymphocyte CD69 cell surface expression was analyzed by flow cytometry. Change in percent CD69 positive was determined by subtracting the percentage of CD69 positive cells in un-stimulated controls from the percentage of CD69 positive cells in stimulated samples. Data represent the mean of three experiments. Error bars represent SEM. B, Intracellular TNF expression was detected by flow cytometry. Data represent mean of three experiments. Error bars represent SEM. **p < 0.01.
Discussion
This study shows that human T and B lymphocytes manifest a relative generalized over-reactivity in response to a variety of methods of stimulation when compared with chimpanzee lymphocytes. Our findings also support the role of Siglec-5, and possibly other inhibitory Siglecs, in dampening an excessive immune response in chimpanzees. Human lymphocytes, lacking a similar level of expression of Siglecs, show a lower threshold for activation, which likely explains the over-reactivity observed in our studies. The results also clearly show that the difference in response between human and chimpanzee T cells is not due to the isotype of the anti-CD3 Ab used, as recently suggested by another group, who studied fewer Abs, and only at a limited concentration range (56). Furthermore, a clear difference in response to stimulation is seen not only with a variety of anti-CD3 isotypes, but also with T cell stimulation methods that do not involve direct Ab-mediated TCR stimulation. Of course, the immune dampening effect from Siglecs can be overcome with the use of higher doses of immune activators, demonstrating that chimpanzee lymphocytes are (as expected for healthy individuals) able to respond but need a higher level of stimulation to do so.
The difference in response to stimulation between human and chimpanzee T cells also does not appear to be due to differences in numbers of lymphocyte subsets, which have been shown by others to be very similar (66), or because of differences in the CD3ε-chain of the TCR. Recent studies have identified and characterized CD25+ Tregs in chimpanzees, and have shown that CD4+CD25+ cells constituted ~5% of the circulating CD4+ T cell population and had a nonactivated (CD69−), central memory (CCR7+CD62L+) phenotype (67). In the future, it will be interesting to further dissect the specific T cells subsets that respond to activation, to see if there any subtle differences beyond the difference in proliferation.
A few mechanisms have been proposed to explain the cytokine storm that was observed in patients treated with the CD28 super-agonist, TGN1412, in 2006 (52). These include protection from higher numbers of Tregs in rodent populations and differences in calcium flux and intracellular cell signaling between human and nonhuman primates (54, 59, 68). Our results using a very similar Ab can offer an alternative and more direct explanation, as these humans may have been missing the Siglec immunoregulatory “brake” on T cell activation. Indeed, others have also recently shown that TGN1412 stimulated large proliferative responses of 3-d cultures of human lymphocytes (69). Overall, although we recognize that other mechanisms are involved in modulating the immune response, we believe that low Siglecs on human lymphocytes could help explain the TGN1412 disaster, and should be considered during such preclinical drug development.
Siglecs may also play an important protective role against the excessive immune activation seen in some viral infections. The inhibitory effect of Siglecs may contribute to the species-specific differences observed in viral infections that cause chronic stimulation of the immune system (17, 19). We are aware of other potential explanations, such as differences in expression of other inhibitory molecules, like PD-1, CTLA-4, and CD160 (70). Also, in the case of HCV infection differences, there are other factors that could determine late complications, including diet, alcohol, recreational drugs, hepatotropic pathogens, etc. In the case of HIV infection and development of AIDS, differences in the activity of molecules, such as APOBEC3G and TRIM5α, have been suggested as potential explanations. However, the latter molecules cannot account for the human-chimpanzee differences in susceptibility to AIDS, as they are essentially identical in sequence and function in the two species (71, 72). Likewise, although many primate lentiviruses use Nef to efficiently down modulate the TCR-CD3 and suppress the responsiveness of infected T cells to activation and activation-induced cell death, this is not the case for the Nef proteins of HIV-1 and its closest simian relative, SIVcpz (73).
Unlike the human species, which recently expanded from a small founder population of ancestors originating ~100,000 y ago, currently living chimpanzees originated from a larger founder population >500,000 y ago, and split into three subspecies, likely because of geographic isolation (74). HIV-1 evolved from a strain of SIVcpz that infects the central African subspecies of the chimpanzee, Pan troglodytes troglodytes, crossing over into humans on at least three separate occasions (75). It is important to note that the SIVcpz strain recently reported to cause a milder AIDS-like disease in chimpanzees in Gombe National Park in Tanzania (75) is not closely related to the one that gave rise to HIV-1. Also, the infected chimpanzees involved in that study are a rather different subspecies (east African Pan troglodytes schweinfurthii) from those that are the ancestors of chimpanzees originally brought to the United States (Western Pan troglodytes verus) and then used for HIV-1 infection studies in the 1980s and 1990s. Thus, although the Gombe National Park data show that one strain of SIVcpz can give rise to a moderate AIDS-like syndrome in one subspecies of chimpanzees, it does not explain why another strain of SIVcpz, which gave rise to HIV-1 in humans, very rarely causes AIDS in another subspecies of chimpanzees in captivity. The latter is the question partly addressed in this study. In this regard, it would be interesting to know what lymphocyte Siglec levels are like in the Gombe chimpanzees.
We also exposed human and chimpanzee PBMCs to RNA oligonucleotides containing a GU-rich sequence of HIV-1 (65), to determine the potential role Siglecs may play in controlling the response of lymphocytes to activation by viral infection. Human lymphocytes showed higher levels of expression of an early activation marker (CD69), and there were higher amounts of intracellular TNF produced in human PBMCs. Increased production of TNF is also seen early in HIV infection, and a correlation exists between serum TNF levels and progression to AIDS (76). Moreover, despite being proapoptotic itself, TNF has also been shown to contribute to Fas upregulation, resulting in increased cell death (77). In this context, Siglecs may play a role in protecting chimpanzee lymphocytes from cell death, by dampening the production of proapoptotic factors. More research is needed to address these issues.
Recently, Mandl et al. have shown that sooty mangabeys have substantially reduced levels of innate immune system activation in vivo during acute and chronic SIV infection and that sooty mangabey plasmacytoid dendritic cells produce markedly less IFN-α in response to SIV and other TLR 7 and 9 ligands ex vivo (78). Interestingly, they propose a similar explanation for the different outcomes after SIV infection in sooty mangabeys (natural hosts) versus in rhesus macaques (non-natural hosts), that is, that chronic stimulation of pDCs by SIV and HIV in non-natural hosts may drive the unrelenting immune system activation and dysfunction underlying AIDS progression. Their findings support our hypothesis of human lymphocyte over-reactivity as a contributing factor for the greater susceptibility to AIDS in humans when compared with chimpanzees. It would be interesting to see if differences in the innate immune response between humans and chimpanzees also exist, and whether Siglecs play a role in such differences as well.
In summary, we have shown that Siglec-5 expression likely plays a role in the dampening of chimpanzee immune responses in both T and B lymphocytes. Siglec expression differences observed in humans and chimpanzees could help to explain the observed differences in disease states involving immunopathology. We are, of course, not suggesting that chimpanzees have, in a species-specific manner, suppressed their immune response to avoid diseases like asthma, psoriasis, and rheumatoid arthritis. Indeed, such diseases would not be likely to impact reproductive fitness. Rather, we document, using multiple independent methods that human lymphocytes are relative over-reactors when compared with chimpanzees. Selective pressures that may account for human over-reactivity are unknown, and the result may have been beneficial, although also having consequences for the development of T cell-mediated diseases in humans pathologies that also happen to be rare in chimpanzees and other great apes. Highlighting such differences in immune response between humans and other closely related primate models of disease is of crucial importance not just to understand human disease, but also so that this can be taken into consideration when these animal models are used as preliminary studies for drug trials.
Further work needs to be performed to explore the regulatory mechanisms differentially controlling Siglec expression in human and chimpanzee lymphocytes. Potentially, clinical therapies could be aimed at increasing Siglec expression in human lymphocytes to treat and control T cell-mediated disorders, such as HIV, hepatitis B and C, autoimmune hepatitis, inflammatory bowel disease, rheumatoid arthritis, asthma, and psoriasis. From a broader evolutionary perspective, this work supports the argument that the evolution of the adaptive immune system came with attendant negative costs, which have become evident as the immune system and pathogens engage in a runaway “Red Queen” coevolution, in which the pathogens always end up with the upper hand (79, 80).
Acknowledgments
This work was supported by Grant P01HL057345 (to A.V). P.C.S. was supported by an Institutional Research and Academic Career Development Award Fellowship, and L.L.S. was supported by a University of California, San Diego Gastroenterology National Institutes of Health National Research Service Award grant (T32DK07202). Chimpanzee blood samples were provided by the Yerkes National Primate Research Center (supported by Grant RR00165).
We thank Frank Chisari, Douglas Richman, and Celsa Spina for helpful comments.
Abbreviations used in this paper
- HCV
hepatitis C virus
- SEB
staphylococcal enterotoxin B
- SHP
Src homology region 2 domain-containing phosphatase
- Siglec
sialic acid-recognizing Ig-superfamily lectin
- Treg
T regulatory cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chimpanzee Sequencing and Analysis Consortium. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. A chimpanzee genome project is a biomedical imperative. Genome Res. 2000;10:1065–1070. doi: 10.1101/gr.10.8.1065. [DOI] [PubMed] [Google Scholar]
- 3.Varki A, Altheide TK. Comparing the human and chimpanzee genomes: searching for needles in a haystack. Genome Res. 2005;15:1746–1758. doi: 10.1101/gr.3737405. [DOI] [PubMed] [Google Scholar]
- 4.Finch CE. The evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0909606106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter HJ, Eichberg JW, Masur H, Saxinger WC, Gallo R, Macher AM, Lane HC, Fauci AS. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984;226:549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- 6.Novembre FJ, Saucier M, Anderson DC, Klumpp SA, O’Neil SP, Brown CRI, 2nd, Hart CE, Guenthner PC, Swenson RB, McClure HM. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol. 1997;71:4086–4091. doi: 10.1128/jvi.71.5.4086-4091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp PM, Shaw GM, Hahn BH. Simian immunodeficiency virus infection of chimpanzees. J Virol. 2005;79:3891–3902. doi: 10.1128/JVI.79.7.3891-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heeney JL, Dalgleish AG, Weiss RA. Origins of HIV and the evolution of resistance to AIDS. Science. 2006;313:462–466. doi: 10.1126/science.1123016. [DOI] [PubMed] [Google Scholar]
- 9.Bassett SE, Brasky KM, Lanford RE. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker CM. Comparative features of hepatitis C virus infection in humans and chimpanzees. Springer Semin Immunopathol. 1997;19:85–98. doi: 10.1007/BF00945027. [DOI] [PubMed] [Google Scholar]
- 11.Gagneux P, Muchmore EA. The chimpanzee model: contributions and considerations for studies of hepatitis B virus. Methods Mol Med. 2004;96:289–318. doi: 10.1385/1-59259-670-3:289. [DOI] [PubMed] [Google Scholar]
- 12.Akari H, Iwasaki Y, Yoshida T, Iijima S. Non-human primate surrogate model of hepatitis C virus infection. Microbiol Immunol. 2009;53:53–57. doi: 10.1111/j.1348-0421.2008.00087.x. [DOI] [PubMed] [Google Scholar]
- 13.Bukh J, Forns X, Emerson SU, Purcell RH. Studies of hepatitis C virus in chimpanzees and their importance for vaccine development. Intervirology. 2001;44:132–142. doi: 10.1159/000050040. [DOI] [PubMed] [Google Scholar]
- 14.Porter BF, Goens SD, Brasky KM, Hubbard GB. A case report of hepatocellular carcinoma and focal nodular hyperplasia with a myelolipoma in two chimpanzees and a review of spontaneous hepatobiliary tumors in non-human primates. J Med Primatol. 2004;33:38–47. doi: 10.1111/j.1600-0684.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- 15.Bettauer RH. Chimpanzees in hepatitis C virus research: 1998–2007. J Med Primatol. 2010;39:9–23. doi: 10.1111/j.1600-0684.2009.00390.x. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon ML, Lecoeur H, Boudet F, Ledru E, Marzabal S, Boullier S, Roué R, Nagata S, Heeney J. Lack of chronic immune activation in HIV-infected chimpanzees correlates with the resistance of T cells to Fas/Apo-1 (CD95)-induced apoptosis and preservation of a T helper 1 phenotype. J Immunol. 1997;158:2964–2976. [PubMed] [Google Scholar]
- 17.Baenziger S, Heikenwalder M, Johansen P, Schlaepfer E, Hofer U, Miller RC, Diemand S, Honda K, Kundig TM, Aguzzi A, Speck RF. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009;113:377–388. doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 18.Franco A, Guidotti LG, Hobbs MV, Pasquetto V, Chisari FV. Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J Immunol. 1997;159:2001–2008. [PubMed] [Google Scholar]
- 19.Schott E, Witt H, Neumann K, Taube S, Oh DY, Schreier E, Vierich S, Puhl G, Bergk A, Halangk J, et al. A Toll-like receptor 7 single nucleotide polymorphism protects from advanced inflammation and fibrosis in male patients with chronic HCV-infection. J Hepatol. 2007;47:203–211. doi: 10.1016/j.jhep.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JI, Moskal T, Shapiro M, Purcell RH. Varicella in Chimpanzees. J Med Virol. 1996;50:289–292. doi: 10.1002/(SICI)1096-9071(199612)50:4<289::AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, Winkler TH, Kneitz B, Crocker PR, Nitschke L. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 25.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornish AL, Freeman S, Forbes G, Ni J, Zhang M, Cepeda M, Gentz R, Augustus M, Carter KC, Crocker PR. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123–2132. [PubMed] [Google Scholar]
- 29.Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J Biol Chem. 2005;280:19843–19851. doi: 10.1074/jbc.M502041200. [DOI] [PubMed] [Google Scholar]
- 30.Carlin AF, Chang YC, Areschoug T, Lindahl G, Hurtado-Ziola N, King CC, Varki A, Nizet V. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med. 2009;206:1691–1699. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis x. J Pharmacol Exp Ther. 2009;330:608–612. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd CR, Orr SJ, Spence S, Burrows JF, Elliott J, Carroll HP, Brennan K, Gabhann JNí, Coulter WA, Johnston JA, Jefferies CA. Siglec-E is up-regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR-driven cytokine production. J Immunol. 2009;183:7703–7709. doi: 10.4049/jimmunol.0902780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 37.Sharpe AH, Abbas AK. T-cell costimulation—biology, therapeutic potential, and challenges. N Engl J Med. 2006;355:973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 38.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 41.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006;20:1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 42.Varki A, Angata T. Siglecs—the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 43.Lanier LL, Bakker AB. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today. 2000;21:611–614. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 44.Yamanaka M, Kato Y, Angata T, Narimatsu H. Deletion polymorphism of SIGLEC14 and its functional implications. Glycobiology. 2009;19:841–846. doi: 10.1093/glycob/cwp052. [DOI] [PubMed] [Google Scholar]
- 45.Tacke M, Hanke G, Hanke T, Hünig T. CD28-mediated induction of proliferation in resting T cells in vitro and in vivo without engagement of the T cell receptor: evidence for functionally distinct forms of CD28. Eur J Immunol. 1997;27:239–247. doi: 10.1002/eji.1830270136. [DOI] [PubMed] [Google Scholar]
- 46.Bischof A, Hara T, Lin CH, Beyers AD, Hünig T. Autonomous induction of proliferation, JNK and NF-alphaB activation in primary resting T cells by mobilized CD28. Eur J Immunol. 2000;30:876–882. doi: 10.1002/1521-4141(200003)30:3<876::AID-IMMU876>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Palmero M, Hara T, Thumbs A, Hünig T. Triggering of T cell proliferation through CD28 induces GATA-3 and promotes T helper type 2 differentiation in vitro and in vivo. Eur J Immunol. 1999;29:3914–3924. doi: 10.1002/(SICI)1521-4141(199912)29:12<3914::AID-IMMU3914>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Hünig T, Dennehy K. CD28 superagonists: mode of action and therapeutic potential. Immunol Lett. 2005;100:21–28. doi: 10.1016/j.imlet.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Beyersdorf N, Hanke T, Kerkau T, Hünig T. Superagonistic anti-CD28 antibodies: potent activators of regulatory T cells for the therapy of autoimmune diseases. Ann Rheum Dis. 2005;64(Suppl 4):iv91–iv95. doi: 10.1136/ard.2005.042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt J, Elflein K, Stienekemeier M, Rodriguez-Palmero M, Schneider C, Toyka KV, Gold R, Hünig T. Treatment and prevention of experimental autoimmune neuritis with superagonistic CD28-specific monoclonal antibodies. J Neuroimmunol. 2003;140:143–152. doi: 10.1016/s0165-5728(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 51.Lin CH, Hünig T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003;33:626–638. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- 52.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 53.Schneider CK, Kalinke U, Löwer J. TGN1412—a regulator’s perspective. Nat Biotechnol. 2006;24:493–496. doi: 10.1038/nbt0506-493. [DOI] [PubMed] [Google Scholar]
- 54.Schraven B, Kalinke U. CD28 superagonists: what makes the difference in humans? Immunity. 2008;28:591–595. doi: 10.1016/j.immuni.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Muul LM, Silvin C, James SP, Candotti F. Curr Protoc Immunol. Unit 7.10.1–7.10.24. Chapter 7. 2008. Measurement of proliferative responses of cultured lymphocytes. [DOI] [PubMed] [Google Scholar]
- 56.Bibollet-Ruche F, McKinney BA, Duverger A, Wagner FH, Ansari AA, Kutsch O. The quality of chimpanzee T-cell activation and simian immunodeficiency virus/human immunodeficiency virus susceptibility achieved via antibody-mediated T-cell receptor/CD3 stimulation is a function of the anti-CD3 antibody isotype. J Virol. 2008;82:10271–10278. doi: 10.1128/JVI.01319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moretta A, Poggi A, Olive D, Bottino C, Fortis C, Pantaleo G, Moretta L. Selection and characterization of T-cell variants lacking molecules involved in T-cell activation (T3 T-cell receptor, T44, and T11): analysis of the functional relationship among different pathways of activation. Proc Natl Acad Sci USA. 1987;84:1654–1658. doi: 10.1073/pnas.84.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cummings RD, Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982;257:11230–11234. [PubMed] [Google Scholar]
- 59.Waibler Z, Sender LY, Merten C, Hartig R, Kliche S, Gunzer M, Reichardt P, Kalinke U, Schraven B. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS ONE. 2008;3:e1708. doi: 10.1371/journal.pone.0001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bavari S, Hunt RE, Ulrich RG. Divergence of human and nonhuman primate lymphocyte responses to bacterial superantigens. Clin Immunol Immunopathol. 1995;76:248–254. doi: 10.1006/clin.1995.1123. [DOI] [PubMed] [Google Scholar]
- 61.Cottrez F, Auriault C, Capron A, Groux H. Analysis of the V beta specificity of superantigen activation with a rapid and sensitive method using RT PCR and an automatic DNA analyser. J Immunol Methods. 1994;172:85–94. doi: 10.1016/0022-1759(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 62.Komanduri KV, Salha MD, Sékaly RP, McCune JM. Superantigen-mediated deletion of specific T cell receptor V beta subsets in the SCID-hu Thy/Liv mouse is induced by staphylococcal enterotoxin B, but not HIV-1. J Immunol. 1997;158:544–549. [PubMed] [Google Scholar]
- 63.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:431–437. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 65.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 66.Stone GA, Johnson BK, Druilhet R, Garza PB, Gibbs CJ., Jr Immunophenotyping of peripheral blood, ranges of serum chemistries and clinical hematology values of healthy chimpanzees (Pan troglodytes) J Med Primatol. 2000;29:324–329. doi: 10.1034/j.1600-0684.2000.290503.x. [DOI] [PubMed] [Google Scholar]
- 67.Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, Piccirillo CA, Rehermann B. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424–4432. doi: 10.1182/blood-2005-09-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gogishvili T, Langenhorst D, Lühder F, Elias F, Elflein K, Dennehy KM, Gold R, Hünig T. Rapid regulatory T-cell response prevents cytokine storm in CD28 superagonist treated mice. PLoS One. 2009;4:e4643. doi: 10.1371/journal.pone.0004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Findlay L, Eastwood D, Stebbings R, Sharp G, Mistry Y, Ball C, Hood J, Thorpe R, Poole S. Improved in vitro methods to predict the in vivo toxicity in man of therapeutic monoclonal antibodies including TGN1412. J Immunol Methods. 2009 doi: 10.1016/j.jim.2009.10.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 71.Mariani R, Chen D, Schröfelbauer B, Navarro F, König R, Bollman B, Münk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 72.Song B, Javanbakht H, Perron M, Park DH, Stremlau M, Sodroski J. Retrovirus restriction by TRIM5alpha variants from Old World and New World primates. J Virol. 2005;79:3930–3937. doi: 10.1128/JVI.79.7.3930-3937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schindler M, Münch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Müller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 74.Gagneux P, Wills C, Gerloff U, Tautz D, Morin PA, Boesch C, Fruth B, Hohmann G, Ryder OA, Woodruff DS. Mitochondrial sequences show diverse evolutionary histories of African hominoids. Proc Natl Acad Sci USA. 1999;96:5077–5082. doi: 10.1073/pnas.96.9.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Badley AD. Cell death during HIV infection. CRC/Taylor & Francis; Boca Raton: 2006. [Google Scholar]
- 77.Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-gamma and tumor necrosis factor-alpha secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 78.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 79.Hedrick SM. The acquired immune system: a vantage from beneath. Immunity. 2004;21:607–615. doi: 10.1016/j.immuni.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Hedrick SM. Immune system: not so superior. Science. 2009;325:1623–1624. doi: 10.1126/science.325_1623a. [DOI] [PMC free article] [PubMed] [Google Scholar]