Abstract
Objective
To compare the distribution of error types across subgroups of primary progressive aphasia and poststroke aphasia in different vascular locations.
Method
We analyzed naming errors in 49 individuals with acute left hemisphere ischemic stroke and 55 individuals with three variants of primary progressive aphasia. Location of atrophy or ischemic stroke was characterized using MRI.
Results
We found that distribution of error types was very similar across all subgroups, irrespective of the site or etiology of the lesion. The only significant difference across groups was the percentage of circumlocutions (F(7, 96) = 3.02, p = .005). Circumlocution errors were highest among logopenic variant PPA (24%) and semantic variant PPA (24%). Semantic coordinate errors were common in all groups, probably because they can arise from disruption of different cognitive processes underlying naming and, therefore, from different locations of brain damage.
Conclusions
Semantic errors are common among all types of primary progressive aphasia and poststroke aphasia, and the type of error depends in part on the location of damage.
Keywords: naming errors, stroke aphasia, primary progressive aphasia
Although impaired naming is among the most common deficits caused by stroke or neurodegenerative disease, the types of errors in naming vary widely. Distinct error types may reflect differences in the cognitive processes underlying naming that are impaired (Jefferies & Lambon Ralph, 2006; Kohn & Good-glass, 1985; Rohrer et al., 2008; Schwartz & Brecher, 2000), the location of neural dysfunction (Cloutman et al., 2009; DeLeon et al., 2007; Rohrer et al., 2008; Schwartz & Brecher, 2000), or even the nature of the disease (e.g., stroke vs. neurodegenerative disease).
Previous authors have reported that semantic errors made by patients with semantic dementia (now termed semantic variant primary progressive aphasia, PPA, or svPPA; Gorno-Tempini et al., submitted) and those made by patients with aphasia with multimodal semantic deficits after either prefrontal and/or parietal-temporal stroke are qualitatively different (Hodgson & Lambon Ralph, 2008; Jefferies & Lambon Ralph, 2006; Jefferies, Patterson, & Lambon Ralph, 2008; Rogers et al., 2004a; Rogers, Lambon Ralph, Hodges, & Patterson, 2004b). Jefferies and Lambon Ralph (2006) found that their patients with chronic poststroke aphasia more commonly produced associative semantic errors (e.g., cow named as “milk”) than patients with svPPA, even though both had multimodality semantic deficits. Jefferies and Lambon Ralph (2006) hypothesized that the semantic errors in their poststroke aphasic patients resulted from “deregulated semantic cognition” (p. 2144) – impaired executive processes that help direct and control semantic activation and rejection of strong irrelevant factors. In contrast, patients with aphasia due to svPPA predominately made coordinate errors (e.g., cow named as “horse”) or superordinate semantic errors (e.g., cow named as “animal”); Jefferies and Lambon Ralph proposed that these semantic errors were due to progressive degradation of amodal semantic representations. Patients with svPPA tend to initially make coordinate errors and progress to superordinate errors and then to nonresponses as semantic concepts further deteriorate (Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001; Rogers et al., 2004a).
We sought to further explore differences in error types between poststroke aphasia and primary progressive aphasia (PPA, including svPPA) by extending the study to patients with other locations of stroke and other variants of PPA. Like Jefferies and Lambon-Ralph (2006), we assumed that the nature of the semantic error depends on the site of tissue dysfunction. However, the differences could also reflect the nature of the disease (stroke vs. neurodegenerative disease). Distinct language profiles characterize different subtypes of PPA, which are associated with different areas of atrophy (Gorno-Tempini et al., 2004; Gorno-Tempini et al., submitted) and provide clues to the particular tissue pathology (Davies, Halliday, Xuereb, Kril, & Hodges, 2008; Hodges et al., 2004; Kertesz, McMonagle, Blair, Davidson, & Munoz, 2005; Snowden, Goulding, & Neary, 1989). Nonfluent variant PPA (nfvPPA, also called the PPA-agrammatic; Mesulam, 2008, or progressive nonfluent aphasia), characterized by nonfluent speech, agrammatism, and preserved single-word comprehension is associated with atrophy in regions of the left posterior frontal and insular cortex. Semantic variant PPA, characterized by fluent and well-articulated speech with little content and with pronounced comprehension deficits, is associated with bilateral (left greater than right) anterior and inferior temporal atrophy (Gorno-Tempini et al., 2004; Knibb, Xuereb, Patterson, & Hodges, 2006; Nestor, Fryer, & Hodges, 2006), an area that is rarely affected by stroke (Wise, 2003). Logopenic variant PPA (lvPPA, also called logopenic progressive aphasia) is characterized by frequent word-finding pauses and phonemic paraphasias in spontaneous speech and disproportionately impaired repetition. LvPPA is associated with left inferior parietal and superior temporal atrophy (see Table 1 for working criteria; see also Gorno-Tempini et al., 2008; Gorno-Tempini et al., submitted; Hillis, 2008; Josephs et al., 2008; Mesulam et al., 2008). These distinctive syndromes reflect overlapping neuropathologies, with tau being most common in nfvPPA, ubiquitin most common in svPPA, and amyloid plaques and neurofibrillary tangles (consistent with Alzheimer’s disease) most common in lvPPA (Davies et al., 2008; Gorno-Tempini et al., 2004; Hodges et al., 2004; Kertesz et al., 2005).
Table 1.
Criteria for Subtypes of Primary Progressive Aphasia (From Gorno-Tempini et al., Submitted)
| Clinical diagnosis of svPPA |
Both of the following core features must be present:
|
At least three of the following other diagnostic features must be present:
|
| Imaging-supported svPPA diagnosis |
Both of the following criteria must be present:
|
| Clinical diagnosis of nfvPPA |
At least one of the following core features must be present:
|
At least two of three of the following other features must be present:
|
| Imaging-supported nfvPPA diagnosis |
Both of the following criteria must be present:
|
| Clinical diagnosis of lvPPA |
Both of the following core features must be present:
|
At least three of the following other features must be present:
|
| Imaging-supported lvPPA diagnosis: |
Both criteria must be present:
|
Note. nfvPPA = Nonfluent variant PPA; svPPA = Semantic variant PPA; lvPPA = Logopenic variant PPA. Adapted with permission from “International Guidelines for the Diagnosis of Primary Progressive Aphasia and Its Variants,” by M. L. Gorno-Tempini, A. E. Hillis, S. Weintraub, et al., 2010, submitted for publication.
We tested our first hypothesis—that error types in object naming depend on location of tissue dysfunction rather than the nature of the disease—by comparing naming errors across these three subtypes of PPA and acute stroke in different vascular distributions to identify any associations between location of tissue damage/dysfunction and particular types of naming errors. We tested our second hypothesis—that semantic coordinate errors would be common in all types of PPA and poststroke aphasia—by examining the distributions of error types across groups. We evaluated stroke patients in the acute period (within 24 hours of onset of symptoms), before the opportunity for reorganization of structure/function relationships or rehabilitation that might result in adoption of specific strategies for naming.
We expected that error types would be similar in patients with nfvPPA and strokes involving the superior division of the left middle cerebral artery territory (hereafter, “anterior MCA”) because these both affect the posterior inferior frontal gyrus and anterior insula. Based on previous lesion studies and functional imaging studies, we would expect both groups of patients to make articulatory planning errors resulting in both phonetic (phoneme distortion) and phonemic (phoneme deletion, substitution, transposition, or insertion) errors of which they are aware (see Dronkers, 1996; Hillis et al., 2004) as well as errors selecting among competing alternatives (see Thompson-Schill, D’Esposito, Aguirre, & Farrah, 1997), such as semantic coordinate errors (e.g., horse named as cow).
We also expected that error profiles would be similar in patients with lvPPA and patients with strokes involving the inferior division of middle cerebral artery territory (hereafter, “posterior MCA”) stroke, because both frequently involve posterior superior temporal cortex and inferior parietal cortex. Based on previous lesion studies and functional imaging studies indicating a role of the posterior superior temporal region in lexical-semantics (e.g., Fridriksson, Fridriksson, & Morrow, 2005; Hart & Gordon, 1990; Hillis et al., 2001; Lesser et al., 1986), we would expect that semantic coordinate errors would also be common in this group, because a degraded lexical-semantic representation might activate all lexical representations (phonological or orthographic representations) to which it corresponds (see below). Whether the correct or incorrect lexical representation is selected might depend on how recently or frequently it has been accessed, resulting in an inconsistency in the accuracy of the response. However, because of a role of the inferior parietal cortex in phonological working memory (Marshuetz, Reuter-Lorenz, Smith, Jonides, & Noll, 2006; Scott, in press), we might expect that these patients would also make phonological errors (phonemic paraphasias). The inferior parietal areas affected in lvPPA and posterior MCA stroke are also important in executive function (and perhaps not in lexical-semantics per se, but in control of semantic memory search), which would also predict semantic coordinate and associative semantic errors when there is tissue dysfunction in this area (Jefferies & Lambon-Ralph, 2006).
We expected that errors in svPPA would be qualitatively distinct because svPPA is associated with bilateral (left greater than right) anterior and inferior temporal atrophy, which does not occur in single strokes. Because these regions bilaterally are thought to be important in amodal representations of concepts as well as visual recognition of objects (Martin, 2007; Wierenga et al., 2009), we expected a relatively high proportion of superordinate semantic errors (resulting from severely degraded concepts) and visual or unrelated errors (resulting from impaired visual recognition of the stimulus as something familiar), in addition to semantic coordinate errors (resulting from degraded concepts).
We hypothesized that semantic coordinate errors would be common in all groups of PPA and poststroke aphasia because several studies have also shown that semantic coordinate errors can result from damage to different components of the naming process (Caramazza & Hillis, 1990; Hillis & Caramazza, 1995a) as well as to different locations of the brain (Cloutman et al., 2009). Previous studies have provided evidence that naming is a complex process that requires a number of cognitive processes that can be selectively disrupted by brain damage (DeLeon et al., 2007; Good-glass & Wingfield, 1997). Semantic coordinate errors might arise if (a) the amodal concept is degraded (as proposed in semantic variant PPA; Jefferies & Lambon-Ralph, 2006); (b) the subset of features in this concept that define the name (“lexical-semantic representation”) is degraded (as proposed in some cases of post-stroke aphasia; see Caramazza & Hillis, 1990); (c) there is impaired access to a subset of semantic features from vision; or (d) there is impaired access to phonological or orthographic lexical representations.
Semantic errors might arise from damage to any of these levels because of the assumed distributed nature of amodal semantic (conceptual) representations and lexical-semantic representations and because of the assumed parallel activation of lexical representations. That is, we assume that normally every feature of an amodal semantic representation (including each of the subset of these features that define a particular word; i.e., the lexical-semantic representation) activates in parallel every lexical representation to which it corresponds. If there is an underspecified amodal semantic or lexical-semantic representation, the spared features will still activate all lexical representations to which they correspond. That is, features <mammal>, <hooves>, <gallops> in the degraded concept (or degraded lexical-semantic representation) of horse would activate both horse and deer; if the feature <mane> were unavailable, the patient might name a picture of a horse as “deer.” This same pattern of performance can be seen if there is impaired access to certain features specifically from vision, as seen in some cases of poststroke aphasia (Hillis & Caramazza, 1995b), following the same logic. However, these errors can also occur as a result of impairment at the level of accessing orthographic or phonological lexical representations, as proposed for some cases of PPA or poststroke aphasia (Caramazza & Hillis, 1990). In these cases, if the phonological representation of “horse” is not available, the features that comprise the intact concept (including lexical-semantic representation) of horse will still activate all of the lexical representations to which they correspond (e.g., <hooves> will activate phonological representations of both horse and deer; <mane> will activate phonological representations of horse, zebra, and lion). Therefore, if the target lexical representation is not accessible, or if the patient has trouble selecting among competing alternative responses (a deficit in executive control), a semantic coordinate error might be produced instead. Therefore, semantic coordinate errors might result from disruption of many different components of the naming process, due to either stroke or PPA.
In short, here we tested the hypotheses that (a) the distribution of error types depends more on location than on the nature of the aphasia (neurodegenerative disease vs. acute ischemic stroke), and (b) semantic coordinate errors are common in all subtypes of PPA and poststroke aphasia.
Method
Participants
We studied a consecutive series of 110 right-handed individuals who were evaluated at the Johns Hopkins Hospital for aphasia due to acute stroke or who were diagnosed in the cognitive neurology outpatient clinic with primary progressive aphasia (svPPA, nfvPPA, or lvPPA). Within 24 hours of onset of symptoms, 50 participants with aphasia due to acute, left hemisphere ischemic stroke were evaluated and imaged. Participants with primary progressive aphasias included 60 outpatients who were selected for this study based on (a) presenting symptoms of isolated language disturbance for at least two years and before other dementia symptoms; (b) disease duration of at least three years; (c) language/speech deficits that were still the main cause of functional deficits; and (d) meeting criteria for the root diagnosis of PPA, as well as criteria for one of the variants svPPA, nfvPPA, or lvPPA (see Table 1; Gorno-Tempini et al., submitted).
Exclusion criteria included (a) education level below 10th grade; (b) premorbid neurological condition other than stroke or primary progressive aphasia; (c) uncorrected hearing or visual loss; (d) lack of proficiency in English prior to onset of stroke or progressive aphasia; (e) diminished level of consciousness; or (f) unable to either give consent or indicate a family member give consent. The following procedures were approved by the Johns Hopkins Institutional Review Board.
Patient Classification
Stroke patients underwent MRI, including diffusion weighted imaging (DWI) to reveal infarct or dense ischemia, perfusion-weighted imaging (PWI) to reveal areas of hypoperfusion corresponding to tissue dysfunction, Fluid Attenuated Inversion Recovery (FLAIR) to rule out old infarcts, and T2*-weighted gradient-echo to rule out hemorrhage, within 24 hours of language testing and within 48 hours of stroke onset. Hypoperfusion was defined as >4 sec delay in time to peak (TTP) arrival of contrast, relative to the homologous regions in the right hemisphere. Two board-certified neurologists (AH and RG) classified each infarct or area of hypoperfusion as (a) superior division middle cerebral artery (MCA), hereafter “anterior MCA” stroke; (b) inferior division MCA, hereafter “posterior MCA” stroke; (c) mixed anterior and posterior MCA stroke; (d) anterior cerebral artery stroke; (e) posterior cerebral artery stroke; or (f) purely subcortical stroke. Some patients with purely subcortical infarct showed hypoperfusion of a small area of cortex but not involving the entire territory of the inferior or superior division of the left MCA. There was 96% point-to-point agreement in classification (agreed upon cases minus disagreed upon cases divided by total cases, Times 100; i.e., 49 – 1/50 × 100). In the case of disagreement, additional review led to 100% agreement. There was only one patient with ACA stroke, so that patient’s data were not compared to the other groups.
Patients with PPA were categorized using clinical criteria for variants of Primary Progressive Aphasia, listed in Table 1, along with characteristic pattern of atrophy from the MRI scans (imaging-support variants of PPA). Patients were classified as (a) nfvPPA; (b) svPPA; or (c) lvPPA on the basis of neurological examination, neuropsychological battery, complete language testing (e.g., Western Aphasia Battery; Kertesz et al., 2005, and supplemental tests), and MRI scans showing the characteristic pattern of atrophy (anterior perisylvian cortex for nfvPPA, posterior perisylvian cortex for lvPPA, and anterior/inferior temporal cortex for svPPA). Any patients whose pattern of atrophy did not match the clinical criteria were to be excluded. However, no patient needed to be excluded on this basis. Five PPA patients were not included because they did not meet criteria for any subtype (e.g., they had only anomia and/or dysgraphia), leaving 55 total participants with PPA for subsequent analyses.
Language Testing
Picture naming consisted of 30 black and white drawings of objects (Berndt, Mitchum, Haendiges, & Sandson, 1997). Each patient was asked, “What is the name of this?” Oral responses were recorded and scored as correct if they were phonemically correct productions of the target word, with allowances for phonemes to be slightly distorted or stiff (due to increased tone/rigidity of the muscles of articulation) if they were recognized as the target phoneme. That is, all the phonemes had to be present and in the correct order but might be imprecise due to dysarthria. Articulatory planning errors that resulted in deletion, insertion, transposition, or substitution of phonemes (e.g., due to apraxia of speech), or similar errors without articulatory planning deficits (phonemic paraphasias) were scored as phonemic/phonological errors. These were combined because they are difficult to distinguish in naming tasks. The first response was coded with the most appropriate classification of error type (see Table 2). Two scorers, blind to the patient diagnosis, independently coded each patient’s response. Point-point percent agreement between these two coders for 3,300 scored items was 95% for the total 110 initial participants. Questionable response codes were discussed with the senior author and a consensus determined the final coding classification.
Table 2.
Error Classification System (Modified From Hodges, Salmon, & Butters, 1991)
|
Note. Adapted with permission from “The Nature of the Naming Deficit in Alzheimer’s and Huntington’s Disease,” by J. R. Hodges, D. P. Salmon, and N. Butters, 1991, Brain, 114, 1547–1558.
Data Analysis
Before conducting the main analyses, data from naming tests and subject data were tested for normality, linearity, and the presence of outliers (Bradley, 1982; Rosner, 2000). Additionally, identification of potential confounding variables (potential group differences on age, education, and naming error rate) was undertaken by conducting a series of ANOVAs. No significant differences were found for these variables. Bonferroni correction for multiple comparisons was applied. An alpha level of p < .05 after correction (e.g., p < .006 for 9 comparisons) was used to identify significant differences between groups.
We compared the distribution of error types across groups. To test hypothesis 1, we first grouped patients with a given etiology (different types of progressive aphasia vs. different types of stroke) to compare PPA versus stroke. We then grouped patients by dysfunction in a particular location (e.g., anterior MCA stroke plus nfvPPA) to determine if there were significant differences across locations (e.g., anterior perisylvian vs. posterior perisylvian) using analysis of variance (ANOVA). To test hypothesis 2, ANOVA was used to determine if the proportion of coordinate semantic errors was different across all eight subgroups (three subgroups of PPA and five subgroups of stroke). If a significant difference were found, we planned to carry out post hoc comparisons between each pair of subgroups to identify which subgroups differed in the rate of coordinate semantic errors. We also carried out ANOVAs for each of the other error types to identify any statistically significant differences between the eight subgroups. We did not carry out this analysis for any error type that represented <5% of errors in all subgroups of PPA and stroke; this was the case for mixed errors.
Results
In the subgroup of anterior cerebral artery stroke, there was only one case. This patient made no errors and so further analyses excluded this subgroup. Results are reported for the remaining 104 patients. ANOVA revealed no significant group differences for age (F7, 96 = 2.28, ns), education (F7, 96 = 2.22, ns), or total errors (F7, 96 = 1.84, ns; see Table 3 for mean total error rates for each subgroup).
Table 3.
Patient Demographics and Mean Total Error Rate for Each Subgroup (N = 104)
| Groups | Age, Mean (SD; Range) | Sex | Education (Years), Mean (SD) | Total errors, Mean (SD) |
|---|---|---|---|---|
| lvPPA (n = 13) | 69.0 (8.2; 60–85) | Males = 8, Females = 5 | 15.8 (2.9) | 10.2 (7.9) |
| dfvPPA (n = 28) | 65.3 (10.4; 40–82) | Males = 11, Females = 17 | 14.4 (2.0) | 7.1 (8.1) |
| svPPA (n = 14) | 67.6 (7.6; 51–78) | Males = 4, Females = 10 | 15.7 (3.1) | 14.3 (8.9) |
| Anterior MCA (n = 8) | 55.5 (22.0; 19–79) | Males = 3, Females = 5 | 15.0 (3.9) | 5.5 (8.8) |
| Posterior MCA (n = 11) | 63.1 (13.6; 41–83) | Males = 6, Females = 5 | 14.3 (3.4) | 11.8 (10.3) |
| Mixed MCA (n = 13) | 60.1 (11.5; 45–80) | Males = 3, Females = 10 | 14.9 (4.1) | 9.7 (9.1) |
| Subcortical (n = 12) | 55.3 (16.8; 22–80) | Males = 8, Females = 4 | 11.7 (2.6) | 4.5 (5.1) |
| Posterior cerebral artery stroke (n = 5) | 62.0 (19.6; 36–81) | Males = 4, Females = 1 | 15.0 (2.0) | 8.4 (8.0) |
Note. lvPPA = Logopenic variant PPA; dfvPPA = Nonfluent variant PPA; svPPA = Semantic variant PPA; MCA = Middle Cerebral Artery.
Hypothesis 1: The distribution of error types depends more on location than etiology of lesion
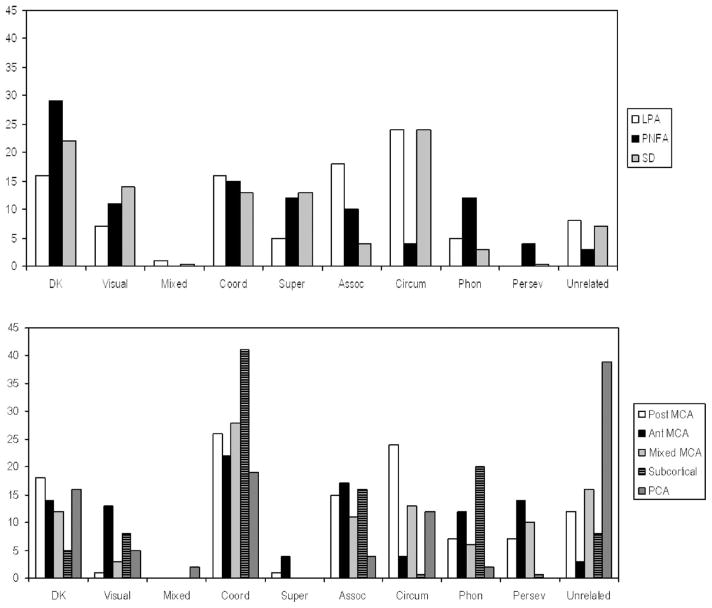
Figure 1 shows the distribution of error types (as a mean percentage of total errors) for each subgroup. There were no striking distinctions between subgroups that were visually apparent. When subgroups were collapsed across locations to compare etiologies (stroke vs. PPA), there were no significant differences across etiologies for any of the error types using ANOVAs after correcting for multiple comparisons (for the nine errors types). When subgroups were collapsed across etiologies to compare error distributions across locations of brain damage/dysfunction (anterior MCA stroke/nfvPPA, posterior MCA stroke/lvPPA, subcortical, svPPA), there was a significant difference only for circumlocutions (F3, 103 =4.55; p = .005), with the smallest proportions of circumlocutions in the anterior MCA stroke/nfvPPA group. There were too few patients in the other stroke locations to include in this analysis. Therefore, there was a difference across locations of brain dysfunction but not across etiology.
Figure 1.
Mean percentage of error types for each subgroup as a function of total errors by that subgroup. Top panel show PPA subtypes. Lower panel show Stroke subtypes. DK = “don’t know” or “nonresponse”; includes vague descriptions; Visual = visual errors; MIX = mixed visual/semantic; COOR = coordinate semantic errors; SUPER = superordinate semantic errors; ASSOC = associative semantic errors; CIRCUM = circumlocutions; PHON = phonemic/phonological errors; PERSEV = perseverative errors.
Hypothesis 2: Semantic coordinate errors are common errors among all subgroups
Semantic coordinate errors were a prominent error type in all subgroups, comprising a mean of 13–41% of total errors for all subgroups. Semantic coordinate errors were the highest proportion of errors in the subcortical stroke subgroup (41% of errors, but these patients made very few errors) and lowest in the progressive aphasia subgroups (13–16% of errors). However, there was not a significant difference between the groups in the rate of semantic coordinate errors, even without any correction factor (F7, 96 =1.105; p = .389).
In fact, the only significant difference among the eight subgroups after Bonferroni correction for nine comparisons (nine error types) was in percentage of circumlocutions (F7, 96 =3.02, p = .005). Circumlocution errors were highest among lvPPA (24%) and svPPA (24%). See Figure 1 for results of all groups. The proportion of associative semantic errors was highest in the lvPPA patients (18% of errors). Phonemic/phonological errors were a high proportion of errors for nfvPPA (12%), anterior MCA stroke (12%) and subcortical stroke (20%). Perseverative errors were typically present only in the three subgroups of MCA stroke (7–14% vs. <5% in all others). Unrelated errors were common after PCA stroke (39% of errors). Visual errors were highest in those with SVPPA (14% of errors).
The main differences across progressive aphasia subtypes were that lvPPA and svPPA patients made more circumlocutions (24% in each) than nfvPPA patients (4%), while nfvPPA patients made more phonemic/phonological errors (12% vs. <5%). The main differences across subgroups of acute stroke were that phonemic/phonological errors were relatively common after anterior MCA (12%) and subcortical stroke (20%) only.
Discussion
We hypothesized that the distribution of naming error types depended more on the subtype of progressive aphasia (and associated location of tissue dysfunction) and location of stroke than the nature of the disease (progressive aphasia vs. stroke). However, we found there was substantial overlap across locations and etiologies, with no marked differences in the distribution of error types by either location or etiology. The “location” of brain damage/dysfunction was, however, very coarsely defined for both groups. We expected similar distributions of error types in lvPPA and left posterior MCA stroke, which both affect left superior temporal cortex and inferior parietal cortex. This area has been implicated in lexical retrieval, auditory comprehension, and working memory, which are impaired in both groups of patients (Hillis et al., 2006; Leff et al., 2002; Wise et al., 2001). We did find this similarity, but their error types were also similar to those of svPPA patients, whose dysfunction is thought to be predominantly anterior temporal, and patients with other stroke locations. We also expected similar distributions of error types in patients with nfvPPA and patients with anterior MCA stroke, which both affect posterior inferior frontal cortex and anterior insula. These areas have been implicated in motor programming and planning of speech articulation (Broca, 1866; Dronkers, 1996; Hillis et al., 2004; Mohr et al., 1978) as well as grammatical sentence production (Grodzinsky, 2000), such that damage or dysfunction in these areas is associated with apraxia of speech and agrammatism (hallmarks of both anterior MCA stroke, Alexander & Hillis, 2008, and nfvPPA, Gorno-Tempini et al., 2004). Again, we found such a similarity, but the patterns of errors were also similar to other subgroups. Finally, we hypothesized that the distribution of errors in svPPA would be rather distinct because unilateral stroke does not affect the areas that are generally damaged in svPPA. In fact, these patients made more visual errors and superordinate errors than the other patients, consistent with the assumption that they have degraded amodal concepts of objects. However, their error types were otherwise very similar to those of other subgroups of PPA and stroke.
The only significant difference across subgroups was in the percentage of errors that were circumlocutions, which was highest among participants with svPPA and those with lvPPA. Because all of the PPA patients were chronic and all of the stroke patients were acute, this difference could well be due to the chronicity, rather than the nature or location, of the brain dysfunction. For example, circumlocutions might reflect chronic compensation for impaired naming. Consistent with this account, these errors are common in chronic anomic aphasia but were not commonly observed in acute stroke.
Our second hypothesis, that coordinate semantic errors would be common in all subgroups, with dysfunction in different locations, was confirmed. This finding is consistent with previous studies showing that semantic coordinate errors can result from damage to several different cognitive components of the naming system (Caramazza & Hillis, 1990, Cloutman et al., 2009; Hillis & Caramazza, 2005a) as well as the reading system (Morton & Patterson, 1980). For example, they might occur, as argued by Jefferies and Lambon Ralph (2006), from damage to an amodal conceptual system with degraded distinctions between related items in svPPA. Patients with svPPA fail to appreciate distinctions between related items, such that they inappropriately use items (e.g., use a knife to eat soup). Patients with poststroke aphasia rarely make such confusions in using objects, but many patients with posterior MCA stroke do point to the wrong item in response to a word (Cloutman et al., 2009). Pointing to a knife when asked to point to a spoon might reveal degradation of lexical-semantic representations (the subset of the conceptual representation that defines the word), while other aspects of the concepts of knife and spoons are intact. Other patients with acute poststroke aphasia also make coordinate semantic errors in naming but do not point to the wrong picture in response to words. In these cases, an intact lexical-semantic representation might activate several related lexical phonological representations (as normally occurs), but when the target is unavailable due to a lexical access deficit (or cannot be selected from competing alternatives due to an executive deficit) another lexical phonological representation, partially activated by the intact lexical-semantic representation, might be accessed instead (see Hillis & Caramazza, 1995a, for discussion). Thus, coordinate semantic errors might reflect at least four distinct deficits in the complex processes and representations underlying naming (degraded amodal semantic representation, degraded lexical-semantic representations, impaired lexical access, or executive deficit in selecting among competing lexical representations).
Jefferies and Lambon Ralph (2006) found that in a subset of chronic stroke patients with multimodal semantic impairment, associative semantic errors were more common than in svPPA. We also found that associative errors were more common in some types of poststroke aphasia (anterior and posterior MCA) than in semantic svPPA but not more common in PCA stroke than svPPA. Associative semantic errors were more common in lvPPA than any other group. Furthermore, they were more common in lvPPA and nfvPPA than in svPPA. One explanation for this difference in our findings and those of previous authors is that they studied only patients with multimodality semantic deficits, whereas we studied patients with a variety of underlying impairments as the cause of their naming errors. Another possible explanation is that we studied patients acutely after stroke, whereas Jefferies and Lambon-Ralph studied patients in the chronic stage of stroke. Associative semantic errors might reflect chronic compensation for naming deficits in patients with relatively intact semantics (as in lvPPA, nfvPPA, and chronic poststroke aphasia), irrespective of the cause of the naming deficit. Computational models of language (e.g., reading) indicate that patterns of errors change over time with experience and “plasticity” (Welbourne & Lambon Ralph, 2007). Another explanation, consistent with the hypotheses of Jefferies and Lambon Ralph, is that associative semantic errors result from executive dysfunction, with poor executive control over semantic processes. This sort of deficit might occur only in patients with left posterior frontal or inferior parietal dysfunction when there is no superimposed lexical-semantic impairment (i.e., nfvPPA, lvPPA, and chronic post-MCA stroke aphasia). In acute left MCA stroke, a lexical-semantic impairment might result in coordinate semantic errors rather than associative semantic errors. Such a lexical-semantic impairment might resolve over time after stroke, leaving just an executive deficit in chronic stroke.
Limitations of this study include the small numbers of patients in some of the acute stroke groups, which limited our power to identify significant differences and significant associations. Second, the lesion location was identified only grossly (by vascular distribution) in the acute stroke patients, and the area of atrophy in progressive aphasia was identified by gross classification (posterior frontal/insula; anterior and inferior temporal; superior temporal/inferior parietal or other) in patients with progressive aphasia. Etiology was also confounded with chronicity; PPA patients were chronic, while stroke patients were acute. Additionally, we did not attempt to match the groups for severity of aphasia or volume of dysfunctional tissue. One can imagine that most patients make semantic errors because they are easy errors to make, even with mild levels of word-finding difficulty. However, as errors rates increase, semantically more distant errors, such as unrelated errors, have greater opportunity to occur. However, unrelated errors were observed most commonly in patients with PCA stroke, who made fewer total errors than patients with lvPPA, svPPA, posterior MCA stroke, or mixed MCA stroke.
In summary, the profile of naming errors in aphasia likely reflects a complex interaction between site of neural dysfunction, time since onset of brain injury, reorganization of structure/function relationships (in patients with chronic damage), and the cognitive process or representation underlying naming that is impaired. The cognitive processes and representations include, at least, the amodal conceptual/semantic representation (impaired only in svPPA), the subset of features within the conceptual representation that define the word (the lexical-semantic representation, impaired in some cases of acute posterior MCA stroke), access to the phonological representation from an intact lexical-semantic representation (impaired in lvPPA and some cases of acute poststroke aphasia), articulatory planning and programming involved in word production (impaired in nfvPPA and anterior MCA stroke), and executive control. These findings are consistent with recent views that language tasks, such as naming, require a distributed network of neural regions in the left frontal, temporal, and parietal cortex (Mesulam, 2008). Identifying the source of semantic errors in naming requires evaluating performance across various tasks and over time, as well as evaluating the characteristics and distribution of naming error types.
Acknowledgments
The research reported in this paper was supported by NIH (NIDCD), through R01 DC 05375. We gratefully acknowledge this support and the participation of the patients. Additional thanks is given to Patricia Rogers, PhD, for assisting with the coding the participants’ responses, and to Matt Lambon Ralph and four anonymous reviewers for their helpful comments on an earlier version.
Contributor Information
Maggi A. Budd, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine
Kathleen Kortte, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine.
Lauren Cloutman, Department of Neurology, Johns Hopkins University School of Medicine.
Melissa Newhart, Department of Neurology, Johns Hopkins University School of Medicine.
Rebecca F. Gottesman, Department of Neurology, Johns Hopkins University School of Medicine
Cameron Davis, Department of Neurology, Johns Hopkins University School of Medicine.
Jennifer Heidler-Gary, Department of Neurology, Johns Hopkins University School of Medicine.
Margaret W. Seay, Department of Neurology, Johns Hopkins University School of Medicine
Argye E. Hillis, Departments of Physical Medicine and Rehabilitation, Neurology, and Cognitive Science, Johns Hopkins University School of Medicine
References
- Alexander M, Hillis AE. Aphasia. In: Miller B, Goldenberg G, Aminoff M, Boller F, Swaab D, editors. Neuropsychology and behavior: A volume to handbook of clinical neurology series. 3. Amsterdam, The Netherlands: Elsevier; 2008. [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN, Sandson J. Verb retrieval in aphasia. Brain and Language. 1997;56:68–106. doi: 10.1006/brln.1997.1727. [DOI] [PubMed] [Google Scholar]
- Bradley JV. The insidious L-shaped distribution. Bulletin of the Psychonomic Society. 1982;20:85–88. [Google Scholar]
- Broca P. Sur la faculté générale du langage, dans ses rapports avec la faculté du langage articulé. Bulletin de la Société d“Anthropologie deuxième série, tome I. 1866:377–382. [Google Scholar]
- Caramazza A, Hillis AE. Where do semantic errors come from? Cortex. 1990;26:95–122. doi: 10.1016/s0010-9452(13)80077-9. [DOI] [PubMed] [Google Scholar]
- Cloutman L, Gottesmann R, Chaudhry P, Davis C, Kleinman JT, Pawlak M, Hillis AE. Where (in the brain) do semantic errors come from? Cortex. 2009;45(5):641–649. doi: 10.1016/j.cortex.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RR, Halliday GM, Xuereb JH, Kril JJ, Hodges JR. The neural basis of semantic memory: Evidence from semantic dementia. Neurobiology of Aging. 2008;16(16):1741–1754. doi: 10.1016/j.neurobiolaging.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Deleon J, Gottesman R, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Hillis AE. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130(5):1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Dronkers NE. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Fridriksson J, Morrow L. Cortical activation and language task difficulty in aphasia. Aphasiology. 2005;19:239–250. doi: 10.1080/02687030444000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Wingfield A. Word-finding deficits in aphasia: Brain-behavior relations and symptomatology. In: Goodglass H, editor. Anomia. London, England: Academic Press; 1997. pp. 3–27. [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa S, Ogar J, Grossman M. International guidelines for the diagnosis of primary progressive aphasia and its variants. 2010. Manuscript submitted for publication. [Google Scholar]
- Grodzinsky Y. The neurology of syntax: Language use without Broca’s area. Behavioral and Brain Sciences. 2000;23:1–21. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Annals of Neurology. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Lost for words. Neurology. 2008;71:1218–1219. doi: 10.1212/01.wnl.0000327100.41096.b3. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. The compositionality of lexical-semantic representations: Clues from semantic errors in object naming. Memory. 1995a;3:333–358. doi: 10.1080/09658219508253156. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Cognitive and neural mechanisms underlying visual and semantic processing. Journal of Cognitive Neuroscience. 1995b;7:457–478. doi: 10.1162/jocn.1995.7.4.457. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kleinman KT, Newhart M, Heidler-Gary J, Gottesman R, Barker PB, Chaudry P. Restoring cerebral blood flow reveals neural regions critical for naming. Journal of Neuroscience. 2006;26:8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Annals of Neurology. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH. Clinicopathological correlates in frontotemporal dementia. Annals of Neurology. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. The nature of the naming deficit in Alzheimer’s and Huntington’s disease. Brain. 1991;114:1547–1558. doi: 10.1093/brain/114.4.1547. [DOI] [PubMed] [Google Scholar]
- Hodgson, Lambon Ralph MA. Mimicking aphasic semantic errors in normal speech production: Evidence from a novel experimental paradigm. Brain and Language. 2008;104:89–101. doi: 10.1016/j.bandl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46:649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, Petersen RC. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain: A Journal of Neurology. 2005;128(9):1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Annals of Neurology. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Kohn SE, Goodglass H. Picture-naming in aphasia. Brain and Language. 1985;24:266–283. doi: 10.1016/0093-934x(85)90135-x. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Leff A, Crinion J, Scott S, Turkheimer F, Howard D, Wise R. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Annals of Neurology. 2002;51:553–558. doi: 10.1002/ana.10181. [DOI] [PubMed] [Google Scholar]
- Lesser R, Luders M, Dinner N, Dinner DS, Klem G, Hahn J, Gurd AR. Electrical stimulation of Wernicke’s area interferes with comprehension. Neurology. 1986;36:658–663. doi: 10.1212/wnl.36.5.658. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Reuter-Lorenz PA, Smith EE, Jonides J, Noll DC. Working memory for order and the parietal cortex: An event-related functional magnetic resonance imaging study. Neuroscience. 2006;139(1):311–316. doi: 10.1016/j.neuroscience.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Annals of Neurology. 2008;68:367–378. doi: 10.1002/ana.21534. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr J, Pessin M, Finkelstein S, Funkenstein H, Duncan G, Davis K. Broca Aphasia: Pathological and clinical. Neurology. 1978;28:311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Morton J, Patterson K. A new attempt at an interpretation, or, an attempt at a new interpretation. In: Coltheart M, Patterson K, Marshall JC, editors. Deep dyslexia. London, England: Routledge and Kegan Paul; 1980. pp. 91–118. [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer’s disease and semantic dementia. Neuroimage. 2006;30:1010–1020. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. The structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review. 2004a;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Hodges JR, Patterson K. Natural selection: The impact of semantic impairment on lexical and object decision. Cognitive Neuropsychology. 2004b;21:331–352. doi: 10.1080/02643290342000366. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Knight WD, Warren JE, Fox NC, Rossor MN, Warren JD. Word-finding difficulty: A clinical analysis of the progressive aphasias. Brain. 2008;131:8–38. doi: 10.1093/brain/awm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of biostatistics. 5. Pacific Grove, CA: Duxbury Publishers; 2000. [Google Scholar]
- Schwartz MF, Brecher A. A model-driven analysis of severity, response characteristics, and partial recovery in aphasics’ picture naming. Brain and Language. 2000;73:62–91. doi: 10.1006/brln.2000.2310. [DOI] [PubMed] [Google Scholar]
- Scott SK. The neurobiology of speech perception. In: Cutler A, editor. Twenty-first century psycholinguistics: Four cornerstones. Mahwah, NJ: Erlbaum; (in press) [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioral Neurology. 1989;2:167–182. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farrah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences, USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbourne SR, Lambon Ralph MA. Using parallel distributed processing models to simulate phonological dyslexia: The key role of plasticity-related recovery. Journal of Cognitive Neuroscience. 2007;19:1125–1139. doi: 10.1162/jocn.2007.19.7.1125. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Perlstein WM, Benjamin M, Leonard CM, Rothi LG, Conway T, Crosson B. Neural substrates of object identification: Functional magnetic resonance imaging evidence that category and visual attribute contribute to semantic knowledge. Journal of the International Neuropsychological Society. 2009;15:169–181. doi: 10.1017/S1355617709090468. [DOI] [PubMed] [Google Scholar]
- Wise RJS. Language systems in normal and aphasic human subjects: Functional imaging studies and inferences form animal studies. British Medical Bulletin. 2003;65:96–119. doi: 10.1093/bmb/65.1.95. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank C, Mummery CJ, Murphy K, Warbuton EA. Separate neural subsystems within “Wernicke’s area. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]